Abstract
Ginsan, a Panax ginseng polysaccharide that contains glucopyranoside and fructofuranoside, has immunomodulatory effects. Although several biologic studies of ginsan have been performed, its effects on dendritic cells (DCs), which are antigen-presenting cells of the immune system, have not been studied. We investigated the immunomodulatory effects of ginsan on DCs. Ginsan had little effect on DC viability, even when used at high concentrations. Ginsan markedly increased the levels of production by DCs of IL-12 and TNF-α, as measured by ELISA. To examine the maturation-inducing activity of ginsan, we measured the surface expression levels of the maturation markers MHC class II and CD86 (B7.2) on DCs. It is interesting that ginsan profoundly enhanced the expression of CD86 on DC surfaces, whereas it increased that of MHC class II only marginally. In 3H-thymidine incorporation assays, ginsan-treated DCs stimulated significantly higher proliferation of allogeneic CD4+ T lymphocytes than did medium-treated DCs. Taken together, our data demonstrate that ginsan stimulates DCs by inducing maturation. Because DCs are critical antigen-presenting cells in immune responses, this study provides valuable information on the activities of ginsan.
Keywords: Ginsan, Dendritic cells, Immunomodulation
INTRODUCTION
Ginsan is an immunomodulatory polysaccharide from Panax ginseng, a medicinal herb. Since its initial purification, ginsan has been shown to have critical effects on immune cells (Lee et al., 1997). Ginsan enhances the production of cytokines and reactive oxygen species (ROS) by macrophages (Shin et al., 2002) and stimulates the phagocytic activity of macrophages (Song et al., 2002). As an immunomodulator, ginsan plays a radioprotective role in hematopoietic and immune cells (Song et al., 2003). It increases the number of bone marrow (BM) cells, splenocytes, and some hematopoietic stem cells and enhances the production of cytokines involved in hematopoietic recovery (Kim et al., 2007). Therefore, ginsan appears to protect the host from ionizing radiation via its immunomodulatory activities.
Dendritic cells (DCs) are specialized antigen-presenting cells (APCs) that prime naïve T lymphocytes in the immune system of the host (Mellman and Steinman, 2001). DCs take up exogenous antigens at the first lines of defense, e.g., the skin, and migrate into nearby lymphoid organs, such as lymph nodes (Yoneyama et al., 2005). In the lymph nodes, antigen-processing DCs present antigenic peptides to naïve or memory lymphocytes in a MHC class-restricted manner (Steinman et al., 1997). Maturation of DCs is essential to achieving appropriate immune responses, as it enhances surface expression of MHC and costimulatory molecules, which reflect antigen-presenting capabilities, as well as the production of a variety of cytokines (Larsen et al., 1994; Mosca et al., 2000).
Although the effects of ginsan on various immune cells have been studied, its influence on DCs remains to be elucidated. In the present study, we investigated the mechanisms through which ginsan exerts immunomodulatory activities on DCs.
METHODS
Animals and reagents
To generate bone marrow-derived DCs, C57BL/6 mice were used. For setup of mixed leukocyte responses (MLRs), Balb/c mice were used. Both strains were purchased from Orient Bio Co. (Seongnam, Korea) and maintained in lab animal facility until use. 7 to 12 week-old female mice were used for all experiments. Animal experiments were performed based on the NIH guide for the care and use of laboratory animals (NIH Publication No. 80-23; revised 1978). Ginsan was purified from Panax ginseng at Korea Institute of Radiological and Medical Sciences (Ahn et al., 2006) and dissolved in sterile phosphate-buffered saline (Invitrogen, Calsbad, CA, USA). Lipopolysaccharide (LPS) and anti-CD40 mAb were purchased from Sigma (St. Louis, MO, USA) and BD Biosciences (San Jose, CA, USA), respectively.
