Abstract
We investigated whether deficiency of inducible nitric oxide synthase (iNOS) could prevent isoproterenol-induced cardiac hypertrophy in iNOS knockout (KO) mice. Isoproterenol was continuously infused subcutaneously (15 mg/kg/day) using an osmotic minipump. Isoproterenol reduced body weight and fat mass in both iNOS KO and wild-type mice compared with saline-infused wild-type mice. Isoproterenol increased the heart weight in both iNOS KO and wild-type mice but there was no difference between iNOS KO and wild-type mice. Posterior wall thickness of left ventricle showed the same tendency with heart weight. Protein level of iNOS in the left ventricle was increased in isoproterenol-infused wild-type mice. The gene expression of interleukin-6 (IL-6) and transforming growth factor-β (TGF-β) in isoproterenol-infused wild-type was measured at 2, 4, 24, and 48-hour and isoproterenol increased both IL-6 (2, 4, 24, and 48-hour) and TGF-β (4 and 24-hour). Isoproterenol infusion for 7 days increased the mRNA level of IL-6 and TGF-β in iNOS KO mice, whereas the gene expression in wild-type mice was not increased. Phosphorylated form of extracellular signal-regulated kinases (pERK) was also increased by isoproterenol at 2 and 4-hour but was not increased at 7 days after infusion in wild-type mice. However, the increased pERK level in iNOS KO mice was maintained even at 7 days after isoproterenol infusion. These results suggest that deficiency of iNOS does not prevent isoproterenol-induced cardiac hypertrophy and may have potentially harmful effects on cardiac hypertrophy.
Keywords: Inducible nitric oxide synthase, Isoproterenol, Cardiac hypertrophy
INTRODUCTION
Pathologic cardiac hypertrophy is an important risk factor for heart failure (Levy et al., 1990). Although hypertension and loss of cardiomyocytes following ischemic insult are the main causes of hypertrophy (Rajabi et al., 2007), there is accumulating evidence that adrenergic overactivation induces cardiac hypertrophy. Patients with pathologic cardiac hypertrophy demonstrate increased circulating noradrenalin levels and enhanced mean discharge frequency in peripheral sympathetic nerves compared with subject without hypertrophy (Greenwood et al., 2001; Schlaich et al., 2003; Strand et al., 2006; Osadchii, 2007). In line with this finding, administration of adrenergic agonists such as isoproterenol induces cardiac hypertrophy in experimental animals (Osadchii, 2007) and isoproterenol- induced cardiac hypertrophy is reliable, reproducible, and well-characterized model of cardiac hypertrophy (Szabo et al., 1975; Krenek et al., 2009).
Nitric oxide (NO) is a gas molecule that plays critical roles in a wide variety of physiological function including vascular dilation, synaptic transmission, and immune regulation (Tsuchiya et al., 2007). NO is produced by three isoforms of nitric oxide synthase (NOS): endothelial NOS (eNOS), neuronal NOS (nNOS), and inducible NOS (iNOS) (Tsuchiya et al., 2007). All three isoforms are present in the heart (Kelly et al., 1996). While eNOS and nNOS are constitutively present, iNOS produces greater amounts of NO compared to eNOS and nNOS and is induced under various pathologic conditions (Kelly et al., 1996). Activation of iNOS has been demonstrated in hypertrophied heart and overexpression of iNOS induces cardiac hypertrophy (CMungrue et al., 2002; Ji et al., 2008). However, it is not known whether deletion of iNOS prevents isoproterenol-induced cardiac hypertrophy. In the present study we investigated whether isoproterenol-induced cardiac hypertrophy is prevented in iNOS knockout mice.
