Abstract
Although extracellular Ca2+ entry through the voltage-dependent Ca2+ channels plays an important role in the spontaneous phasic contractions of the pregnant rat myometrium, the role of the T-type Ca2+ channels has yet to be fully identified. The aim of this study was to investigate the role of the T-type Ca2+ channel in the spontaneous phasic contractions of the rat myometrium. Spontaneous phasic contractions and [Ca2+]i were measured simultaneously in the longitudinal strips of female Sprague-Dawley rats late in their pregnancy (on day 18~20 of gestation: term=22 days). The expression of T-type Ca2+ channel mRNAs or protein levels was measured. Cumulative addition of low concentrations (<1 µM) of nifedipine, a L-type Ca2+ channel blocker, produced a decrease in the amplitude of the spontaneous Ca2+ transients and contractions with no significant change in frequency. The mRNAs and proteins encoding two subunits (α1G, α1H) of the T-type Ca2+ channels were expressed in longitudinal muscle layer of rat myometrium. Cumulative addition of mibefradil, NNC 55-0396 or nickel induced a concentration-dependent inhibition of the amplitude and frequency of the spontaneous Ca2+ transients and contractions. Mibefradil, NNC 55-0396 or nickel also attenuated the slope of rising phase of spontaneous Ca2+ transients consistent with the reduction of the frequency. It is concluded that T-type Ca2+ channels are expressed in the pregnant rat myometrium and may play a key role for the regulation of the frequency of spontaneous phasic contractions.
Keywords: Calcium channels, Nickel, Mibefradil, NNC 55-0396, Spontaneous contractility
INTRODUCTION
The uterus maintains a sustained muscle tone to support the growing fetus without coordinated contractions during pregnancy (quiescence phase). At the end of gestation, it undergoes many changes regarding hormone activities, density and activity of ion channels, and of gap junctions, which result in rhythmic, forceful, and highly coordinated spontaneous contractions to labor (Riemer and Heymann, 1998; Challis et al., 2000; Parkington and Coleman, 2001).
It is well known that spontaneous phasic contraction of uterine smooth muscle - myometrium - is related to an increase in the concentration of intracellular free Ca2+ ([Ca2+]i) and that voltage-dependent Ca2+ channels represent the major machinery for [Ca2+]i elevation (Wray et al., 2003). Two types of Ca2+ channels, L (long-lasting)-type and T (transient)-type Ca2+ channel, have been described in the myometrium. L-type Ca2+ channel has been identified in myometrium by electrophysiological (Parkington and Coleman, 1988), pharmacologic (Chien et al., 1996; Collins et al., 1996), and molecular studies (Mershon, 1994). It is also known that Ca2+ entry during the action potential is via L-type Ca2+ channel which is opened by spontaneous pacemaker activity and is an essential component for excitation-contraction coupling in uterine smooth muscle (Riemer and Heymann, 1998; Parkington and Coleman, 2001). On the other hand, the presence and the functional significance of T-type Ca2+ channels in the myometrium are less well defined.
It has been previously demonstrated in electrophysiological studies that T-type Ca2+ channels are present in human (Young et al., 1993; Knock and Aaronson, 1999) myometrium but not evidenced in rat myometrium (Ohya and Sperelakis, 1989; Inoue and Sperelakis, 1991). It has been also demonstrated that the mRNAs of T-type Ca2+ channel are expressed in human myometrium (Blanks et al., 2007). However, in a recent molecular study on the rat, it was demonstrated that both Cav 3.1 (α1G) and Cav 3.2 (α1H), α-subunits of T-type Ca2+ channels, were expressed in circular and longitudinal layers of myometrium and that the relative expression profile of these channels differed, dependent on gestational age and layer (Ohkubo et al., 2005).
As the T-type Ca2+ channel may respond to the pacemaker potential and depolarize the plasma membrane sufficiently to allow for activation of other voltage-dependent ion channels such as L-type Ca2+ channels, elucidation of the role of T-type Ca2+ channels in spontaneous contractions may provide important clues to the nature of the molecular mechanism responsible for the generation of spontaneous contractions. In a recent study, it was demonstrated that treatment of nickel, a T-type Ca2+ channel inhibitor, reduced frequency without changing the force of spontaneous contractions in the human myometrium (Blanks et al., 2007). This indeed suggests that the T-type Ca2+ channels may be involved in the initiation of action potentials in myometrium, but the functional significance is not fully understood.
The aim of the present study was to investigate whether the T-type Ca2+ channels are present in rat myometrium and what the role of the T-type Ca2+ channels is in the spontaneous phasic contractions of the rat myometrium.
METHODS
The investigation conforms with the Guide for the Care and use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Simultaneous measurement of [Ca2+]i and force
Female Sprague-Dawley rats in their late pregnancy (on 18~20 days) were killed by cervical dislocation. All procedures were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee. The uterine horns were isolated and immediately placed in an ice-cold, oxygenated normal Tyrode solution composed of (in mmol/l): Glucose 12; NaCl 135; KCl 5.4; MgCl2 1.2; HEPES 10; CaCl2 2.5. Blood, placental tissue, endometrium and the circular smooth muscle layer were gently removed and longitudinal myometrial strips, approximately 1.5×3 mm from each horn, were dissected out with a fine scissor under a binocular microscope. One end of the tissue strip was tied by a thin thread to connect to the transducer.
