Abstract
In the peripheral nerves, injury-induced cytokines and growth factors perform critical functions in the activation of both the MEK/ERK and JAK/STAT3 pathways. In this study, we determined that nerve injury-induced ERK activation was temporally correlated with STAT3 phosphorylation at the serine 727 residue. In cultured Schwann cells, we noted that ERK activation is required for the serine phosphorylation of STAT3 by neuropoietic cytokine interleukin-6 (IL-6). Serine phosphorylated STAT3 by IL-6 was transported into Schwann cell nuclei, thereby indicating that ERK may regulate the transcriptional activity of STAT3 via the induction of serine phosphorylation of STAT3. Neuregulin-1 (NRG) also induced the serine phosphorylation of STAT3 in an ERK-dependent fashion. In contrast with the IL-6 response, serine phosphorylated STAT3 induced by NRG was not detected in the nucleus, thus indicating the non-nuclear function of serine phosphorylated STAT3 in response to NRG. Finally, we determined that the inhibition of ERK prevented injury-induced serine phosphorylation of STAT3 in an ex-vivo explants culture of the sciatic nerves. Collectively, the results of this study show that ERK may be an upstream kinase for the serine phosphorylation of STAT3 induced by multiple stimuli in Schwann cells after peripheral nerve injury.
Keywords: STAT3, ERK, Schwann cells, Interleukin-6, Neuregulins-1, Nerve injury
INTRODUCTION
In the peripheral nervous system, the differentiation of Schwann cells (SCs) during postnatal development involves the loss of immature phenotypes and axonal myelination (Jessen and Mirsky, 2005). In the peripheral nerves of adult animals, myelinating SCs are a type of regenerative cells, which undergo a dramatic alteration after nerve injury (Jessen and Mirsky, 2008). They lose their myelinating phenotypes via downregulation of myelin gene expression and acquire the properties of immature SCs, which can be identified by the upregulation of several proteins including glial fibrillary acidic protein and the p75 neurotrophin receptor. This dedifferentiation of SCs in response to nerve damage is a crucial component of Wallerian degeneration, and is an essential reaction of SCs to myelin removal and axonal regeneration (Fu and Gordon, 1997). Although a great many factors are capable of inducing the phenotype changes of SCs in vitro, the intracellular signaling associated with SC dedifferentiation has yet to be thoroughly elucidated. It was reported recently that the Ras/extracellular signal-regulated kinase (ERK) pathway might drive the dedifferentiation of SCs in vitro (Harrisingh et al., 2004). A forceful activation of ERK led to the downregulation of markers of myelinating SCs, including as P0 and periaxin. ERK activation in the injured nerves in vivo further supports the notion that ERK signaling plays an important role in the phenotype changes occurring in myelinating SCs after peripheral nerve injury (Sheu et al., 2000; Harrisingh et al., 2004).
Signal transducer and activator of transcription 3 (STAT3) is a critical regulator of gene expression in response to a variety of cytokines (Battle and Frank, 2002). The exposure of cells to interleukin-6 (IL-6) or ciliary neurotrophic factors results in STAT3 activation via STAT3 phosphorylation at the tyrosine 705 and serine 727 residues (Kamimura et al., 2003). Phosphorylated STAT3 dimerizes and translocates to the nucleus, where it regulates the transcription of several apoptosis- and cell-cycle-associated genes (Reich and Liu, 2006). The tyrosine phosphorylation of STAT3 has been shown to be increased in the peripheral nerves following injury, and the activation of STAT3 in the dorsal root ganglion neurons performs a significant function in regenerative axonal growth (Cafferty et al., 2001; Lee et al., 2004; Qui et al., 2005). STAT3 activation has also been detected in SCs following injury (Lee et al., 2004), and we recently reported that STAT3 activation in SCs might be involved in the induction of a marker for dedifferentiated SCs, glial fibrillary acidic protein (Lee et al., 2009).
Many factors can activate the Ras/ERK pathway in SCs after nerve injury. For example, neuregulins (NRG) may activate ERK for demyelination (Harrisingh et al., 2004). We recently reported that IL-6 activated ERK in SC-derived RT4 cells and primary SCs (Lee et al., 2009), even though the role of IL-6-induced ERK activation currently remains unknown. In this study, we placed our focus on the possible role of the Ras/ERK pathway in STAT3 phosphorylation in primary SCs and RT4 cells (Hai et al., 2002), because it has already been established that the Ras/ERK pathway is either positively or negatively involved in STAT3 activation. We determined that ERK is an upstream kinase for the serine 727 phosphorylation of STAT3 in SCs in vitro and in vivo.
METHODS
Chemicals
Media and sera for cell cultures were obtained from Life Technologies (Grand Island, NY). All of the phospho-specific antibodies and recombinant IL-6 utilized herein were purchased from Cell Signaling Technology (Beverly, MA). STAT3, ERK and beta-tubulin antibodies were acquired from Santa Cruz Biotechnology (Santa Cruz, CA). An antibody against S100 was purchased from Chemicon (Temecula, CA). Electrophoresis and Western blot reagents were obtained from BioRad (Richmond, VA). Neuregulin-1 (NRG) was purchased from R&D systems (Minneapolis, MN). U0126 and PD98059 were purchased from Calbiochem (San Diego, CA), and all other reagents were acquired from Sigma (St. Louis, MO).
