Abstract
Purpose
The National Cancer Institute has completed a first-in-human clinical pharmacodynamic (PD) trial of the targeted agent ABT-888, a poly (ADP-ribose) polymerase (PARP) inhibitor, under the auspices of the US Food and Drug Administration's Exploratory Investigational New Drug Application. Performance of the study design, needle biopsy procedure, and validated PD assay were evaluated in human tumor xenograft models.
Experimental Design
A validated, enzyme-linked immunoassay was used to quantify PAR, a product of the PARP 1/2 enzyme activity. Sampling variability from tumor heterogeneity was determined by comparing PAR content in multiple tumors, and in different areas of the same tumor in a particular animal, collected under anesthesia by needle biopsy or resection before and after administration of non-toxic doses of ABT-888. The degree of PARP inhibition following single-dose treatment was evaluated in the time frame anticipated for biopsy in humans.
Results
Sampling variability around the mean (∼50%) for untreated and vehicle-treated animals was random and due to specimen heterogeneity. PAR levels in initial and repeat tumor biopsies, separated by 1 week, were not altered by the stress induced by daily handling of the animals. A single ABT-888 dose (3 mg/kg or 12.5 mg/kg) reduced intra-tumor PAR levels by >95%. ABT-888 (1.56 mg/kg to 25 mg/kg) significantly decreased PAR levels at 2 hours post dosing.
Conclusion
The detailed methodologies developed for this study facilitated the design of a Phase 0, first-in-human clinical trial of ABT-888 and could serve as a model for developing proof-of-principle clinical trials of molecularly targeted anticancer agents.
Keywords: pharmacodynamic assays, Phase 0, ABT-888, animal model, translational research
Introduction
Barely 5% of all Investigational New Drug (IND) applications for novel molecular agents in oncology advance from the investigational phase to registration6 (1). Questionable interpretation and validation of existing preclinical models for early phase clinical trials may contribute to this statistic. Furthermore, early phase trials rarely incorporate robust pharmacodynamic (PD) assay methodologies designed to measure a drug's effect on its presumed target. PD assays can provide critical information on the probability of a drug's overall success in advancing through the developmental pipeline and could be very useful in eliminating future clinical failures. A PD assay that provides meaningful scientific results can also preclude exposing study participants to invasive procedures unnecessarily. Thus, “humanizing” preclinical models with validated PD assays and clinically relevant methodologies for tissue sampling, coupled with better statistical design and analysis, could substantially improve early phase clinical trials. This philosophy is critical to the success of clinical PD trials in oncology.
The National Cancer Institute (NCI) has initiated a series of Phase 0 trials under the purview of an Exploratory IND (xIND) application from the FDA6 (2) to explore their value in accelerating clinical evaluation of investigational agents in oncology. Some Phase 0 trials will be designed to evaluate the PD effects of non-toxic doses of new agents at a molecular level; tumor biopsies and established surrogate tissues determine whether an investigational agent acts on its presumed target, thus providing evidence for its mechanism of action and pharmaceutical properties. Essential to this process is a PD assay that has been validated for analytical performance and proven to be therapeutically relevant in preclinical studies(3). Rigorous proof of clinical readiness is predicated on preclinical data showing drug effectiveness as measured in repeat tumor biopsies, a drug time-effect window that is clinically feasible to study, and some insight into the drug exposure likely to produce a measurable PD effect(3). Furthermore, questions that have confounded the interpretation of correlative studies in Phase I and II trials can be answered because they affect the primary endpoint of a Phase 0 trial, for example, whether clinical assessment of drug action can be better assessed by comparing biopsies from two lesions or sequential biopsies of the same lesion.
Poly (ADP-ribose) polymerase (PARP) detects and facilitates repair of single-stranded DNA breaks; expression is upregulated in tumor cells, possibly as a mechanism to escape apoptosis(4, 5). PARP activity is also important in inflammation, necrosis, and in apoptotic pathways in the presence of DNA damage(6-11). Thus, PARP inhibitors are being investigated for a number of disease indications(12-14). Theoretically, inhibition of PARP via small molecule agents such as ABT-888 (NSC 737664) should sensitize tumor cells to a variety of cytotoxic drugs and radiation. In a series of recent studies, ABT-888 potentiated treatment with temozolomide, platinum-containing agents, cyclophosphamide, irinotecan plus temozolomide, topotecan, indenoisoquinolines, camptothecin, and ionizing radiation in peripheral blood mononuclear cells (PBMCs) and syngeneic and xenograft tumor models(9-11, 15, 16). The product of PARP 1/2 enzymatic activity, PAR, was selected as a PD endpoint in the current study in an effort to minimize complicating factors associated with measuring drug activity by enzyme assays of tissue extracts. Specimen collection and handling methods were also designed to stabilize PAR, allowing measurement of PARP activity inside the target tissue at the time of tissue excision.
Our laboratory has developed and cross-validated an enzyme immunoassay, in collaboration with Abbott Laboratories and the National Clinical Target Validation Laboratory at the NCI, to measure PAR levels in human tumor xenograft models and in PBMCs isolated from healthy human subjects; assay validation details are provided with the Supplementary Materials of this report. Preclinical modeling of the planned Phase 0 trial design tested whether non-toxic doses of ABT-888 would result in a statistically significant reduction of PAR levels in tumor needle biopsies despite sampling variability due to intra- and inter-tumor heterogeneity. The Phase 0 clinical protocol was mirrored in the current preclinical study in athymic nude (nu/nu [NCr]) mice bearing Colo829 and A375 human tumor (melanoma) xenografts by using clinical procedures for collecting needle biopsies over a time frame achievable in the clinical setting, and by implementing standard operating procedures for specimen handling and storage transferable to a clinical laboratory. Inter- and intra- tumor variability of PAR levels was assessed both in needle biopsies and resected tumors of live animals under general anesthesia, including biopsies from two different tumor nodules in the same animal. Stability was also evaluated between repeat biopsy procedures separated by 1 week. The dose- and time-effect of ABT-888 on PAR levels in tumor samples established the minimum dose required to elicit a PD effect and the optimal time after drug administration to schedule a biopsy for PD assessment in the Phase 0 clinical trial. Preclinical modeling not only informed the design of the trial, but also served as a useful preclinical paradigm for developing future clinical PD studies of molecularly targeted anticancer agents.