[Ca2+]i was measured according to the method described by Yeon et al. (2002) using fluorescent Ca2+ indicator, Fura-2. The longitudinal strips were exposed to acetoxymethyl ester of Fura-2 (Fura-2/AM, 5 µM) and 0.02% cremophor EL in normal Tyrode solution for 3~4 hr at room temperature. At the end of the loading period, the muscle strips were washed with normal Tyrode solution for 30 min to remove extracellular Fura-2/AM and were held horizontally in a temperature-controlled 5 ml organ chamber. The normal Tyrode solution was maintained at 37℃ and was continuously aerated with 100% O2. After 30 min of washing in normal Tyrode solution, one end of the muscle strip was connected to force-displacement transducer (Harvard, Holliston, MA, USA) to monitor the muscle contraction. Muscle strips were stretched passively to the optimal length by imposing a stretch of 140% of resting length and equilibrated for 60 min. Muscle strips were illuminated alternately (48 Hz) at two excitation wavelengths (340 and 380 nm). The intensity of 500 nm fluorescence (F340 and F380) was measured by using a fluorimeter (CAF110, JASCO, Tokyo, Japan). The ratio of F340 to F380 (F340/F380) was calculated as an indicator of [Ca2+]i. After regular spontaneous phasic contractions had been established (0~60 min), several inhibitors were added to the strips to determine their effects on Ca2+ transient and force. In some experiments, 0-Ca2+ solution was used: normal Tyrode solution in which CaCl2 had been omitted and 1 mM EGTA added.
Reverse transcription - polymerase chain reaction
For the isolation of total RNA, the dissected myometrial tissue was broken down using a pestle in 1 ml easy-BLUE™ (Intron Biotechnology, South Korea) and isolation was achieved by means of the manufacturer's instructions. RNA concentration was measured by ultraviolet absorbance at 260 nm using a spectrophotometer. First-strand complementary DNA was synthesized by incubating 2 µg of RNA at 42℃ for 60 min in a final volume of 20 µl containing 5× RT buffer, 10 U/µl of AMV Reverse Transcriptase, 0.2 mM of oligo dT, 2.5 mM of deoxynucleoside triphosphate (dNTP) mixture, and 10 U/µl RNase inhibitor (Power cDNA Synthesis Kit, Intron Biotechnology, South Korea). Complementary DNA (2 µg) was amplified using primers for α1G and α1H in a final volume of 20 µl, containing 5 U/µl of Taq DNA polymerase (i-MAX™ DNA polymerase, Intron Biotechnology, South Korea), 2.5 mM of each dNTP, 10× PCR buffer, 20 pmol of α1G and α1H primers, and sufficient water. The PCR reaction mixtures were heated to 94℃ for 5 min and amplified in 35 cycles. Each cycle consisted of denaturation at 94℃ for 30 sec, annealing at 55.4℃ for 30 sec, and extension at 72℃ for 30 sec. The primers used were as follows: forward, 5'-gdaaagtctccaagcacatc-3'; reverse, 5'-ctgacagcaatggagtgtct-3' for the α1G subunit (with an expected PCR product of 262 base pairs); forward, 5'-ggacagtgaccaaagtgtga-3'; reverse, 5'-ccagctacaggctcatct-3' for the α1H subunit (with an expected PCR product of 218 base pairs). Mixtures were separated on a 1% agarose gel and after staining with ethidium bromide, PCR products were visualized under UV light.
Western blot
Longitudinal strips were dissected and quick-frozen in dry ice and homogenized in buffer containing Triton × 100 1 ml, NaCl 0.088 g, Tris base 0.012 g, NP40 50 µl, and water in final volume of 10 ml. Protein-matched samples (100 µg protein/lane) were subjected to electrophoresis on 6% SDS-polyacrylamide gels and then were transferred to nitrocellulose membranes. Reversible Ponceau staining of the membranes was performed to confirm the equal loading of protein. Membranes were incubated in 5% skim milk in PBS-Tween 20 buffer for 1 hr at room temperature and then were incubated for 2 hr at room temperature in the presence of primary antibodies to α1G (1:200; Alomone Labs, Jerusalem, Israel) and α1H (1:200; Alomone Labs, Jerusalem, Israel). Membranes were washed and then incubated with horseradish peroxidase conjugated secondary antibody (1:5,000; Calbiochem, Darmstadt, Germany) for 1 hr at room temperature. Immunoreactive bands were visualized by enhanced chemiluminescence (ECL; Amersham, Uppsala, Sweden). Developed films from ECL were scanned.
Drugs and chemicals
The following drugs were used: nifedipine (Sigma, St Louis, MO, USA), NNC 55-0396 ([(1S,2S)-2-(2-(N-[(3-Benzimidazol-2-yl)propyl]-N-methylamino)ethyl)-6-fluoro-1,2,3,4-tetrahydro-1-isopropyl-2-naphtyl cyclopropanecarboxylate dihydrochloride]) (Sigma, St Louis, MO, USA), mibefradil (Sigma, St Louis, MO, USA), nickel (Sigma, St Louis, MO, USA), Fura-2/AM (Molecular Probes, Eugene, OR, USA). General laboratory reagents were used analytical grade or better.
Statistics
Data are expressed as the mean±SEM and n indicates the number of strips. Force was expressed as a relative percentage of the amplitude of spontaneous phasic contractions or of the 70 mM K+ solution. Differences between means tested using ANOVA. Significant differences were taken at the p<0.05 level.
