Abstract
This study aimed to investigate whether selective serotonin reuptake inhibitors (SSRIs) attenuate brain injury and facilitate recovery following photothrombotic cortical ischemia in mice. Male ICR mice were anesthetized and systemically administered Rose Bengal. Permanent focal ischemia was induced in the medial frontal and somatosensory cortices by irradiating the skull with cold light laser. The animals were treated with fluoxetine or sertraline once a day for 14 d starting 1 h after ischemic insult. Treatment with fluoxetine and sertraline significantly reduced the infarct size. The Evans blue extravasation indices of the fluoxetine- and sertraline-treated groups were significantly lower than that of the vehicle group. Treatment with fluoxetine and sertraline shifted the lower limit of the mean arterial blood pressure for cerebral blood flow autoregulation toward normal, and significantly increased the expression of heme oxygenase-1 (HO-1) and hypoxia-inducible factor-1α (HIF-1α) proteins in the ischemic region. These results suggest that SSRIs, such as fluoxetine and sertraline, facilitate recovery following photothrombotic cortical ischemia via enhancement of HO-1 and HIF-1α proteins expression, thereby providing a benefit in therapy of cerebral ischemia.
Keywords: Cerebral blood flow autoregulation, Focal cerebral ischemia, Heme oxygenase-1, Hypoxia-inducible factor-1α, Selective serotonin reuptake inhibitors
INTRODUCTION
Depression is a frequent and important problem among patients with stroke and presents as post-stroke depression in 20~50% of stroke survivors (Starkstein and Robinson, 1989; Andersen et al., 1994b; Bhogal et al., 2005). Post-stroke depression has negative effects on functional recovery (Parikh et al., 1990; Kotila et al., 1999), and these effects can be overcome by pharmacological treatment of depression (Gainotti et al., 2001). Although antidepressants have been effectively used to treat post-stroke depression (Primeau, 1988; Gainotti et al., 2001; Paolucci et al., 2001), their prescription is critically influenced by their safety, tolerability, and their impact on co-morbid conditions (Rampello et al., 2005).
Selective serotonin reuptake inhibitors (SSRIs) are widely used antidepressants, and may prove to be effective in the treatment of post-stroke depression because previous studies have reported that the symptoms of post-stroke depression are related to serotonergic dysfunction in the brain (Kim et al., 2002). However, it is essential to understand the cerebrovascular effects of SSRIs, since serotonin is a potent vasoactive amine with complex actions on the cerebral arteries. SSRIs induce vasoconstriction of large cerebral arteries and vasodilatation of small cerebral vessels (Bonvento et al., 1991; Muhonen et al., 1997).
SSRI use may have a protective effect against the depression-related increase in the risk of ischemic stroke (Jakovljevic and Tuomilehto, 2002). This possibility is based on the evidence that depression may increase the risk of ischemic stroke (Ramasubbu and Patten, 2003) and on the assumption that depression is a common indication for treatment with SSRIs.
The expression of hypoxia-inducible factor (HIF)-1α (HIF-1α), a specific oxygen-regulated subunit of HIF-1, is tightly regulated by the cellular O2 concentration (Semenza, 2001). HIF-1 has been suggested to represent a novel therapeutic target in cerebrovascular disease, and HIF-1α gene therapy or treatment with HIF-1 activity inducers could provide protection against ischemia-induced cell death (Semenza, 2001). Emerging evidence suggests that after ischemia, HIF-1α exerts beneficial effects that favor neuronal survival (Semenza, 2000; Acker and Acker, 2004).
Heme oxygenase (HO)-1 (HO-1) is an inducible form of HO, which is the rate-limiting enzyme in the degradation of heme to yield equimolar quantities of biliverdin, carbon monoxide and iron (Marks et al., 1991; Verma et al., 1993; Maines, 1997), and has been demonstrated to be an HIF-1α-regulated gene (Lee et al., 1997; Dawn and Bolli, 2005). A growing body of evidence suggests that HO may play an important role in protection against cerebral ischemia, confirming that induction of the HO-1 protein following cerebral ischemia can lead to a beneficial outcome (Geddes et al., 1996; Nimura et al., 1996; Panahian et al., 1999; Bidmon et al., 2001; Fu et al., 2006).
Thus, it was speculated that postischemic treatment with SSRIs would exert a favorable influence on progressive ischemic brain damage and alterations in cerebral hemodynamics through the induction of HIF-1α and HO-1. In the present study, we investigated whether SSRIs such as fluoxetine and sertraline reduce brain injury and facilitate recovery following photothrombotic cortical ischemia in mice.
METHODS
Animals
The experiment was conducted in accordance with the Guidelines for Animal Experiments in Pusan National University School of Medicine. Male ICR mice weighing 25~30 g were obtained from Samtako (Osan, Gyeonggi-do, Korea). They were housed in an air-conditioned room at 22~25℃. Light was provided on a 12-h light/dark cycle with lights being switched on at 6:00 a.m. Food and water were provided ad libitum.
