Abstract
The striatum receives glutamatergic afferents from the cortex and thalamus, and these synaptic transmissions are mediated by α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) and N-methyl-D-aspartate (NMDA) receptors. The purpose of this study was to characterize glutamate receptors by analyzing NMDA/AMPA ratio and rectification of AMPA and NMDA excitatory postsynaptic currents (EPSCs) using a whole-cell voltage-clamp method in the dorsal striatum. Receptor antagonists were used to isolate receptor or subunit specific EPSC, such as (DL)-2-amino-5-phosphonovaleric acid (APV), an NMDA receptor antagonist, ifenprodil, an NR2B antagonist, CNQX, an AMPA receptor antagonist and IEM-1460, a GluR2-lacking AMPA receptor blocker. AMPA and NMDA EPSCs were recorded at -70 and +40 mV, respectively. Rectification index was calculated by current ratio of EPSCs between +50 and -50 mV. NMDA/AMPA ratio was 0.20±0.05, AMPA receptor ratio of GluR2-lacking/GluR2-containing subunit was 0.26±0.05 and NMDA receptor ratio of NR2B/NR2A subunit was 0.32±0.03. The rectification index (control 2.39±0.27) was decreased in the presence of both APV and combination of APV and IEM-1460 (1.02±0.11 and 0.93±0.09, respectively). These results suggest that the major components of the striatal glutamate receptors are GluR2-containing AMPA receptors and NR2A-containing NMDA receptors. Our results may provide useful information for corticostriatal synaptic transmission and plasticity studies.
Keywords: Striatum, AMPA, Glutamate receptor, NMDA, Patch clamp
INTRODUCTION
The striatum plays a key role in movement control and habitual formation. It receives glutamatergic afferents from the cortex and thalamus. These glutamatergic synaptic transmissions are mediated by α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptors and N-methyl-D-aspartate (NMDA) receptors. The NMDA receptors are heteromeric assemblies of NR1 and NR2 subunits; the NR2 subunit is an essential factor in the biophysical and pharmacological properties of NMDA receptors, and can influence NMDA receptor assembly, ion conductance and synaptic plasticity (Bliss and Collingridge, 1993; Lau and Zukin, 2007). The NR2A subunit confers a lower affinity for both glutamate and NMDA, distinctly faster kinetics and higher channel open probability than does the NR2B subunit, which confers slower channel kinetics and reduced open probability (Parsons et al., 1998; Lau and Zukin, 2007). Earlier reports showed that long-term potentiation (LTP) is dependent on NR2A subunit, whereas NR2B subunit is involved in the induction of long-term depression (LTD) in the hippocampus and cortex (Liu et al., 2004; Massey et al., 2004). Interestingly, another report suggested that NR2A subunit was not required for NMDA receptor dependent LTP in the whole brain region (Weitlauf et al., 2005). However, a different report suggested that activation of both NR2A and NR2B subunits was required for LTP in nucleus accumbens (Schotanus and Chergui, 2008). AMPA receptors consist of GluR1-4 subunits, and the presence or absence of GluR2 subunit characterizes physiological properties of AMPA receptors. For example, GluR2-containing AMPA receptors are Ca2+ impermeable and have linear rectification pattern, whereas GluR2-lacking AMPA receptors are Ca2+ permeable and have inward rectification pattern (Adesnik and Nicoll, 2007; Derkach et al., 2007).
The NMDA/AMPA ratio was reported using the electrophysiological method (McDermott et al., 2006; Kreitzer and Malenka, 2007; Du et al., 2008), and glutamatergic receptor subunit component ratio was shown through immunohistochemical studies in several brain regions (Nansen et al., 2000; Greger et al., 2002). However, the composition of the NMDA and AMPA receptor subunits has not been well established using electrophysiological methods in the dorsal striatum. Thus, the purpose of the present study was to characterize the AMPA and NMDA receptors including their subunit ratio, NMDA/AMPA ratio, and rectification pattern within the dorsal striatum using electrophysiological methods with specific receptor antagonists.