RESULTS
Effect of removing external Ca2+ and nifedipine on spontaneous Ca2+ transients and contractions
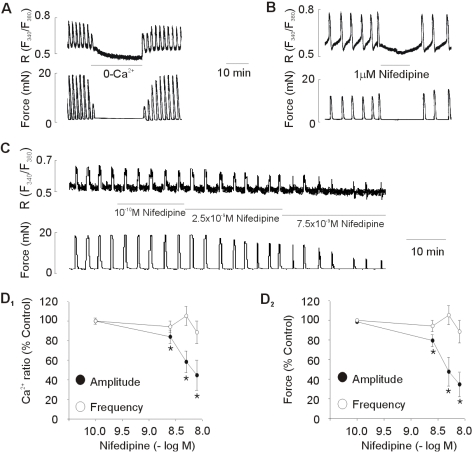
The myometrial strips from pregnant rats exhibited spontaneous rhythmic Ca2+ transients and contractions in normal Tyrode solution, with a mean contractile amplitude of 12.16±2.30 mN and mean frequency of 0.69±1.14 contractions/ min (n=10). Under control conditions, spontaneous Ca2+ transients and contractions of consistent amplitude and frequency could be recorded for several hours. The effect of removing external Ca2+ (0-Ca2+ solution) or 1 µM nifedipine, a blocker of L-type Ca2+ channels, on the spontaneous Ca2+ transients and contractions of uterus strips is shown in Fig. 1. The spontaneous contractions stopped and [Ca2+]i fell upon changing to 0-Ca2+ solution or adding 1 µM nifedipine (Fig. 1A, B).
Fig. 1.
Effect of removing external Ca2+ and nifedipine on spontaneous Ca2+ transients and contractions. (A, B) Effect of removing external Ca2+ (0-Ca2+) and 1 µM nifedipine on spontaneous Ca2+ transients and contractions. Representative recoding (C) and statistical evaluation (D) showing the concentration-response curve obtained by cumulative addition of low concentrations of nifedipine. Data are expressed as relative percentage of the control (amplitude before treatment of nifedipine). Results are expressed as mean±SEM of six experiments. *Control vs Nifedipine (p<0.05).
Effect of low concentration of nifedipine on spontaneous Ca2+ transients and contractions
To determine the role of L-type Ca2+ channels on the frequency and amplitude of spontaneous Ca2+ transients and contractions, effect of low concentration of nifedipine, a blocker of L-type Ca2+ channels, was tested. As shown in Fig. 1C, D, cumulative addition of low concentrations, which did not completely abolished spontaneous contractions, produced a decrease in the amplitude of spontaneous Ca2+ transients and contractions. However, in contrast, the frequency of spontaneous Ca2+ transients and contractions was not significantly changed by these concentrations of nifedipine.
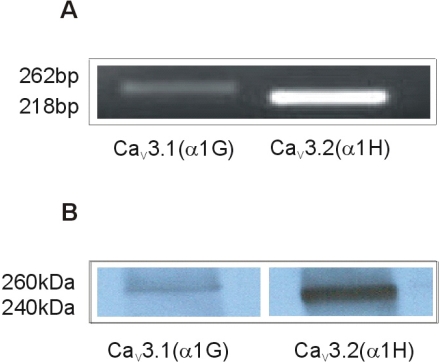
Expression of T-type Ca2+ channels in rat myometrium
Expression of the mRNAs and proteins encoding two subunits (α1G and α1H) of T-type Ca2+ channel was examined using comparative kinetic RT/PCR and western blot in longitudinal muscle layer. As shown in Fig. 2, two subunits were found to be expressed in longitudinal muscle layer of rat uterus.
Fig. 2.
Expression of mRNAs and proteins for α subunits (α1G and α1H) of T-type Ca2+ channel in longitudinal muscle layer of pregnant rat myometrium. Representative data of RT/PCR (A) and western blot (B). Immunoblots are representative of four independent preparations. The PCR was performed with 35 cycles and PCR products were followed by electrophoresis on a 1% agarose gel.
