Abstract
In human studies, low vitamin C intake has been associated with more severe Helicobacter pylori gastritis and a higher incidence of gastric cancer. However, vitamin C supplementation has not been definitively shown to protect against gastric cancer. Using vitamin C-deficient B6.129P2-Gulotm1Umc/mmcd (gulo−/−) mice lacking L-gulono-γ-lactone oxidase, we compared gastric lesions and Th1 immune responses in H. pylori-infected gulo−/− mice supplemented with low (33 mg/L) or high (3,300 mg/L) vitamin C in drinking water for 16 or 32 weeks. Vitamin C levels in plasma and gastric tissue correlated with the vitamin C supplementation levels in gulo−/− mice. H. pylori infection resulted in comparable gastritis and premalignant lesions in wildtype C57BL/6 and gulo−/− mice supplemented with high vitamin C, but lesions were less severe in gulo−/− mice supplemented with low vitamin C at 32 weeks post infection. The reduced gastric lesions in infected gulo−/− mice supplemented with low vitamin C correlated with reduced Th1-associated IgG2c, gastric IFN-γ and TNF-α mRNA and higher H. pylori colonization levels. These results in the H. pylori-infected gulo−/− mouse model suggest that although supplementation with a high level of vitamin C achieved physiologically normal vitamin C levels in plasma and gastric tissue, this dose of vitamin C did not protect gulo−/− mice from H. pylori-induced premalignant gastric lesions. In addition, less severe gastric lesions in H. pylori infected gulo−/− mice supplemented with low vitamin C correlated with an attenuated Th1 inflammatory response.
Keywords: Helicobacter pylori, vitamin C, gulo−/− mouse model
Helicobacter pylori infects the human stomach1 and has been definitively linked to chronic gastritis, which in some individuals results in serious gastric disease such as peptic ulcer, gastric adenocarcinoma or gastric MALToma.2 Multiple factors have been evaluated for impact on helicobacter-associated gastric disease.2,3 Dietary factors, including nitrosamines, high salt, and low dietary vitamin C (ascorbic acid), have been proposed to negatively influence the clinical outcome of H. pylori infection in epidemiological and animal studies.4–8
Vitamin C, a water-soluble antioxidant, reduces the formation of carcinogenic N-nitroso compounds in gastric juice and scavenges reactive oxygen species in the gastric mucosa.9,10 Vitamin C is also important for carboxyamidation of gastrin and cross linkage of collagen and elastin.11,12 Epidemiological studies in humans have linked vitamin C deficiency to more severe H. pylori-associated gastritis and a higher risk for gastric cancer.10,13 It has also been reported that reduced vitamin C levels in gastric juice and plasma in H. pylori-infected patients returned to normal levels after H. pylori eradication.10,13–16 Supplementation of vitamin C has been associated with reduced gastric cancer risk in some human studies.7,13 Despite initial promising results in a prospective trial in a very high-risk population supplemented with vitamin C at 2 g per day for 6 years,17 a followup study on this population indicated that vitamin C supplementation over a 12-year period did not provide any lasting protection against gastric cancer.18 These results are consistent with other studies that did not observe a correlation between severity of chronic gastritis or gastric cancer risk and vitamin C levels.19–21
In human vitamin C intervention studies, confounding variables include diet, vitamin C status, genetic polymorphisms, duration of infection with specific or unknown H. pylori strains, and degree of gastritis. Therefore, animal models have been used to analyze the effects of vitamin C on H. pylori gastritis and gastric cancer.22,23 Mice and Mongolian gerbils have been used to evaluate H. pylori gastritis and vitamin C oral intake.24–26 However, like most laboratory rodents, a major limitation for using these animal models for vitamin C studies is their ability to endogenously synthesize vitamin C. Thus results in these rodents are difficult to interpret. In contrast, the vitamin C-deficient gulo−/− mouse on a C57BL/6 background (B6.129P2-Gulotm1Umc/mmcd) lacks L-gulono-γ-lactone oxidase, and thus cannot endogenously synthesize vitamin C.27 Using this model, we were able to modulate low and high vitamin C levels in plasma and gastric tissue during H. pylori infection to specifically analyze whether dietary vitamin C would influence the outcome of H. pylori infection. We hypothesized that high dietary levels of vitamin C would reduce the severity of H. pylori gastritis, while physiologically lower levels of vitamin C would exacerbate disease.