METHODS
Slice preparation
Brain slices were prepared from 15 to 21-day-old Sprague-Dawley rats using a previously described technique (Choi et al., 2006; Cho et al., 2008). A coronal slice (300 µm thick) containing the cerebral cortex and striatum was produced using a manual vibratome (Campden Insruments, Loughborough, UK). The rats were anesthetized with pentobarbital (50 mg/kg, i.p.) and decapitated in conformity with the National Institutes of Health Guide for The Care and Use of Laboratory Animals. The brain slices were removed and placed in ice-cold, modified artificial cerebrospinal fluid (aCSF) containing (in mM) 194 sucrose, 30 NaCl, 4.5 KCl, 1 MgCl2, 26 NaHCO3, 1.2 NaH2PO4 and 10 D-glucose adjusted to pH 7.4 by bubbling with 95% O2/5% CO2. The brain slices were then transferred to aCSF containing (in mM) 124 NaCl, 4.5 KCl, 2 CaCl2, 1 MgCl2, 26 NaHCO3, 1.2 NaH2CO3 and 10 D-glucose adjusted to pH 7.4 by bubbling with 95% O2/5% CO2. The slices were allowed to recover for at least 1 h in aCSF at room temperature. A hemi-slice, containing the cortex and striatum, was submerged in a recording chamber and constantly superfused with aCSF bubbled with 95% O2/5% CO2. The flow rate was maintained at 2~3 ml/min using a peristaltic pump (Miniplus 2, Gilson, France). The temperature of the bath solution was maintained at 31±1℃.
EPSC recording
Whole-cell voltage-clamp recordings were performed as described previously (Sung et al., 2001; Cho et al., 2008). Electrical stimuli (0.05 Hz) were delivered through a bipolar, Teflon®-coated tungsten electrode placed in the white matter dorsal to the striatum and in close proximity to the recording electrode. The stimulus intensity was set to yield excitatory postsynaptic current (EPSC) amplitudes from 100 pA to 300 pA. Tight-seal whole-cell recordings were obtained using pipettes made from borosilicate glass capillaries pulled on a P-97 micropipette puller (Sutter Instruments, Novato, CA). Pipette resistance ranged from 3 MΩ to 5 MΩ, filled with an internal solution containing (in mM) 120 CsMeSO3, 5 NaCl, 10 tetraethylammonium chloride, 10 HEPES, 5 lidocaine N-ethyl bromide (QX-314) (Br2+ salt), 1.1 EGTA, 4 ATP (Mg2+ salt), 0.3 GTP (Na+ salt), pH adjusted to 7.2 with CsOH, osmolarity adjusted to 300±10 mOsm with sucrose. Medium-sized neurons within two or three layers below the surface of the slice were identified using an Olympus BX50WI (Tokyo, Japan) differential interference contrast microscope. Neurons were voltage-clamped at -70 mV applying APV, an NMDA receptor antagonist, IEM-1460, a GluR2-lacking AMPA receptor blocker for AMPA receptor characterization. NMDA receptors were characterized by voltage clamp method at +40 mV holding potential with CNQX, an AMPA receptor antagonist. Also ifenprodil, an NMDA receptor subunit NR2B antagonist was used to further characterize the NR2A subunit specificity. All EPSCs were recorded from the dorsal striatum in the presence of 50 µM picrotoxin, a GABAA receptor antagonist, to suppress GABAA-mediated currents. The rectification was measured at peak amplitude between -70 mV and +50 mV by 20 mV intervals. EPSCs were recorded with an Axopatch 1D patch-clamp amplifier (Molecular Device, Sunnyvale, CA), filtered at 5 kHz, digitized at 10 kHz using a Digidata 1322A (Molecular Device), and stored on a personal computer using pClamp 9.2 software (Molecular Device). The series resistance was <25 MΩ and was not compensated. Both series and input resistance were monitored throughout the experiment by delivering 5 mV voltage steps. If the series resistance changed more than 20% during the course of an experiment, the data was discarded.
Data analysis
AMPA and NMDA-mediated EPSCs were analyzed by Clampfit 10.2 software (Molecular Device). All averaged data were presented as means±S.E.M.. Statistical significance was determined by the Student's t-test using Prism 4.0 software (Graph Pad Software, Avenida, CA).
Drugs and chemicals
(DL)-2-amino-5-phosphonovaleric acid (APV), IEM-1460 and CNQX were purchased from Tocris Cookson (Avonmouth, UK). All other chemicals used in this study were purchased from Sigma-Aldrich (St. Louis, MO). Drugs were diluted with aCSF immediately before use from stock solutions prepared according to the manufacturer's instructions.
