Abstract
Apolipoprotein E4 (ApoE4) is a risk factor for Alzheimer's disease (AD). Whether this risk arises from a deficient function of E4 or the lack of protection provided by E2 ir E3 is unclear. Previous studies demonstrate that deprivation of folate and vitamin E, coupled with dietary iron as a pro-oxidant, for 1 month displayed increased PS-1 expression, gamma-secretase and Abeta generation in mice lacking ApoE (ApoE-/- mice). While ApoE-/- mice are a model for ApoE deficiency, they may not reflect the entire range of consequences of E4 expression. We therefore compared herein the impact of the above deficient diet on mice expressing human E2, E3 or E4. Since folate deficiency is accompanied by a decrease in the major methyl donor, S-adenosyl methionine (SAM), additional mice received the deficient diet plus SAM. E2 was more protective than murine ApoE or E3 and E4. Surprisingly, PS-1 and gamma-secretase were over-expressed in E3 to the same extent as in E4 even under a complete diet, and were not alleviated by SAM supplementation. Abeta increased only in E4 mice maintained under the complete diet, and was alleviated by SAM supplementation. These findings suggest dietary compromise can potentiate latent risk factors for AD.
Keywords: Abeta, S-Adenosylmethionine, folate, Apolipoprotein E, presenilin, gamma-secretase, Alzheimer's disease
Introduction
The E4 allele of apolipoprotein E (ApoE) increases the incidence and can hasten the onset of Alzheimer's disease (AD; Cedazo-Minguez, 2007; Growdon, 2001; Rebeck et al., 2002). In addition to impaired lipid homeostasis, the E4 allele is associated with multiple risk factors and hallmarks of AD, including Abeta deposition, neurofibrillary tangle formation, impaired synaptic plasticity, cholinergic dysfunction and increased oxidative damage (for review, see Cedazo-Minguez, 2007). ApoE deficiency can augment the deleterious consequences of presenilin 1 (PS-1) overexpression (Pastor et al., 2003), increase gamma-secretase activity (Irizarry et al., 2004) and impair clearance of Abeta (Hone et al., 2003). This apparent pivotal role is consistent with the notion that oxidative stress represents an early event in AD pathology (Berr, 2002; Perry et al., 2002). The extent of brain oxidative damage in AD is correlated with E4 (Ramassamy et al., 1999).
AD has a multifactoral etiology encompassing nutritional, environmental and genetic risk factors (Brouwers et al., 2008; Lahiri et al., 2007; Migliore and Coppede, 2008; Pratico, 2008; Solfrizzi et al., 2008). No one known factor can account for all cases of the disease, suggesting that the interaction of two or more risk factors may be necessary to promote clinical manifestation. The combined impact of genetic and dietary risk factors have been examined in mouse models, where key dietary deficiency and/or oxidative challenge have been demonstrated to provoke neurodegeneration in mice harboring known genetic neurodegenerative risk factors under conditions where neither dietary nor genetic factors alone exhibited detectable impact. Dietary folate and vitamin E deficiency, coupled with iron-induced oxidative stress, increased neuronal oxidative damage, homocysteine levels, PS-1 expression, gamma-secretase activity, Abeta and phospho-tau levels, inhibited glutathione S-transferase activity, acetylcholine production, synaptic activity and spatial memory, and increased aggression in mice lacking ApoE (ApoE-/-) or in some cases expressing human E4 (Chan and Shea, 2006, 2007a,b; Chan et al., 2008a,b; Tchantchou et al., 2006, 2008). Notably, all of these deleterious effects were attenuated by supplementation with S-adenosyl methionine (SAM), which is noteworthy since SAM declines during aging and AD (Kennedy et al., 2004; Morrison, 1996; Shea and Chan, 2008).
While cognitive impairment and increased aggression were documented in both ApoE-/- and mice expressing E4, the majority of the above studies were examined only in ApoE-/- mice. ApoE-/- mice represent a useful model for the impact of oxidative stress, but do not necessarily encompass the full range of consequences of E4 expression (e.g., Huang et al., 2000; Ramassamy et al., 1999). We therefore compared herein the impact of the above deficient diet ± SAM on PS-1, gamma-secretase and Abeta in mice expressing human E2, E3 or E4 alleles.
Materials and Methods
Mouse strains and Diets
Mice used in this study were C57B/6 mice 9-12 months of age lacking murine ApoE and expressing human ApoE2 (b6.129P2-TgH/ApoE2/N8), E3 (b6.129P2-TgH/Apoe3/N8) or E4 (b6.129P2-TgH/Apoe4/N8), obtained from Taconic Farms (Germantown, NY;Chan and Shea, 2007a).
Mice were maintained for 1 month on a diet (“AIN-76”; Purina/Mother Hubbard, Inc.) either lacking folate and vitamin E (previously defined as a “deficient diet”) or supplemented with folic acid (2 mg/kg), and vitamin E (50IU/kg total diet wet weight; the “complete diet”). The deficient diet was also supplemented in all cases with a high concentration of iron (50g/500g total diet) as a pro-oxidant (Chan and Shea, 2007a, b; Shea and Rogers, 2002). Additional groups on the deficient diet also received SAM (100mg/kg diet) for the entire month (Chan and Shea, 2007a, b). Mice were sacrificed by cervical dislocation, and the frontal portion of the brain (encompassing cortex and hippocampus) was immediately removed. All animal procedures were approved by our Institutional Animal Care and Use Committee.