Photothrombotic cortical ischemia
Permanent focal ischemia was induced by cortical photothrombotic vascular occlusion by the method of Schroeter et al. (2002) with slight modifications. The mice were anesthetized with chloral hydrate (450 mg/kg, i.p.) and were allowed spontaneous respiration throughout the surgical procedure. The mice were placed in a head-holding adaptor (SG-4N; Narishige, Tokyo, Japan), and the rectal temperature was monitored and maintained at 37±0.5℃ using a heating pad (Homeothermic blanket system; Harvard Apparatus Inc., Edenbridge, Kent, UK). After midline scalp incision and pericranial tissue dissection, the bregma and lambda were identified. A fiber-optic bundle from a cold light source (KL 1500 LCD; Carl Zeiss, Göttingen, Germany) with a 4-mm aperture was centered at 2 mm lateral to the bregma using a micromanipulator. The aperture of the cold light source was placed as close to the skull as possible in order to avoid scattering of light, which could cause variability. The skull was then irradiated for 15 min starting at 5 min after i.p. injection of rose bengal (0.1 ml of 10 mg/ml; Sigma, St. Louis, MO, USA). Subsequently, the skin was sutured, and the mice were allowed to awaken. The sham group was treated in a manner similar to that described above except the irradiation and rose bengal injection. All mice tolerated the entire procedure very well, showed no visible neurological or behavioral deficits, and survived brain ischemia.
Study design and drug treatment
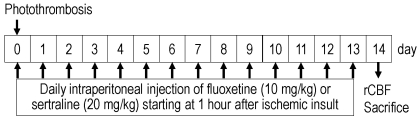
The study design and timing of drug treatment are shown in Fig. 1. Fluoxetine HCl (Eli Lilly and Co. Ltd., Hampshire, UK) and sertraline HCl (Pfizer Inc., New York, NY, USA) were dissolved in saline and administered daily at doses of 10 and 20 mg/kg, respectively, starting at 1 h after ischemia induction and continuing for 14 d. The ischemic control and sham-operated control rats were injected with saline. All drugs were injected i.p. in a volume of 1 ml/100 g.
Fig. 1.
Study design and drug treatment. rCBF, regional cerebral blood flow.
Measurement of the infarct size
The mice were anesthetized with urethane (1 g/kg, i.p.) and sacrificed by decapitation. The brain was quickly removed and chilled in ice-cold saline for 10 min. Five coronal sections (thickness, 2-mm) were cut using a mouse brain matrix (RBM-2000C; ASI Instruments Inc., Warren, MI, USA) beginning 2 mm posterior to the anterior pole. The sections were then immersed in a saline solution containing 2% 2,3,5-triphenyltetrazolium chloride (Sigma) at 37℃ for 30 min and were fixed by immersion in a 10% neutral buffered formalin solution (Bederson et al., 1986). The dorsal surfaces of the sections were scanned with a digital camera, and the infarct area in each section was quantified using an image analyzing system (AxioVision LE; Carl Zeiss). The total infarct volume was determined by summation of the infarct areas of the 5 sections. The infarct volume was quantified by a standard computer-assisted image analysis technique.
Assessment of blood-brain barrier permeability
The degree of blood-brain barrier (BBB) disruption was assessed by the measurement of Evans blue dye leakage (Uyama et al., 1988; Demougeot et al., 2004) with slight modifications. Briefly, 1% Evans blue (Sigma) in saline was injected (3 ml/kg, i.v.) through the tail vein as a BBB permeability tracer 1 h before sacrifice. The mice were then anesthetized with urethane (1 g/kg, i.p.) and transcardially perfused with 200 ml of heparinized saline (10 U/ml heparin in saline) through the left ventricle at a pressure of 100 mmHg till colorless perfusion fluid was obtained from the right atrium. The ipsilateral and contralateral cortices were then dissected, weighed, and homogenized in 1 ml of 0.1 M PBS. After centrifugation for 5 min at 1,000 g, 0.7 ml of the supernatant was taken and 0.7 ml of 100% (w/v) trichloroacetic acid was added to it. The mixture was then incubated at 4℃ for 18 h. After centrifugation for 30 min at 1,000 g, the absorbance of Evans blue in the supernatant was measured at 610 nm using a spectrophotometer (UV-1201, UV-VIS Spectrophotometer; Shimadzu, Kyoto, Japan). The amount of Evans blue was quantified from a linear standard curve, and the changes in BBB permeability were expressed as the ratio of the ipsilateral to the contralateral cortex (Evans blue extravasation index).
Measurement of regional cerebral blood flow
A catheter was placed in the common carotid artery for measurement of the systemic arterial blood pressure (Statham P23D pressure transducer; Gould, Cleveland, OH, USA) and a femoral arterial catheter was inserted for withdrawing and sampling of arterial blood. The blood was collected 10 min before and 10 min after ischemia for the purpose of blood gas and pH determination (i-STAT Portable Clinical Analyzer; i-STAT Corporation, East Windsor, NJ, USA). The respiration was modulated and maintained to keep the changes in PaCO2 within 3~4% of the baseline during the entire experimental period. Changes in the regional cerebral blood flow (rCBF) were evaluated using a laser tissue blood flowmeter (FLO-N1; Omegawave, Tokyo, Japan). The laser Doppler probe (type ST-N; Omegawave) was placed on the same site as the cold light irradiation. The mean arterial blood pressure (MABP), which was required to maintain 80% of the baseline rCBF of the sham-operated group, expressed as the lower limit (LL) of autoregulation, was employed to analyze the CBF response.