METHODS
Animals
Mice harboring a selectively disrupted gene encoding iNOS (Jax/tm1) and wild type C57BL/6J mice were housed in the animal unit of the College of Medicine at Yeungnam University. Mice were housed in a group cage in a room on an alternating 12 hours light/dark cycle (lights-on at 7:00 and off at 19:00). The mice were fed a standard chow diet and given ad libitum access to water. This study was conducted in accordance with the guidelines for the care and use of laboratory animals provided by Yeungnam University. The mice received a continuous administration of isoproterenol (15 mg/kg/day) or 0.9% saline for 1 week through a subcutaneously implanted osmotic minipump (Alzet, Cupertino, CA, USA). The minipump was inserted into skin in the interscapular region under anesthesia (25 mg/kg body weight, tiletamine and zolezepam; 10 mg/kg body weight, xylazine), and the small wound was closed with silk suture. After 1 week, the mice were anesthetized and blood was withdrawn from the orbital sinus. Heart was weighed and the left ventricles was excised and stored at -80℃ for the measurement of expression of genes and proteins. Percentage of heart weight to body weight was used as an indicator of cardiac hypertrophy.
Echocardiography
Echocardiograms of iNOS knockout mice and wild-type mice were performed on mice anesthetized with intraperitoneal injection of anesthetics after 1 week of isoproterenol or saline administration. Echo imaging was acquired using a Sequoia C512 (Acuson, Mountainview, CA, USA) platform equipped with a 15 MHz linear transducer. Measurements were performed in triplicate using the leading edge convention for myocardial borders, as defined by the American Society of Echocardiography. Posterior wall thickness in diastole standardized with body weight was used as an indicator of cardiac hypertrophy.
Real-time polymerase chain reaction (PCR)
Left ventricle of approximately 25 mg was homogenized in TRI reagent (Sigma-Aldrich, St. Louis, MO, USA) using an Ultra-Turrax T25 (Janke & Kunkel, IKA-Labortechnik, Staufel, Germany). RNA was reverse transcribed to cDNA from 1 µg of total RNA using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Quantitative real-time PCR was performed using the Real-Time PCR 7500 Software system and Power SYBR Green PCR master mix (Applied Biosystems), according to the manufacture's instructions. Expression levels of β-actin were used for sample normalization. The reactions were incubated at 95℃ for 10 min, followed by 45 cycles at 95℃ for 15 s, 55℃ for 20 s, and 72℃ for 35 s for interleukin-6 (IL-6). Primers for mouse β-actin and IL-6 were based on NCBI's nucleotide database and designed using the Primer Express program (Applied Biosystems):β-actin (121 bp: forward, 5'-TGG ACA GTG AGG CAA GGA TAG-3'; reverse, 5'-TAC TGC CCT GGC TCC TAG CA-3'), IL-6 (71 bp: forward, 5'-AAA TGA TGG ATG CTA CCA AAC T-3'; reverse, 5'-CCA GAA GAC CAG AGG AAA TTT T-3'). The reactions for transforming growth factor-β (TGF-β) used the same condition as with IL-6, except for the annealing temperature, which was 52℃ instead of 55℃. Primers for mouse β-actin and TGF-β were also based on NCBI's nucleotide database and designed using the Primer Express program (Applied Biosystems):β-actin (71 bp: forward, 5'-CCA ACC GTG AAA AGA TGA-3'; reverse, 5'- CTG GAT GGC TAC GTA CAT G-3'), TGF-β (72 bp: forward, 5'-CAA CGC CAT CTA TGA GAA AA-3'; reverse, 5'-CGA ATG TCT GAC GTA TTG AAG A-3').
Western blotting
Left ventricle was used for measurement of protein level of phosphorylated extracellular signal-regulated kinase (pERK), ERK, and iNOS. Left ventricle of approximately 25 mg was homogenized in a lysis buffer (Invitrogen, Carlsbad, CA, USA) containing 1% NP40, 150 mM NaCl, 5 mM MgCl, 10 mM HEPES, leupeptin, and pepstatin A. Total protein was separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophosresis. The protein was then transferred to a 0.45 µm polyvinylidene fluoride membrane (Gelman Sciences, East Hill, NY, USA). After blocking with 5% skin milk/10 mM Tris-HCl, pH 7.4/150 mM NaCl/0.1% Tween 20, the membrane was incubated overnight at 4℃ with the primary antibodies (diluted 1 : 1,000). The specific antibody binding was detected using 1 : 2,000 dilution of sheep anti-rabbit IgG horseradish peroxidase (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 1 hour at room temperature and visualized using an enhanced chemiluminescence detection regent (Amersham, Buckinghamshire, UK).