Materials and Methods
Xenograft models
Cell lines
Cells were purchased from the American Type Culture Collection (ATCC; Manassas, VA) and grown in humidified incubators supplemented with 5% CO2; the cell-culture media were maintained according to ATCC recommendations. The assay controls were produced from the Colo829 tumor cell line and grown to super-confluence in T75 flasks. After washing in Hank's balanced salt solution, cells were harvested by scraping in lysis buffer (Biosource; Camarillo, CA), supplemented with protease inhibitor cocktail tablets (Roche Applied Science; Indianapolis, IN) and 1% phenylmethanesulfonyl fluoride (Sigma-Aldrich; St Louis, MO). Cell culture media and reagents were purchased from Invitrogen (Carlsbad, CA).
Animals
Xenografts were established in female athymic nu/nu (NCr) mice (NCI Animal Production Program; Frederick, MD) with the human melanoma cell lines A375 and Colo829 by subcutaneous injection (1.0 × 107 cells/0.1 mL/mouse) on the lateral body wall, just caudal to the axilla. All mice developed tumors, and the tumors were maintained by serial in vivo passage using tumor fragment transplantation when the donor tumors reached 10 mm to 15 mm in diameter. Tumors were staged to a pre-selected size (weight) using the following formula: weight [mg = (tumor length × tumor width2)/2](17). Mice were housed in sterile, filter-capped, polycarbonate cages (Allentown Caging; Allentown, NJ) maintained in a barrier facility on a 12-hour light/dark cycle, and they were provided sterilized food and water ad libitum(17). Mice were randomized prior to initiation of treatment using a commercial software program (StudyLog; San Francisco, CA).
NCI-Frederick is accredited by AAALAC International and follows the Public Health Service Policy for the Care and Use of Laboratory Animals. Animal care was provided in accordance with the procedures outlined in the “Guide for Care and Use of Laboratory Animals” (National Research Council; 1996; National Academy Press; Washington, DC). All the studies were conducted according to an approved animal care and use committee protocol.
Study agents
ABT-888 (NSC 737664; Abbott; Abbott Park, IL) was solubilized to the required strength, not exceeding 5 mg per mL, in a clinically relevant vehicle consisting of sorbitol (105 mg/mL) and citric acid (monohydrate; 5.17 mg/mL) in sterile water. ABT-888 was administered orally by gavage as a single 3 mg/kg or 12.5 mg/kg dose. Dose volume was defined as 0.1 mL/10 g body weight. Topotecan was administered intraperitoneally at the single maximally tolerated dose (MTD) in mice of 15 mg/kg.
Anesthesia
Mice were anesthetized by isoflurane gas inhalation prior to biopsy or tumor resection.
Tumor biopsies
When surgical anesthesia was reached (no toe pinch), the skin was disinfected with Nolvasan® (Fort Dodge Lab Inc.; Fort Dodge, IA) and a 2 mm to 5 mm incision was made through the skin adjacent to the subcutaneous tumor being biopsied. An approved human biopsy needle (Temno 18-gauge; Allegiance Healthcare Corp.; McGaw Park, IL) was passed through the skin incision into the tumor. Once the needle was maximally in the tumor, a biopsy was collected, and the biopsy needle was retracted. The collected material (∼1 mm × 5 mm) was immediately flash frozen in an O-ring sealed, screw-capped, Sarstedt cryovial (Newton, NC) by touching the biopsy to the inside of the vial that was pre-cooled in liquid nitrogen (a critical step for stabilizing specimen PAR content). The vial was then sealed and returned to liquid nitrogen. Frozen specimens were stored at −80°C until use. After sample collection, the wound was closed with a surgical wound clip. Biopsies were performed at baseline/0, 2, 4, 7, and 24 hours after dosing. Repeat biopsies in untreated animals were separated by a 1-week recovery period, during which time animals were handled daily to model patient assessment anticipated during the clinical trial. Standard operating procedures were developed for stabilizing PAR content in the needle biopsies using the tumor xenograft models in an interventional radiology setting.
Tumor resection
Xenograft tumors were collected on the same schedule as tumor biopsies by standard dissection methods. Specimens were left intact or cut into two to four equal pieces with fine-point scissors and placed into Sarstedt microfuge tubes that were pre-cooled in liquid nitrogen as described above.
Tumor extract preparation
All tissue samples were processed by adding lysis buffer to the frozen tissue (0.5 mL/biopsy). Tissue was minced with fine-point scissors, vortexed, minced and vortexed again, and then kept on an ice bath. Extracts were disrupted by sonication, vortexed, allowed to stand on an ice bath for 15 minutes, vortexed again, and then supplemented with 1% SDS by adding 20% SDS concentrate (Ambion Inc.; Austin, TX). Specimens were vortexed and then immersed in a boiling water bath for 5 minutes; subsequently, they were snap-cooled for 1 minute in an ice bath and then moved to ambient temperature. After vortexing again, specimens were clarified by centrifugation at 10,000 × g for 2 minutes at 4°C. Specimens could be subjected to at least 3 freeze-thaw cycles without a detectable loss of antigen binding.
Statistical analysis
Regression analysis and descriptive statistics were conducted with Microsoft Excel. The significance level for the confidence interval (CI) was set at 5% (α=0.05) for a 1-sided test. In this study, coefficient of determination (R2) values <0.55 were considered random, while all higher values were indicative of systematic variability (i.e., the correlation coefficient [R] >0 at the 1-sided, α=0.05, significance level). This was justified by the fact that, for a data set of 6 pairs (n=6), R=0.74 (the square root of 0.55) was the smallest R, such that the lower 95% confidence bound was >0 by use of Fisher's transformation(18). An n of 6 was appropriate for all comparisons, even for those involving 12 data pairs, because there were only 6 animals. A 1-sided significance level was appropriate because all correlations were expected to be non-negative. Also, an R2 value of 0.55 indicated that 55% of the variation associated with either of the two variables was accounted for by the linear fit to the other variable.