Biochemical analyses
To analyze PS-1 expression, total mRNA was extracted from the above homogenate using an RNA isolation kit (Ambion, Inc.) and stored at -20° C as described (Chan and Shea, 2007a). PS-1 transcription was analyzed using of the primers 5′-ACGGTTTCCAACATCCATCG-3″ and 5′-GATGACAGGGACTGTTGAGCAA-3,″ respectively, which yielded a product of 557 base pairs (bp). As in our prior studies, the “housekeeping gene” glyceraldehyde phosphate dehydrogenase (GADPH), used as a normalization factor, was amplified using the primers 5′- ACCACAGTCCATGCCATCAC-3″, and 5′-TCCACCACCCTGTTGCTGTA-3′ respectively, which yielded a product of 451bp. Sequences were verified using the BLAST algorithm function (available on the GenBank website). RT-PCR was carried out in a single tube using the Access RT-PCR kit (Promega, CA). Purified mRNA (2μg in 3μl) and 0.5 mM each of forward and reverse primers were used in each reaction, with amplification for 35 cycles. RT-PCR products were electrophoresed, visualized under UV and analyzed using Quantity One software. The 557bp and 451bp products were eluted from the gel, cloned, and verified as PS-1 and GAPDH via sequencing.
Gamma secretase activity was quantified by fluorescent spectroscopy using a kit according to the manufacturer's instructions (R&D Systems, Minneapolis, MN) as described previously (Chan and Shea, 2007a).
As in our prior studies (Chan and Shea, 2007a), a portion (40-50mg) of the above cortical/hippocampal sample was homogenized with 4 volumes of Tris-saline (50mmol/L Tris HCl, pH 7.4, 150mmol/L NaCl containing 1mmol/L EGTA, 0.5 mmol diisopropyl fluorophosphate, 0.5mmol phenylmethylsulfonyl fluoride, 1ug/ml N-p-tosyl-L-lysine chloromethylketone, 0.1 ug/ml pepstatin, 1 ug/ml antipain, and 1 ug/ml leupeptin). The homogenate was centrifuged at 500,000RPM for 50 minutes at 4oC. Pellets were resuspended by brief sonication in 10 volumes of 6 mol/L guanidine HCl in 50mmol/L Tris-HCl, pH 7.6, followed by centrifugation at 450,000 RPM for 22 minutes at 4 C. The insoluble pellet was dilapidated with chloroform/methanol (2:1) and then with chloroform/methanol/water (1:2:0.8). The residue was extracted with 70% formic acid, and dried using a SpeedVac (Savant Instuments, Farmingdale, NY). Replicate samples of the resulting pellet, normalized for protein content, were subjected to SDS-gel electrophoresis, transferred to PVDF membrane, and visualized by chemiluminescent reaction with anti-Abeta antibody BC05 (Morishima-Kawashima et al., 2000).
NIH Image software (v1.62) was utilized to determine the density of the RT-PCR PS-1 and GADPH housekeeping gene and levels of Abeta. PS-1 expression was normalized for each sample according to GADPH expression from the same mouse in adjacent lanes of the same gel using the formula (PS-1 densitometric units/GADPH densitometric units). Statistical analyses were completed with ANOVA and Student's t test (Chan et al., 2006).
Results
Expression of PS-1, activity of gamma-secretase and levels of Abeta differed among the genotypes and diets utilized herein, and were differentially affected by dietary manipulation.
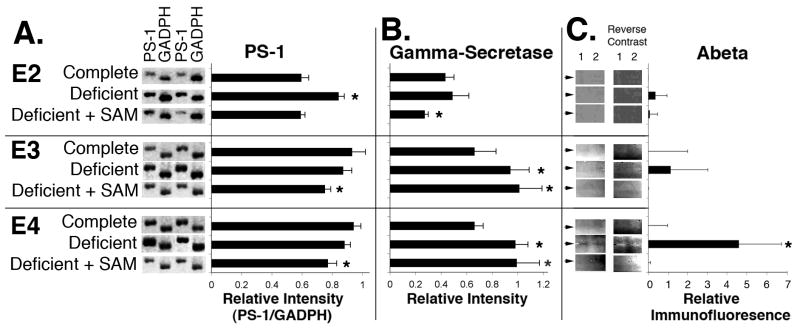
Expression of PS-1 in E2 mice was virtually identical to that previously demonstrated for ApoE+/+ mice on the complete diet (Chan et al., 2007a). Maintenance of E2 mice on the deficient diet statistically increased expression, which was prevented in all cases by supplementation with SAM (Fig. 1). By contrast, PS-1 expression was significantly elevated to an identical extent in E3 and E4 mice versus E2 mice prior to dietary challenge, and was not further elevated following maintenance on the deficient diet. Supplementation of the deficient diet with SAM statistically-reduced PS-1 expression in E3 and E4 mice versus their respective levels on the complete diet; however, these levels remained statistically-increased relative to E2 mice maintained on the complete diet (Fig. 1).