Statistical analyses
The results are expressed as mean±SE. Differences among the groups were assessed via one-way analysis of variance followed by LSD test. All statistical analyses were conducted using the SPSS system (SPSS, Chicago, IL, USA).
RESULTS
Body composition and heart weight
Body weight and epididymal fat mass were lower in iNOS knockout (KO) mice than wild-type mice in both saline- and isoproterenol-infused groups, which may be caused by increased locomotor activity in iNOS KO mice (Cha, 2009). Isoproterenol infusion reduced epididymal fat mass in both iNOS KO and wild-type mice (Fig. 1). Isoproterenol increases lipolytic activity (Pelat et al., 2003) and chronic activation of adrenergic receptors leads to reduced fat mass (Collins and Surwit, 2001). Heart weight presented as percentage of heart weight to body weight was increased in isoproterenol-infused iNOS KO and wild-type mice compared with saline-infused wild-type mice, but there was no difference in heart weight between iNOS KO and wild-type mice. Posterior wall thickness of left ventricle was measured with echocardiography under anesthesia; the wall thickness was not different between the two groups (Fig. 1).
Fig. 1.
Body weight (A), epididymal fat mass (B), heart weight (C) and posterior wall thickness (PWT) of left ventricle (D) in inducible nitric oxide synthase (iNOS) knockout (black bar) and wild-type (white bar) mice infused with saline or isoproterenol. The experimental cases in each group are 6 to 9. The results are presented as mean±SE. *p<0.05 vs. saline-infused corresponding control in wild-type and iNOS knockout and #p<0.05 vs. corresponding wild-type in saline and isoproterenol groups.
Effect of isoproterenol on cardiac iNOS expression
The mRNA level of iNOS was significantly lower in iNOS KO mice compared with wild-type mice. Isoproterenol infusion using an osmotic minipump did not affect iNOS mRNA expression both in wild-type and iNOS KO mice. However, iNOS protein level was increased in isoproterenol-infused wild-type mice while iNOS protein was not detected in iNOS KO mice both in saline- and isoproterenol-infused groups (Fig. 2). Isoproterenol has no effect on iNOS mRNA in wild-type mice that may be caused by period of time isoproterenol is infused. Since isoproterenol has been infused for 7 days, the increased mRNA level of NOS in early period may return to normal at 7 days after isoproterenol infusion.
Fig. 2.
The effect of isoporterenol infusion on the mRNA expression (A) and protein level (B) of left ventricle in inducible nitric oxide synthase (iNOS) knockout (black bar) and wild-type mice (white bar). The experimental cases in each group are 4 to 6. The results are presented as mean±SE. *p<0.05 vs. saline-infused corresponding control in wild-type and iNOS knockout and #p<0.05 vs. corresponding wild-type in saline and isoproterenol groups.
Expression of genes and protein involved in cardiac hypertrophy
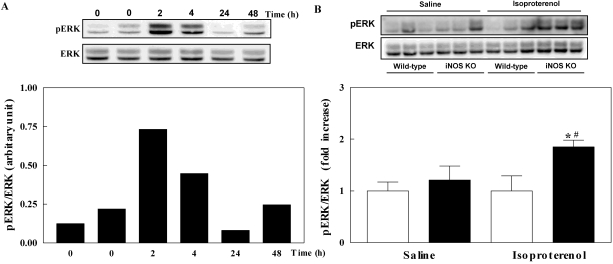
IL-6 and TGF-β has been known to be associated with isoproterenol-infused cardiac hypertrophy. Firstly, we investigated whether these two genes are increased in isoproterenol-infused wild-type mice in a time dependent manner at 2, 4, 24, and 48 hours. Isoproterenol infusion increased the mRNA level of IL-6 and TGF-β that peaked at 2 and 4 hours after infusion, respectively and reduced after that. Next, we measured the gene expression of IL-6 and TGF-β in the hypertrophied heart after 7-day isoproterenol infusion in wild-type and iNOS KO mice. The mRNA level of IL-6 and TGF-β was increased in isoproterenol-infused iNOS KO mice compared with saline-infused wild-type mice. The mRNA level of both genes in isoproterenol-infused wild-type mice was not increased (Fig. 3). Since the mitogen activated protein kinase (MAPK) subfamily ERK is also associated with cardiac hypertrophy (Bueno and Molkentin, 2002), we investigated the ERK activities by measuring the phosphorylated form of this protein. Phosphorylated ERK (pERK) was increased at 2 and 4 hours in isoproterenol-infused wild-type mice and was returned to normal at 24 and 48 hours. Isoproterenol infusion for 7 days increased pERK in iNOS KO mice but did not increase pERK in wild-type mice (Fig. 4). Like iNOS gene expression, the stimulatory effect of isoproterenol on the gene expression of IL-6 and TGF-β and pERK level in early period in wild-type mice has been vanished at 7 days after infusion.