Throughout this paper, error bars for individual tumor measurements are not visible because they are covered by the symbols on the graphs. Thus, the significant variability in PAR values between two tumors within a particular animal was due to local or regional differences in xenograft composition, plus any differences in PAR preservation through the tissue collection and extraction steps.
Biomarker development
Assay methodology
The PAR immunoassay uses a purified monoclonal antibody (clone 10H; Catalog no. 4335; Trevigen Inc.; Gaithersburg, MD) to PAR as the capture reagent, a rabbit anti-PAR antiserum (Catalog no. 4336-BPC-100; Trevigen) as the detecting agent, and an anti-rabbit horseradish peroxidase (HRP) conjugate reporter (Catalog no. 074−15−061; KPL; Gaithersburg, MD). Assay methodology and validation are detailed in the Supplementary Materials. Units of measure were pg/mL PAR normalized to 100 μg/assay well protein load, abbreviated in the Tables and Figures as “PAR level.”
Assay validation
The PAR immunoassay was subject to a validation protocol for analytical performance (see Supplementary Materials).
Results
Random inter-tumor variability in PAR levels of untreated xenografts
Variability in PAR levels across resected tumors in individual animals was assessed in a bilateral tumor Colo829 xenograft model. Large (≥300 mg) and small (150−200 mg) tumors occurred randomly on the left and right flanks of the animal. Large tumors were included as a surrogate for degree of necrosis within individual tumors. Mean PAR content was 5584 units (95% CI, 4102−7066 units) in the large tumors and 4146 units (95% CI, 3087−5205 units) in the small tumors (Fig. 1A). PAR levels in extracts from one to two pieces of each tumor varied by a 5- and 4-fold margin in the large and small nodule, respectively. Variability in PAR levels was random (i.e., not systematic; R2 <0.55), and there was no correlation in PAR levels between the large and small nodules in the individual animals.
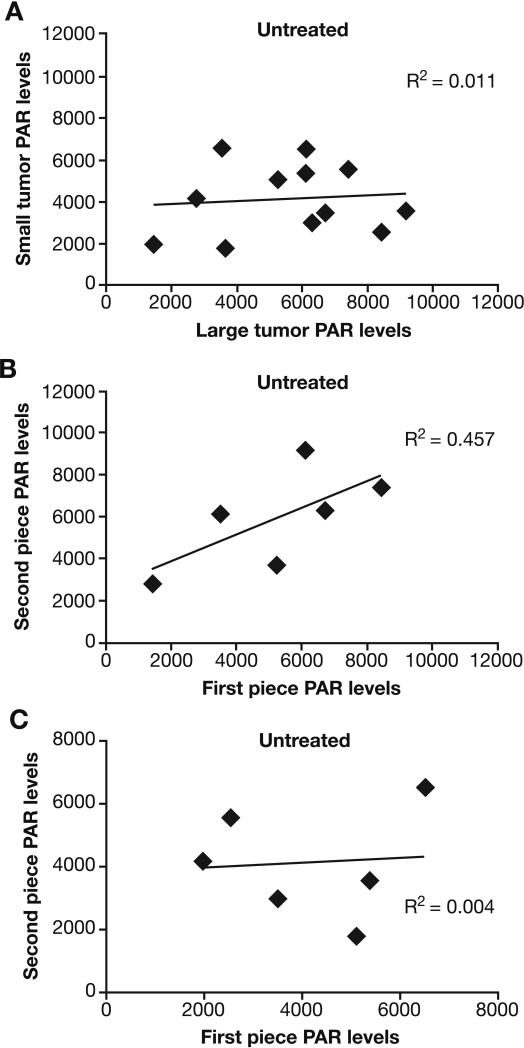
Fig. 1.
Inter- and intra-tumor variability of PAR levels in untreated-Colo829 xenografts. (A) Correlation in PAR levels between the large and small bilateral tumors of individual mice; (B) Correlation in PAR levels between two quadrants cut from each resected large tumor of the bilateral pair; (C) Correlation in PAR levels between two quadrants cut from each resected small tumor of the bilateral pair. Samples were collected 4 hours post dose. Values reported as pg PAR/mL normalized to 100 μg protein. Solid diamond, measured point; line, linear regression fit.
Random intra-tumor variability in PAR levels of untreated xenografts
Experiments were conducted both with large and small tumors in the Colo829 xenograft model to determine whether intra-tumor variability of PAR levels was greater than inter-tumor variability. As with inter-tumor variability, large tumors were evaluated as a surrogate for levels of necrosis. Two quadrants (“first piece” and “second piece”) of each resected large and small tumor were selected for analysis. Random intra-tumor variability of PAR levels was observed from pieces of both large and small tumors (Fig. 1B and C). PAR levels in the first and second pieces of the large or small tumors were not significantly different (Supplementary Table S3). Four of the 24 tumors had PAR levels >6000 units; these were all large tumors (Fig. 1), perhaps reflecting tumor necrosis. However, variability in PAR levels due to heterogeneity within a particular tumor nodule was not different from variability in PAR levels between different tumor nodules of the same or different sizes.
PAR levels from 18-gauge needle biopsies
Specimens obtained using the needle biopsy procedure ranged from 5 mm to 20 mm in length and 3 mg to 12 mg in mass, with good cellular content (Fig. 2). The feasibility of using needle biopsies for measuring PAR levels with the validated immunoassay was assessed using two tumor needle biopsies obtained from each of six Colo829 xenograft tumors. Combining the biopsy procedure with the sample handling SOP resulted in evaluable specimens from all 12 attempts. PAR levels in the individual biopsy specimens are presented in Supplementary Table S4.
Fig. 2.