Culture of DCs
Bone marrow cells were harvested from femur and tibia of C57BL/6 mice and used for culture of DCs as described before (Kim and Joo, 2008). Briefly, after the lysis of red blood cells, bone marrow cells were cultured in the presence of 10 ng/ml recombinant mouse granulocyte macrophagecolony stimulating factor (GM-CSF; Biosource International, Camarillo, CA, USA) for 6 to 10 days. Culture media was replaced with fresh media containing GM-CSF every two days. To minimize lymphocytes and granulocytes in DC culture, the floating cells were removed at 2nd, 4th day of culture and the adherent cells containing DC precursors were cultured. After 6 day culture, the floating cells were harvested and used as bone marrow-derived DCs. For the identification of DCs, cells were stained for a surface marker, CD11c using specific antibody.
Measurement of cell viability
DCs were cultured at a concentration of 5×104 cells/well in 96-well culture plates. Cells were treated with ginsan for 2 days and then 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT; Sigma) solution was added into each well. The crystal violet generated by viable cells was dissolved with 10% sodium dodecyl sulfate (SDS; Sigma) solution and optical density (O.D.) was measured at 570 nm using a microplate reader (Molecular devices, Sunnyvale, CA, USA).
Measurement of cytokines and nitric oxide production
After treatment, the culture supernatants were harvested and used for the determination of cytokine and nitric oxide (NO) amounts. For measuring the amount of cytokines based on ELISA, IFN-gamma, IL-4, IL-10, IL-12, and TNF-alpha specific CytoSet or kits (all from Biosource International) were used as followed by manufacturer's manuals. For measuring the amount of NO, modified Griess reagent and as standard, sodium nitrite (all from Sigma) were used.
Flow cytometric analysis
Cell staining was performed as described in a previous report (Joo, 2003). Briefly, cells were treated with 1 µg/sample anti-CD16/32 monoclonal antibody (mAb) for blocking Fc receptors on DC surface. For checking the purity of cultured DCs, phycoerythrin (PE)-labeled anti-CD11c mAb was used. And also, biotin-labeled anti-MHC class II (I-Ab), CD86 mAb and subsequently streptavidin-fluorescein isothiocyanate (FITC; all from BD Biosciences) were used for measuring the maturation status of DCs. The stained cells were analyzed by FACSCalibur™ flow cytometer and CellQuest™ software (All from BD Biosciences).
Estabilishment of mixed leukocyte responses (MLRs)
C57BL/6 mice-originated DCs were co-cultured with Balb/c mice-originated CD4+ T lymphocytes for MLRs. Splenocytes were harvested from Balb/c mice as described before (Joo et al., 2001). CD4+ T lymphocytes were purified from splenocytes by using CD4+ magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. Cells in co-culture were incubated for 5 days and pulsed with 1 µCi/well 3H-thymidine (PerkinElmer, Wellesley, MA, USA) for last 18 hr. Incorporated radioactivity in cells was measured by a scintillation counter, Wallac Microbeta® TriLux (PerkinElmer).
Statistics
In MTT assay and MLRs, data were obtained from 3, 5 wells respectively and represented as mean±standard deviation (SD). Data were analyzed with Tukey-Kramer multiple comparison tests and data with p<0.05 was recognized as significant.
RESULTS
Viability of ginsan-treated DCs
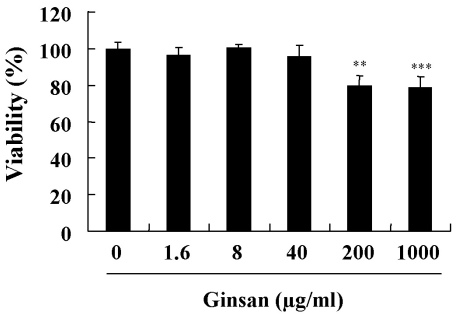
An MTT assay was used to investigate whether ginsan affects the viability of DCs. DCs were cultured in the presence of ginsan (0~1,000 µg/ml) for 2 days. Ginsan added at concentrations of 0~40 µg/ml did not affect DC viability, although viability was affected at higher concentrations (Fig. 1). We used ginsan at concentrations up to 10 µg/ml.
Fig. 1.