Sciatic nerve injury and immunohistochemistry
In accordance with the protocols approved by the Dong-A University Committee on animal research that followed the guide of animal experiments established by The Korean Academy of Medical Sciences, adult male Sprague-Dawley rats (150~300 gm) were anesthetized with an intraperitoneal injection of 10% ketamine hydrochloride (Sanofi-Ceva, Düsseldorf, Germany; 0.1 ml/100 g body weight) and 0.1 ml of Rompun (Bayer, Leverkusen, Germany). For sciatic nerve lesions, the right sciatic nerve was exposed at the mid-thigh level and was crushed twice in succession using a fine forceps. After recovery for the indicated time, the rats were sacrificed with a high dose of a mixture of 10% ketamine and Rompun, and then the control and crushed nerves were removed and frozen with dryice. Frozen nerve sections (12 µm) were prepared using a cryostat (Leica CM3050), air-dried, and fixed for 20 min with methanol at 4℃. The immunostaining of phosphorylated STAT3 was conducted in accordance with the previously described protocols (Lee et al., 2009). In brief, the sections were blocked with 4% goat serum in phosphate-buffered saline (PBS) containing 0.3% Triton X-100 for 15 min at room temperature, and then incubated overnight with a rabbit anti-phospho-STAT3 antibody (1:200) in PBS containing 0.2% Triton X-100 at 4℃ followed by 3 washings with PBS. Next, the sections were incubated for 2 h with Alexa 488-conjugated anti-rabbit IgG (1:800, Jackson Immunoresearch Laboratories) at room temperature, and visualized with a laser confocal microscope (LSM510, Carl Zeiss, Germany). The sections were labeled with DAPI nuclear stain to visualize nuclei in the sections.
Western blot analysis
In order to prepare protein lysates from the injured nerves, the proximal and distal stumps (10 mm length) were removed and homogenized with a polytron homogenizer in modified radioimmune precipitation assay (RIPA) lysis buffer (150 mM NaCl, 1% Nonidet P-40, 1 mM EDTA, 0.5% deoxycholic acid, 2 µg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride, 5 mM benzamidine, 1 mM sodium orthovanadate). The lysates were centrifuged for 15 min at 9,000 g at 4℃, and then the supernatant was employed for Western blot analysis. The proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred onto a nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ). After 1 h of blocking with 0.1% Tween 20 and 5% nonfat dry milk in Tris-buffered saline (TBS, 25 mM Tris-HCl pH 7.5, 140 mM NaCl) at room temperature, the membrane was incubated with primary antibodies (1:500~1,000) at 4℃ overnight. After three 15 min washes with TBS containing 0.1% Tween 20 (TBST), the membranes were incubated with a a horseradish peroxidase-conjugated secondary antibody (1:3,000) for 1 h at room temperature. The signals were detected with an Enhanced Chemiluminescence System (ECL Advance kit, Amersham Biosciences).
For quantification, the X-ray films were then subsequently scanned with an HP scanner and analyzed using an LAS image analysis system (Fujifilm, Japan). The intensities of the bands were normalized to those of beta-tubulin or non-phosphorylated STAT3 in three independent experiments.
Primary Schwann cell cultures and RT4 cells
For the experiment using SCs, primary SCs from adult sciatic nerves and RT4 cells were employed. Primary SCs were obtained from adult rat sciatic nerves as previously reported (Lee et al., 2007). Briefly, the sciatic nerves of adult Sprague-Dawley rats were axotomized to enhance the SC population. Sciatic nerves were sectioned 5 mm proximal to the tibioperoneal bifurcation with a fine iris scissor (FST Inc, Foster City, CA, USA) and the animals were housed in plastic cages for 3~4 days after injury. The sciatic nerves were removed and subjected to chemical digestion in 0.2% collagenase for 2 h in calcium/magnesium-free Hank's buffered solution at 37℃. The nerves were then dissociated via 2 min of gentle shaking followed by two or three triturations performed with a flame-polished Pasteur pipette. The cell pellets obtained after centrifugation were then resuspended in Dulbecco's modified Eagle's medium (DMEM) containing 10% (vol/vol) heat-inactivated fetal bovine serum (FBS), plated at a density of 20,000 cells/cm2 and grown for 2 days at 37℃ in a humidified atmosphere containing 5% CO2. After two or three subcultures using the coldjet method (Jirsova et al., 1997), the identity and purity of SCs were evaluated via immunostaining with an antibody raised against S100 (data not shown).
The rat schwannoma cell line (RT4, CRL-2768) was obtained from the American Type Culture Collection (Rockville, MD) and maintained as previously described (Lee et al., 2009).