Fig. 3.
The mRNA levels of interleukin-6 (IL-6) and transforming growth factor-β (TGF-β) in the left ventricle of mice. The mRNA level of IL-6 (A) and TGF-β (B) in isoproterenol-infused wild-type mice in a time dependent manner. The mRNA level of IL-6 (C) and TGF-β (D) in inducible nitric oxide synthase (iNOS) knockout (black bar) and wild-type (white bar) mice infused with saline or isoproterenol. The experimental cases in C and D are 6 to 9 in each group. The results are presented as mean±SE. *p<0.05 vs. saline-infused iNOS knockout and #p<0.05 vs. isoproterenol-infused wild-type.
Fig. 4.
Phosphorylation of extracellular signal-regulated kinases (ERK) in the left ventricle of mice. Phosphorylation of ERK in isoproterenol-infused wild-type mice in a time dependent manner (A). Phosphorylation of ERK in inducible nitric oxide synthase (iNOS) knockout (black bar) and wild-type (white bar) mice infused with saline or isoproterenol (B). The experimental cases in B are 6 to 9 in each group. The results are presented as mean±SE. *p<0.05 vs. saline-infused iNOS knockout and #p<0.05 vs. isoproterenol-infused wild-type.
DISCUSSION
This study demonstrates the isoproterenol increases heart weight in both wild-type and iNOS knockout mice. This result suggests that lack of iNOS does not prevent isoproterenol-induced cardiac hypertrophy. Interestingly, the increased expression of genes involved in cardiac hypertrophy and ERK activity was maintained in iNOS knockout mice at 7 days after infusion, while they returned to normal in wild-type mice.
Induction of iNOS in cardiac hypertrophy has been demonstrated previously. The level of mRNA for vascular endothelial growth factor and iNOS are increased in hypertrophied heart induced by abdominal aortic banding (Ji et al., 2008). Administration of isoproterenol increases iNOS immunoreactivity in the heart of Sprague Dawley rats (Zhang et al., 2008). Consistent with these previous results, the present study showed that chronic infusion of isoporterenol increased the protein level of iNOS in the left ventricle. Increased iNOS expression in heart causes cardiac hypertrophy in previous study. Chronic cardiac-specific upregulation of iNOS in transgenic mice leads to cardiac fibrosis, hypertrophy, and dilation that are accompanied with increased production of peroxynitrite (Cmungrue et al., 2002). While myoglobin knockout mice or iNOS transgenic mice display normal heart weight and cardiac function, mice deficient in myoglobin with a concomitant high level of cardiac specific iNOS expression shows increased heart weight and reduced heart function (Godecke et al., 2003). Therefore, we hypothesized that a deficiency of iNOS could prevent isoproterenol-induced cardiac hypertrophy. However, presently, a lack of iNOS did not affect isoproterenol-induced cardiac hypertrophy.
The effect of lack of iNOS on cardiac hypertrophy has been investigated previously in another experimental animal model and the results have been inconsistent. Deficiency of iNOS partially prevents cardiac hypertrophy induced by chronic transverse aortic constriction (Zhang et al., 2007). However, cardiac hypertrophy induced by tumor necrosis factor-α (TNF-α) overexpression was not prevented in iNOS knockout mice (Funakoshi et al., 2002). Additionally, a lack of iNOS was shown to induce cardiac hypertrophy and increases collagen synthesis (Kundu et al., 2009) and iNOS-derived NO production mediates the antihypertrophic effect of brain natriuretic pepetide (BNP) (Wang et al., 2007). In the present study, we showed for the first time that disruption of iNOS did not prevent isoproterenol- induced cardiac hypertrophy. Currently, the basis of these inconsistent results is unclear, although it is conceivable that the basis may be differences in experimental conditions and animal models.