Viability of DCs treated with ginsan. DCs were seeded at 5×104 cells/well in 96-well culture plates and treated with different concentrations of ginsan for 2 days. After treatment, an MTT assay was performed, as described in Methods. The representative data, shown as mean±SD, are from three independent experiments that gave similar results. **p<0.01, ***p<0.001.
NO production from DCs treated with ginsan
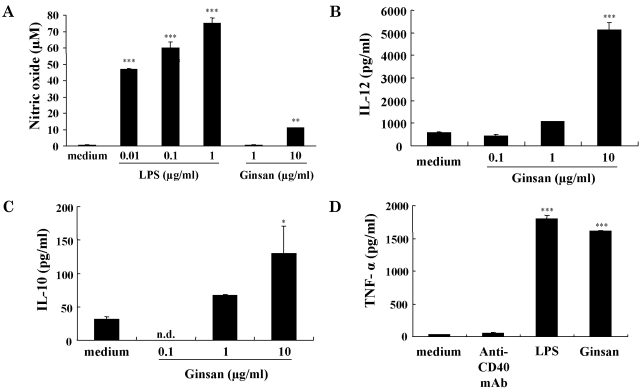
DCs play critical roles as potent APCs. However, some maturing agents, such as LPS, induce the production of NO as well as the maturation of DCs (Lu et al., 1996). NO is an immunosuppressor of DC-mediated immune responses (Bonham et al., 1996). To determine whether ginsan induces the production of NO, we measured the amount of NO released from DCs that were incubated with ginsan for 2 days (Fig. 2A). LPS was used as a positive control. Ginsan at 1 µg/ml induced a trace amount of NO from DCs, which was similar to the level of NO detected from medium alone-treated DCs (ContDCs). In addition, the production of NO in response to 10 µg/ml ginsan was profoundly lower than that in response to 0.01 µg/ml LPS.
Fig. 2.
Production of NO and cytokines by DCs treated with ginsan. For the NO assay (A), DCs were established in culture as described in Fig. 1. LPS or ginsan was added to the wells, and the culture supernatants were harvested 2 days later. The NO assay was performed as described in Methods. For cytokine measurements (B~D), DCs were seeded at 5×105 cells/well in 24-well culture plates and treated for 48 h. Concentrations of IL-12 (B), IL-10 (C), and TNF-α (D) in the culture media were measured using ELISA. In D, 1 µg/ml anti-mouse CD40 mAb, 1 µg/ml LPS, and 10 µg/ml ginsan were used. *p<0.05, **p<0.01, ***p<0.001.
Cytokine production by DCs treated with ginsan
We investigated the effects of ginsan on the production by DCs of cytokines IL-12, IL-10, and TNF-α. IL-12 and IL-10 are involved in cell-mediated and regulatory immunity, respectively (Heufler et al., 1996; Corinti et al., 2001), whereas TNF-α induces DC maturation and survival (Esche et al., 2001). According to ELISA results, ginsan significantly increased the production of IL-12 and IL-10 in a concentration-dependent manner (Fig. 2B, C). The level of TNF-α production induced by ginsan was similar to that induced by LPS in DCs (Fig. 2D).
Ginsan increases the expression of maturation markers on the surfaces of DCs
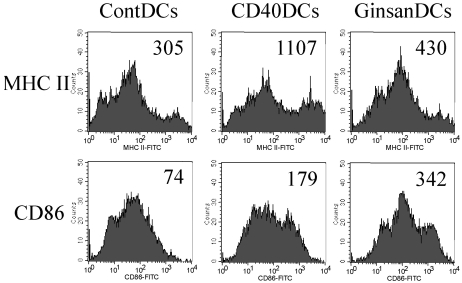
The maturation status of DCs plays a critical role in the stimulation of immune responses. MHC class II and CD86 (B7.2) are maturation markers for BM-derived DCs. To assess the ability of ginsan to induce DC maturation, we measured the expression levels of MHC class II and CD86 on the surfaces of DCs (Fig. 3). In this experiment, an anti-CD40 mAb was used as a positive control, because it binds to CD40 on the DC surface, thereby generating strong cell maturation signals. Flow cytometric analysis revealed higher expression of MHC class II and CD86 molecules on the surfaces of ginsan-treated DCs (GinsanDCs) than on control DCs (ContDCs). It is interesting that the highest level of CD86 was detected on the GinsanDCs, whereas the highest level of MHC class II was detected on the anti-CD40 mAb-treated DCs (CD40DCs).