Measurement of PAR levels from 18-gauge needle biopsies. (A) Direct placement of the 18-gauge biopsy needle into the subcutaneous tumor nodule via a skin flap; animals were under general anesthesia. (B) Typical needle biopsy yield from a subcutaneous tumor nodule. Typical specimen sizes ranged from 5 mm to 20 mm in length and 3 mg to 12 mg in mass (see Table 2). (C) Hematoxylin-stained frozen sections from a needle biopsy.
No systematic variability observed in PAR levels between extracts from biopsy samples and A375 tumor xenograft pieces
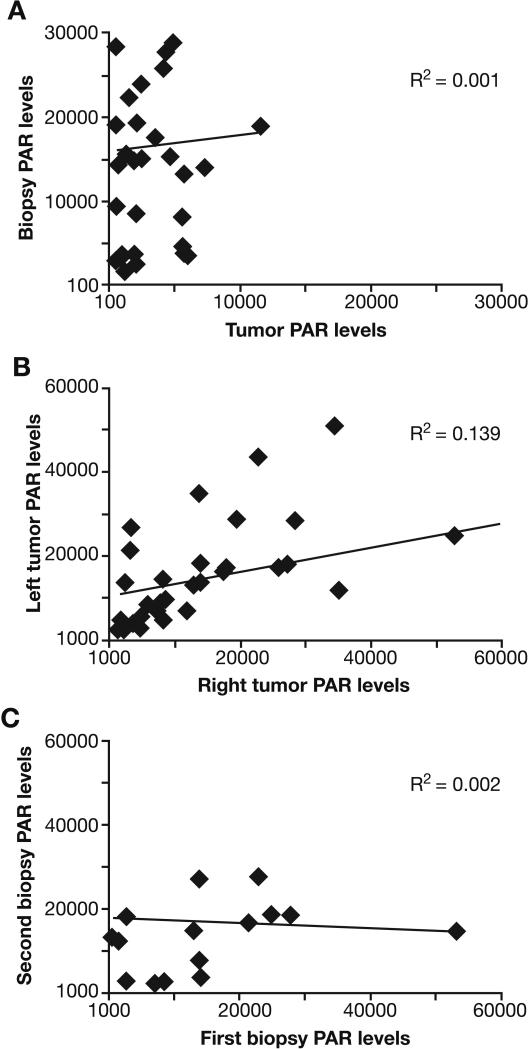
PAR levels in the biopsy specimens were compared with those in the residual tumor that was resected immediately after the biopsy procedure (Fig. 3). No systematic variability was detected in PAR levels between biopsies and tumor xenograft pieces (R2=0.001). However, biopsy PAR levels were generally higher than those obtained from the corresponding resected tumor. Furthermore, the 5-fold range in PAR level variability across individual biopsies was not greater than that found in the excised tumor quadrants, indicating that a similar drug effect level will be required to demonstrate significant target inhibition using either the 18-gauge needle or excisional biopsy procedures. Thus, smaller specimen size did not increase sampling variability in PAR levels.
Fig. 3.
Correlation of PAR levels in A375 xenografts. (A) PAR levels in 18-gauge needle biopsies versus surgically excised tumor pieces. Needle biopsies were collected from anesthetized animals, placed in pre-tared, pre-cooled vials, and flash frozen in liquid nitrogen; the remaining xenograft was surgically excised and flash frozen. (B) PAR levels in left versus right side tumors. Biopsies and tumor pieces (left versus right) from the same animal were graphed together. Data points represent either samples from needle biopsies or xenograft tumor pieces. (C) First versus second repeat biopsy samples (collected from 16 xenografts in 8 animals). Values were reported as pg PAR/mL normalized to 100 μg protein. Solid diamond, measured point; line, linear regression fit.
Although PAR levels in the A375 tumors were generally higher than in the Colo829 tumors, the variability in PAR levels observed with the needle biopsy procedure in untreated A375 xenografts was similar to that of Colo829 xenografts. PAR levels from the needle biopsies of A375 tumors implanted on the left or right flank showed large variations around the mean (R2=0.139), which appeared to be random (Fig. 3B). Individual PAR values from A375 needle biopsies were not normally distributed around the mean (2 standard deviations below the mean resulted in negative PAR values). Acquiring A375 biopsy specimens was made difficult by the softness of the tumor tissue, especially with repeat biopsies. Low PAR values in all the panels were associated with higher levels of extract loaded into the immunoassay. Subsequent to this work, optimum protein load for assaying xenografts was determined to be 5 μg per well with a range of 1 μg to 10 μg per well. This proved to be a critical parameter for the success of the assay. Analytical experiments indicated that the lower PAR levels at protein loads above 5 μg per well were associated with passive interference in the assay. High levels of cellular DNA (increasing extract viscosity) yielded a similar interference effect.
Repeat biopsy sampling produced random variability of PAR levels in A375 xenografts
A375 xenografts were used for comparing PAR levels in repeat 18-gauge needle biopsies of individual tumor, separated by a 1-week recovery period. Individual animals were handled during the 1-week interval to control for the effects of stress on PAR levels and attempt to replicate conditions encountered by Phase 0 trial participants. No statistically significant effect was observed in PAR levels between the first biopsy and the second biopsy (first biopsy mean ± 95% CI, 15620 ± 7245; second biopsy mean ± 95% CI, 17703 ± 8950). Regression analysis of sequential biopsies failed to show a strong correlation between PAR levels and the biopsy sequence (Fig. 3C), demonstrating random variation around the mean.
ABT-888-induced suppression of PAR levels was maintained over time in Colo829 xenografts
ABT-888 significantly decreased PAR levels 2 hours post dose in all treatment groups (Table 1). At the lowest dose of 1.56 mg/kg, ABT-888 significantly reduced PAR levels compared with the paired controls. Higher doses (12.5 mg/kg) suppressed PAR levels by >99%. After 5 hours, significant suppression of PAR levels persisted in the three highest dose levels of ABT-888 (6.25, 12.5, and 25 mg/kg), despite some indication of partial recovery. At 24 hours post dose, PAR levels recovered in all ABT-888-treated groups, although they remained suppressed by >50% in the 12.5 mg/kg and 25 mg/kg dose levels. PAR levels in the vehicle and untreated control groups were comparable and similar to the results observed in the A375 and Colo829 experiments described above. However, the mean PAR values in these two control groups were higher in this experiment, possibly because smaller tumors (100−150 mg) were collected or because of differences in the storage time of frozen tumors prior to extraction and assay testing. Nevertheless, the 95% CIs of the treatment groups in this and the other Colo829 experiments overlap.