Immunofluorescent staining
The cells were starved for 4 h or overnight in serum-free DMEM supplemented with 0.5% FBS prior to the addition of cytokines or trophic factors at the concentrations indicated in the Results section. For ERK inhibition, the cells were pretreated with U0126 (10 µM) or PD98059 (20 µM) for 30 min before the addition of IL-6 or growth factors. After stimulation with IL-6 or NRG, the cells were fixed for 15 min with ice-cold methanol at 4℃ for pSTAT3 immunostaining and then washed three times with PBS. The cells were blocked with PBS containing 0.2% Triton X-100 and 2% bovine serum albumin for 1 h. Primary SCs were double immunostained with a mouse anti-S100 antibody (1:200) and a rabbit anti-phospho-STAT3 antibody (1:200) for 16 h at 4℃. Next, the cells were incubated for 2 h with Alexa 488-conjugated anti-rabbit IgG and Texas Red-labeled donkey anti-mouse IgG at room temperature, then viewed under a laser confocal microscope.
Ex-vivo explant culture
Sciatic nerves from adult rats were removed and the connective tissues surrounding the nerves were carefully removed in calcium/magnesium-free Hank's buffered solution under a stereomicroscope. The sciatic nerves were longitudinally cut into 2 or 3 nerve explants and were then cut into small explants of 3 mm length. The explants were maintained for 6 h or 24 h in DMEM containing 10% FBS at 37℃ in a humidified atmosphere containing 5% CO2.
RESULTS
Serine phosphorylation of STAT3 in the injured sciatic nerves
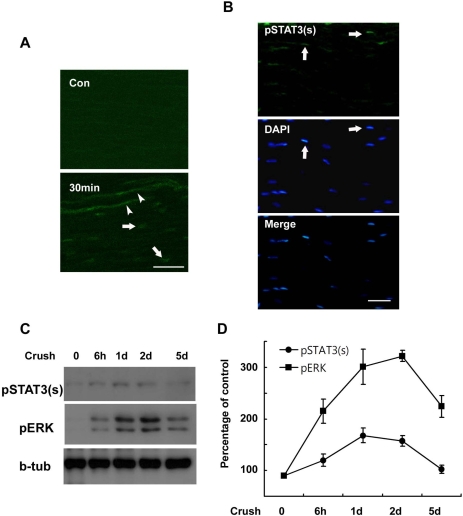
The results of previous studies have demonstrated STAT3 activation in injured peripheral nerves by detecting phosphorylation of STAT3 at tyrosine residue 705 (pSTAT3(y), Sheu et al., 2000; Lee et al., 2004). However, phosphorylation of STAT3 at serine residue 727 (pSTAT3(s)) has yet to be investigated in the sciatic nerve after injury. We initially assessed the serine phosphorylation of STAT3 in injured sciatic nerves using immunofluorescent staining. pSTAT3(s) was not detected in the control sciatic nerves, but was clearly observed in the nuclei of SC-like cells and damaged axoplasm within 30 min at lesion sites after sciatic nerve injury (Fig. 1A). Increased pSTAT3(s) staining was also noted in the nuclei of SC-like cells in the proximal and distal stump of the injured nerves within 60 min of injury (Fig. 1B), thereby indicating that serine phosphorylation of STAT3 occurs in damaged axons and SC-like cells of injured nerves in vivo. In order to know determine the temporal profile of serine phosphorylation of STAT3 in the sciatic nerve after injury, we conducted Western blot analysis (Fig. 1C). This experiment demonstrated that the serine phosphorylation of STAT3 peaked at 1 day and persisted until 2 days after the induced crush injury. Because ERK is known to be activated in injured sciatic nerves (Harrisingh et al., 2004), we assessed the activation profile of ERK after the crush injury. Interestingly, the temporal profile of ERK activation observed after nerve injury was similar to that of STAT3 serine phosphorylation, particularly during the early post-injury period (Fig. 1D), thereby suggesting that ERK may be associated with the serine phosphorylation of STAT3 following nerve injury.
Fig. 1.
Serine phosphorylation of STAT3 in the sciatic nerve following crush injury. (A, B) Frozen sections from control and injured sciatic nerves were immunostained with an antibody against serine phosphorylated STAT3 (pSTAT3(s)). Axons (arrowheads) and nuclei of SC-like cells (arrows) display a rapid induction in the serine phosphorylation of STAT3 at 30 min (A) and 60 min (B) after lesion. Scale bar; 30 µm. (C) Protein lysates were prepared from the injured sciatic nerves and analyzed with Western blot analysis for detection of serine phosphorylated STAT3 and pERK. (D) A quantitative analysis for result (C).