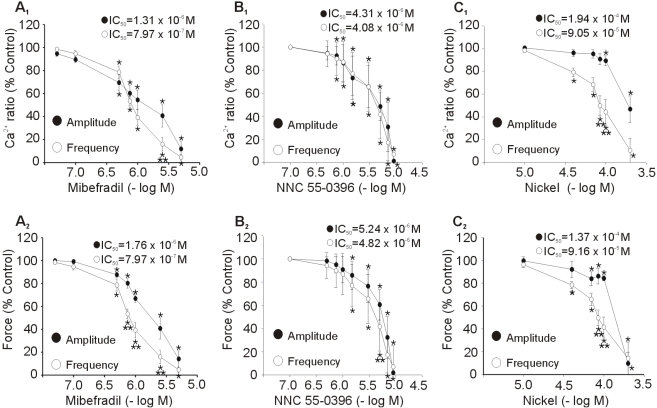
Effect of T-type Ca2+ channel blockers on spontaneous Ca2+ transients and contractions
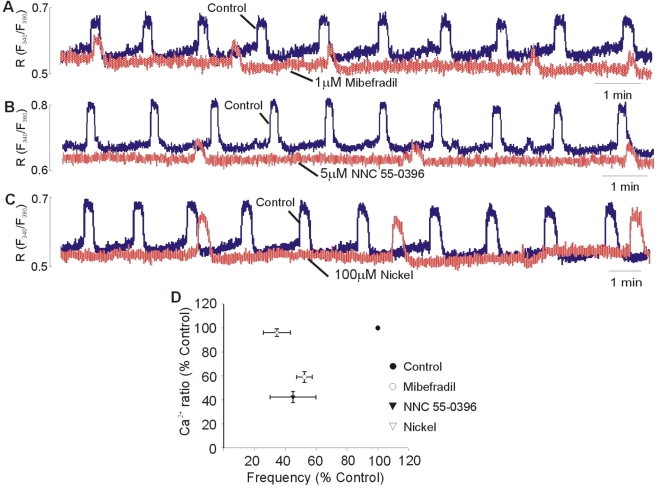
Effects of three different T-type Ca2+ channel blockers on spontaneous Ca2+ transients and contractions of uterus strips are shown in Fig. 3. Cumulative addition of mibefradil produced concentration-dependent inhibition of amplitude and frequency of spontaneous Ca2+ transients and contractions. The threshold concentration of mibefradil to produce an inhibitory effect on the amplitude and frequency for the Ca2+ transients and contractions was 0.5 µM. The mean IC50 values for mibefradil to inhibit the amplitude and frequency were 1.31×10-6 M and 7.97×10-7 M for Ca2+ transients, and 1.76×10-6 M and 7.97×10-7 M for contractions. NNC 55-0396 had similar inhibitory effect with mibefradil. The mean IC50 values for NNC 55-0396 to inhibit the amplitude and frequency were 4.31×10-6 M and 4.08×10-6 M for Ca2+ transients, and 5.24×10-6 M and 4.82×10-6 M for contractions. Cumulative addition of nickel produced a concentration-related inhibitory effect of the amplitude and frequency of spontaneous Ca2+ transients and contractions. The mean IC50 values for nickel inhibition of the amplitude and frequency were 1.94×10-4 M and 9.05×10-5 M for Ca2+ transients, and 1.37×10-4 M, 9.16×10-5 M for contractions. Mibefradil and NNC 55-0396 produced a similar concentration-response curve for inhibition of the amplitude and frequency, although the IC50 for frequency was lower than it for amplitude. However, nickel produced a steeper concentration-response curve for inhibition of the frequency than that of the amplitude.
Fig. 3.
Dose-response curve for the effect of T-type Ca2+ channel blockers on the spontaneous Ca2+ transients and contractions. (A1, B1, C1) Concentration-related reduction of the amplitude and frequency of spontaneous Ca2+ transients. (A2, B2, C2) concentration-related reduction of the amplitude and frequency of spontaneous contractions. Mibefradil (A), NNC 55-0396 (B), and nickel (C) were added cumulatively. Data are expressed as relative percentage of control (amplitude before treatment of blockers). Results are expressed as mean±SEM of seven experiments. *Control vs Blockers, **Amplitude vs Frequency (p<0.05).
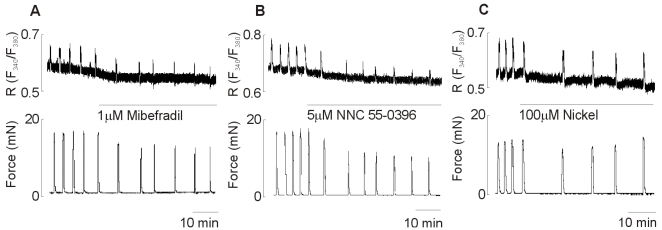
To investigate the blockers-related reduction of the amplitude and frequency of spontaneous Ca2+ transients and contractions, effects of each IC50 of T-type Ca2+ channel blockers on spontaneous Ca2+ transients and contractions were tested. As shown in Fig. 4, 1 µM mibefradil (Fig. 4A) and 5 µM NNC 55-0396 (Fig. 4B) reduced the amplitude as well as frequency for the spontaneous Ca2+ transients and contractions, respectively. However, 100 µM nickel (Fig. 4C) reduced the frequency of spontaneous Ca2+ transients and contractions but not the amplitude of them.
Fig. 4.
Representative recording for the effect of T-type Ca2+ channel blockers on spontaneous Ca2+ transients and contractions. Spontaneous Ca2+ transients (top) and contractions (bottom) before and during 1 µM mibefradil (A), 5 µM NNC 55-0396 (B), and 100 µM nickel (C) application. Data are representative of ten independent preparations.
Comparison of frequency and slope of rising phase of spontaneous Ca2+ transients in the presence and absence of T-type Ca2+ channel blockers
To clarify the role of the T-type Ca2+ channels on the frequency of the spontaneous Ca2+ transients and contractions, effects of the T-type Ca2+ channel blockers on the frequency and slope of the rising phase of Ca2+ transients were tested. Fig. 5 showed the spontaneous Ca2+ transients in the presence and absence of blockers. Mibefradil (Fig. 5A), NNC 55-0396 (Fig. 5B), and nickel (Fig. 5C) reduced the frequency of the spontaneous Ca2+ transients and attenuated the slope of rising phase of the spontaneous Ca2+ transient. Especially, nickel has more sensitive inhibitory effect on the inhibition of frequency compared than other inhibitors, mibefradil or NNC 55-0396 (Fig. 5D).