Material and methods
Mice
Mice were maintained in an Association for Assessment and Accreditation of Laboratory Animal Care-accredited facility in static microisolator cages under specific-pathogen-free (SPF) status including free of Helicobacter spp. as previously described.28 Wildtype (WT) helicobacter-free C57BL/6 mice were obtained from Taconic Farms (Germantown, NY). Gulo+/− mice (B6.129P2-Gulotm1Umc/mmcd, back-crossed to C57BL/6 for 10 generations) were obtained from the Mutant Mouse Regional Resource Center (University of California at Davis, CA), rederived to SPF status, and bred to maintain a homozygous state.27 For breeding and maintenance, gulo−/− mice were weaned at 3 weeks of age and fed ad libitum with regular mouse chow (Prolab RMH 3,000 PMI Nutrition International, Richmond, IN) and water supplemented with 330 mg/L of L-ascorbic acid (Sigma-Aldrich Co., St. Louis, MO) and 0.01 mM EDTA (Sigma-Aldrich Co.). Supplemented water was changed weekly.27 Animal experiments were approved by the Committee on Animal Care of the Massachusetts Institute of Technology.
Experimental design
Male and female, 6–8-week old gulo−/− mice supplemented with vitamin C (330 mg/L) were experimentally infected with H. pylori and then randomly subdivided into low and high vitamin C supplemented groups. The low vitamin C group was supplemented with vitamin C in water at 33 mg/L and the high vitamin C group was supplemented with vitamin C in water at 3,300 mg/L.29 Control uninfected gulo−/− mice were supplemented with low or high vitamin C. Age-matched control uninfected WT mice and WT mice dosed with the same inoculum of H. pylori were used to confirm the mouse-adapted strain induced robust gastritis in WT mice. Approximately half of the mice of each group were euthanatized with CO2 at 16- or 32-weeks post infection (WPI) (Tables I and II). To confirm the results observed at 32 WPI, a second experiment evaluated the same vitamin C treatment groups of uninfected and H. pylori-infected gulo−/− mice along with uninfected and H. pylori-infected WT mice. In addition, a group of H. pylori-infected WT mice were supplemented with high vitamin C in the water (Table II).
TABLE I.
H. pylori INFECTION AT 16 WPI
| Genotype | H. pylori | Vitamin C | Number of mice |
|---|---|---|---|
| Gulo−/− | − | Low | 5 |
| Gulo−/− | + | Low | 13 |
| Gulo−/− | − | High | 5 |
| Gulo−/− | + | High | 8 |
| WT | + | None | 6 |
TABLE II.
H. pylori INFECTION AT 32 WPI
| Genotype | H. pylori | Vitamin C | Number of mice1 |
|---|---|---|---|
| Gulo−/− | − | Low | 7/13 |
| Gulo−/− | + | Low | 11/15 |
| Gulo−/− | − | High | 7/13 |
| Gulo−/− | + | High | 12/14 |
| WT | − | None | 3/9 |
| WT | + | None | 5/10 |
| WT | + | High | 0/6 |
First experiment/second experiment.
Experimental infection with H. pylori
H. pylori Sydney strain (SS1) was used for oral inoculation as described previously.28,30 After incubation for 24 hr at 37°C while shaking under microaerobic conditions in Brucella broth with 10% fetal bovine serum, H. pylori was harvested, resuspended in PBS and assessed by Gram stain and phase microscopy for purity, morphology, and motility. The bacterial concentration was adjusted to OD600 = 1.000 in PBS. This was ~109 organisms/mL. Mice were dosed with 0.2 mL of the H. pylori suspension in PBS by gavage every other day for 3 doses. Control mice were dosed with PBS.
Vitamin C measurements
Total vitamin C levels were measured by high performance liquid chromatography and UV detection as described previously with some modifications.31 In brief, blood was collected at necropsy in EDTA and centrifuged immediately at 4°C. Vitamin C was extracted from plasma by adding an equal volume of cold perchloric acid (PCA) solution (1 L contained 50 mL of PCA and 95 mg of EDTA in ddH2O), followed by vortexing and centrifugation at 2,500g at 4°C for 10 min. The supernatant was frozen at −70°C pending analysis. To measure vitamin C levels in tissue, a longitudinal strip of the gastric greater curvature was weighed, frozen in liquid nitrogen, and stored at −70°C. Frozen tissue was added to cold PCA solution at a ratio of 1:9 (weight/weight) followed by homogenization and centrifugation at 2,500g at 4°C for 10 min, and then frozen at −70°C until analysis. Throughout the vitamin C extraction process, samples were kept on ice, protected from light, and measured within 1-week post processing.