IL-6, a member of IL-6 family of cytokines, plays a major role in cardiac hypertrophy and exerts its effect through glycoprotein 130 receptor (Barry et al., 2008). IL-6 transgenic mice display increased left ventricular wall thickness and the expression of atrial natriuretic peptides and BNP (Tanaka et al., 2001). These latter two peptides are known to be increased in hypertrophied heart (Gardner et al., 2007). Isoproterenol increases IL-6 expression in the heart of Wistar rats previously (Mikaelian et al., 2008). ERK, a member of the MAPK subfamily, is involved in cardiac hypertrophy (Bueno and Molkentin, 2002) and mediates the induction of cardiac hypertrophy by IL-6 family (IKodama et al., 2000). In the present study, we showed that isoproterenol increased IL-6 and ERK only in early period in wild-type mice. However, lack of iNOS showed increased gene expression of IL-6 and ERK activity even at 7 days after infusion. The blunted response of chronically infused isoproterenol on gene expression and pERK in wild-type may be caused by the period of time isoproterenol was infused. Chronic administration of isoproterenol abolishes isoproterenol-mediated early response in the heart (Zhang et al., 2005) that is consistent with the present study. Nevertheless, isoproterenol-mediated response was maintained in chronically infused iNOS knockout mice. These results suggest that deficiency of iNOS has a potentially harmful effect on cardiac hypertrophy. This notion is supported by the fact that mRNA expression of TGF-β was presently increased in chronically infused iNOS knockout mice.
The mRNA expression of TGF-β is increased in the left ventricle of patients with idiopathic cardiomyopathy and dilated cardiomyopathy (Li et al., 1997; Pauschinger et al., 1999) and in an animal model of pressure overload hypertrophy (Villarreal and Dillmann, 1992). TGF-β is particularly expressed in hypertrophic myocardium during transition from stable hypertrophy to heart failure (Boluyt et al., 1994). Like IL-6 and ERK, the current study showed that the increased expression of TGF-β by isoproterenol in early period has been abolished 48 hours after infusion in wild-type mice, whereas isoproterenol-mediated response was maintained in chronically infused iNOS knockout mice. Moreover, the mRNA expression of TGF-β in isoproterenol-infused iNOS knockout mice was increased more than 10 times suggesting that a deficiency of iNOS may make subjects more vulnerable to isoproterenol-induced heart failure. Sustained increases of IL-6 and TGF-β in iNOS knockout mice may be caused by modulation of signal transduction mediated by nuclear factor-κB or/and activator protein 1 in iNOS knockout mice (Zingarelli et al., 2002). Cytokines expression such as TNF-α and IL-6 in the heart following ischemia and reperfusion damage was more augmented in iNOS knockout mice than wild-type mice (Zingarelli et al., 2002).
Overall, iNOS deficiency does not affect isoproterenol-induced increased heart weight but increases the expression of genes involved in cardiac hypertrophy and ERK activity. These results suggest that iNOS does not play determinate roles in isoproterenol-induced cardiac hypertrophy, but that a lack of iNOS may have harmful effects rather than protective effects on cardiac hypertrophy. A pathological role of iNOS is well-known in heart, but a cardioprotective function of iNOS in the heart has previously been shown (Xi et al., 1999; Imamura et al., 2002). In line with these findings, although it is known that overproduction of NO from iNOS produces harmful effect on cardiac hypertrophy, the present results indicates that deletion of iNOS may also be potentially harmful. These complicated findings may also imply dual promotion/inhibition role of iNOS in cardiac hypertrophy under specific pathological conditions. Clearly, further studies are required to characterize the role of iNOS in cardiac hypertrophy.