Fig. 1. Dietary challenge differentially affects PS-1 expression, gamma-secretase activity and Abeta levels depending upon ApoE genotype.
Panel A presents RT-PCR products generated from total mRNA of 2 representative samples as listed using PS-1 and GADPH primers. Accompanying graphs present the mean increase ± standard error in PS-1 expression (normalized according to corresponding GADPH levels) for ≥7 mice of each group compiled from ≥2 separate experiments.
Panel B presents gamma-secretase activity quantified in the same homogenates normalized for total protein as described in Methods.
PanelC presents the 14.3-3.5kDa region of representative immunblots of the same homogenates probed with anti-Abeta antibody BC05; duplicate samples are presented. Immunblots are also presented in reverse contrast for clarity. Arrows denote the migratory position of endogenous murine Abeta (4kDa), which is not reproducibly detectable under all conditions. Accompanying graphs present the mean chemiluminiscent intensity (± standard error); values represent the relative immunofluoresence versus that observed for E2 mice maintained on the complete diet.
An asterisk indicates a statistically-significant difference (p<0.05) for the indicated diet versus its respective genotype on the complete diet. See text for description of results.
Gamma-secretase activities were statistically-identical among all genotypes prior to dietary challenge, and were statistically increased in E3 and E4 following maintenance on the deficient diet (Fig. 1). Supplementation of the deficient diet with SAM decreased levels in E2 mice below those observed for the complete diet alone. By contrast, SAM did not attenuate the impact of the deficient diet gamma-secretase levels in on E3 or E4 mice.
Endogenous Abeta was monitored by immunoblot analyses. Notably, while Abeta is readily detectable in transgenics that overexpress APP (e.g., Kruman et al., 2002), endogenous levels remain more difficulty to quantify. Little Abeta was detected in any genotype prior to dietary challenge (Fig. 1). The deficient diet significantly increased Abeta levels in E4 mice. Supplementation with SAM in all cases restored Abeta levels to those observed on the complete diet.
Discussion
We provide further evidence of the interaction of dietary and genetic risk factors for AD, demonstrating that a deficient diet can potentiate an apparent otherwise latent genetic predisposition, and, conversely, that key supplementation may attenuate an otherwise critical genetic deficiency.
The findings herein are consistent with E2 as being the most neuroprotective allele. However, they leave open the possibility that E3 may not provide superior neuroprotection under all parameters as compared to that provided by E4. Notably, while E4 is most strongly associated with AD, it can at best only account for a minority of the total incidence of AD, which leaves the vast majority of AD associated with the other alleles. While one reasonable interpretation is that all other cases are independent of any association with ApoE, the findings of the present and previous (Chan and Shea, 2007b; Chan et al., 2008b) studies demonstrate that the combined impact of dietary deficiency and oxidative challenge can provoke similar neuropathogenic effects in mice expressing E3 or E4. This may represent a more widespread phenomenon than previously appreciated. For example, E2 was more effective at suppressing fibrillar Abeta deposition than E3, which in turn was more protective than E4 (Fagan et al., 2002; Holtzman, 2004). Our demonstration herein that only E2 prevented increased PS-1 and gamma-secretase (both of which can contribute to Abeta generation) leaves open the possibility that additional, undisclosed factors might preclude Abeta clearance by E3 (Hone et al., 2003). Moreover, mice expressing E3 demonstrated deficits as severe as those expressing E4 in certain memory tasks (Grootendorst et al., 2005) and demonstrated levels of aggression comparable to that of mice expressing E4 while mice expressing E2 displayed no aggression (Chan et al., 2008a). The generally-accepted notion that E3 is neuroprotective is largely derived from studies which compare only the E3 and E4 allele; individuals heterozytogic and homozygotic for E4 are typically pooled out of necessity due to limiting numbers, which unfortunately precludes direct comparison of E3/4 and E4/4 genotypes. In this regard, while E3 is more neuroprotective than E4, it is less neuroprotective than E2; indeed, E3/3 is associated with a higher incidence of AD than is 2/4 (Ashford, 2004; Crutcher, 2004; Roses, 1997).
Importantly, these findings in transgenic mouse models cannot simply be extrapolated to the human population. Moreover, the dietary regimen utilized herein is extreme compared to what would normally be encountered; e.g., individuals may consume a diet with suboptimal folate and/or vitamin E, but are unlikely to ingest no folate or vitamin E at all. We have previously demonstrated that supplementation with vitamin E alone was unable to prevent neuronal oxidative damage in mice maintained on the deficient diet (Shea et al., 2003). The month-long experimental regimen carried out herein is also extreme, since one month of murine life span corresponds to a substantial length of time for humans. Nevertheless, these findings underscore that, while E3 may be benign and perhaps relatively beneficial in isolation, it may provide insufficient neuroprotection in the presence of other mitigating factors. This highlights the potential importance of considering the interaction of ApoE alleles with other risk factors (Lahiri et al., 2004), and developing therapeutic strategies based on improving the potential beneficial effects of E3 (e.g., Cedazo-Minguez, 2007).