Interleukin-6 induces serine phosphorylation of STAT3 in an ERK-dependent manner
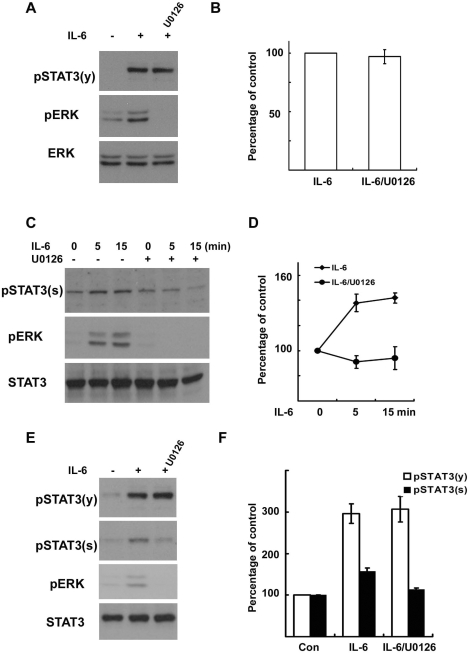
In order to characterize the relationship between ERK and the serine phosphorylation of STAT3 in SCs, we employed RT4 schwannoma cells and primary SCs cultured from adult rat sciatic nerves. Many stimuli can be involved in the activation of ERK in SCs, and we previously reported that IL-6 activated ERK in RT4 cells and primary SCs (Lee et al., 2009). These findings led us to evaluate the role of ERK in phosphorylation of STAT3 in response to IL-6. Consistent with the previous result (Lee et al., 2009), we found an activation of ERK by IL-6 within 5 min in the RT4 cells (Fig. 2A, C). When the RT4 cells were pretreated with the mitogen-activated protein kinase/extracellular signalregulated kinase kinase (MEK) inhibitors, U0126 (10 µM) or PD980519 (20 µM, data not shown), before the stimulation with IL-6, a complete reduction in the phosphorylation of ERK was noted (Fig. 2A, C). However, the tyrosine phosphorylation of STAT3 by IL-6 was not altered by the inhibition of MEK (Fig. 2A, B), suggesting that ERK activation is not involved in the IL-6-induced tyrosine phosphorylation of STAT3. In the RT4 cells, IL-6 induced an increase in the level of serine phosphorylation of STAT3 even though this serine phosphorylation was not as robust as the tyrosine phosphorylation (Fig. 2C). The temporal profile of STAT3 serine phosphorylation was similar to that of ERK activation, and treatment with the specific MEK inhibitors resulted in the blockage of IL-6-induced STAT3 serine phosphorylation (Fig. 2C, D). The involvement of ERK in the serine phosphorylation, but not the tyrosine phosphorylation, of STAT3 was also detected in primary SCs cultured from adult sciatic nerves (Fig. 2E, F). This finding shows that IL-6-mediated ERK activation is involved in STAT3 phosphorylation at the serine 727 residue in RT4 cells and primary SCs.
Fig. 2.
IL-6 induces serine phosphorylation of STAT3 in Schwann cells. RT4 schwannoma cells (A~D) and primary SCs (E, F) were serum starved overnight and then treated with IL-6 (50 ng/ml) for the indicated times. Total cell lysates were subjected to Western blot analysis using antibodies specific for phosphorylated proteins. (A, B) RT4 cells were treated with IL-6 for 15 min, and the cellular lysates were analyzed using the indicated antibodies. (B) A quantitative analysis of tyrosine phosphorylated STAT3 in result (A). (C, D) RT4 cells were treated with IL-6 for the indicated time in the presence or absence of U0126. (D) A quantitative analysis of serine phosphorylated STAT3 in result (C). (E, F) Primary SCs were treated with IL-6 for 15 min, and the total lysates were analyzed by Western blot analysis using the indicated antibodies. (F) A quantitative analysis of phosphorylated STAT3 in result (E).
Neuregulin-1 induces serine phosphorylation of STAT3 in an ERK-dependent manner
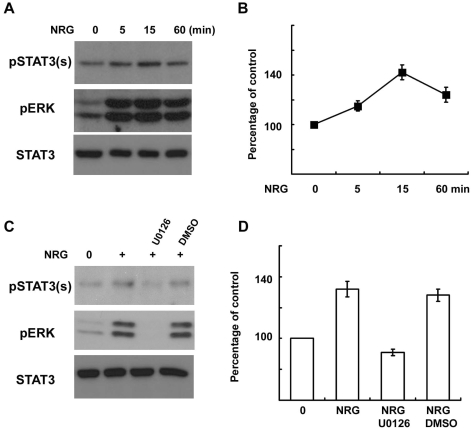
It was previously reported that NRG is an important activator of the MEK/ERK pathway in SCs (Harrisingh et al., 2004). However, the role of NRG-induced ERK activation in the serine phosphorylation of STAT3 in SCs had, until present, not yet been thoroughly investigated. Thus, we attempted to determine whether NRG-1 induces serine phosphorylation of STAT3 through ERK activation using primary SCs. NRG-1 (200 ng/ml) induced not only ERK activation but also serine phosphorylation of STAT3 in a time-dependent manner (Fig. 3A, B). However, the tyrosine phosphorylation of STAT3 was not changed after NRG-1 treatment as previously reported (data not shown, Lee et al., 2009). The serine phosphorylation appeared within 5 min and peaked 15 min after NRG treatment. MEK inhibition with U0126 completely inhibited serine phosphorylation of STAT3 induced by NRG-1 (Fig. 3C, D). These findings show that the NRG-1-induced activation of the MEK/ERK pathway is involved in the serine phosphorylation of STAT3 in primary SCs.