Fig. 3.
Ginsan increases the expression of maturation markers on the surfaces of DCs. DCs were established in culture and treated as described in Fig. 2B~D. Data shown are representative values from three separate experiments. ContDCs, medium alone-treated DCs; CD40DCs, DCs treated with 1 µg/ml anti-mouse CD40 mAb; GinsanDCs, DCs treated with 10 µg/ml ginsan.
Enhanced antigen-presenting capacity of ginsan-treated DCs
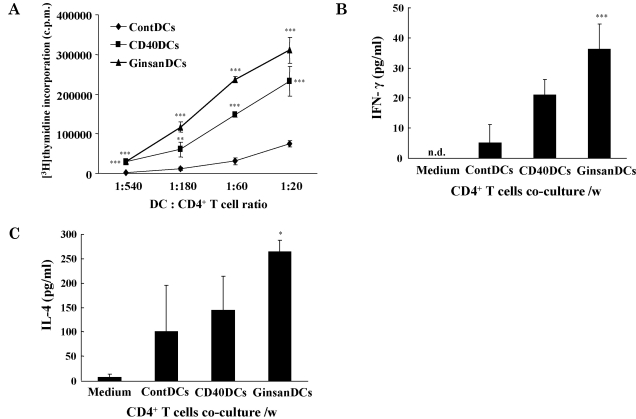
To confirm the maturation-inducing capability of ginsan, we assessed the antigen-presenting capabilities of the GinsanDCs (Fig. 4A). Depending on their maturation status, the DCs of C57BL/6 mice can stimulate the proliferation of CD4+ T lymphocytes from BALB/c mice, as allogeneic responder cells. In the 3H-thymidine incorporation assay, the GinsanDCs stimulated higher proliferation of allogeneic CD4+ T lymphocytes than the ContDCs. And also, the CD40DCs stimulated higher proliferation of allogeneic CD4+ T lymphocytes than the ContDCs.
Fig. 4.
Increased antigen-presenting capacities of ginsan-treated DCs. DCs were established in culture as described in Methods. For the MLR assay, DCs were co-cultured with 1×105 cells/well allogeneic CD4+ lymphocytes for 5 days. As described in Methods, the incorporated radioactivity was measured using a scintillation counter. The mean±SD level of radioactivity was derived from five individual wells (A). For the cytokine assay (B, C), 5×103 cells/well DCs were cocultured with 1×105 cells/well allogeneic CD4+ lymphocytes. The culture supernatants were harvested and used to measure IFN-γ (B) and IL-4 (C) levels. Shown are representative data from three independent experiments that gave similar results. *p<0.05, **p<0.01, ***p<0.001.
Ginsan induces cytokine production by activated lymphocytes
Using the same experimental setup as depicted in Fig. 4A, we examined the production of CD4+ T-lymphocyte-derived cytokines. IFN-γ induces Th1-shifted immune responses, including the activation of CD8+ cytotoxic T lymphocytes, whereas IL-4 induces Th2-shifted immune responses. According to ELISA results, the CD4+ T lymphocytes activated by the GinsanDCs produced higher levels of IFN-γ and IL-4 than those activated by the ContDCs (Fig. 4B, C). It is interesting that the ratio of IFN-γ produced by GinsanDCs vs ContDCs was higher than that of IL-4 produced by GinsanDCs vs ContDCs.