The results of this and prior studies (Chan and Shea, 2007a,b; Chan et al., 2008a,b; Fuso et al., 2005; Kuzuya et al., 2007; Scarpa et al., 2003; Serra et al., 2008; Sontag et al., 2007) demonstrate that dietary deficiency and oxidative challenge can foster a cascade of deleterious events related to AD including increased PS-1 expression, gamma-secretase activity, beta-secretase activity and Abeta generation, increased tau phosphorylation, decreased phosphatase activity, decreased acetylcholine levels, decreased synaptic activity, impaired cognitive performance, and increased aggression, and that this cascade is exacerbated under conditions of impaired ApoE function. The inter-relationship/convergence of the various elements of this cascade remain unclear and are the subject of debate, but these findings remain consistent with the notion that oxidative stress may be a pivotal early event in AD (Berr, 2002; Perry et al., 2002; Pratico, 2008); resultant increase in HC has been shown to potentiate the impact of Abeta-induced oxidative damage (Ho et al., 2001, 2002, 2003) as well as impair hydrolysis of SAM, which will further potentiate oxidative damage by diminishing glutathione-mediated quenching of reactive oxygen species (Tchantchou et al., 2006, 2008).
Folate deficiency induces a deleterious cascade of events relevant to AD. The decline in SAM that accompanies folate deficiency promotes overexpression of PS-1, due to impaired DNA methylation, leading to increased activity of BACE and production of Abeta (Fuso et al., 2005, 2007; Scarpa et al., 2003). B vitamin deprivation, which also resulted in decreased SAM, had similar effects (Fuso et al., 2008). HC, which increases following folate deprivation and decreases SAM-dependent methylation via feedback inhibition, was recently demonstrated to increasePS-1 expression of PS-1 and Abeta generation in rats that could be prevented by folate supplementation (Zhang et al., 2009). SAM deficiency has a far-reaching impact with relation to AD in that resultant impaired methylation of protein phosphatase 2A reduces its activity, and fosters an increase in phospho-tau levels and Abeta (Sontag et al., 2007). In addition, HC-induced increase in calcium influx, which leads to tau phosphorylation via calcium-dependent kinases, could be prevented by SAM supplementation (Chan et al., 2008b).
These findings also underscore the potential influence of nutritional compromise in manifestation of AD (Donini et al., 2007; Dosunmu et al., 2007; Joseph et al., 2000; Mattson and Shea, 2003). Of interest would be to initiate supplementation at an early age (I.e., perhaps before E4 mice have accumulated age-related oxidative damage).
It is noteworthy that supplementation with SAM was effective against all of the above parameters with the exception of increased gamma-secretase. The nature of increased activity of gamma-secretase observed in E3 and E4 mice (as well as ApoE-/- mice; Chan et al., 2007a) prior to any dietary compromise, and the inability of SAM to attenuate these increases despite decreasing PS-1, are unclear, but are consistent with the demonstration that gamma-secretase activity is not dependent upon PS-1 alone (e.g., Crystal et al., 2004; Herranz et al., 2006). SAM was nevertheless still able to prevent the increase in Abeta in all mice regardless of genotype. SAM levels decline in brain tissue of mice maintained on the deficient diet utilized herein, and are restored by supplementation of the deficient diet with SAM (Chan et al., 2008b; Chishty et al., 2002). In this regard, SAM levels, and activity of the enzyme responsible for its generation (methionine-S-adenosyltransferase), are decreased in brain tissue of individuals exhibiting neurodegeneration (Bottiglieri et al. 1990; Trolin et al. 1994; Kennedy et al., 2004; Morrison et al. 1996; Muller et al., 2001). These findings therefore underscore the potential usefulness of dietary supplementation with SAM as part of a therapeutic approach to minimize neurodegeneration (Shea and Chan, 2008). In this regard, pilot studies demonstrated that a combinatorial formulation including SAM was maintained or improved cognitive performance and behavioral parameters in AD (Chan et al., 2008c; Remington et al., 2008). In particular, since SAM supplementation reduced generation of Abeta and phosphoryation of tau (Chan and Shea, 2006, 2007a), of interest would be to examine whether or not it can reduce ultimate plaque and/or neurofibrillary tangle burden in mouse models in the presence and absence of dietary challenge.