Fig. 5.
Effect of the T-type Ca2+ channel blockers on the frequency and slope of rising phase of spontaneous Ca2+ transients. Superimposed spontaneous Ca2+ transients under control conditions and in presence of 1 µM Mibefradil (A), 5 µM NNC 55-0396 (B), and 100 µM nickel (C), respectively. Each blocker was added in the bath solution after spontaneous Ca2+ transients were stable. Statistical evaluation (D) showing the Ca2+ ratio and frequency obtained by adding mibefradil, NNC 55-0396, and nifedipine. Data are expressed as relative percentage of control. Results are expressed as mean±SEM of six experiments.
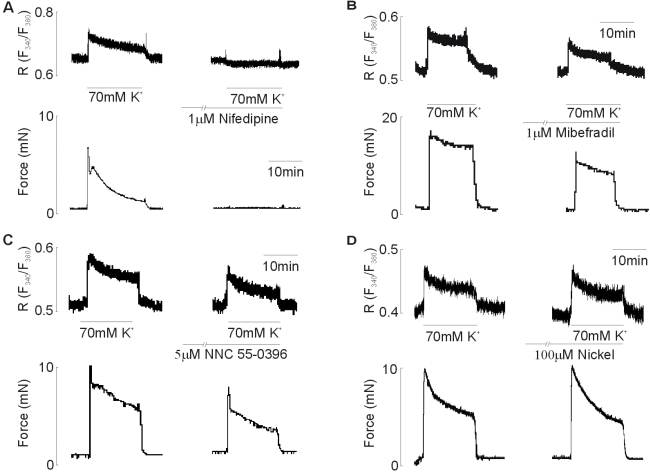
Effect of T-type Ca2+ channel blockers on the 70 mM KCl-induced contractions
From the above data it is clear that mibefradil and NNC 55-0396 reduce both the frequency and amplitude of the spontaneous Ca2+ transients and contractions. To determine whether T-type Ca2+ channel blockers used in the present study affect the L-type Ca2+ channels, effects of T-type Ca2+ channel blockers on the 70 mM KCl-induced contraction were examined. As shown in Fig. 6A, 1 µM nifedipine completely inhibited the 70 mM KCl-induced increase in the [Ca2+]i and contraction. 1 µM Mibefradil and 5 µM NNC55-0396 inhibited the 70 mM KCl-induced increase in the [Ca2+]i and force, respectively. Mibefradil caused 47.48±5.53% (p<0.05) decrease in [Ca2+]i and 16.15±6.54% decrease in force compared to that induced by 70 mM KCl (n=7, Fig. 6B). NNC 55-0396 also caused 22.56±2.08% (p<0.05) decrease in [Ca2+]i and 13.18±6.54% decrease in force compared to that induced by 70 mM KCl (n=8, Fig. 6C). However, in contrast, there was little effect on [Ca2+]i and force in response to 100 µM nickel. The decrease in [Ca2+]i and force was 4.73±2.05% and 3.1±2.93% (n=9), respectively, of the rise in [Ca2+]i and force produced by 70 mM KCl.
Fig. 6.
Effect of (A) nifedipine (1 µM), (B) mibefradil (1 µM), (C) NNC 55-0396 (5 µM), and (D) nickel (100 µM) on the 70 mM KCl-induced increase in [Ca2+]i and force. All drugs were added for 10 min before 70 mM KCl-induced contraction. Data are representative of seven~nine independent preparations.
DISCUSSION
In this study, it has been shown that the T-type Ca2+ channels are expressed on the pregnant rat myometrium and the T-type Ca2+ channels play an important role in the generation of the spontaneous phasic Ca2+ transients and contractions. Furthermore, our data suggests that Ca2+ influx through T-type Ca2+ channels may regulate the frequency of spontaneous phasic contractions.
The generation of the spontaneous phasic contractions is due to the ability of a cell to fire a regenerative action potential. Thus, it is important to understand the mechanisms underlying the spontaneous depolarization between action potentials in the uterine smooth muscle. It has been known that L-type Ca2+ channel is the major source of Ca2+ influx for contraction in both human and rat (Mironneau, 1973; Ohya and Sperelakis, 1989; Young et al., 1993). It is consistent with our results that the spontaneous Ca2+ transients and contractions were abolished by the removal of external Ca2+ and treatment of 1 µM nifedipine, L-type Ca2+ channel blocker.
The membrane potential in uterine smooth muscle cells is not stable, and in some cells, termed pacemakers, a spontaneous depolarization of the membrane occurs. The exact nature of the membrane currents and channels leading this depolarization in the myometrium is not known (Parkington and Coleman, 1988; Coleman and Parkington, 1990; Wray et al., 2003). In the present study, although nifedipine completely abolished the spontaneous Ca2+ transients and contractions, L-type Ca2+ channels may be not involved in the generation of the slow depolarization. L-type Ca2+ channels have a high voltage activation threshold (around -40 mV) (Jmari et al., 1986; Honore et al., 1989). Parkington et al. (1999) have shown that the value of the resting membrane potential recorded in the myometrium ranged from -80 to -55 mV between species. Taken together, these previous results represent that L-type Ca2+ channel may be involved in the firing of action potentials, but not in the generation of slow depolarization. In the present study, we also determined the role of L-type Ca2+ channel on the spontaneous Ca2+ transients and contractions by treatment of low concentration of nifedipine, L-type Ca2+ channel blockers. Cumulative addition of low concentration of nifedipine (Fig. 1), which did not completely abolished spontaneous contractions, produced a decrease in the amplitude of spontaneous contractions. However, in contrast, the frequency of spontaneous contractions did not significantly changed by nifedipine. This means that there should be other types of Ca2+ channels, which may be involved in the slow membrane depolarization to aid in the opening of the L-type Ca2+ channel.