RESULTS
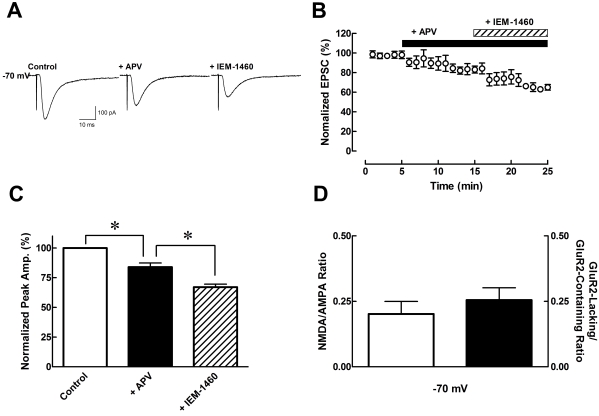
To calculate the ratio of AMPA receptor, NMDA receptor and their subunits on striatal glutamatergic synapses, we first isolated AMPA receptor-mediated currents at -70 mV (Fig. 1). In the presence of APV (50 µM), the normalized EPSCs were decreased by 16.12±3.49% (n=6, p<0.05) of the control and in the presence of IEM-1460 (100 µM), the EPSCs were decreased by 32.96±2.53% (n=6, p<0.05) of the control (Fig. 1). The NMDA/AMPA ratio was calculated by the subtraction of EPSCs in the presence of APV from normal condition, and the GluR2-lacking/GluR2-containing ratio was calculated by the subtraction of EPSCs in the presence of APV and IEM-1460 from APV-treated only. The NMDA/AMPA ratio and the GluR2-lacking/GluR2-containing ratio was 0.20±0.05 and 0.26±0.05 at -70 mV, respectively. The NMDA/AMPA ratio and subunit ratio were calculated from Fig. 1C (Fig. 1D).
Fig. 1.
Pharmacological isolation of AMPA receptors. (A) Sample traces of EPSCs with continuously added specific antagonist such as APV (NMDA receptor antagonist, 50 µM), and IEM-1460 (GluR2-lacking AMPA receptor blocker, 100 µM), respectively. (B) Plot graph shows baseline, addition of APV, and IEM-1460 by 10 min interval, tendency decreased EPSCs by APV and IEM-1460 (n=6). (C) Bar graph shows average peak data normalized to control (open bar) currents. *p<0.05, when compared to control versus APV and APV versus IEM-1460. (D) Bar graph shows NMDA/AMPA ratio and GluR2-lacking AMPA receptor/GluR2-containing AMPA receptor ratio calculated by data from Fig. 1C. Data are expressed as mean±S.E.M.
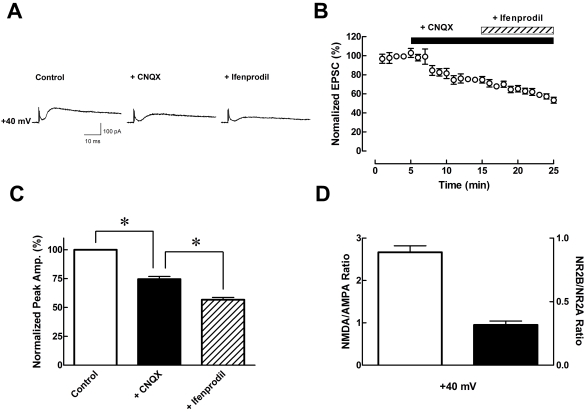
We then isolated NMDA receptor-mediated currents at +40 mV (Fig. 2). EPSCs in the presence of CNQX (10 µM) were decreased by 25.48±2.36% (n=6, p<0.05) of control, and in the presence of ifenprodil (3 µM), the EPSCs were decreased by 43.31±2.05% (n=6, p<0.05) of the control (Fig. 2). The NMDA/AMPA ratio was calculated by subtraction of EPSCs in the presence of CNQX from absence of CNQX. The NR2B/NR2A ratio was calculated by the subtraction of EPSCs in the presence of CNQX and ifenprodil from CNQX-treated only. The NMDA/AMPA and NR2B/NR2A ratios were 2.67±0.15 and 0.32±0.03 at +40 mV, respectively. Both these ratios were calculated from Fig. 2C (Fig. 2D).
Fig. 2.