Fig. 3.
NRG-1 induces serine phosphorylation of STAT3 in Schwann cells. (A, B) Primary SCs were serum starved overnight and then treated with NRG-1 (200 ng/ml) for the indicated times. Total cell lysates were subjected to Western blot analysis using antibodies specific for phosphorylated proteins. (B) A quantitative analysis of serine phosphorylated STAT3 in result (A). (C, D) Primary SCs were treated with NRG-1 for 15 min in the presence or absence of U0126 or DMSO, and cellular lysates were analyzed with Western blot analysis. (D) A quantitative analysis of serine phosphorylated STAT3 in result (C).
Differential localization of serine phosphorylated STAT3 by IL-6 and neuregulin-1
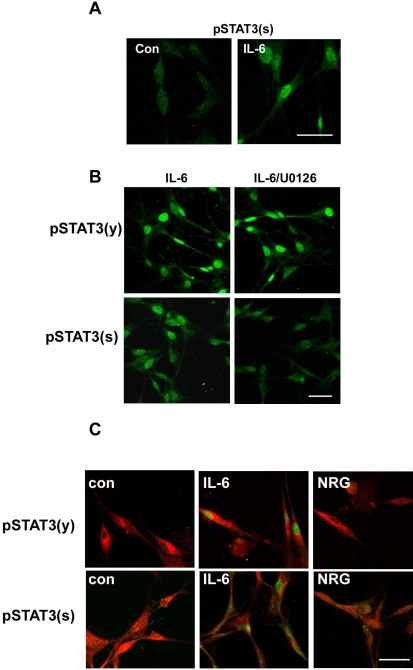
We then attempted to determine whether IL-6 induces the nuclear translocation of serine phosphorylated STAT3, and did indeed detect serine-phosphorylated STAT3 in the nucleus within 20 min of IL-6 treatment (Fig. 4A). Treatment with the MEK inhibitor U0126 did not affect a marked increase in pSTAT3(y) by IL-6 in the nucleus, whereas it prevented the appearance of serine phosphorylated STAT3 (Fig. 4B), in a finding consistent with the biochemical results (Fig. 2C). Additionally, the identical results were observed with the cultured primary SCs (Fig. 4C).
Fig. 4.
Differential localization of phosphorylated STAT3 by IL-6 and NRG-1. (A) After 20 min of stimulation with IL-6, RT4 cells were fixed and immunostained for serine phosphorylated STAT3. Con; untreated control. (B) RT4 cells were pretreated with U0126 for 30 min and then stimulated with IL-6 for 20 min. Cells were immunostained for tyrosine or serine phosphorylated STAT3. Scale bar; 30 µm. (C) Primary SCs were treated with IL-6 or NRG for 20 min, and the cells were fixed and double immunostained for a SC marker (S100) and phosphorylated STAT3. Scale bar; 30 µm.
Although the tyrosine phosphorylation of STAT3 is a prerequisite for the nuclear translocation of STAT3 in many situations, the nuclear translocation of serine phosphorylated STAT3 (without tyrosine phosphorylation) has also been reported in previous studies (Ng et al., 2006b; Reich and Liu, 2006). Thus, we examined the possibility of that the nuclear translocation of serine phosphorylated STAT3 by NRG-1 might occur in primary SCs (Fig. 4C). Double immunofluorescent staining with an antibody against a SC marker (S100) and an antibody against pSTAT3(y) or pSTAT3(s) showed obvious nuclear translocation of pSTAT3(y) and pSTAT3(s) by IL-6 in S100-positive cells. However, NRG-1 treatment was not shown to induce an increase in the nuclear staining of either pSTAT3(y) or pSTAT3(s). Rather, NRG generally increased the immunolabeling of pSTAT3(s) in the cytoplasm without distinct nuclear staining. Thus, our data suggest that NRG-1 cannot induce the nuclear translocation of pSTAT3(s).
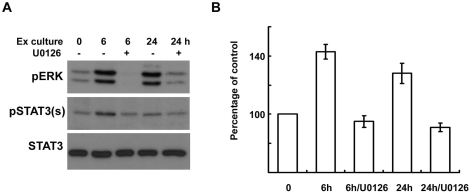
ERK inhibition reduces serine phosphorylation of STAT3 in an ex-vivo sciatic nerve injury model
We have determined that ERK is a crucial upstream kinase for the IL-6 and NRG-induced serine phosphorylation of STAT3 in cultured SCs. In order to know the function of ERK in the serine phosphorylation of STAT3 in the injured nerve itself, we utilized an ex-vivo sciatic nerve injury model. When sciatic nerves are removed from animals and cultured as explants, the nerve explants are known to undergo Wallerian degeneration in a manner very similar to that seen under in vivo conditions (Thomson et al., 1993; Bleuel and Monard, 1995). We cultured sciatic nerve explants in the presence or absence of the MEK inhibitor, U0126 (20 µM), for 6 h and 24 h, after which the protein lysates from the explants were assessed with Western blot analysis (Fig. 5A). This experiment showed that ERK and STAT3 were activated during explant culture, and that the inhibition of ERK significantly reduced the serine phosphorylation of STAT3 (Fig. 5), thus suggesting that ERK performs an important function in the serine phosphorylation of STAT3 after nerve injury.