Histological evaluation
At necropsy, the stomach and proximal duodenum were removed and opened along the greater curvature. Linear gastric strips from the lesser curvature were fixed overnight in 10% neutral-buffered formalin, embedded, cut at 4 μm, and stained with hematoxylin and eosin (H&E). Using criteria described previously,32 gastric lesions were scored for inflammation, epithelial defects, oxyntic atrophy, foveolar hyperplasia, pseudopyloric metaplasia and dysplasia by board certified veterinary pathologists (BHR and ABR) blinded to sample identity. The sum of individual scores was used to define the gastric disease index.30 Mucous metaplasia was scored separately from total gastric indices because this lesion is not clearly understood, having been observed to develop spontaneously in some strains of mice as well as result from a variety of pathological processes in humans and mice that are independent of H. pylori infection.30
RNA extraction and quantitative PCR for cytokine mRNA
A longitudinal strip of gastric tissue from the anterior wall was harvested and snap-frozen in liquid nitrogen. Total RNA was extracted with Trizol reagent (Invitrogen, Carlsbad, CA). cDNA was synthesized from 5 μg of total RNA with the High Capacity cDNA Archive kit (Applied Biosystems, Forster City, CA). mRNA levels of interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) were quantified with TaqMan gene expression assays and TaqMan Fast Universal PCR Master Mix in a 7500 Fast Real-Time PCR system (Applied Biosystems) per manufacturer’s instructions. mRNA levels of each cytokine were normalized to the mRNA level of internal control glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and compared to data from uninfected WT mice using the ΔΔCT method (Bulletin 2, Applied Biosystems).
Plasma IgG isotypes measurement
Plasma was evaluated for H. pylori-specific IgG2c and IgG1 by ELISA using an outer membrane protein preparation from H. pylori (SS1 strain) as described previously.33 In brief, 96-well flat-bottom plates were coated with 100 μL of antigen (10 μg/mL) overnight at 4°C, and sera were diluted 1:100. Biotinylated secondary antibodies for detecting IgG2c and IgG1 were from clone 5.7 and A85-1 (BD Pharmingen, San Jose, CA). Incubation with extravidin peroxidase (Sigma-Aldrich) was followed by treatment with 2,2′-azinobis (3-ethylbenzthiazolinesulfonic acid) (ABTS) substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD) for color development. The optical density was recorded by a plate reader per manufacturer’s protocol (Power WaveX Select, Biotek Instruments, Winooski, VT).
Quantitative PCR for H. pylori colonization
A longitudinal strip of gastric tissue from the greater curvature was proteinase K digested at 55°C for 8 hr followed by DNA extraction with phenol:chloroform:isoamyl alcohol (25:24:1) and ethanol precipitation. H. pylori colonization levels (DNA copy numbers) were quantified by a fluorogenic quantitative PCR assay using urease B primers and probe.34 H. pylori copy numbers were normalized to the amount of murine genomic DNA as determined by quantitative PCR using a eukaryotic 18S endogenous control (Applied Biosystems) (Bulletin 2, Applied Biosystems). Data is presented and compared as log-transformed copy numbers per μg host DNA.
Quantification of plasma levels of gastrin
Plasma concentrations of glycine-extended gastrin (G-gly) and amidated gastrin were determined by radioimmunoassay using the antibodies 109-21 and L2 specific to G-gly and amidated gastrin, respectively, as described previously.35
Statistical analysis
Histological scores from both 32 WPI experiments were pooled for analysis because they were performed under identical conditions. Pathology scores were analyzed by variance (ANOVA) followed by post-hoc comparison when ANOVA results were significant. Because cytokine mRNA levels increased progressively across the H. pylori-infected groups from low vitamin C to high vitamin C to WT, we assigned a numerical value from 1 to 3 to each group in the model and estimated the significance level of gastric lesions using a general linear model that tests for trends. Pathology scores between different genders were compared by the Mann-Whitney test. Levels of gastric cytokine, plasma IgG isotypes, H. pylori colonization, and amidated gastrin were compared by the Student t test. All statistical analyses were two-sided tests at a significance level of 0.05 performed with SAS software version 9.1 (SAS Institute, Cary, NC). The mean and standard error of all data are presented in the figures using Graphpad Prism 4.0 (Graphpad software, San Diego, CA).