Western blot analysis of HIF-1α and HO-1 protein expression
The brain cortical samples were homogenized in 5 volumes of an ice-cold lysis buffer (50 mM Tris-HCl, pH 8.0; 150 mM NaCl; 1 mM EDTA; 1% Triton X-100; 10% glycerol; 100 µg/ml phenylmethylsulfonyl fluoride; 1 mM sodium orthovanadate; and 5 mM sodium fluoride). Following centrifugation at 14,000 g for 7 min, 50 µg of total protein from each sample was loaded into a 10% SDS-polyacrylamide electrophoresis gel and transferred onto a nitrocellulose membrane (Amersham Biosciences Inc., Piscataway, NJ, USA). After blocking with non-fat milk, the membrane was incubated with the following primary antibodies: Anti-HIF-1α mouse monoclonal antibody (1:1,000, Clone H1α67; Biomol, Hamburg, Germany), rabbit HO-1 polyclonal antibody (1:1,000; Stressgen Biotechnologies Co., Victoria, BC, Canada), and mouse monoclonal β-actin antibody (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA). The immunoreactive bands were visualized using the chemiluminescent reagent of the Supersignal West Dura Extended Duration Substrate Kit (Pierce Chemical, Rockford, IL, USA). The band signals were quantified using a GS-710 calibrated imaging densitometer (Bio-Rad, Hercules, CA, USA). The values were expressed as the ratio of HO-1 or HIF-1α protein expression to β-actin.
Statistical analysis
The data are expressed as the mean±S.E.M. The statistical differences between the groups were determined either by nonlinear regression analysis for changes in the rCBF in response to the changes in MABP or by one-way analysis of variance followed by Tukey's multiple comparisons test using a statistical software (Prism, version 3.03; GraphPad Software Inc., San Diego, CA). The significance was defined as p<0.05.
RESULTS
Infarct size
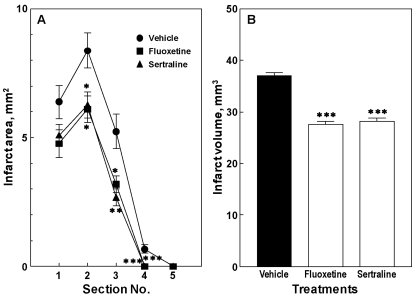
The infarct size, including the infarct area and volume, was measured at 14 d after the first dose of fluoxetine or sertraline. At 14 d after the first dose of fluoxetine, the infarct area was significantly reduced in sections 2, 3, and 4 (p<0.05, p<0.05, and p<0.01, respectively; Fig. 2A), thereby bringing about significant reductions in the infarct volume (p<0.001; Fig. 2B). Even in case of sertraline, the infarct size, including the infarct area and volume, showed a similar reduction (Fig. 2).
Fig. 2.
Effects of fluoxetine and sertraline on infarct area (A) and volume (B) in mice. Animals were treated daily with fluoxetine (10 mg/kg, i.p.) or sertraline (20 mg/kg, i.p.) for 14 d. Data are presented as mean±S.E.M. from 6 independent experiments. *p<0.05, **p<0.01, ***p<0.001 vs. vehicle group.
Evans blue extravasation
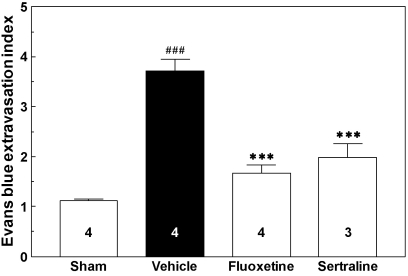
The effect of fluoxetine and sertraline on BBB permeability after photothrombotic cortical ischemia is shown in Fig. 3. The mice were sacrificed at 1 h after Evans blue injection, at 24 h after drug treatment, and at 14 d after photothrombotic cortical ischemia. In the ischemic control group, photothrombotic cortical ischemia induced a 3.3-fold increase in the Evans blue extravasation index, which was expressed as the ratio of the Evans blue content in the ipsilateral cortex to that in the contralateral cortex, implying enhanced BBB permeability (p<0.001). The Evans blue extravasation indices of the fluoxetine- and sertraline-treated groups were significantly lower than that of the vehicle-treated group (p<0.001 in both cases).
Fig. 3.
Evans blue extravasation in cerebral cortex after photothrombotic cortical ischemia in mice. Mice were sacrificed at 1 h after Evans blue injection, at 24 h after drug treatment, and at 14 d after photothrombotic cortical ischemia. The numbers in columns indicate the numbers of animals. ###p<0.001 vs. sham group. ***p<0.001 vs. vehicle group.
Autoregulation of cerebral blood flow
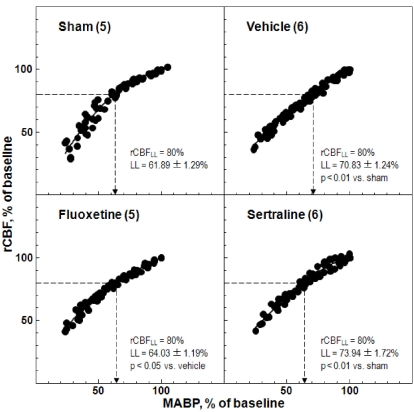
CBF was measured by laser-Doppler flowmetry while graded hypotension was being induced by controlled hemorrhage. The CBF response to bleeding hypotension was measured at 14 d after the first dose of fluoxetine and sertraline. The LL of CBF autoregulation in the sham-operated group was 61.89±1.29% of the baseline MABP and that in the ischemic control group was 70.83±1.24% of the baseline MABP (p<0.01). Treatment with fluoxetine and sertraline shifted the LL toward a lower MABP (64.03±1.19% and 73.94±1.72%, respectively; p<0.05 and p<0.01, respectively; Fig. 4). The arterial blood gas analysis values did not differ significantly between the before and after graded hypotension groups, and were within normal limits (data not shown).