Table 1.
Temporal effects of single-dose ABT-888 on PAR levels in Colo829 xenografts.
| ABT-888* + 2 hours | |||||||
| Vehicle | 1.56 | 3.13 | 6.25 | 12.5 | 25 | TPT 15* | |
| Mean | 18029 | 1361 | 454 | 293 | 159 | 143 | 32701 |
| SD (±) | 6711 | 874 | 786 | 457 | 319 | 299 | 19583 |
| 95% CI | 10986−25072 | 444−2278 | LLQ-1279 | LLQ-773 | LLQ-666 | LLQ-393 | 12150−53252 |
| ABT-888* + 5 hours | |||||||
| Vehicle | 1.56 | 3.13 | 6.25 | 12.5 | 25 | TPT 15* | |
| Mean | 20002 | 11392 | 13274 | 10606 | 7907 | 4023 | 19904 |
| SD (±) | 6076 | 6375 | 10913 | 9062 | 3899 | 2332 | 12658 |
| 95% CI | 13626−26378 | 3477−19307 | 1822−24726 | LLQ-21858 | 3066−12748 | 1128−6918 | 6620−33188 |
| ABT-888* + 24 hours | |||||||
| Vehicle | 1.56 | 3.13 | 6.25 | 12.5 | 25 | TPT 15* | |
| Mean | 18866 | 33927 | 11353 | 10404 | 8342 | 8794 | 1917 |
| SD (±) | 5185 | 17651 | 3358 | 4173 | 7753 | 4957 | 2332 |
| 95% CI | 6070−31662 | 12010−55844 | 4062−18644 | 37−20771 | LLQ-17969 | LLQ-21108 | LLQ-4365 |
All doses are mg/kg. n=6 animals per group; whole xenografts were surgically excised and half of the excised specimen was measured in the PAR immunoassay at protein loads of 10 μg to 20 μg per well. Single-dose topotecan was administered by intraperitneal injection as an additional control. Collection time points were selected to mimic the time points in the clinical trial. All units are pg PAR/mL per 100 μg protein. TPT=topotecan. LLQ= lower limit of quantitation of the assay.
Topotecan did not significantly reduce PAR levels until 24 hours after administration, consistent with the inhibition associated with a cytotoxic agent rather than a molecularly targeted agent, such as ABT-888. Topotecan has been shown to reduce the tumor growth rate of Colo829 and A375 xenografts in other studies (data not shown). The apparent increase in mean PAR levels 2 hours after topotecan dosing was not statistically significant but may be a real effect.
PD response to ABT-888 in excised Colo829 xenografts—dose-dependent suppression
PAR levels from “average” tumor samples (quadrants from small and large tumors) were suppressed by >95% and 99% in the 3 mg/kg and 12.5 mg/kg ABT-888 groups, respectively, compared with vehicle-treated controls (Table 2). At the 12.5 mg/kg dose level, the modal value of PAR levels in the assay readout was zero; all but one value was lower than the lowest assay standard of 15.6 pg PAR/mL. Similar dose-dependent reductions in PAR levels were observed in the first and second tumor pieces from the resected small and large tumors. PAR levels were suppressed >95% at 3mg/kg and ∼100% at 12.5 mg/kg in large tumors, and suppressed >98% in small tumors at 3mg/kg. All but one specimen from the smaller tumor at 12.5 mg/kg exhibited PAR levels below the nominal LLQ of the assay, and the modal PAR level for the group was zero.
Table 2.
PAR levels in two quadrants (“first piece” and “second piece”) from resected large and small tumors of bilateral Colo829 xenografts treated with vehicle or ABT-888.
| Group | Sample type | Averaged tumor | Larger tumor | Smaller tumor | |||
|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | ||
| Vehicle Treated | First piece* | – | – | 4146 | 816−7476 | 3901 | 1953−5849 |
| Second piece* | – | – | 4747 | 2049−7445 | 3990 | 2513−5467 | |
| Combined pieces† | 4475 | 3008−5942 | – | – | – | – | |
| | |||||||
| ABT-888 (3 mg/kg) | First piece* | – | – | 182 | 113−252 | 23 | LLQ-154 |
| Second piece* | – | – | 151 | 116−187 | 226 | 87−365 | |
| Combined pieces† | 198 | 156−239 | – | – | – | – | |
| | |||||||
| ABT-888 (12.5 mg/kg) | First piece* | – | – | 18.1 | LLQ-54 | 23 | LLQ-55 |
| Second piece* | – | – | 34.2 | LLQ-66.8 | 56 | LLQ-143 | |
| Combined pieces† | 33‡ | 12−54 | – | – | – | – | |
n=6 animals per group, two quadrants were dissected 4 hours post dosing from large or small xenografts, which occurred randomly in the right or left flank of each mouse
n=6 animals per group × 2 tumors per animal × one half of each tumor (from large and small tumors)
the modal value for this group was zero (12/24 specimens). ABT-888 and vehicle were administered as a single dose. All units are pg PAR/mL per 100 μg protein.
CI=confidence interval.
No correlation was observed between PAR levels of large and small tumors following ABT-888 dosing, and only the vehicle-treated groups showed a modest correlation (R2=0.758) between large and small tumor PAR levels (Fig. 4A). No correlation in PAR response to ABT-888 was detected between the first and second piece cut from the excised large tumors at either the 3 mg/kg or 12.5mg/kg dose (Fig. 4B). The vehicle-treated group for excised large tumors was also considered to exhibit random variability. The higher coefficient of determination (R2=0.644) was due to a single outlier which drove the regression coefficient from the randomly clustered data points around the mean value of 4000 pg/mL per 100 μg protein.