ACKNOWLEDGEMENTS
This work was supported by the Korea Science and Engineering Foundation (KOSEF) through the Aging-associated Vascular Disease Research Center at Yeungnam University (R13-2005-005-01003-0).
ABBREVIATIONS
- eNOS
endothelial nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- IL-6
interleukin-6
- KO
knockout
- MAPK
mitogen activated protein kinase
- nNOS
neuronal nitric oxide synthase
- pERK
phosphorylated extracellular signal-regulated kinase
- TGF-β
transforming growth factor-β
- TNF-α
tumor necrosis factor-α
References
- 1.Barry SP, Davidson SM, Townsend PA. Molecular regulation of cardiac hypertrophy. Int J Biochem Cell Biol. 2008;40:2023–2039. doi: 10.1016/j.biocel.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 2.Boluyt MO, O'Neill L, Meredith AL, Bing OH, Brooks WW, Conrad CH, Crow MT, Lakatta EG. Alterations in cardiac gene expression during the transition from stable hypertrophy to heart failure. Marked upregulation of genes encoding extracellular matrix components. Circ Res. 1994;75:23–32. doi: 10.1161/01.res.75.1.23. [DOI] [PubMed] [Google Scholar]
- 3.Bueno OF, Molkentin JD. Involvement of extracellular signal-regulated kinases 1/2 in cardiac hypertrophy and cell death. Circ Res. 2002;91:776–781. doi: 10.1161/01.res.0000038488.38975.1a. [DOI] [PubMed] [Google Scholar]
- 4.Cha H. The Effect of iNOS deficiency on age-associated insulin resistance. 2009. MS Thesis. [Google Scholar]
- 5.Cmungrue IN, Gros R, You X, Pirani A, Azad A, Csont T, Schulz R, Butany J, Stewart DJ, Husain M. Cardiomyocyte overexpression of iNOS in mice results in peroxynitrite generation, heart block, and sudden death. J Clin Invest. 2002;109:735–743. doi: 10.1172/JCI13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins S, Surwit RS. The beta-adrenergic receptors and the control of adipose tissue metabolism and thermogenesis. Recent Prog Horm Res. 2001;56:309–328. doi: 10.1210/rp.56.1.309. [DOI] [PubMed] [Google Scholar]
- 7.Funakoshi H, Kubota T, Kawamura N, Machida Y, Feldman AM, Tsutsui H, Shimokawa H, Takeshita A. Disruption of inducible nitric oxide synthase improves beta-adrenergic inotropic responsiveness but not the survival of mice with cytokine-induced cardiomyopathy. Circ Res. 2002;90:959–965. doi: 10.1161/01.res.0000017632.83720.68. [DOI] [PubMed] [Google Scholar]
- 8.Gardner DG, Chen S, Glenn DJ, Grigsby CL. Molecular biology of the natriuretic peptide system: implications for physiology and hypertension. Hypertension. 2007;49:419–426. doi: 10.1161/01.HYP.0000258532.07418.fa. [DOI] [PubMed] [Google Scholar]
- 9.Godecke A, Molojavyi A, Heger J, Flogel U, Ding Z, Jacoby C, Schrader J. Myoglobin protects the heart from inducible nitric-oxide synthase (iNOS)-mediated nitrosative stress. J Biol Chem. 2003;278:21761–21766. doi: 10.1074/jbc.M302573200. [DOI] [PubMed] [Google Scholar]
- 10.Greenwood JP, Scott EM, Stoker JB, Mary DA. Hypertensive left ventricular hypertrophy: relation to peripheral sympathetic drive. J Am Coll Cardiol. 2001;38:1711–1717. doi: 10.1016/s0735-1097(01)01600-x. [DOI] [PubMed] [Google Scholar]
- 11.IKodama H, Fukuda K, Pan J, Sano M, Takahashi T, Kato T, Makino S, Manabe T, Murata M, Ogawa S. Significance of ERK cascade compared with JAK/STAT and PI3-K pathway in gp130-mediated cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2000;279:H1635–H1644. doi: 10.1152/ajpheart.2000.279.4.H1635. [DOI] [PubMed] [Google Scholar]