DISCUSSION
Ginsan is an acidic polysaccharide from Panax ginseng, a well-known medicinal herb. Although ginsan has been studied with regard to its immunologic properties, its immunomodulatory effects on DCs have not yet been elucidated. Because ginsan alters cytokine production by macrophages, we first investigated whether ginsan alters cytokine production by DCs. Indeed, ginsan profoundly enhanced the production by DCs of IL-12 and IL-10 in a concentration-dependent manner. In addition, ginsan induced significant production of the DC-maturing cytokine TNF-α. The results of the present study demonstrate that ginsan clearly enhances cytokine production by DCs. Therefore, ginsan may modulate DC function by altering cytokine levels.
A previous report demonstrated that ginsan induced the production of significant amounts of NO by macrophages. It is interesting that, in the present study, ginsan induced a negligible amount of NO from DCs (Fig. 2). This discrepancy may be attributed to the different cell types used and also regulating system for NO production. The detailed mechanism through which ginsan acts on macrophages and DCs needs to be investigated. In fact, NO acts as an immunosuppressor of DC-mediated immune responses.
In the present study, phenotypic and functional analyses showed that ginsan induces the maturation of murine BM-derived DCs. Ginsan enhanced the production of cytokines from DCs and the expression of maturation markers on the surfaces of DCs. Furthermore, GinsanDCs markedly increased the proliferation of allogeneic CD4+ T lymphocytes, as compared to ContDCs. It is of interest that ginsan increased the expression of CD86 rather than that of MHC class II (Fig. 3). CD86 is a major costimulatory molecule that plays an essential role in the interaction between APCs and T lymphocytes. Generally, the maturing agents, such as anti-CD40 mAb and LPS strongly increase the expression of MHC class II. It is possible that ginsan differentially regulates DC maturation and the increased CD86 molecules may potentiate the costimulatory signal in the DC-T lymphocyte interaction.
This study demonstrated that GinsanDCs increased the production of both IFN-γ and IL-4 of allogeneic lymphocytes compared to ContDCs (Fig. 4). However, the ratio of IFN-γ produced by GinsanDCs vs ContDCs was higher than that of IL-4 produced by GinsanDCs vs ContDCs. This result thus suggests that ginsan may prefer Th1 DC-mediated immune responses.
To the best of our knowledge, this is the first study to demonstrate the immunostimulatory effect of ginsan on DCs. Although we provide evidence of the effects of ginsan on DCs, important questions remain to be answered: 1) Which receptors on the DC surface are involved in the stimulatory activity of ginsan? and 2) How does ginsan transduce the activation signals in DCs? Taken together, the results of the present study provide valuable information on ginsan and on the application of ginsan in clinical medicine.
ACKNOWLEDGEMENTS
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (KRF-2008-313-E00609).
ABBREVIATIONS
- IL
interleukin
- TNF
tumor necrosis factor
- ELISA
enzyme-linked immunosorbent assay
- MHC
major histocompatibility complex
- CD
cluster of differentiation
- IFN
interferon
References
- 1.Ahn JY, Choi IS, Shim JY, Yun EK, Yun YS, Jeong G, Song JY. The immunomodulator ginsan induces resistance to experimental sepsis by inhibiting Toll-like receptor-mediated inflammatory signals. Eur J Immunol. 2006;36:37–45. doi: 10.1002/eji.200535138. [DOI] [PubMed] [Google Scholar]
- 2.Bonham CA, Lu L, Li Y, Hoffman RA, Simmons RL, Thomson AW. Nitric oxide production by mouse bone marrow-derived dendritic cells: implications for the regulation of allogeneic T cell responses. Transplantation. 1996;62:1871–1877. doi: 10.1097/00007890-199612270-00033. [DOI] [PubMed] [Google Scholar]