Pharmacological isolation of NMDA receptors. (A) Sample traces of EPSCs with continuously added specific antagonist such as CNQX (AMPA receptor antagonist, 10 µM), and ifenprodil (NMDA receptor subunit NR2B blocker, 3 µM), respectively. (B) Plot graph shows baseline, addition of CNQX, and ifenprodil by 10 min interval, tendency decreased EPSCs by CNQX, and ifenprodil (n=6). (C) Bar graph shows average peak data normalized to control (open bar) currents. *p<0.05, when compared to control versus CNQX and CNQX versus ifenprodil. (D) Bar graph shows NMDA/AMPA ratio and NR2B NMDA receptor subunit/NR2A NMDA receptor subunit ratio calculated by data from Fig. 2C. Data are expressed as mean±S.E.M..
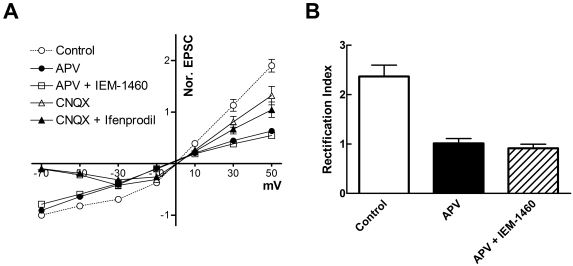
The rectification pattern of AMPA receptor containing GluR2 subunit and NMDA receptor containing NR2A subunit was calculated using voltage clamp method (holding level was between -70 mV and +50 mV, 20 mV interval; Fig. 3A). The rectification index was calculated by the current/voltage relationship as the ratio of EPSCs amplitudes between -50 and +50 mV. The rectification pattern of AMPA receptors was linear by APV-treated currents. Also, the GluR2-containing rectification pattern was similar to APV-treated currents (current ratio between +50 mV and -50 mV; control, 2.39±0.27; APV, 1.02±0.11; combination of APV and IEM-1460, 0.93±0.09; n=7, Fig. 3B). Generally, it is well known that GluR2 has a linear rectification pattern, and this was confirmed by our results showing that the GluR2-containing AMPA receptor exhibited a linear rectification pattern. To indicate the rectification pattern of the NMDA receptor, we studied the current/voltage relationship of NMDA receptors (Fig. 3A). The rectification pattern of CNQX-treatment was not significantly different from that of CNQX and ifenprodil-treatment (current ratio between +50 mV and -50 mV: control, 2.32±0.24, CNQX, 5.92±0.31; combination of CNQX and ifenprodil, 5.46±0.46; n=7). These results suggest that the major components of AMPA and NMDA receptors in the dorsal striatum were GluR2-containing AMPA receptors and NR2A-containing NMDA receptors.
Fig. 3.
Rectification pattern of synaptic responses in rat striatum. Current/voltage curve and bar graph shows the rectification pattern and the rectification index. (A) Current/voltage curve showing the rectification pattern of control, APV, combination of IEM-1460 and APV, CNQX and combination of ifenprodil and CNQX. (B) Bar graph showing the rectification index (current ratio between +50 mV and -50 mV) of control, APV and combination of APV and IEM-1460 (n=7). Data are expressed as mean±S.E.M.
DISCUSSION
We isolated the glutamate receptor and subunit on striatal neurons. Our results showed that the predominant composition of AMPA receptors were GluR2-containing AMPA receptors at -70 mV (Fig. 1). Also other immunological studies reported that GluR2 expression was higher than other subunits (Nansen et al., 2000; Greger et al., 2002). Furthermore, a study using IEM-1460 reported that the GluR2-containing AMPA receptor was the major component of the hippocampus (Buldakova et al., 2007). We isolated NMDA receptors and subunit currents at +40 mV (Fig. 2). Our data showed that NR2A mediated currents were higher than NR2B at +40 mV, when NMDA receptor activation was higher. An autoradiography study reported that the predominant NMDA receptor subunits were NR2A and NR2B in the striatum (Standaert et al., 1994). Recently other studies also reported that NR2A was expressed at higher levels than NR2B using electrophysiological and immunohistochemical methods in the striatum (Wang et al., 1995; Ding et al., 2008). Our results indicate that NR2A might be the functional property of the major NMDA receptor composition.
To investigate the rectification pattern of AMPA and NMDA receptors and their subunits, we first isolated the AMPA receptor and GluR2-containing AMPA receptor. Generally, the GluR2-containing AMPA receptor has a linear rectification pattern in comparison to the GluR2-lacking AMPA receptor on the hippocampal neuron (Adesnik and Nicoll, 2007; Derkach et al., 2007). Our results show that the GluR2-containing AMPA receptor has a linear pattern on striatal neurons (Fig. 3). Furthermore our data shows that the APV-treated pattern was linear. These data suggest that GluR2-containing AMPA receptor is the major component of the striatal AMPA receptor. The rectification pattern of NMDA receptors in the presence or absence of ifenprodil was not significantly different because the currents were very small at negative holding level. These finding suggest that the major component of NMDA receptors in the dorsal striatum are NR2A-containing NMDA receptors.