As a candidate, the T-type Ca2+ channel has a low activation threshold (around -60 mV) and a rapid inactivation (Perez-Reyes, 2003). In addition, a number of studies have been reported that the T-type Ca2+ channel is involved in the regulation of the frequency of action potential and the spontaneous contractions in various types of muscles such as the sinoarterial node of a rabbit heart (Doerr et al., 1989) and the detrusor smooth muscle of a guinea pig (Chow et al., 2003).
To determine the expression of the T-type Ca2+ channel in the pregnant rat myometrium, we examined the expression of the two T-type α subunits (α1G and α1H) by methods of RT/PCR and western blot. We observed that the mRNAs and proteins of α1G and α1H subunits are expressed in longitudinal strips of rat myometrium. These results are consistent with a previous study that both α1G and α1H are differentially expressed throughout gestation in the different layers of rat myomerium (Ohkubo et al., 2005).
To elucidate the role of the T-type Ca2+ channel in the spontaneous Ca2+ transients and contractions of rat myometrium, we observed the effect of the T-type Ca2+ channel blockers on the change of the spontaneous Ca2+ transients and contractions. In the present study, mibefradil, NNC 55-0396 and nickel were used as T-type Ca2+ channel blockers. Until recently, the lack of selective T-type Ca2+ channel blockers has hindered the attempts to investigate the role of T-type Ca2+ channels. Mibefradil has been known that a novel Ca2+ channel antagonist from the new chemical structural class of bensimidazolyl-substituted teraline derivatives (Billman and Hermsmeyer, 1992). In the vascular smooth muscle, a low concentration of mibefradil selectively blocked T-type Ca2+ channels (Mishra and Hermsmeyer, 1994). However, in contrast, the recent investigation reported that mibefradil also blocked the L-type Ca2+ channel by active metabolite produced via intracellular hydrolysis. Therefore, non-hydrolyzable analogue of mibefradil, NNC 55-0396, was developed as a selective blocker of the T-type Ca2+ channel (Huang et al., 2004). We showed that cumulative addition of mibefradil and NNC 55-0396 produced concentration-dependent inhibition of frequency as well as amplitude of spontaneous Ca2+ transients and contractions, respectively. These blockers also inhibited both the frequency and amplitude of Ca2+ transients and contractions at IC50 of these blockers. The results are consistent with a previous study that mibefradil inhibited the frequency as well as amplitude of uterine contractility (Asokan et al., 2002). These results suggested that mibefradil and NNC 55-0396 have an other side effect beside the inhibition of T-type Ca2+ channels. To evaluate whether mibefradil and NNC 55-0396 block L-type Ca2+ channels, we determined the effect of mibefradil and NNC 55-0396 on the high K+-induced contractions. Mibefradil and NNC 55-0396 significantly inhibited the amplitude of high K+-induced increase in [Ca2+]i and force. According to the previous report, high K+-induced contraction is due to Ca2+ influx through L-type Ca2+ channel by membrane depolarization (Shmigol et al., 1998; Coleman et al., 2000). In the present study, we also showed that 1 µM nifedipine, L-type Ca2+ channel blocker, completely inhibited high K+-induced contraction. Therefore, these blockers not only block Ca2+ influx through T-type Ca2+ channels more selectively but also block it through L-type Ca2+ channel.
To further determine the role of T-type Ca2+ channels on the spontaneous Ca2+ transients and contractions, we used nickel as a blocker of T-type Ca2+ channel. Nickel has been proposed as a selective blocker of the T-type Ca2+ channel depending on concentration (Lee et al., 1999). In the present study, cumulative addition of nickel produced a concentration-related inhibitory effect on frequency and amplitude of spontaneous Ca2+ transients and contractions, but the inhibition was more sensitive in frequency than in amplitude. In IC50 of 100 µM nickel produced an inhibition of frequency of the spontaneous Ca2+ transients and contractions. However, nickel has little effect on the amplitude of them. IC50 of nickel for the amplitude and frequency of spontaneous contractions was around 100 µM (91~137 µM). In some study, 100~200 µM nickel inhibits preferentially T-type Ca2+ channels (Tytgat et al., 1990; Sui et al., 2001). It is similar to the IC50 in oocytes (Lee et al., 1999). We also showed that 100 µM nickel had no effect on the high K+-induced contractions. Therefore, the inhibitory effect of nickel to the frequency of spontaneous Ca2+ transients and contractions may be due to inhibition of Ca2+ influx through T-type Ca2+ channels.
Finally, to determine whether T-type Ca2+ channels are involved in the generation of spontaneous slow depolarization in rat myometrium, we compared the effect of three blockers on the slope of rising phase of spontaneous Ca2+ transients. All three different T-type Ca2+ channel blockers decreased the slope of the initial rising phase of Ca2+ transients and frequency. Although we did not measure the membrane potential for the change of rising phase of slow depolarization in the present study, the change of Ca2+ transients can represent the change of membrane potentials. Furthermore, the t-type window current (the balance between the voltage-dependence of activation and inactivation) may be around about the resting membrane potential of myometrial cells and therefore able theoretically to contribute to action potential firing (Taggart and Tribe, 2007). Therefore, T-type Ca2+ channels may be involved in the generation of spontaneous Ca2+ transients and the modulation of the frequency of spontaneous Ca2+ transients.