Fig. 5.
ERK inhibition reduces the serine phosphorylation of STAT3 in explant cultures of sciatic nerves. (A, B) Sciatic nerve explants were cultured for 6 or 24 h in the presence or absence of U0126, and then the protein lysates were analyzed with Western blot analysis using an antibody against serine phosphorylated STAT3 and anti-pERK antibody. (B) A quantitative analysis of serine phosphorylated STAT3 in result (A).
DISCUSSION
It was previously reported that STAT3 in axons and SC-like cells is activated by peripheral nerve lesion (Sheu et al., 2000; Lee et al., 2004; Lee et al., 2009). The activation of STAT3 at lesion sites was demonstrated by the observed tyrosine phosphorylation of STAT3 and occurred within 30 min of injury. The present data demonstrate, for the first time, that the serine phosphorylation of STAT3 also occurred in axons and SC-like cells in the peripheral nerves following lesion. According to previous studies that demonstrated STAT3 activation in the peripheral nerves after the development of a lesion, the temporal and spatial profiles of tyrosine phosphorylation of STAT3 in the injured nerves were similar to that associated with the serine phosphorylation of STAT3. Thus, it appears that STAT3 can be phosphorylated simultaneously at tyrosine 705 and serine 727 residues in the injured peripheral nerves. It has been previously reported that the tyrosine phosphorylation of STAT3 is an essential step for the dimerization of STAT3. Because dimerization is required for the binding of STAT3 to DNA promoter regions, tyrosine phosphorylation is a prerequisite for STAT3-dependent transcriptional activation (Battle and Frank, 2002). In addition to the tyrosine phosphorylation of STAT3, phosphorylation of STAT3 at the serine 727 residue has been suggested to play an important role in the regulation of transcriptional activity of STAT3. For example, it was reported that the serine phosphorylation of STAT3 results in the maximal transcriptional activation of tyrosine phosphorylated STAT3 (Wen et al., 1995; Plaza-Menacho et al., 2007). On the contrary, several papers have demonstrated that the serine phosphorylation of STAT3 exerts a negative effect on the transcriptional activity and tyrosine phosphorylation of STAT3 (Chung et al., 1997; Jain et al., 1998). Thus, the functional significance of serine phosphorylation of STAT3 may differ from cell to cell. Considering the role of serine phosphorylation of STAT3 in its transcriptional activity, we think that the serine phosphorylation of STAT3 in SCs may play a significant role in STAT3 function in the injured sciatic nerves. Because the role of STAT3 in SCs after nerve injury remains largely unknown at present, further studies into the function of STAT3 in SCs are required for a great understanding of the functional significance of serine phosphorylation of STAT3 in SCs and in peripheral nerve regeneration.
After nerve injury, there is an initial degradation of myelin sheath and subsequent phenotype changes of SCs into immature states. This process is called dedifferentiation (Jessen and Mirsky, 2008). This regenerative change of SCs is essential for successful nerve regeneration. The dedifferentiation of SCs after injury involves the activation of several signaling pathways to alter gene expression profiles. It was previously reported that ERK is activated within 30 min of injury and persisted for more than one week (Sheu et al., 2000; Harrisingh et al., 2004). Even though numerous ligands might be involved in the induction of this long lasting ERK activation, the initial activation of ERK after injury might be caused by the release of certain factors that are already present in the uninjured nerve. For example, NRG, which is derived from damaged axons and SCs, may be a promising candidate because NRG signaling is acutely activated after nerve injury, and has been implicated in the dedifferentiation of SCs via ERK activation (Harrisingh et al., 2004; Guertin et al., 2005). Later on, ERK activation may be induced by injury-induced growth factors and cytokines. For example, cytokines such as IL-6 could activate ERK in SCs as we have demonstrated in other studeies. The ERK activation by multiple ligands after nerve injury has been suggested to be associated with the initiation of demyelination and proliferation (Harrisingh et al., 2004; Monje et al., 2006), two important characteristics of dedifferentiation, of SCs. However, the molecular mechanism underlying the function of ERK in dedifferentiation of SCs remains largely uncharacterized. In this study, we have found that ERK activation induced by NRG and IL-6 is involved in the serine phosphorylation of the transcription factor STAT3 in SCs, thereby suggesting that ERK might play a role in the dedifferentiation of SCs, in part through the modulation of STAT3 activity.