Acknowledgments
This research was supported by the National Institute on Aging
List of Abbreviations
- ApoE
Apolipoprotein E
- PS-1
Presenilin 1
- Abeta
Beta amyloid
- SAM
S-Adenosylmethionine
References
- Ashford JW. APOE genotype effects on Alzheimer's disease onset and epidemiology. J Mol Neurosci. 2004;23:157–165. doi: 10.1385/JMN:23:3:157. [DOI] [PubMed] [Google Scholar]
- Bentahir M, Nyabi O, Verhamme J, Tolia A, Horre K, Wiltfang J, Esselmann H, De Strooper B. Presenilin clinical mutations can affect gamma-secretase activity by different mechanisms. J Neurochem. 2006;96:732–742. doi: 10.1111/j.1471-4159.2005.03578.x. [DOI] [PubMed] [Google Scholar]
- Berr C. Oxidative stress and cognitive impairment in the elderly. J Nutr Health Aging. 2002;6:261–266. [PubMed] [Google Scholar]
- Bottiglieri T, Godfrey P, Flynn T, Carney MW, Toone BK, Reynolds EH. Cerebrospinal fluid S-adenosylmethionine in depression and dementia: effects of treatment with parenteral and oral S-adenosylmethionine. J Neurol Neurosurg Psychiatry. 1990;53:1096–1098. doi: 10.1136/jnnp.53.12.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht WJ, Harris FM, Chang S, Tesseur I, Yu GQ, Xu Q, Dee Fish J, Wyss-Coray T, Buttini M, Mucke L, Mahley RW, Huang Y. Neuron-specific apolipoprotein e4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J Neurosci. 2004;24:2527–2534. doi: 10.1523/JNEUROSCI.4315-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers N, Sleegers K, Van Broeckhoven C. Molecular genetics of Alzheimer's disease: an update. Ann Med. 2008;40:562–583. doi: 10.1080/07853890802186905. [DOI] [PubMed] [Google Scholar]
- Brunkan AL, Goate AM. Presenilin function and gamma-secretase activity. J Neurochem. 2005;93:769–792. doi: 10.1111/j.1471-4159.2005.03099.x. [DOI] [PubMed] [Google Scholar]
- Cedazo-Minguez A. Apolipoprotein E and Alzheimer's disease: molecular mechanisms and therapeutic opportunities. J Cell Mol Med. 2007;11:1227–1238. doi: 10.1111/j.1582-4934.2007.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A, Shea TB. Dietary and genetically-induced oxidative stress alter tau phosphorylation: influence of folate and apolipoprotein E deficiency. J Alzheimers Dis. 2006;9:399–405. doi: 10.3233/jad-2006-9405. [DOI] [PubMed] [Google Scholar]
- Chan A, Shea TB. Folate deprivation increases presenilin expression, gamma-secretase activity, and Abeta levels in murine brain: potentiation by ApoE deficiency and alleviation by dietary S-adenosyl methionine. J Neurochem. 2007a;102:753–760. doi: 10.1111/j.1471-4159.2007.04589.x. [DOI] [PubMed] [Google Scholar]
- Chan A, Shea TB. Effects of dietary supplementation with N-acetyl cysteine, acetyl-L-carnitine and S-adenosyl methionine on cognitive performance and aggression in normal mice and mice expressing human ApoE4. Neuromolecular Med. 2007b;9:264–269. doi: 10.1007/s12017-007-8005-y. [DOI] [PubMed] [Google Scholar]
- Chan A, Tchantchou F, Graves V, Rozen R, Shea TB. Dietary and genetic compromise in folate availability reduces acetylcholine, cognitive performance and increases aggression: critical role of S-adenosyl methionine. J Nutr Health Aging. 2008a;12:252–261. doi: 10.1007/BF02982630. [DOI] [PubMed] [Google Scholar]
- Chan AY, Alsaraby A, Shea TB. Folate deprivation increases tau phosphorylation by homocysteine-induced calcium influx and by inhibition of phosphatase activity: Alleviation by S-adenosyl methionine. Brain Res. 2008b;1199:133–137. doi: 10.1016/j.brainres.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Chan A, Paskavitz J, Remington R, Rasmussen S, Shea TB. Efficacy of a Vitamin/Nutriceutical Formulation for Early-stage Alzheimer's Disease: A 1-year, Open-label Pilot Study With an 16-Month Caregiver Extension. Am J Alzheimers Dis Other Demen. 2008c;23:571–585. doi: 10.1177/1533317508325093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Karaplis AC, Ackerman SL, Pogribny IP, Melnyk S, Lussier-Cacan S, Chen MF, Pai A, John SW, Smith RS, Bottiglieri T, Bagley P, Selhub J, Rudnicki MA, James SJ, Rozen R. Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum Mol Genet. 2001;10:433–443. doi: 10.1093/hmg/10.5.433. [DOI] [PubMed] [Google Scholar]
- Chishty M, Reichel A, Abbott NJ, Begley DJ. S-adenosylmethionine is substrate for carrier mediated transport at the blood-brain barrier in vitro. Brain Res. 2002;942:46–50. doi: 10.1016/s0006-8993(02)02654-9. [DOI] [PubMed] [Google Scholar]