Generally, GluR2-containing AMPA receptors show properties of Ca2+ impermeability, low channel conductance and a linear rectification pattern, whereas GluR2-lacking AMPA receptors exhibit Ca2+ permeability, high channel conductance and an inward rectification pattern (Jonas and Burnashev, 1995; Adesnik and Nicoll, 2007; Derkach et al., 2007; Panicker et al., 2008). Moreover, the presence or absence of the GluR2 subunit can alter AMPA receptor properties and synaptic transmission (Derkach et al., 2007). These subunits relate to receptor trafficking (Tardin et al., 2003) and determine synaptic plasticity (Adesnik and Nicoll, 2007; Derkach et al., 2007). Another report suggested that GluR2-lacking AMPA receptors do not play a key role in LTP in the most commonly used preparation for studying hippocampal synaptic plasticity (Adesnik and Nicoll, 2007). Our results show that the predominant AMPA receptor component was the GluR2-containing AMPA receptor in the striatum at near resting membrane potential. Although it has not yet been proven, these results suggest the possibility that the GluR2-containing AMPA receptor may contribute to modulation of the striatal synaptic plasticity such as LTP and LTD, though its high Ca2+ permeability and involvement in AMPA receptor trafficking.
Meanwhile, NR2A of the NMDA receptor subunit confers a lower affinity for glutamate and NMDA, distinctly faster kinetics and higher channel open probability than NR2B. It has also been established that the NMDA receptor subunits play a key role in synaptic plasticity (Parsons et al., 1998; Massey et al., 2004; Weitlauf et al., 2005; Lau and Zukin, 2007). Another report suggested that the NR2A subunit was not required for NMDA receptor dependent LTP in the whole brain region (Weitlauf et al., 2005). However, NR2A-containing NMDA receptors have more prominent Ca2+-dependent desensitization and slower receptor trafficking than NR2B-containing NMDA receptors (Lau and Zukin, 2007), and also our results showed that NR2A-containing NMDA receptors are the major component of the striatum. These result suggested that NR2A-containing NMDA receptors might have an important role in modulating striatal synaptic plasticity though its higher Ca2+ conductance, and lower receptor trafficking compared to NR2B-containing NMDA receptors. However, further studies are necessary to verify whether the distinctive differences in striatal NMDA and AMPA receptor subunit composition relate to synaptic plasticity, because it is unclear that which AMPA and NMDA receptor subunits relate to synaptic plasticity in the striatum. In conclusion, our results suggest that GluR2-containing AMPA receptors and NR2A-containing NMDA receptors are principal glutamatergic receptors in the dorsal striatum, and these subunits specificity might contribute to the characteristic pattern of synaptic transmission and plasticity in this brain region.
ACKNOWLEDGEMENT
This research was supported by a grant from the Medical Research Center, Korea Science and Engineering Foundation, Republic of Korea (R13-2002-005-04001-0).