- 3.Corinti S, Albanesi C, la Sala A, Pastore S, Girolomoni G. Regulatory activity of autocrine IL-10 on dendritic cell functions. J Immunol. 2001;166:4312–4318. doi: 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- 4.Esche C, Shurin GV, Kirkwood JM, Wang GQ, Rabinowich H, Pirtskhalaishvili G, Shurin MR. Tumor necrosis factor-alpha-promoted expression of Bcl-2 and inhibition of mitochondrial cytochrome c release mediate resistance of mature dendritic cells to melanoma-induced apoptosis. Clin Cancer Res. 2001;7:974s–979s. [PubMed] [Google Scholar]
- 5.Heufler C, Koch F, Stanzl U, Topar G, Wysocka M, Trinchieri G, Enk A, Steinman RM, Romani N, Schuler G. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur J Immunol. 1996;26:659–668. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- 6.Joo HG. Altered maturation of dendritic cells by taxol, an anticancer drug. J Vet Sci. 2003;4:229–234. [PubMed] [Google Scholar]
- 7.Joo HG, Goedegebuure PS, Sadanaga N, Nagoshi M, von Bernstorff W, Eberlein TJ. Expression and function of galectin-3, a beta-galactoside-binding protein in activated T lymphocytes. J Leukoc Biol. 2001;69:555–564. [PubMed] [Google Scholar]
- 8.Kim HJ, Kim MH, Byon YY, Park JW, Jee Y, Joo HG. Radioprotective effects of an acidic polysaccharide of Panax ginseng on bone marrow cells. J Vet Sci. 2007;8:39–44. doi: 10.4142/jvs.2007.8.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim MH, Joo HG. Immunostimulatory effects of fucoidan on bone marrow-derived dendritic cells. Immunol Lett. 2008;115:138–143. doi: 10.1016/j.imlet.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Larsen CP, Ritchie SC, Hendrix R, Linsley PS, Hathcock KS, Hodes RJ, Lowry RP, Pearson TC. Regulation of immunostimulatory function and costimulatory molecule (B7-1 and B7-2) expression on murine dendritic cells. J Immunol. 1994;152:5208–5219. [PubMed] [Google Scholar]
- 11.Lee YS, Chung IS, Lee IR, Kim KH, Hong WS, Yun YS. Activation of multiple effector pathways of immune system by the antineoplastic immunostimulator acidic polysaccharide ginsan isolated from Panax ginseng. Anticancer Res. 1997;17:323–331. [PubMed] [Google Scholar]
- 12.Lu L, Bonham CA, Chambers FG, Watkins SC, Hoffman RA, Simmons RL, Thomson AW. Induction of nitric oxide synthase in mouse dendritic cells by IFN-gamma, endotoxin, and interaction with allogeneic T cells: nitric oxide production is associated with dendritic cell apoptosis. J Immunol. 1996;157:3577–3586. [PubMed] [Google Scholar]
- 13.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 14.Mosca PJ, Hobeika AC, Clay TM, Nair SK, Thomas EK, Morse MA, Lyerly HK. A subset of human monocyte-derived dendritic cells expresses high levels of interleukin-12 in response to combined CD40 ligand and interferon-gamma treatment. Blood. 2000;96:3499–3504. [PubMed] [Google Scholar]
- 15.Shin JY, Song JY, Yun YS, Yang HO, Rhee DK, Pyo S. Immunostimulating effects of acidic polysaccharides extract of Panax ginseng on macrophage function. Immunopharmacol Immunotoxicol. 2002;24:469–482. doi: 10.1081/iph-120014730. [DOI] [PubMed] [Google Scholar]
- 16.Song JY, Han SK, Son EH, Pyo SN, Yun YS, Yi SY. Induction of secretory and tumoricidal activities in peritoneal macrophages by ginsan. Int Immunopharmacol. 2002;2:857–865. doi: 10.1016/s1567-5769(01)00211-9. [DOI] [PubMed] [Google Scholar]
- 17.Song JY, Han SK, Bae KG, Lim DS, Son SJ, Jung IS, Yi SY, Yun YS. Radioprotective effects of ginsan, an immunomodulator. Radiat Res. 2003;159:768–774. doi: 10.1667/0033-7587(2003)159[0768:reogai]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 18.Steinman RM, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev. 1997;156:25–37. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 19.Yoneyama H, Matsuno K, Matsushima K. Migration of dendritic cells. Int J Hematol. 2005;81:204–207. doi: 10.1532/IJH97.04164. [DOI] [PubMed] [Google Scholar]