ACKNOWLEDGEMENTS
This study was supported by a faculty research grant of Yonsei University of Medicine for 2007 (No. 6-2007-0180).
ABBREVIATIONS
- [Ca2+]i
concentration of intracellular free Ca2+
- Fura-2/AM
acetoxymethyl ester of Fura-2
- HEPES
[4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid]
- dNTP
deoxynucleoside triphosphate
- RT
reverse transcriptase
- PCR
polymerase chain reaction
- ECL
enhanced chemiluminescence
- NNC 55-0396
([(1S,2S)-2-(2-(N-[(3-Benzimidazol-2-yl)propyl]-N-methylamino)ethyl)-6-fluoro-1,2,3,4-tetrahydro-1-isopropyl-2-naphtyl cyclopropanecarboxylate dihydrochloride]
- ANOVA
analysis of variance
- L-type Ca2+ channel
long-lasting-type Ca2+ channel
- T-type Ca2+ channel
transient-type Ca2+ channel
References
- 1.Asokan KT, Sarkar SN, Mishra SK, Raviprakash V. Effects of mibefradil on uterine contractility. Eur J Pharmacol. 2002;455:65–71. doi: 10.1016/s0014-2999(02)02487-1. [DOI] [PubMed] [Google Scholar]
- 2.Billman GE. Ro 40-5967, a novel calcium channel antagonist, protects against ventricular fibrillation. Eur J Pharmacol. 1992;229:179–187. doi: 10.1016/0014-2999(92)90553-g. [DOI] [PubMed] [Google Scholar]
- 3.Blanks AM, Zhao ZH, Shmygol A, Bru-Mercier G, Astle S, Thornton S. Characterization of the molecular and electrophysiological properties of the T-type calcium channel in human myometrium. J Physiol. 2007;581(Pt 3):915–926. doi: 10.1113/jphysiol.2007.132126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocrine Rev. 2000;21:514–550. doi: 10.1210/edrv.21.5.0407. [DOI] [PubMed] [Google Scholar]
- 5.Chien EK, Saunders T, Phillippe M. The mechanisms underlying Bay K 8644-stimulated phasic myometrial contractions. J Soc Gynecol Invest. 1996;3:106–112. [PubMed] [Google Scholar]
- 6.Chow KY, Wu C, Sui GP, Fry CH. Role of the T-type Ca2+ current on the contractile performance of guinea pig detrusor smooth muscle. Neurourol Urodyn. 2003;22:77–82. doi: 10.1002/nau.10081. [DOI] [PubMed] [Google Scholar]
- 7.Coleman HA, Hart JDE, Tonta MA, Parkington HC. Changes in the mechanisms involved in uterine contractions during pregnancy in guinea-pigs. J Physiol. 2000;523:785–798. doi: 10.1111/j.1469-7793.2000.00785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coleman HA, Parkington HC. The role of membrane potential in the control of uterine motility. In: Carsten ME, Miller JD, editors. Uterine function: Molecular and cellular aspects. New York: Plenum Press; 1990. pp. 195–248. [Google Scholar]
- 9.Collins PL, Moore JJ, Idriss E, Kulp TM. Human fetal membranes inhibit calcium L-channel activated uterine contractions. Am J Obstet Gynecol. 1996;175:1173–1179. doi: 10.1016/s0002-9378(96)70024-8. [DOI] [PubMed] [Google Scholar]
- 10.Doerr T, Denger R, Trautwein W. Calcium currents in single SA nodal cells of the rabbit heart studied with action potential clamp. Pflugers Arch. 1989;413:599–603. doi: 10.1007/BF00581808. [DOI] [PubMed] [Google Scholar]
- 11.Honore E, Amedee T, Martin C, Dacquet C, Mironneau C, Mironneau J. Calcium channel current and its sensitivity to (+) isradipine in cultured pregnant rat myometrial cells. Pflugers Arch. 1989;414:477–483. doi: 10.1007/BF00585060. [DOI] [PubMed] [Google Scholar]
- 12.Huang L, Keyser BM, Tagmose TM, Hansen JB, Taylor JT, Zhuang H, Zhang M, Ragsdale DS, Li M. NNC 55-0396[(1S,2S)-2-(2-(N-[(3-benzimidazol-2-yl)propyl]-N-methylamino)ethyl)-6-fluoro-1,2,3,4-tetrahydro-1-isopropyl-2-naphytyl cyclopropanecarboxylate dihydrochloride]: a new selective inhibitor of T-type calcium channels. J Pharmacol Exp Therap. 2004;309:193–199. doi: 10.1124/jpet.103.060814. [DOI] [PubMed] [Google Scholar]
- 13.Inoue Y, Sperelakis N. Gestational change in Na+ and Ca2+ current densities in rat myometrial smooth muscle cells. Am J Physiol. 1991;260:C658–C663. doi: 10.1152/ajpcell.1991.260.3.C658. [DOI] [PubMed] [Google Scholar]