- Crutcher KA. Apolipoprotein E is a prime suspect, not just an accomplice, in Alzheimer's disease. J Mol Neurosci. 2004;23:181–188. doi: 10.1385/JMN:23:3:181. [DOI] [PubMed] [Google Scholar]
- Crystal AS, Morias VA, Fortna RR, Carlin D, Pierson TC, Wilson CA, Lee VM, Doms RW. Presenilin modulates Pen-2 levels posttranslationally by protecting it from proteasomal degradation. Biochem. 2004;30:3555–63. doi: 10.1021/bi0361214. [DOI] [PubMed] [Google Scholar]
- Donini LM, De Felice MR, Cannella C. Nutritional status determinants and cognition in the elderly. Arch Gerontol Geriatr. 2007;44 1:143–153. doi: 10.1016/j.archger.2007.01.022. [DOI] [PubMed] [Google Scholar]
- Dosunmu R, Wu J, Basha MR, Zawia NH. Environmental and dietary risk factors in Alzheimer's disease. Expert Rev Neurother. 2007;7:887–900. doi: 10.1586/14737175.7.7.887. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Watson M, Parsadanian M, Bales KR, Paul SM, Holtzman DM. Human and murine ApoE markedly alters A beta metabolism before and after plaque formation in a mouse model of Alzheimer's disease. Neurobiol Dis. 2002;9:305–318. doi: 10.1006/nbdi.2002.0483. [DOI] [PubMed] [Google Scholar]
- Fuso A, Seminara L, Cavallaro RA, D'Anselmi F, Scarpa S. S-adenosylmethionine/homocysteine cycle alterations modify DNA methylation status with consequent deregulation of PS1 and BACE and beta-amyloid production. Mol Cell Neurosci. 2005;28:195–204. doi: 10.1016/j.mcn.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Fuso A, Cavallaro RA, Zampelli A, D'Anselmi F, Piscopo P, Confaloni A, Scarpa S. gamma-Secretase is differentially modulated by alterations of homocysteine cycle in neuroblastoma and glioblastoma cells. J Alz Dis. 2007;11:275–90. doi: 10.3233/jad-2007-11303. [DOI] [PubMed] [Google Scholar]
- Fuso A, Nicolia V, Cavallaro RA, Ricceri L, D'Anselmi F, Coluccia P, Calamandrei G, Scarpa S. B-vitamin deprivation induces hyperhomocysteinemia and brain S-adenosylhomocysteine, depletes brain S-adenosylmethionine, and enhances PS1 and BACE expression and amyloid-beta deposition in mice. Mol Cell Neurosci. 2008;37:731–46. doi: 10.1016/j.mcn.2007.12.018. [DOI] [PubMed] [Google Scholar]
- Grootendorst J, Bour A, Vogel E, Kelche C, Sullivan PM, Dodart JC, Bales K, Mathis C. Human apoE targeted replacement mouse lines: h-apoE4 and h-apoE3 mice differ on spatial memory performance and avoidance behavior. Behav Brain Res. 2005;159:1–14. doi: 10.1016/j.bbr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Growdon JH. Incorporating biomarkers into clinical drug trials in Alzheimer's disease. J Alzheimers Dis. 2001;3:287–292. doi: 10.3233/jad-2001-3303. [DOI] [PubMed] [Google Scholar]
- Herranz H, Stamataki E, Feiguin F, Milan M. Self-refinement of Notch activity through the transmembrane protein Crumbs: modulation of gamma-secretase activity. EMBO Rep. 2006;7:297–302. doi: 10.1038/sj.embor.7400617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho PI, Collins SC, Dhitavat S, Ortiz D, Ashline D, Rogers E, Shea TB. Homocysteine potentiates beta-amyloid neurotoxicity: role of oxidative stress. J Neurochem. 2001;78:249–253. doi: 10.1046/j.1471-4159.2001.00384.x. [DOI] [PubMed] [Google Scholar]
- Ho PI, Ortiz D, Rogers E, Shea TB. Multiple aspects of homocysteine neurotoxicity: glutamate excitotoxicity, kinase hyperactivation and DNA damage. J Neurosci Res. 2002;70:694–702. doi: 10.1002/jnr.10416. [DOI] [PubMed] [Google Scholar]
- Ho PI, Ashline D, Dhitavat S, Ortiz D, Collins SC, Shea TB, Rogers E. Folate deprivation induces neurodegeneration: roles of oxidative stress and increased homocysteine. Neurobiol Dis. 2003;14:32–42. doi: 10.1016/s0969-9961(03)00070-6. [DOI] [PubMed] [Google Scholar]
- Holtzman DM. In vivo effects of ApoE and clusterin on amyloid-beta metabolism and neuropathology. J Mol Neurosci. 2004;23:247–254. doi: 10.1385/JMN:23:3:247. [DOI] [PubMed] [Google Scholar]
- Hone E, Martins IJ, Fonte J, Martins RN. Apolipoprotein E influences amyloid-beta clearance from the murine periphery. J Alzheimers Dis. 2003;5:1–8. doi: 10.3233/jad-2003-5101. [DOI] [PubMed] [Google Scholar]
- Huang GS, Yang SM, Hong MY, Yang PC, Liu YC. Differential gene expression of livers from ApoE deficient mice. Life Sci. 2000;68:19–28. doi: 10.1016/s0024-3205(00)00912-7. [DOI] [PubMed] [Google Scholar]