Fig. 4.
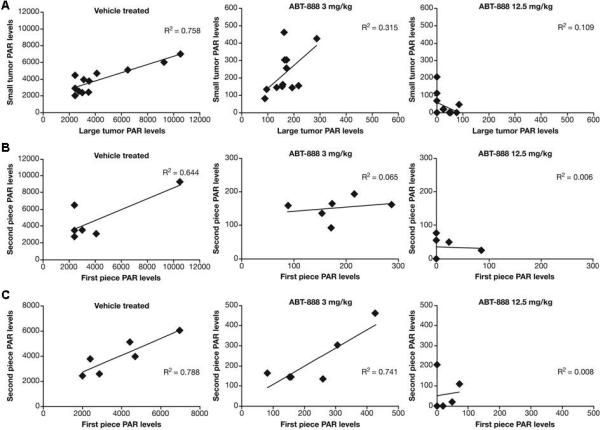
Inter- and intra-tumor variability of PAR levels in vehicle- and ABT-888-treated Colo829 xenografts. PAR levels were measured 4 hours following drug administration by combining 1 to 2 quadrants cut from each resected tumor. ABT-888 was administered at doses of 3 mg/kg or 12.5 mg/kg as indicated (n=6 animals/group). (A) Correlation of PAR levels between small and large tumors following ABT-888 dosing in mice bearing Colo829 xenografts. Large and small tumors occurred randomly on the right or left flank, thus, xenograft size was randomized. The difference in scale of PAR values in vehicle compared to ABT-888 treatment was due to significant drug suppression of PAR. (B) Correlation of PAR levels between first and second quadrant dissected from resected large tumors (n=6 animals/group). (C) Correlation of PAR levels between first and second quadrant dissected from resected small tumors (n=6 animals/group). Values were reported as pg PAR/mL normalized to 100 μg protein. Solid diamond, measured point; line, linear regression fit.
The vehicle-treated group in small tumors again demonstrated a stronger correlation than the ABT-888-treated groups (less random variability; R2=0.788) in PAR levels between the first and second tumor pieces (Fig. 4C). Modest correlation (R2=0.741) in PAR levels was also observed between the pieces from the same tumor in the animals treated with ABT-888 at the 3 mg/kg dose. The lack of apparent correlation in intra-tumor PAR levels at the 12.5 mg/kg dose (R2=0.008) may be associated with nearly complete suppression of PAR levels in the specimens. Sampling variability in PAR levels detected at baseline and following a single dose of vehicle or ABT-888 relative to the magnitude of the drug effect dictates a PD response of 42%−95% suppression to reach statistical significance.
PD response to ABT-888 in needle-biopsy xenograft samples—dose-dependent suppression
Four hours following administration of 12.5 mg/kg ABT-888, PAR levels had decreased by 89% in the Colo829 model and 99% in the A375 model compared with the controls. The A375 model exhibited higher baseline tumor PAR levels than the Colo829 model (Supplementary Table S5). PAR levels in the control groups did not change significantly following vehicle or topotecan administration.
Discussion
To our knowledge, this study is unique in that it tested human procedures in mice to measure inhibition of a molecular target in xenograft tumors collected from living animals. Two human tumor xenograft models were used to evaluate the variability of PAR levels in tissue from surgically excised tumor pieces and biopsies, to test whether a single dose of ABT-888 could suppress PARP activity, and to evaluate dose-escalation effects of ABT-888 on PAR levels. Human melanoma cell lines Colo829 and A375 were selected for modeling on the basis of experiments performed at Abbott using the syngeneic mouse B16 melanoma model; B16 tumors are not readily assessable by the live animal biopsy methods this model was designed to evaluate. Sampling was consistent with the Phase 0 clinical trial plan, which specified that consenting patients be biopsied on the same day as drug administration. This time restriction imposed a 4- to 7-hour post-dose sampling window, which is important clinically (i.e., allows sufficient travel time) and because PAR levels recover 24 hours after a single dose of ABT-888. Use of needle biopsy material also prompted an investigation of the feasibility of conducting a PD immunoassay on limited tumor quantities. Wet-weight measurements from 18-gauge needle biopsies of Colo829 xenografts showed significant differences in the quantity of material recovered. Partial biopsies were associated with low weight because the needle passed completely through the tumor. Protein levels were also variable, probably reflecting the amount of plasma present as the animals were not perfused prior to sampling, a step omitted to mimic the clinical design.
The expected imprecision in PAR immunoassay results from all sources was quantified as <9%. This precision enabled a detailed analysis of the sources of variability encountered in the current experiments. For example, variability documented within different treatment and control groups could be attributed to the sampling method and inherent heterogeneity of the tumor itself, as most groups exhibited random variability (R2 <0.55). This trend was similar for both surgically excised tumor pieces and sequential biopsies. Higher PAR concentrations in biopsy samples than in tumor pieces was attributed to better preservation of the PAR antigen as biopsy samples freeze and thaw faster in lysis buffer and are more easily and rapidly extracted. Also, biopsy specimens were more readily solubilized by the extraction method than the tumor xenograft sections such that values obtained from matched pairs of xenograft sections appeared to be as discrepant as the values obtained for treatment groups from different animals.
Although the variability observed in surgically excised tumor and sequential biopsy samples was random in most groups, an apparently higher (R2 >0.55), non-random correlation of PAR levels was observed in the vehicle-treated groups in contralateral tumors (R2=0.758) and in large (R2=0.644) and small (R2=0.788) xenograft tumors. A possible explanation is that the high correlation was an artifact of the unusual heterogeneity of the groups, each containing one or two animals with very high levels of PAR, compared with the rest of the animals in those groups, thus driving the regression coefficient. A higher (R2 >0.55), non-random correlation of PAR levels was also measured within tumor pieces of the small xenograft tumors in the 3 mg/kg group (R2=0.741), which was not directly attributable to an artifact of the methods employed. This value was significantly positive at the 1-sided CI (α=0.01 significance level); however, this somewhat isolated example of positive correlation between the samples from the same animal detected in a treated group must be viewed with some skepticism, due to the other multiple analyses performed demonstrating different results. Baseline PAR levels also varied significantly in Colo829 xenografts between different experiments. PAR levels in the untreated and vehicle-treated groups varied in the same manner, and the means, standard deviations, and 95% CIs in those groups always overlapped, as was the case with PAR levels across experiments. Additional experiments have been planned to address whether the variability observed is inherent to the Colo829 model or whether it is associated with specimen storage.