Interestingly, our results suggested that the serine phosphorylation of STAT3 induced by IL-6 may have a different meaning than that induced by NRG. IL-6 induced a dramatic increase in the nuclear transport of double-phosphorylated STAT3, whereas NRG induced only the serine phosphorylation of STAT3. Furthermore, the nuclear transport of serine phosphorylated STAT3 was not observed following NRG treatment. It may be possible that serine phosphorylated STAT3 by NRG may play a cytoplasmic function, as was reported previously (Ng et al., 2006a) or NRG may induce genes with a very low level of serine phosphorylated STAT3 in the nucleus (undetectable level with the immunostaining method). Recently, Ng et al (2006b) reported that nerve growth factor stimulates the expression of STAT3 target genes such as JunB and egr-1 by inducing solely via the induction of serine phosphorylation of STAT3 in PC12 pheochromocytoma cells. Further studies will be required to more clearly elucidate the functional significance of serine phosphorylated STAT3 in response to NRG in SCs.
On the other hand, it has been reported that the kinases that phosphorylate STAT3 on the serine 727 residue are diverse, and are dependent on both extracellular stimuli and cell types (Gartsbein et al., 2006; Ng et al., 2006b; Shi et al., 2006). However, we determined that the inhibition of MEK nearly completely inhibited the induction of serine phosphorylation of STAT3 in ex-vivo explant cultures. This finding demonstrates that ERK may constitute the principal component of the serine/threonine kinase pathways that result in the phosphorylation of the serine 727 residue of STAT3 in SCs. Further studies will be required in order to elucidate the serine kinases that directly phosphorylate STAT3 in Schwann cells.
In conclusion, we have determined in this study that ERK is important in modulating the activity of STAT3 in cultured SCs and in injured nerves. Because ERK plays an important role in the dedifferentiation of SCs after nerve injury, our findings have important implications for our understanding of the molecular mechanism of degenerative and regenerative processes occurring after nerve injury.
ACKNOWLEDGEMENTS
This work was supported by Research fund from Dong-A University.
ABBREVIATIONS
- SCs
Schwann cells
- DMEM
Dulbecco's modified Eagle's medium
- FBS
fetal bovine serum
- TBS
tris-buffered saline
- ERK
extracellular signal-regulated kinase
- IL-6
interleukin-6
- JAK
janus kinase
- STAT
signal transducer and activator of transcription
- PBS
phosphate-buffered saline
- NRG
neuregulin
- MEK
mitogen-activated protein kinase/extracellular signal-regulated kinase kinase
References
- 1.Battle TE, Frank DA. The role of STATs in apoptosis. Curr Mol Med. 2002;2:381–392. doi: 10.2174/1566524023362456. [DOI] [PubMed] [Google Scholar]
- 2.Bleuel A, Monard D. Regulation of protease nexin-1 and angiotensin II receptor subtype 1 expression: inverse relationship in experimental models of nerve injury. J Neurosci Res. 1995;42:562–570. doi: 10.1002/jnr.490420414. [DOI] [PubMed] [Google Scholar]
- 3.Cafferty WB, Gardiner NJ, Gavazzi I, Powell J, McMahon SB, Heath JK, Munson J, Cohen J, Thompson SW. Leukemia inhibitory factor determines the growth status of injured adult sensory neurons. J Neurosci. 2001;21:7161–7170. doi: 10.1523/JNEUROSCI.21-18-07161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung J, Uchida E, Grammer TC, Blenis J. STAT3 serine phosphorylation by ERK-dependent and -independent pathways negatively modulates its tyrosine phosphorylation. Mol Cell Biol. 1997;17:6508–6516. doi: 10.1128/mcb.17.11.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- 6.Gartsbein M, Alt A, Hashimoto K, Nakajima K, Kuroki T, Tennenbaum T. The role of protein kinase C delta activation and STAT3 Ser727 phosphorylation in insulin-induced keratinocyte proliferation. J Cell Sci. 2006;119:470–481. doi: 10.1242/jcs.02744. [DOI] [PubMed] [Google Scholar]
- 7.Guertin AD, Zhang DP, Mak KS, Alberta JA, Kim HA. Microanatomy of axon/glial signaling during Wallerian degeneration. J Neurosci. 2005;25:3478–3487. doi: 10.1523/JNEUROSCI.3766-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hai M, Muja N, DeVries GH, Quarles RH, Patel PI. Comparative analysis of Schwann cell lines as model systems for myelin gene transcription studies. J Neurosci Res. 2002;69:497–508. doi: 10.1002/jnr.10327. [DOI] [PubMed] [Google Scholar]