- Irizarry MC, Deng A, Lleo A, Berezovska O, Von Arnim CA, Martin-Rehrmann M, Manelli A, LaDu MJ, Hyman BT, Rebeck GW. Apolipoprotein E modulates gamma-secretase cleavage of the amyloid precursor protein. J Neurochem. 2004;90:1132–1143. doi: 10.1111/j.1471-4159.2004.02581.x. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Denisova NA, Bielinski D, Fisher DR, Shukitt-Hale B. Oxidative stress protection and vulnerability in aging: putative nutritional implications for intervention. Mech Ageing Dev. 2000;116:141–153. doi: 10.1016/s0047-6374(00)00128-7. [DOI] [PubMed] [Google Scholar]
- Kamboh MI. Apolipoprotein E polymorphism and susceptibility to Alzheimer's disease. Hum Biol. 1995;67:195–215. [PubMed] [Google Scholar]
- Kennedy BP, Bottiglieri T, Arning E, Ziegler MG, Hansen LA, Masliah E. Elevated S-adenosylhomocysteine in Alzheimer brain: influence on methyltransferases and cognitive function. J Neural Transm. 2004;111:547–567. doi: 10.1007/s00702-003-0096-5. [DOI] [PubMed] [Google Scholar]
- Kruman II, Kumaravel TS, Lohani A, Pedersen WA, Cutler RG, Kruman Y, Haughey N, Lee J, Evans M, Mattson MP. Folic acid deficiency and homocysteine impair DNA repair in hippocampal neurons and sensitize them to amyloid toxicity in experimental models of Alzheimer's disease. J Neurosci. 2002;22:1752–1762. doi: 10.1523/JNEUROSCI.22-05-01752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzuya A, Uemura K, Kitagawa N, Aoyagi N, Kihara T, Ninomiya H, Ishiura S, Takahashi R, Shimohama S. Presenilin 1 is involved in the maturation of beta-site amyloid precursor protein-cleaving enzyme 1 (BACE1) J Neurosci Res. 2007;85:153–165. doi: 10.1002/jnr.21104. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Sambamurti K, Bennett DA. Apolipoprotein gene and its interaction with the environmentally driven risk factors: molecular, genetic and epidemiological studies of Alzheimer's disease. Neurobiol Aging. 2004;25:651–660. doi: 10.1016/j.neurobiolaging.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Maloney B, Basha MR, Ge YW, Zawia NH. How and when environmental agents and dietary factors affect the course of Alzheimer's disease: the “LEARn” model (latent early-life associated regulation) may explain the triggering of AD. Curr Alzheimer Res. 2007;4:219–228. doi: 10.2174/156720507780362164. [DOI] [PubMed] [Google Scholar]
- Lleo A, Berezovska O, Growdon JH, Hyman BT. Clinical, pathological, and biochemical spectrum of Alzheimer disease associated with PS-1 mutations. Am J Geriatr Psychiatry. 2004;12:146–156. doi: 10.1097/00019442-200403000-00006. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Shea TB. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 2003;26:137–146. doi: 10.1016/S0166-2236(03)00032-8. [DOI] [PubMed] [Google Scholar]
- Migliore L, Coppede F. Genetics, environmental factors and the emerging role of epigenetics in neurodegenerative diseases. Mutat Res. 2008 doi: 10.1016/j.mrfmmm.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Morishima-Kawashima M, Oshima N, Ogata H, Yamaguchi H, Yoshimura M, Sugihara S, Ihara Y. Effect of apolipoprotein E allele epsilon4 on the initial phase of amyloid beta-protein accumulation in the human brain. Am J Pathol. 2000;157:2093–2099. doi: 10.1016/s0002-9440(10)64847-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison LD, Smith DD, Kish SJ. Brain S-adenosylmethionine levels are severely decreased in Alzheimer's disease. J Neurochem. 1996;67:1328–1331. doi: 10.1046/j.1471-4159.1996.67031328.x. [DOI] [PubMed] [Google Scholar]
- Muller T, Woitalla D, Hauptmann B, Fowler B, Kuhn W. Decrease of methionine and S-adenosylmethionine and increase of homocysteine in treated patients with Parkinson's disease. Neurosci Lett. 2001;308:54–56. doi: 10.1016/s0304-3940(01)01972-3. [DOI] [PubMed] [Google Scholar]
- Pastor P, Roe CM, Villegas A, Bedoya G, Chakraverty S, Garcia G, Tirado V, Norton J, Rios S, Martinez M, Kosik KS, Lopera F, Goate AM. Apolipoprotein Eepsilon4 modifies Alzheimer's disease onset in an E280A PS1 kindred. Ann Neurol. 2003;54:163–169. doi: 10.1002/ana.10636. [DOI] [PubMed] [Google Scholar]
- Perry G, Nunomura A, Hirai K, Zhu X, Perez M, Avila J, Castellani RJ, Atwood CS, Aliev G, Sayre LM, Takeda A, Smith MA. Is oxidative damage the fundamental pathogenic mechanism of Alzheimer's and other neurodegenerative diseases? Free Radic Biol Med. 2002;33:1475–1479. doi: 10.1016/s0891-5849(02)01113-9. [DOI] [PubMed] [Google Scholar]