Patients eligible to participate in oncology trials often have multiple tumors, presenting a dilemma to clinicians over whether to biopsy different lesions at baseline and post-dose, or biopsy the same lesion following a recovery period. These results illustrate the feasibility of re-biopsying the same lesion. The data also indicate that, at least in the xenograft models examined, the variation across PAR measurements from the same animal at different times or sites is comparable to the variation across measurements from different animals. Therefore, little advantage may be gained from multiple pre-treatment PAR measurements among individual tumor nodules or study participants. Decreases in PAR levels following ABT-888 treatment may have to be evaluated against the variation found in pre-treatment PAR levels and across patients to determine statistical significance. Furthermore, the bilateral tumor model demonstrated no bias in PAR measurements attributable to order of sampling or to the degree of necrosis or other features of tumor heterogeneity present in the xenografts.
The effect of a single dose of ABT-888 on PAR levels was impressive, with significant inhibition of PAR synthesis within 2 hours at all dose levels tested. A strong tendency toward complete (>99%) inhibition of PAR levels was observed at the higher dose levels. By the 5-hour time point, significant inhibition of PAR levels was detected only at the 12.5 mg/kg and 25 mg/kg dose levels, and by 24 hours, PAR levels had all recovered. These data suggest that the dose effects induced by 3 mg/kg to 25 mg/kg doses of ABT-888 were distinguishable primarily by the duration of the response and not by the magnitude of inhibition observed.
Two technical aspects of tumor needle biopsy sample collection are essential to a successful PD study. First, sampling variability that is sufficiently small at baseline (or in vehicle-treated groups) to demonstrate a drug-induced change in the target function, and second, a sufficiently low limit of quantitation in the validated assay to quantify a drug effect in a 1-mg to 2-mg tissue specimen. The high coefficients of variation around the means in the treatment groups (50% at high PAR levels to 150% at low PAR levels), regardless of the tissue collection procedure, have important implications for the design of clinical trials with ABT-888, as they predict the minimum amount of PARP inhibition necessary to achieve a statistically significant effect. Due to the high variability within treatment groups, at least a 50% inhibition of PARP levels was required to demonstrate a significant PD response to ABT-888 with statistical confidence. Therefore, the success of these modeling experiments was in no small measure due to the effectiveness of ABT-888 at inhibiting PAR synthesis in vivo.
The animal models presented in this study provided a good foundation for the design of the first oncology Phase 0 clinical trial at the NCI. Furthermore, the detailed evaluation of target variability and proof-of-principle concepts used in this study are important steps to complete prior to conducting any Phase 0 trial, in which the PD endpoint serves as the primary objective justifying the collection of biopsies from study participants. The availability of a validated immunoassay to measure PAR levels in real-time analysis was critical for identifying dose levels and time points anticipated to show biochemical effects in human tumors. Equally important were the development and validation of tissue handling procedures that could be used clinically with biopsy specimens to stabilize the PD endpoint. Biopsy procedures used in early clinical trial assessments were “reverse translated” into the animal models as closely as possible. This step allowed proof of feasibility for using the validated assay to assess PD response in needle biopsy samples prior to entering the clinic. Furthermore, biopsy of live animals under anesthesia replaced the traditional use of necropsy tissue from dying or dead animals to assess dynamic, drug-induced molecular target responses that often utilize energy-dependent substances like adenosine 5’-triphosphate (ATP) or NAD+.
Despite these improvements in preclinical modeling it was not feasible to model the Phase 0 clinical trial in the mouse with complete accuracy. For example, using general isoflurane inhalation anesthesia in the mice is quite different from using local lidocaine anesthesia for percutaneous biopsy procedures, especially since the variable use of epinephrine in the lidocaine by interventional radiologists could influence PAR levels in the tumor biopsies(19). Furthermore, excisional biopsy procedures are conducted more quickly in mice than in the clinical setting, and the impact of the elapsed time after initial tissue trauma and possibly hypoxia on PAR levels is not well understood.
In conclusion, combining the use of clinical tissue-acquisition procedures with validated PD assays and clinically relevant SOPs for specimen handling is expected to lead to more accurate preclinical modeling. These factors should be carefully considered for future drug developmental trials of novel, molecularly targeted anticancer therapies. Strict assay performance requirements are justified if the PD endpoint serves as the primary objective in a Phase 0 clinical trial.
Supplementary Material
Acknowledgements
We would like to thank Drs. Brad Wood and James Pingpank for very helpful discussions about biopsy procedures, and how these might be replicated with some degree of clinical fidelity in the preclinical models; Vali Sevastita, M.S., Yvonne A. Evrard, Ph.D., and Gina Uhlenbrauck, for writing and editing support; and Carrie Bonomi, Suzanne Borgel, John Carter, Kelly Dougherty, and Howard Stotler for their assistance with tissue culture, and tumor generation and passage.
We would also like to thank Drs. Vincent Giranda, Joann Palma, and Xuesong Liu at Abbott Laboratories, whose close collaboration made this study possible.
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
This research was supported [in part] by the Developmental Therapeutics Program in the Division of Cancer Treatment and Diagnosis of the National Cancer Institute.
Grant support: Funded by NCI Contract N01-CO-12400.