Fig. 4.
Effects of fluoxetine and sertraline on regional cerebral blood flow (rCBF) response to changes in mean arterial blood pressure (MABP). Animals were treated daily with fluoxetine (10 mg/kg, i.p.) or sertraline (20 mg/kg, i.p.) for 14 d. The numbers in parentheses indicate the numbers of animals.
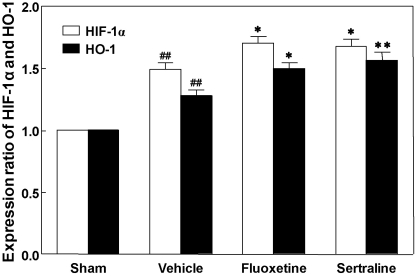
Expression of HIF-1α and HO-1 proteins
Western blot analysis revealed a significant increase in HIF-1α protein expression in the ipsilateral cortex in response to photothrombotic ischemia as compared to the sham-operated group (p<0.01; Fig. 5). Treatment with fluoxetine and sertraline elevated HIF-1α expression to significantly higher levels as compared to the vehicle group (p<0.05 and p<0.05, respectively; Fig. 5).
Fig. 5.
Effects of fluoxetine and sertraline on hypoxia-inducible factor-1α (HIF-1α) and heme oxygense-1 (HO-1) protein expression in photothrombotic ischemic cortex. Animals were treated daily with fluoxetine (10 mg/kg, i.p.) or sertraline (20 mg/kg, i.p.) for 14 d. Data are presented as mean±S.E.M. from 6 independent experiments. *p<0.05, **p<0.01 vs. vehicle, ##p<0.01 vs. sham.
HO-1 protein expression in the ischemic cortex increased greatly after photothrombosis as compared to the sham-operated group (p<0.01; Fig. 5). Treatment with fluoxetine and sertraline elevated HO-1 expression to significantly higher levels as compared to the vehicle group (p<0.05 and p<0.01, respectively; Fig. 5).
DISCUSSION
The present study examined the influence of postischemic treatment with SSRIs, including fluoxetine and sertraline, on recovery from brain tissue damage and impaired autoregulation of CBF following photothrombotic cortical ischemia in mice. Postischemic treatment with fluoxetine and sertraline significantly reduced the infarct size and the Evans blue extravasation index, restored CBF autoregulation, and elevated the expression of HIF-1α and HO-1 proteins.
In focal ischemia, the ischemic vascular bed comprises an area with severe CBF reduction consisting of an ischemic core and a more distal ischemic penumbra and includes regions that are marginally perfused and might be served by collateral vascular channels (Harukuni and Bhardwaj, 2006). A critical determinant of the severity of brain injury is the duration and severity of the ischemic insult and early restoration of CBF. The histopathological outcome following focal ischemia largely depends on the severity and duration of ischemia (Pulsinelli, 1985; Bhardwaj et al., 2003).
Most stroke patients show some degree of spontaneous functional recovery from their initial disabling state (Nakayama et al., 1994; Arboix et al., 2003). The extent of recovery depends primarily on the size and location of the cerebral infarct. Early recovery following stroke is thought to be due to the resolution of cerebral edema, absorption of the damaged tissue, or reperfusion of the ischemic penumbra. In the later stages of recovery, neurite growth and synaptogenesis occur, leading to functional and structural reorganization of the remaining intact brain tissue. Brain imaging studies have proven that the intact brain tissue can compensate for the functions lost after brain damage (Dijkhuizen et al., 2001; Hanlon et al., 2005).
SSRIs may possibly have a preventive effect on the recurrence of stroke in patients with post-stroke depression or depression associated with multi-infarct dementia. With regard to risk-benefit assessment, SSRIs such as citalopram and fluoxetine have been shown to be effective in the treatment of post-stroke depression in randomized controlled trials (Robinson et al., 2000). In addition, sertraline has been found to be effective in the prevention of depression in stroke patients (Rasmussen, 2003), and fluoxetine appears to facilitate stroke recovery, even in nondepressed stroke patients (Dam et al., 1996). Neuronal recovery in stroke might be mediated through the effects exerted by SSRIs on brain-derived neurotrophic factor and neurogenesis (Duman et al., 1997).
In the present study, postischemic treatment with fluoxetine and sertraline significantly reduced the infarct size. This finding can be explained by the fact that fluoxetine interferes with the Ca2+ signaling mechanisms in the vascular smooth muscle of small cerebral arteries (Ungvari et al., 1999). Fluoxetine-mediated dilatation of the cerebral vessels can result in an increase in the CBF in vivo, which might benefit the collateral circulation in the ischemic penumbra and may be clinically important because considerable data support the existence of impaired regional cerebral blood flow in major depression (Bonne et al., 1996).
Brain edema is a major and often life-threatening complication of a variety of brain injuries. Clinically, late stage edema, as defined by BBB disruption, is the most common form of brain edema (Betz, 1997). Serotonin has been reported that participate in the development of ischemic brain edema (Ishii et al., 1994). In the present study, the Evans blue extravasation indices of the fluoxetine- and sertraline-treated groups were significantly lower than that of the ischemic control group. This result suggests that SSRIs may protect against BBB dysfunction after photothrombotic cortical ischemia.