- Pratico D. Oxidative stress hypothesis in Alzheimer's disease: a reappraisal. Trends Pharmacol Sci. 2008;29:609–615. doi: 10.1016/j.tips.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Ramassamy C, Averill D, Beffert U, Bastianetto S, Theroux L, Lussier-Cacan S, Cohn JS, Christen Y, Davignon J, Quirion R, Poirier J. Oxidative damage and protection by antioxidants in the frontal cortex of Alzheimer's disease is related to the apolipoprotein E genotype. Free Radic Biol Med. 1999;27:544–553. doi: 10.1016/s0891-5849(99)00102-1. [DOI] [PubMed] [Google Scholar]
- Rebeck GW. Confirmation of the genetic association of interleukin-1A with early onset sporadic Alzheimer's disease. Neurosci Lett. 2000;293:75–77. doi: 10.1016/s0304-3940(00)01487-7. [DOI] [PubMed] [Google Scholar]
- Remington R, Chan A, Paskavitz J, Shea TB. Efficacy of a Vitamin/Nutriceutical Formulation for Moderate-stage to Later-stage Alzheimer's Disease: A Placebo-controlled Pilot Study. Am J Alzheimers Dis Other Demen. 2008 doi: 10.1177/1533317508325094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roses AD, Saunders AM. ApoE, Alzheimer's disease, and recovery from brain stress. Ann N Y Acad Sci. 1997;826:200–212. doi: 10.1111/j.1749-6632.1997.tb48471.x. [DOI] [PubMed] [Google Scholar]
- Scarpa S, Fuso A, D'Anselmi F, Cavallaro RA. Presenilin 1 gene silencing by S-adenosylmethionine: a treatment for Alzheimer disease? FEBS Lett. 2003;541:145–148. doi: 10.1016/s0014-5793(03)00277-1. [DOI] [PubMed] [Google Scholar]
- Serra M, Chan A, Dubey M, Gilman V, Shea TB. Folate and S-adenosylmethionine modulate synaptic activity in cultured cortical neurons: acute differential impact on normal and apolipoprotein-deficient mice. Phys Biol. 2008;5:44002. doi: 10.1088/1478-3975/5/4/044002. [DOI] [PubMed] [Google Scholar]
- Shea TB, Rogers E. Folate quenches oxidative damage in brains of apolipoprotein E-deficient mice: augmentation by vitamin E. Brain Res Mol Brain Res. 2002;108:1–6. doi: 10.1016/s0169-328x(02)00412-6. [DOI] [PubMed] [Google Scholar]
- Shea TB. Folate, the methionine cycle, and Alzheimer's disease. J Alzheimers Dis. 2006;9:359–360. doi: 10.3233/jad-2006-9401. [DOI] [PubMed] [Google Scholar]
- Shea TB, Chan A. S-adenosyl methionine: a natural therapeutic agent effective against multiple hallmarks and risk factors associated with Alzheimer's disease. J Alzheimers Dis. 2008;13:67–70. doi: 10.3233/jad-2008-13107. [DOI] [PubMed] [Google Scholar]
- Shea TB, Rogers E, Ashline D, Ortiz D, Duarte N, Wilson TA, Nicolosi RJ, Sheu MH. Vitamin E deficiency does not induce compensatory antioxidant increases in central nervous system tissue of apolipoprotein E-deficient mice. J Alzheimers Dis. 2003;4:1–6. doi: 10.3233/jad-2003-5102. [DOI] [PubMed] [Google Scholar]
- Solfrizzi V, Capurso C, D'Introno A, Colacicco AM, Santamato A, Ranieri M, Fiore P, Capurso A, Panza F. Lifestyle-related factors in predementia and dementia syndromes. Expert Rev Neurother. 2008;8:133–158. doi: 10.1586/14737175.8.1.133. [DOI] [PubMed] [Google Scholar]
- Sontag E, Nunbhakdi-Craig V, Sontag JM, Diaz-Arrastia R, Ogris E, Dayal S, Lentz SR, Arning E, Bottiglieri T. Protein phosphatase 2A methyltransferase links homocysteine metabolism with tau and amyloid precursor protein regulation. J Neurosci. 2007;27:2751–2759. doi: 10.1523/JNEUROSCI.3316-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchantchou F, Graves M, Ortiz D, Chan A, Rogers E, Shea TB. S-adenosyl methionine: A connection between nutritional and genetic risk factors for neurodegeneration in Alzheimer's disease. J Nutr Health Aging. 2006;10:541–544. [PubMed] [Google Scholar]
- Tchantchou F, Graves M, Falcone D, Shea TB. S-adenosylmethionine mediates glutathione efficacy by increasing glutathione S-transferase activity: implications for S-adenosyl methionine as a neuroprotective dietary supplement. J Alzheimers Dis. 2008;14:323–328. doi: 10.3233/jad-2008-14306. [DOI] [PubMed] [Google Scholar]
- Trolin CG, Lofberg C, Trolin G, Oreland L. Brain ATP:L-methionine S-adenosyltransferase (MAT), S-adenosylmethionine (SAM) and S-adenosylhomocysteine (SAH): regional distribution and age-related changes. Eur Neuropsychopharmacol. 1994;4:469–477. doi: 10.1016/0924-977x(94)90295-x. [DOI] [PubMed] [Google Scholar]
- Zhang CE, Wei W, Liu YH, Peng JH, Tian Q, Liu GP, Zhang Y, Wang JZ. Hyperhomocysteinemia increases beta-amyloid by enhancing expression of gamma-secretase and phosphorylation of amyloid precursor protein in rat brain. Am J Pathol. 2009;174:1481–91. doi: 10.2353/ajpath.2009.081036. [DOI] [PMC free article] [PubMed] [Google Scholar]