- 14.Jmari K, Mironneau C, Mironneau J. Inactivation of calcium channels current in rat uterine smooth muscle: evidence for calcium and voltage-mediated mechanisms. J Physiol. 1986;380:111–126. doi: 10.1113/jphysiol.1986.sp016275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knock GA, Aaronson PI. Calcium antagonistic properties of the cyclooxygenase-2 inhibitor nimesulide in human myometrial myocytes. Br J Pharmacol. 1999;127:1470–1478. doi: 10.1038/sj.bjp.0702685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JH, Gomora JC, Cribbs LL, Perez-Reyes E. Nickel block of three cloned T-type calcium channels: low concentrations selectively block α1H. Biophys J. 1999;77:3034–3042. doi: 10.1016/S0006-3495(99)77134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mershon JL, Mikala G, Schwartz A. Changes in the expression of the L-type voltage-dependent calcium channel during pregnancy and parturition in the rat. Biol Reprod. 1994;51:993–999. doi: 10.1095/biolreprod51.5.993. [DOI] [PubMed] [Google Scholar]
- 18.Mironneau J. Excitation-contraction coupling in voltage clamped uterine smooth muscle. J Physiol. 1973;233:127–141. doi: 10.1113/jphysiol.1973.sp010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mishra SK, Hermsmeyer K. Selective inhibition of T-type Ca2+ channels by Ro 40-5967. Circ Res. 1994;75:144–148. doi: 10.1161/01.res.75.1.144. [DOI] [PubMed] [Google Scholar]
- 20.Ohkubo T, Kawarabayashi T, Inoue Y, Kitamura K. Differential expression of L-and T-type calcium channels between longitudinal and circular muscles of the rat myometrium during pregnancy. Gynecol Obstet Invest. 2005;59:80–85. doi: 10.1159/000082333. [DOI] [PubMed] [Google Scholar]
- 21.Ohya Y, Sperelakis N. Fast Na+ and slow Ca2+ channels in single uterine muscle cells from pregnant rats. Am J Physiol. 1989;257:C408–C412. doi: 10.1152/ajpcell.1989.257.2.C408. [DOI] [PubMed] [Google Scholar]
- 22.Parkington HC, Coleman HA. Ionic mechanisms underlying action potentials in myometrium. Clin Exp Pharmacol Physiol. 1988;15:657–665. doi: 10.1111/j.1440-1681.1988.tb01125.x. [DOI] [PubMed] [Google Scholar]
- 23.Parkington HC, Coleman HA. Excitability in uterine smooth muscle. Front Horm Res. 2001;27:179–200. doi: 10.1159/000061026. [DOI] [PubMed] [Google Scholar]
- 24.Parkington HC, Tonta MA, Brennecke SP, Coleman HA. Contractile activity, membrane potential, and cytoplasmic calcium in human uterine smooth muscle in the third trimester of pregnancy and during labor. Am J Obstet Gynecol. 1999;181:1145–1151. doi: 10.1016/s0002-9378(99)70390-x. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Reyes E. Molecular physiology of low-voltage-activated T-type calcium channels. Physiol Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- 26.Riemer RK, Heymann MA. Regulation of uterine smooth muscle function during gestation. Pediatrics Res. 1998;44:615–627. doi: 10.1203/00006450-199811000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Shmigol AV, Eisner DA, Wray S. Properties of voltage-activated [Ca2+]i transients in single smooth muscle cells isolated from pregnant rat uterus. J Physiol. 1998;511:803–811. doi: 10.1111/j.1469-7793.1998.803bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sui GP, Wu C, Fry CH. Inward calcium currents in cultured and freshly isolated detrusor muscle cells: evidence of a T-type calcium current. J Urol. 2001;165:621–626. doi: 10.1097/00005392-200102000-00084. [DOI] [PubMed] [Google Scholar]
- 29.Taggart MJ, Tribe RM. Cellular ionic mechanisms controlling uterine smooth muscle contraction: effects of gestational state. In: Savineau JP, editor. New Frontiers in Smooth Muscle Biology and Physiology. India: Research Signpost; 2007. pp. 523–549. [Google Scholar]
- 30.Tytgat J, Vereecke J, Carmeliet E. Combined study of sodium current and T-type calcium current in isolated cardiac cells. Pflugers Arch. 1990;417:142–148. doi: 10.1007/BF00370691. [DOI] [PubMed] [Google Scholar]
- 31.Wray S, Jones K, Kupittayanant S, Li Y, Matthew A, Monir-Bishty E, Noble K, Pierce SJ, Quenby S, Shmygol AV. Calcium signaling and uterine contractility. J Soc Gynecol Invest. 2003;10:252–264. doi: 10.1016/s1071-5576(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 32.Yeon DS, Kim JS, Ahn DS, Kwon SC, Kang BS, Morgan KG, Lee YH. Role of protein kinase C- or RhoA-induced Ca2+ sensitization in stretch-induced myogenic tone. Cardiovas Res. 2002;53:431–438. doi: 10.1016/s0008-6363(01)00496-5. [DOI] [PubMed] [Google Scholar]
- 33.Young RC, Smith LH, McLaren MD. T-type and L-type calcium currents in freshly dispersed human uterine smooth muscle cells. Am J Obstet Gynecol. 1993;169:785–792. doi: 10.1016/0002-9378(93)90006-5. [DOI] [PubMed] [Google Scholar]