- 12.Imamura G, Bertelli AA, Bertelli A, Otani H, Maulik N, Das DK. Pharmacological preconditioning with resveratrol: an insight with iNOS knockout mice. Am J Physiol Heart Circ Physiol. 2002;282:H1996–H2003. doi: 10.1152/ajpheart.01013.2001. [DOI] [PubMed] [Google Scholar]
- 13.Ji K, Minakawa M, Fukui K, Suzuki Y, Fukuda I. Increased superoxide radical with a decrease in vascular endothelial growth factor and inducible nitric oxide synthase level leads to the progression of left ventricular hypertrophy in a pressure-overload rat heart model. Ann Thorac Cardiovasc Surg. 2008;14:210–217. [PubMed] [Google Scholar]
- 14.Kelly RA, Balligand JL, Smith TW. Nitric oxide and cardiac function. Circ Res. 1996;79:363–380. doi: 10.1161/01.res.79.3.363. [DOI] [PubMed] [Google Scholar]
- 15.Krenek P, Kmecova J, Kucerova D, Bajuszova Z, Musil P, Gazova A, Ochodnicky P, Klimas J, Kyselovic J. Isoproterenol-induced heart failure in the rat is associated with nitric oxide-dependent functional alterations of cardiac function. Eur J Heart Fail. 2009;11:140–146. doi: 10.1093/eurjhf/hfn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kundu S, Kumar M, Sen U, Mishra PK, Tyagi N, Metreveli N, Lominadze D, Rodriguez W, Tyagi SC. Nitrotyrosinylation, remodeling and endothelial-myocyte uncoupling in iNOS, cystathionine beta synthase (CBS) knockouts and iNOS/CBS double knockout mice. J Cell Biochem. 2009;106:119–126. doi: 10.1002/jcb.21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the framingham heart study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 18.Li RK, Li G, Mickle DA, Weisel RD, Merante F, Luss H, Rao V, Christakis GT, Williams WG. Overexpression of transforming growth factor-beta1 and insulin-like growth factor-I in patients with idiopathic hypertrophic cardiomyopathy. Circulation. 1997;96:874–881. doi: 10.1161/01.cir.96.3.874. [DOI] [PubMed] [Google Scholar]
- 19.Mikaelian I, Coluccio D, Morgan KT, Johnson T, Ryan AL, Rasmussen E, Nicklaus R, Kanwal C, Hilton H, Frank K, Fritzky L, Wheeldon EB. Temporal gene expression profiling indicates early up-regulation of interleukin-6 in isoproterenol-induced myocardial necrosis in rat. Toxicol Pathol. 2008;36:256–264. doi: 10.1177/0192623307312696. [DOI] [PubMed] [Google Scholar]
- 20.Osadchii OE. Cardiac hypertrophy induced by sustained beta-adrenoreceptor activation: pathophysiological aspects. Heart Fail Rev. 2007;12:66–86. doi: 10.1007/s10741-007-9007-4. [DOI] [PubMed] [Google Scholar]
- 21.Pauschinger M, Knopf D, Petschauer S, Doerner A, Poller W, Schwimmbeck PL, Kuhl U, Schultheiss HP. Dilated cardiomyopathy is associated with significant changes in collagen type I/III ratio. Circulation. 1999;99:2750–2756. doi: 10.1161/01.cir.99.21.2750. [DOI] [PubMed] [Google Scholar]
- 22.Pelat M, Verwaerde P, Galitzky J, Lafontan M, Berlan M, Senard JM, Montastruc JL. High isoproterenol doses are required to activate beta3-adrenoceptor-mediated functions in dogs. J Pharmacol Exp Ther. 2003;304:246–253. doi: 10.1124/jpet.102.040691. [DOI] [PubMed] [Google Scholar]
- 23.Rajabi M, Kassiotis C, Razeghi P, Taegtmeyer H. Return to the fetal gene program protects the stressed heart: a strong hypothesis. Heart Fail Rev. 2007;12:331–343. doi: 10.1007/s10741-007-9034-1. [DOI] [PubMed] [Google Scholar]