Postischemic treatment with fluoxetine and sertraline shifted the LL of CBF auroregulation toward a lower MABP, displaying the ability to restore LL. This finding suggests that fluoxetine and sertraline facilitate recovery from the impairment of CBF autoregulation following photothrombotic cortical ischemia.
Accumulating evidence suggests that chronic administration of SSRIs results in the desensitization of 5-HT1A somatodendritic autoreceptor function in the dorsal raphe (Blier and de Montigny, 1994; Kreiss and Lucki, 1995; Le Poul et al., 2000). Chronic SSRI treatment also results in desensitization of the physiological responses mediated by the postsynaptic 5-HT1A receptors (Goodwin et al., 1987; Hensler et al., 1991; Li et al., 1996). The beneficial effects of fluoxetine and sertraline on the recovery from brain tissue damage and impaired autoregulation of CBF following photothrombotic cortical ischemia might be related to the changes in receptor sensitivity. Future investigations are needed to clarify whether the association between the changes in the receptor sensitivity and morphofunctional improvement found in the present study with fluoxetine and sertraline is observed with other antidepressant drugs.
The mechanism underlying the facilitative effect of serotonergic stimulation on functional recovery after brain damage remains to be clarified. Nevertheless, illustration of the mechanism (s) by which fluoxetine and sertraline affect the serotonin levels in the central presynaptic sites extends beyond the scope of the present in vivo study.
Serotonin has been reported to progressively decrease only in the occluded hemisphere of ischemic animals, and preischemic depletion of brain serotonin with p-chlorophenylalanine has been reported to decrease the incidence of ischemia (Welch et al., 1977). The improvement of photothrombotic cortical ischemia with fluoxetine and sertraline is also in accordance with the results of previous studies that have emphasized on serotonergic dysfunction as an etiology of aggression in patients with depression (Van Praag, 1998) or stroke (Kim et al., 2002). It has been shown that chronic antidepressant treatment (e.g., with fluoxetine) has been shown to up-regulate neurogenesis (Malberg, 2004). Fluoxetine has previously been reported to prevent MDMA ("ecstasy")-induced degeneration of serotonergic nerve endings (Sanchez et al., 2001) and to increase the proliferation of neuronal stem cells derived from the hippocampus (Chiou et al., 2006).
It is generally accepted that HIF-1α exerts beneficial effects that favor neuronal survival after ischemia (Semenza, 2000; Acker and Acker, 2004), and the induction of HO-1 protein may protect cerebral tissues from ischemic damage (Fu et al., 2006).
HO-1, the inducible isoform of HO, catalyzes the rate-limiting step of heme oxidation to biliverdin, carbon monoxide, and the free ferrous iron (Otterbein and Choi, 2000; Ryter et al., 2002). Biliverdin is then rapidly converted by biliverdin reductase to bilirubin, a molecule with antioxidant properties, and free iron is sequestered by ferritin (Otterbein and Choi, 2000; Ryter et al., 2002). HO-1 is a heat-shock protein (HSP-32) that is induced in the brain in response to permanent focal ischemia (Geddes et al., 1996; Panahian et al., 1999; Bidmon et al., 2001) and has been demonstrated to be an HIF-1α regulated gene (Lee et al., 1997; Dawn and Bolli, 2005). The increase in HO-1 expression in ischemic brain tissue is probably a physiological consequence in the recovery of neuronal tissue following focal cerebral infarction.
In the present study, both fluoxetine and sertraline brought about a greater enhancement in the expression of HO-1 protein as compared to the vehicle group along with HIF-1α protein expression in the photothrombotic ischemic cortex, indicative of a neuroprotective action of these drugs. Our findings also suggest that SSRIs protect against ischemic brain damage and facilitate recovery following photothrombotic cortical ischemia.
Taken together, it is suggested that SSRIs, such as fluoxetine and sertraline, facilitate recovery following photothrombotic cortical ischemia through the enhancement of HO-1 and HIF-1α protein expression, thereby providing a benefit in the therapy of postischemic brain injury.
ACKNOWLEDGEMENTS
This work was supported for two years by Pusan National University Research Grant.