- 9.Harrisingh MC, Perez-Nadales E, Parkinson DB, Malcolm DS, Mudge AW, Lloyd AC. The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. EMBO J. 2004;23:3061–3071. doi: 10.1038/sj.emboj.7600309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain N, Zhang T, Fong SL, Lim CP, Cao X. Repression of Stat3 activity by activation of mitogen-activated protein kinase (MAPK) Oncogene. 1998;17:3157–3167. doi: 10.1038/sj.onc.1202238. [DOI] [PubMed] [Google Scholar]
- 11.Jessen KR, Mirsky R. Negative regulation of myelination: relevance for development, injury, and demyelinating disease. Glia. 2008;56:1552–1565. doi: 10.1002/glia.20761. [DOI] [PubMed] [Google Scholar]
- 12.Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nat Rev Neurosci. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- 13.Jirsova K, Sodaar P, Mandys V, Bar PR. Cold jet: a method to obtain pure Schwann cell cultures without the need for cytotoxic, apoptosis-inducing drug treatment. J Neurosci Methods. 1997;78:133–137. doi: 10.1016/s0165-0270(97)00146-5. [DOI] [PubMed] [Google Scholar]
- 14.Kamimura D, Ishihara K, Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol. 2003;149:1–38. doi: 10.1007/s10254-003-0012-2. [DOI] [PubMed] [Google Scholar]
- 15.Lee HK, Seo IA, Park HK, Park YM, Ahn KJ, Yoo YH, Park HT. Nidogen is a prosurvival and promigratory factor for adult Schwann cells. J Neurochem. 2007;102:686–698. doi: 10.1111/j.1471-4159.2007.04580.x. [DOI] [PubMed] [Google Scholar]
- 16.Lee HK, Seo IA, Suh DK, Hong JI, Yoo YH, Park HT. Interleukin-6 is required for the early induction of glial fibrillary acidic protein in Schwann cells during Wallerian degeneration. J Neurochem. 2009;108:776–786. doi: 10.1111/j.1471-4159.2008.05826.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee N, Neitzel KL, Devlin BK, MacLennan AJ. STAT3 phosphorylation in injured axons before sensory and motor neuron nuclei: potential role for STAT3 as a retrograde signaling transcription factor. J Comp Neurol. 2004;474:535–545. doi: 10.1002/cne.20140. [DOI] [PubMed] [Google Scholar]
- 18.Monje PV, Bartlett Bunge M, Wood PM. Cyclic AMP synergistically enhances neuregulin-dependent ERK and Akt activation and cell cycle progression in Schwann cells. Glia. 2006;53:649–659. doi: 10.1002/glia.20330. [DOI] [PubMed] [Google Scholar]
- 19.Ng DC, Lin BH, Lim CP, Huang G, Zhang T, Poli V, Cao X. Stat3 regulates microtubules by antagonizing the depolymerization activity of stathmin. J Cell Biol. 2006a;172:245–257. doi: 10.1083/jcb.200503021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng YP, Cheung ZH, Ip NY. STAT3 as a downstream mediator of Trk signaling and functions. J Biol Chem. 2006b;281:15636–15644. doi: 10.1074/jbc.M601863200. [DOI] [PubMed] [Google Scholar]
- 21.Plaza-Menacho I, van der Sluis T, Hollema H, Gimm O, Buys CH, Magee AI, Isacke CM, Hofstra RM, Eggen BJ. Ras/ERK1/2-mediated STAT3 Ser727 phosphorylation by familial medullary thyroid carcinoma-associated RET mutants induces full activation of STAT3 and is required for c-fos promoter activation, cell mitogenicity, and transformation. J Biol Chem. 2007;282:6415–6424. doi: 10.1074/jbc.M608952200. [DOI] [PubMed] [Google Scholar]
- 22.Qiu J, Cafferty WB, McMahon SB, Thompson SW. Conditioning injury-induced spinal axon regeneration requires signal transducer and activator of transcription 3 activation. J Neurosci. 2005;25:1645–1653. doi: 10.1523/JNEUROSCI.3269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reich NC, Liu L. Tracking STAT nuclear traffic. Nat Rev Immunol. 2006;6:602–612. doi: 10.1038/nri1885. [DOI] [PubMed] [Google Scholar]
- 24.Sheu JY, Kulhanek DJ, Eckenstein FP. Differential patterns of ERK and STAT3 phosphorylation after sciatic nerve transection in the rat. Exp Neurol. 2000;166:392–402. doi: 10.1006/exnr.2000.7508. [DOI] [PubMed] [Google Scholar]
- 25.Shi X, Zhang H, Paddon H, Lee G, Cao X, Pelech S. Phosphorylation of STAT3 serine-727 by cyclin-dependent kinase 1 is critical for nocodazole-induced mitotic arrest. Biochemistry. 2006;45:5857–5867. doi: 10.1021/bi052490j. [DOI] [PubMed] [Google Scholar]
- 26.Thomson CE, Griffiths IR, McCulloch MC, Kyriakides E, Barrie JA, Montague P. In vitro studies of axonally-regulated Schwann cell genes during Wallerian degeneration. J Neurocytol. 1993;22:590–602. doi: 10.1007/BF01181486. [DOI] [PubMed] [Google Scholar]
- 27.Wen Z, Zhong Z, Darnell JE., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]