ABBREVIATIONS
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate
- NMDA
N-methyl-D-aspartate
- EPSC
excitatory post-synaptic current
- APV
(DL)-2-amino-5-phosphonovaleric acid
- LTP
long-term potentiation
- LTD
long-term depression
References
- 1.Adesnik H, Nicoll RA. Conservation of glutamate receptor 2-containing AMPA receptors during long-term potentiation. J Neurosci. 2007;27:4598–4602. doi: 10.1523/JNEUROSCI.0325-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 3.Buldakova SL, Kim KK, Tikhonov DB, Magazanik LG. Selective blockade of Ca2+ permeable AMPA receptors in CA1 area of rat hippocampus. Neuroscience. 2007;144:88–99. doi: 10.1016/j.neuroscience.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Cho HS, Lee HH, Choi SJ, Kim KJ, Jeun SH, Li QZ, Sung KW. Forskolin enhances synaptic transmission in rat dorsal striatum though NMDA receptors and PKA in different phases. Korean J Physiol Pharmacol. 2008;12:293–297. doi: 10.4196/kjpp.2008.12.6.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi SJ, Kim KJ, Cho HS, Kim SY, Cho YJ, Hahn SJ, Sung KW. Acute inhibition of corticostriatal synaptic transmission in the rat dorsal striatum by ethanol. Alcohol. 2006;40:95–101. doi: 10.1016/j.alcohol.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- 7.Ding J, Peterson JD, Surmeier DJ. Corticostriatal and thalamostriatal synapses have distinctive properties. J Neurosci. 2008;28:6483–6492. doi: 10.1523/JNEUROSCI.0435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du J, Creson TK, Wu LJ, Ren M, Gray NA, Falke C, Wei Y, Wang Y, Blumenthal R, Machado-Vieira R, Yuan P, Chen G, Zhuo M, Manji HK. The role of hippocampal GluR1 and GluR2 receptors in manic-like behavior. J Neurosci. 2008;28:68–79. doi: 10.1523/JNEUROSCI.3080-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greger IH, Khatri L, Ziff EB. RNA editing at arg607 controls AMPA receptor exit from the endoplasmic reticulum. Neuron. 2002;34:759–772. doi: 10.1016/s0896-6273(02)00693-1. [DOI] [PubMed] [Google Scholar]
- 10.Jonas P, Burnashev N. Molecular mechanisms controlling calcium entry through AMPA-type glutamate receptor channels. Neuron. 1995;15:987–990. doi: 10.1016/0896-6273(95)90087-x. [DOI] [PubMed] [Google Scholar]
- 11.Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- 12.Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- 13.Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- 14.Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDermott CM, Hardy MN, Bazan NG, Magee JC. Sleep deprivation-induced alterations in excitatory synaptic transmission in the CA1 region of the rat hippocampus. J Physiol. 2006;570:553–565. doi: 10.1113/jphysiol.2005.093781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nansen EA, Jokel ES, Lobo MK, Micevych PE, Ariano MA, Levine MS. Striatal ionotropic glutamate receptor ontogeny in the rat. Dev Neurosci. 2000;22:329–340. doi: 10.1159/000017457. [DOI] [PubMed] [Google Scholar]
- 17.Panicker S, Brown K, Nicoll RA. Synaptic AMPA receptor subunittrafficking is independent of the C terminus in the GluR2-lacking mouse. Proc Natl Acad Sci U S A. 2008;105:1032–1037. doi: 10.1073/pnas.0711313105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parsons CG, Danysz W, Quack G. Glutamate in CNS disorders as a target for drug development: an update. Drug News Perspect. 1998;11:523–569. doi: 10.1358/dnp.1998.11.9.863689. [DOI] [PubMed] [Google Scholar]
- 19.Schotanus SM, Chergui K. Long-term potentiation in the nucleusaccumbens requires both NR2A- and NR2B-containing N-methyl-D-aspartate receptors. Eur J Neurosci. 2008;27:1957–1964. doi: 10.1111/j.1460-9568.2008.06173.x. [DOI] [PubMed] [Google Scholar]
- 20.Standaert DG, Testa CM, Young AB, Penney JB., Jr Organization of N-methyl-D-aspartate glutamate receptor gene expression in the basal ganglia of the rat. J Comp Neurol. 1994;343:1–16. doi: 10.1002/cne.903430102. [DOI] [PubMed] [Google Scholar]
- 21.Sung KW, Choi S, Lovinger DM. Activation of group I mGluRs is necessary for induction of long-term depression at striatal synapses. J Neurophysiol. 2001;86:2405–2412. doi: 10.1152/jn.2001.86.5.2405. [DOI] [PubMed] [Google Scholar]
- 22.Tardin C, Cognet L, Bats C, Lounis B, Choquet D. Direct imaging of lateral movements of AMPA receptors inside synapses. EMBO J. 2003;22:4656–4665. doi: 10.1093/emboj/cdg463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang YH, Bosy TZ, Yasuda RP, Grayson DR, Vicini S, Pizzorusso T, Wolfe BB. Characterization of NMDA receptor subunit-specific antibodies: distribution of NR2A and NR2B receptor subunits in rat brain and ontogenic profile in the cerebellum. J Neurochem. 1995;65:176–183. doi: 10.1046/j.1471-4159.1995.65010176.x. [DOI] [PubMed] [Google Scholar]
- 24.Weitlauf C, Honse Y, Auberson YP, Mishina M, Lovinger DM, Winder DG. Activation of NR2A-containing NMDA receptors is not obligatory for NMDA receptor-dependent long-term potentiation. J Neurosci. 2005;25:8386–8390. doi: 10.1523/JNEUROSCI.2388-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]