ABBREVIATIONS
- SSRIs
serotonin reuptake inhibitors
- HO-1
heme oxygenase-1
- HIF-1α
hypoxia-inducible factor-1α
- BBB
blood-brain barrier
- rCBF
regional cerebral blood flow
- MABP
mean arterial blood pressure
- LL
lower limit
- HSP-32
heat-shock protein
References
- 1.Acker T, Acker H. Cellular oxygen sensing need in CNS function:physiological and pathological implications. J Exp Biol. 2004;207:3171–3188. doi: 10.1242/jeb.01075. [DOI] [PubMed] [Google Scholar]
- 2.Andersen G, Vestergaard K, Riis J, Lauritzen L. Incidence of post-stroke depression during the first year in a large unselected stroke population determined using a valid standardized rating scale. Acta Psychiatr Scand. 1994b;90:190–195. doi: 10.1111/j.1600-0447.1994.tb01576.x. [DOI] [PubMed] [Google Scholar]
- 3.Arboix A, Garcia-Eroles L, Comes E, Oliveres M, Balcells M, Pacheco G, Targa C. Predicting spontaneous early neurological recovery after acute ischemic stroke. Eur J Neurol. 2003;10:429–435. doi: 10.1046/j.1468-1331.2003.00630.x. [DOI] [PubMed] [Google Scholar]
- 4.Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17:1304–1308. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- 5.Betz AL. Vasogenic brain edema. In: Welch KMA, Caplan LR, Reis DJ, Siesj BK, Weir B, editors. Primer on cerebrovascular diseases. San Diego: Academic Press; 1997. pp. 56–159. [Google Scholar]
- 6.Bhardwaj A, Alkayed NJ, Kirsch JR, Hurn PD. Mechanisms of ischemic brain damage. Curr Cardiol Rep. 2003;5:160–167. doi: 10.1007/s11886-003-0085-1. [DOI] [PubMed] [Google Scholar]
- 7.Bhogal SK, Teasell R, Foley N, Speechley M. Heterocyclics and selective serotonin reuptake inhibitors in the treatment and prevention of post-stroke depression. J Am Geriatr Soc. 2005;53:1051–1057. doi: 10.1111/j.1532-5415.2005.53310.x. [DOI] [PubMed] [Google Scholar]
- 8.Bidmon HJ, Emde B, Oermann E, Kubitz R, Witte OW, Zilles K. Heme oxygenase-1 (HSP-32) and heme oxygenase-2 induction in neurons and glial cells of cerebral regions and its relation to iron accumulation after focal cortical photothrombosis. Exp Neurol. 2001;168:1–22. doi: 10.1006/exnr.2000.7456. [DOI] [PubMed] [Google Scholar]
- 9.Blier P, de Montigny C. Current advances and trends in the treatment of depression. Trends Pharmacol Sci. 1994;15:220–226. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- 10.Bonne O, Krausz Y, Gorfine M, Karger H, Gelfin Y, Shapira B, Chisin R, Lerer B. Cerebral hypoperfusion in medication resistant, depressed patients assessed by Tc99m HMPAO SPECT. J Affect Disord. 1996;41:163–171. doi: 10.1016/s0165-0327(96)00058-4. [DOI] [PubMed] [Google Scholar]
- 11.Bonvento G, MacKenzie ET, Edvinsson L. Serotonergic innervation of the cerebral vasculature: relevance to migraine and ischaemia. Brain Res Brain Res Rev. 1991;16:257–263. doi: 10.1016/0165-0173(91)90009-w. [DOI] [PubMed] [Google Scholar]
- 12.Chiou SH, Chen SJ, Peng CH, Chang YL, Ku HH, Hsu WM, Ho LL, Lee CH. Fluoxetine up-regulates expression of cellular FLICE-inhibitory protein and inhibits LPS-induced apoptosis in hippocampus-derived neural stem cell. Biochem Biophys Res Commun. 2006;343:391–400. doi: 10.1016/j.bbrc.2006.02.180. [DOI] [PubMed] [Google Scholar]
- 13.Dam M, Tonin P, De Boni A, Pizzolato G, Casson S, Ermani M, Freo U, Piron L, Battistin L. Effects of fluoxetine and maprotiline on functional recovery in post-stroke hemiplegic patients undergoing rehabilitation therapy. Stroke. 1996;27:1211–1214. doi: 10.1161/01.str.27.7.1211. [DOI] [PubMed] [Google Scholar]
- 14.Dawn B, Bolli R. HO-1 induction by HIF-1: a new mechanism for delayed cardioprotection? Am J Physiol Heart Circ Physiol. 2005;289:H522–H524. doi: 10.1152/ajpheart.00274.2005. [DOI] [PubMed] [Google Scholar]
- 15.Demougeot C, Van Hoecke M, Bertrand N, Prigent-Tessier A, Mossiat C, Beley A, Marie C. Cytoprotective efficacy and mechanisms of the liposoluble iron chelator 2,2'-dipyridyl in the rat photothrombotic ischemic stroke model. J Pharmacol Exp Ther. 2004;311:1080–1087. doi: 10.1124/jpet.104.072744. [DOI] [PubMed] [Google Scholar]
- 16.Dijkhuizen RM, Ren J, Mandeville JB, Wu O, Ozdag FM, Moskowitz MA, Rosen BR, Finklestein SP. Functional magnetic resonance imaging of reorganization in rat brain after stroke. Proc Natl Acad Sci U S A. 2001;98:12766–12771. doi: 10.1073/pnas.231235598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 18.Fu R, Zhao ZQ, Zhao HY, Zhao JS, Zhu XL. Expression of heme oxygenase-1 protein and messenger RNA in permanent cerebral ischemia in rats. Neurol Res. 2006;28:38–45. doi: 10.1179/016164106X91852. [DOI] [PubMed] [Google Scholar]