Footnotes
Statement of Clinical Relevance
This article demonstrates how “humanizing” preclinical models with validated pharmacodynamic assays and clinically relevant methodologies for tissue sampling, coupled with better statistical design and analysis, can substantially improve early phase clinical trials. Pharmacodynamic assays provide critical information on whether an investigational agent acts on its presumed target; this knowledge is invaluable to evaluate the probability of an agent's overall success in advancing through the clinical developmental pipeline. This report describes the use of a validated pharmacodynamic assay to support the design of a proof-of-principle, first-in-human, Phase 0 clinical trial of the molecularly targeted anticancer agent ABT-888. Assessing the analytical performance of a validated assay in preclinical models using clinically relevant procedures is essential to demonstrate that the assay is clinically ready, and thus satisfy review boards that pharmacodynamic results (e.g., minimal biologically effective dose) can serve as the clinical trial's primary endpoint. These critical evaluation procedures distinguish the development of an assay for a Phase 0 trial from the correlative studies generally performed as secondary endpoints during early phase clinical trials.
US Department of Health and Human Services, US Food and Drug Administration, Center for Drug Evaluation and Research. Guidance for Industry, Investigators, and Reviewers, Exploratory IND Studies. [Issue date: January 12, 2006]. Available at http://www.fda.gov/cder/guidance/7086fnl.pdf
Note: Supplementary data for this article are available at Clinical Cancer Research Online.
References
- 1.Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3:711–5. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- 2.Kummar S, Kinders RJ, Rubinstein L, Parchment RE, Murgo AJ, Collins J. Compressing drug development timelines in oncology using phase '0' trials. Nat Rev Cancer. 2007;7:131–9. doi: 10.1038/nrc2066. [DOI] [PubMed] [Google Scholar]
- 3.Parchment RE, Kinders RJ, Hollingshead MG. Technical criteria of “clinical readiness” to qualify validated pharmacodynamic (PD) assays for clinical trial use. J Clin Oncol. 2007;25 Abstract 14068. [Google Scholar]
- 4.Memisoglu A, Samson L. Base excision repair in yeast and mammals. Mutat Res. 2000;451:39–51. doi: 10.1016/s0027-5107(00)00039-7. [DOI] [PubMed] [Google Scholar]
- 5.Schreiber V, Ame JC, Dolle P, et al. Poly(ADP-ribose) polymerase-2 (PARP-2) is required for efficient base excision DNA repair in association with PARP-1 and XRCC1. J Biol Chem. 2002;277:23028–36. doi: 10.1074/jbc.M202390200. [DOI] [PubMed] [Google Scholar]
- 6.Ha HC, Snyder SH. Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc Natl Acad Sci U S A. 1999;96:13978–82. doi: 10.1073/pnas.96.24.13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jagtap P, Szabo C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4:421–40. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- 8.Virag L, Szabo C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- 9.Tentori L, Leonetti C, Scarsella M, et al. Inhibition of poly(ADP-ribose) polymerase prevents irinotecan-induced intestinal damage and enhances irinotecan/temozolomide efficacy against colon carcinoma. Faseb J. 2006;20:1709–11. doi: 10.1096/fj.06-5916fje. [DOI] [PubMed] [Google Scholar]
- 10.Albert JM, Cao C, Kim KW, et al. Inhibition of poly(ADP-ribose) polymerase enhances cell death and improves tumor growth delay in irradiated lung cancer models. Clin Cancer Res. 2007;13:3033–42. doi: 10.1158/1078-0432.CCR-06-2872. [DOI] [PubMed] [Google Scholar]
- 11.Ji JJ, Redon C, Pommier Y. Poly-adeninosinediphosphate-ribose polymerase inhibitors as sensitizers for therapeutic treatments in human tumor and blood mononuclear cells. J Clin Oncol. 2007;25 Abstract 14024. [Google Scholar]
- 12.Park EM, Cho S, Frys K, et al. Interaction between inducible nitric oxide synthase and poly(ADP-ribose) polymerase in focal ischemic brain injury. Stroke. 2004;35:2896–901. doi: 10.1161/01.STR.0000147042.53659.6c. [DOI] [PubMed] [Google Scholar]
- 13.Black JH, Casey PJ, Albadawi H, Cambria RP, Watkins MT. Poly adenosine diphosphate-ribose polymerase inhibitor PJ34 abolishes systemic proinflammatory responses to thoracic aortic ischemia and reperfusion. J Am Coll Surg. 2006;203:44–53. doi: 10.1016/j.jamcollsurg.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Virag L, Bai P, Bak I, et al. Effects of poly(ADP-ribose) polymerase inhibition on inflammatory cell migration in a murine model of asthma. Med Sci Monit. 2004;10:BR77–83. [PubMed] [Google Scholar]
- 15.Donawho CK, Luo Y, Luo Y, et al. ABT-888, an Orally Active Poly(ADP-Ribose) Polymerase Inhibitor that Potentiates DNA-Damaging Agents in Preclinical Tumor Models. Clin Cancer Res. 2007;13:2728–37. doi: 10.1158/1078-0432.CCR-06-3039. [DOI] [PubMed] [Google Scholar]
- 16.Calabrese CR, Almassy R, Barton S, et al. Anticancer chemosensitization and radiosensitization by the novel poly(ADP-ribose) polymerase-1 inhibitor AG14361. J Natl Cancer Inst. 2004;96:56–67. doi: 10.1093/jnci/djh005. [DOI] [PubMed] [Google Scholar]
- 17.Plowman J, Dykes DJ, Hollingshead M, Simpson-Herren L, Alley MC. Human tumor xenograft models in NCI drug development. Totowa Humana Press Inc.; 1997. [Google Scholar]
- 18.Snedecor GW, Cochran WG. Statistical methods. 7th edition Ames The Iowa State University Press; 1980. [Google Scholar]
- 19.Dincer EH, Gangopadhyay N, Wang R, Uhal BD. Norepinephrine induces alveolar epithelial apoptosis mediated by alpha-, beta-, and angiotensin receptor activation. Am J Physiol Lung Cell Mol Physiol. 2001;281:L624–L30. doi: 10.1152/ajplung.2001.281.3.L624. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.