- 24.Schlaich MP, Kaye DM, Lambert E, Sommerville M, Socratous F, Esler MD. Relation between cardiac sympathetic activity and hypertensive left ventricular hypertrophy. Circulation. 2003;108:560–565. doi: 10.1161/01.CIR.0000081775.72651.B6. [DOI] [PubMed] [Google Scholar]
- 25.Strand AH, Gudmundsdottir H, Os I, Smith G, Westheim AS, Bjornerheim R, Kjeldsen SE. Arterial plasma noradrenaline predicts left ventricular mass independently of blood pressure and body build in men who develop hypertension over 20 years. J Hypertens. 2006;24:905–913. doi: 10.1097/01.hjh.0000222761.07477.7b. [DOI] [PubMed] [Google Scholar]
- 26.Szabo J, Csaky L, Szegi J. Experimental cardiac hypertrophy induced by isoproterenol in the rat. Acta Physiol Acad Sci Hung. 1975;46:281–285. [PubMed] [Google Scholar]
- 27.Tanaka T, Kanda T, Itoh T, Tsugawa H, Takekoshi N, Yamakawa J, Kurimoto M, Kurabayashi M. Increased cardiac weight in interleukin-6 transgenic mice with viral infection accompanies impaired expression of natriuretic peptide genes. Res Commun Mol Pathol Pharmacol. 2001;110:275–283. [PubMed] [Google Scholar]
- 28.Tsuchiya K, Sakai H, Suzuki N, Iwashima F, Yoshimoto T, Shichiri M, Hirata Y. Chronic blockade of nitric oxide synthesis reduces adiposity and improves insulin resistance in high fat-induced obese mice. Endocrinology. 2007;148:4548–4556. doi: 10.1210/en.2006-1371. [DOI] [PubMed] [Google Scholar]
- 29.Villarreal FJ, Dillmann WH. Cardiac hypertrophy-induced changes in mRNA levels for TGF-beta 1, fibronectin, and collagen. Am J Physiol. 1992;262:H1861–H1866. doi: 10.1152/ajpheart.1992.262.6.H1861. [DOI] [PubMed] [Google Scholar]
- 30.Wang T, Yan M, Li J, Zheng X. The role of iNOS-derived NO in the antihypertrophic actions of B-type natriuretic peptide in neonatal rat cardiomyocytes. Mol Cell Biochem. 2007;302:169–177. doi: 10.1007/s11010-007-9438-1. [DOI] [PubMed] [Google Scholar]
- 31.Xi L, Jarrett NC, Hess ML, Kukreja RC. Essential role of inducible nitric oxide synthase in monophosphoryl lipid A-induced late cardioprotection: evidence from pharmacological inhibition and gene knockout mice. Circulation. 1999;99:2157–2163. doi: 10.1161/01.cir.99.16.2157. [DOI] [PubMed] [Google Scholar]
- 32.Zhang GX, Kimura S, Nishiyama A, Shokoji T, Rahman M, Yao L, Nagai Y, Fujisawa Y, Miyatake A, Abe Y. Cardiac oxidative stress in acute and chronic isoproterenol-infused rats. Cardiovasc Res. 2005;65:230–238. doi: 10.1016/j.cardiores.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, Knapton A, Lipshultz SE, Weaver JL, Herman EH. Isoproterenol-induced cardiotoxicity in sprague-dawley rats: correlation of reversible and irreversible myocardial injury with release of cardiac troponin T and roles of iNOS in myocardial injury. Toxicol Pathol. 2008;36:277–278. doi: 10.1177/0192623307313010. [DOI] [PubMed] [Google Scholar]
- 34.Zhang P, Xu X, Hu X, van Deel ED, Zhu G, Chen Y. Inducible nitric oxide synthase deficiency protects the heart from systolic overload-induced ventricular hypertrophy and congestive heart failure. Circ Res. 2007;100:1089–1098. doi: 10.1161/01.RES.0000264081.78659.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zingarelli B, Hake PW, Yang Z, O'Connor M, Denenberg A, Wong HR. Absence of inducible nitric oxide synthase modulates early reperfusion-induced NF-kappaB and AP-1 activation and enhances myocardial damage. Faseb J. 2002;16:327–342. doi: 10.1096/fj.01-0533com. [DOI] [PubMed] [Google Scholar]