- 19.Gainotti G, Antonucci G, Marra C, Paolucci S. Relation between depression after stroke, antidepressant therapy, and functional recovery. J Neurol Neurosurg Psychiatry. 2001;71:258–261. doi: 10.1136/jnnp.71.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geddes JW, Pettigrew LC, Holtz ML, Craddock SD, Maines MD. Permanent focal and transient global cerebral ischemia increase glial and neuronal expression of heme oxygenase-1, but not heme oxygenase-2 protein in rat brain. Neurosci Lett. 1996;210:205–208. doi: 10.1016/0304-3940(96)12703-8. [DOI] [PubMed] [Google Scholar]
- 21.Goodwin GM, de Souza RJ, Green AR. Attenuation by electroconvulsive shock and antidepressant drugs of the 5-HT1A receptor-mediated hypothermia and serotonin syndrome produced by 8-OH-DPAT in the rat. Psychopharmacology. 1987;91:500–505. doi: 10.1007/BF00216018. [DOI] [PubMed] [Google Scholar]
- 22.Hanlon CA, Buffington AL, McKeown MJ. New brain networks are active after right MCA stroke when moving the ipsilesional arm. Neurology. 2005;64:114–120. doi: 10.1212/01.WNL.0000148726.45458.A9. [DOI] [PubMed] [Google Scholar]
- 23.Harukuni I, Bhardwaj A. Mechanisms of brain injury after global cerebral ischemia. Neurol Clin. 2006;24:1–21. doi: 10.1016/j.ncl.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Hensler JG, Kovachich GB, Frazer A. A quantitative autoradiographic study of serotonin1A receptor regulation. Effect of 5,7-dihydroxytryptamine and antidepressant treatments. Neuropsychopharmacology. 1991;4:131–144. [PubMed] [Google Scholar]
- 25.Ishii H, Bertrand N, Stanimirovic D, Strasser A, Mrsulja BB, Spatz M. The relationship between cerebral ischemic edema and monoamines: revisited. Acta Neurochir Suppl (Wien) 1994;60:238–241. doi: 10.1007/978-3-7091-9334-1_63. [DOI] [PubMed] [Google Scholar]
- 26.Jakovljevic D, Tuomilehto J. Use of selective serotonin reuptake inhibitors and the risk of stroke: is there reason for concern? Stroke. 2002;33:1448–1449. doi: 10.1161/01.str.0000018582.96060.3e. [DOI] [PubMed] [Google Scholar]
- 27.Kim JS, Choi S, Kwon SU, Seo YS. Inability to control anger or aggression after stroke. Neurology. 2002;58:1106–1108. doi: 10.1212/wnl.58.7.1106. [DOI] [PubMed] [Google Scholar]
- 28.Kotila M, Numminen H, Waltimo O, Kaste M. Post-stroke depression and functional recovery in a population-based stroke register. The Finnstroke study. Eur J Neurol. 1999;6:309–312. doi: 10.1046/j.1468-1331.1999.630309.x. [DOI] [PubMed] [Google Scholar]
- 29.Kreiss DS, Lucki I. Effects of acute and repeated administration of antidepressant drugs on extracellular levels of 5-hydroxytryptamine. J Pharmacol Exp Ther. 1995;274:866–867. [PubMed] [Google Scholar]
- 30.Le Poul E, Boni C, Hanoun N, Laporte AM, Laaris N, Chauveau J, Hamon M, Lanfumey L. Differential adaption of brain 5-HT1A and 5-HT1B receptors and 5-HT transporter in rats treated chronically with fluoxetine. Neuropharmacology. 2000;39:110–122. doi: 10.1016/s0028-3908(99)00088-x. [DOI] [PubMed] [Google Scholar]
- 31.Lee PJ, Jiang BH, Chin BY, Iyer NV, Alam J, Semenza GL, Choi AM. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J Biol Chem. 1997;272:5371–5381. [PubMed] [Google Scholar]
- 32.Li Q, Muma NA, Van de Kar LD. Chronic fluoxetine induces a gradual desensitization of 5-HT1A receptors: reductions in hypothalamic and midbrain Gi and Go proteins and in neuroendocrine responses to a 5-HT1A agonist. J Pharmacol Exp Ther. 1996;279:1035–1042. [PubMed] [Google Scholar]
- 33.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 34.Malberg JE. Implications of adult hippocampal neurogenesis in antidepressant action. J Psychiatry Neurosci. 2004;29:196–205. [PMC free article] [PubMed] [Google Scholar]
- 35.Marks GS, Brien JF, Nakatsu K, McLaughlin BE. Does carbon monoxide have a physiological function? Trends Pharmacol Sci. 1991;12:185–188. doi: 10.1016/0165-6147(91)90544-3. [DOI] [PubMed] [Google Scholar]
- 36.Muhonen MG, Robertson SC, Gerdes JS, Loftus CM. Effects of serotonin on cerebral circulation after middle cerebral artery occlusion. J Neurosurg. 1997;87:301–306. doi: 10.3171/jns.1997.87.2.0301. [DOI] [PubMed] [Google Scholar]
- 37.Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS. The influence of age on stroke outcome. The Copenhagen Stroke Study. Stroke. 1994;25:808–813. doi: 10.1161/01.str.25.4.808. [DOI] [PubMed] [Google Scholar]
- 38.Nimura T, Weinstein PR, Massa SM, Panter S, Sharp FR. Heme oxygenase-1 (HO-1) protein induction in rat brain following focal ischemia. Brain Res Mol Brain Res. 1996;37:201–208. doi: 10.1016/0169-328x(95)00315-j. [DOI] [PubMed] [Google Scholar]
- 39.Otterbein LE, Choi AM. Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1029–L1037. doi: 10.1152/ajplung.2000.279.6.L1029. [DOI] [PubMed] [Google Scholar]
- 40.Panahian N, Yoshiura M, Maines MD. Overexpression of heme oxygenase-1 is neuroprotective in a model of permanent middle cerebral artery occlusion in transgenic mice. J Neurochem. 1999;72:1187–1203. doi: 10.1111/j.1471-4159.1999.721187.x. [DOI] [PubMed] [Google Scholar]
- 41.Paolucci S, Antonucci G, Grasso MG, Morelli D, Troisi E, Coiro P, De Angelis D, Rizzi F, Bragoni M. Post-stroke depression, anti-depressant treatment and rehabilitation results. A case-control study. Cerebrovasc Dis. 2001;12:264–271. doi: 10.1159/000047714. [DOI] [PubMed] [Google Scholar]
- 42.Parikh RM, Robinson RG, Lipsey JR, Starkstein SE, Fedoroff JP, Price TR. The impact of post-stroke depression on recovery in activities of daily living over a 2-year follow-up. Arch Neurol. 1990;47:785–789. doi: 10.1001/archneur.1990.00530070083014. [DOI] [PubMed] [Google Scholar]
- 43.Primeau F. Post-stroke depression: a critical review of the literature. Can J Psychiatry. 1988;33:757–765. doi: 10.1177/070674378803300816. [DOI] [PubMed] [Google Scholar]
- 44.Pulsinelli WA. Selective neuronal vulnerability: morphological and molecular characteristics. Prog Brain Res. 1985;63:29–37. doi: 10.1016/S0079-6123(08)61973-1. [DOI] [PubMed] [Google Scholar]
- 45.Ramasubbu R, Patten SB. Effect of depression on stroke morbidity and mortality. Can J Psychiatry. 2003;48:250–257. doi: 10.1177/070674370304800409. [DOI] [PubMed] [Google Scholar]
- 46.Rampello L, Battaglia G, Raffaele R, Vecchio I, Alvano A. Is it safe to use antidepressants after a stroke? Expert Opin Drug Saf. 2005;4:885–897. doi: 10.1517/14740338.4.5.885. [DOI] [PubMed] [Google Scholar]
- 47.Rasmussen A, Lunde M, Poulsen DL, Sørensen K, Qvitzau S, Bech P. A doubleblind, placebo-controlled study of sertraline in the prevention of depression in stroke patients. Psychosomatics. 2003;44:216–221. doi: 10.1176/appi.psy.44.3.216. [DOI] [PubMed] [Google Scholar]
- 48.Robinson RG, Schultz SK, Castillo C, Kopel T, Kosier JT, Newman RM, Curdue K, Petracca G, Starkstein SE. Nortriptyline versus fluoxetine in the treatment of depression and in short-term recovery after stroke: a placebo-controlled, double-blind study. Am J Psychiatry. 2000;157:351–359. doi: 10.1176/appi.ajp.157.3.351. [DOI] [PubMed] [Google Scholar]
- 49.Ryter SW, Otterbein LE, Morse D, Choi AM. Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol Cell Biochem. 2002;234-235:249–263. doi: 10.1023/A:1015957026924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanchez V, Camarero J, Esteban B, Peter MJ, Green AR, Colado MI. The mechanisms involved in the long-lasting neuroprotective effect of fluoxetine against MDMA ('ecstasy')-induced degeneration of 5-HT nerve endings in rat brain. Br J Pharmacol. 2001;134:46–57. doi: 10.1038/sj.bjp.0704230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schroeter M, Jander S, Stoll G. Non-invasive induction of focal cerebral ischemia in mice by photothrombosis of cortical microvessels: characterization of inflammatory responses. J Neurosci Methods. 2002;117:43–49. doi: 10.1016/s0165-0270(02)00072-9. [DOI] [PubMed] [Google Scholar]
- 52.Semenza GL. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med. 2001;7:345–350. doi: 10.1016/s1471-4914(01)02090-1. [DOI] [PubMed] [Google Scholar]
- 53.Semenza GL. Surviving ischemia: adaptive responses mediated by hypoxia-inducible factor 1. J Clin Invest. 2000;106:809–812. doi: 10.1172/JCI11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Starkstein SE, Robinson RG. Affective disorders and cerebral vascular disease. Br J Psychiatry. 1989;154:170–182. doi: 10.1192/bjp.154.2.170. [DOI] [PubMed] [Google Scholar]
- 55.Ungvari Z, Pacher P, Kecskemeti V, Koller A. Fluoxetine dilates isolated small cerebral arteries of rats and attenuates constrictions to serotonin, norepinephrine, and a voltage-dependent Ca2+ channel opener. Stroke. 1999;30:1949–1954. doi: 10.1161/01.str.30.9.1949. [DOI] [PubMed] [Google Scholar]
- 56.Uyama O, Okamura N, Yanase M, Narita M, Kawabata K, Sugita M. Quantitative evaluation of vascular permeability in the gerbil brain after transient ischemia using Evans blue fluorescence. J Cereb Blood Flow Metab. 1988;8:282–284. doi: 10.1038/jcbfm.1988.59. [DOI] [PubMed] [Google Scholar]
- 57.Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: a putative neural messenger. Science. 1993;259:381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- 58.Welch KM, Chabi E, Buckingham J, Bergin B, Achar VS, Meyer JS. Cathecholamine and 5-hydroxytryptamine levels in ischemic brain. Influence of p-chlorophenylalanine. Stroke. 1977;8:341–346. doi: 10.1161/01.str.8.3.341. [DOI] [PubMed] [Google Scholar]