Results
Vitamin C levels in plasma and gastric tissues correlated with vitamin C supplementation levels in gulo−/− mice
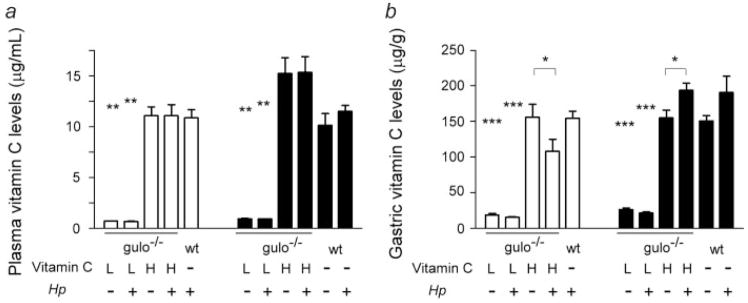
Both uninfected and H. pylori-infected gulo−/− mice supplemented with low vitamin C had significantly lower plasma (p < 0.01) and gastric tissue (p < 0.001) vitamin C levels relative to the mice supplemented with high vitamin C at 16 and 32 weeks (Fig. 1). Uninfected and H. pylori-infected gulo−/− mice supplemented with high vitamin C had plasma and gastric tissue vitamin C levels comparable to those in unsupplemented WT mice at the same time points. H. pylori infection had no significant effect on plasma vitamin C levels in gulo−/− mice supplemented with low or high vitamin C or in WT mice (p = 0.37 and higher). At 16 and 32 WPI, there was a trend for H. pylori infection to reduce gastric tissue vitamin C levels in gulo−/− mice supplemented with low vitamin C (p = 0.071, p = 0.069, respectively). This reduction in gastric tissue vitamin C associated with H. pylori was significant in gulo−/− mice supplemented with high vitamin C at 16 WPI (p < 0.05), however, paradoxically, H. pylori infection was associated with increased gastric tissue vitamin C levels at 32 WPI (p =0.05). Infection in WT mice did not significantly affect gastric tissue vitamin C levels at 32 WPI (p = 0.24).
Figure 1.
Vitamin C levels in plasma (a) and gastric tissue (b). White bars are 16 WPI; Black bars are 32 WPI. Compared to gulo−/− mice supplemented with high vitamin C (H), gulo−/− mice supplemented with low vitamin C (L) had significantly lower plasma and gastric tissue vitamin C levels (**, p < 0.01; ***, p < 0.001). Vitamin C levels in plasma and gastric tissue in gulo−/− mice supplemented with high vitamin C were comparable to those in C57BL/6 (wt) mice. H. pylori (Hp) infection had no significant effect on plasma vitamin C levels or gastric vitamin C levels in gulo−/− mice supplemented with low vitamin C or in wt mice. However, gastric tissue vitamin C levels were reduced at 16 WPI but increased at 32 WPI by H. pylori infection in gulo−/− mice supplemented with high vitamin C (*, p < 0.05).
High vitamin C supplementation did not reduce H. pylori gastritis
Gastritis was not observed in uninfected WT mice and gulo−/− mice supplemented with low or high vitamin C. Consistent with previous reports,8,23 H. pylori-infected WT mice developed robust gastritis at 16 and 32 WPI characterized by corpus inflammation with lymphocytic and granulocytic infiltration, epithelial defects, foveolar hyperplasia, pseudopyloric metaplasia, and oxyntic atrophy (Figs. 2 and 3).
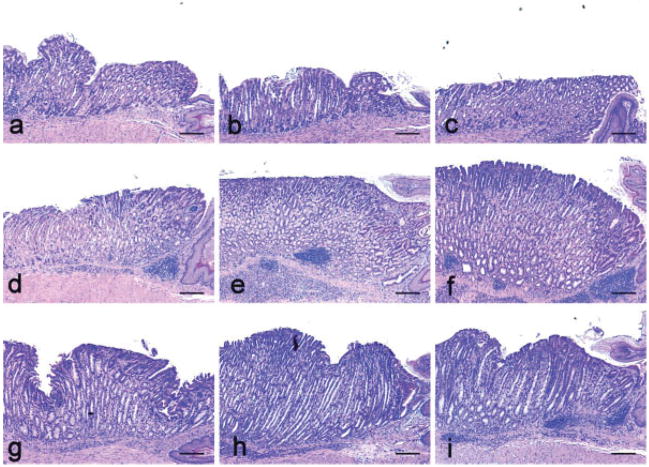
Figure 2.
Gastric histopathology of H. pylori infection in gulo−/− with low vitamin C supplementation (a, d, g) or high vitamin C supplementation (b, e, h) versus C57BL/6 (WT) controls (c, f, i). (a–c) No significant lesions developed in uninfected mice in any group at 32 weeks. (d–f) Equivalent gastric lesions developed in all H. pylori-infected groups at 16 WPI, although gulo−/− mice with low vitamin C supplementation (d) exhibited a trend for less severe epithelial defects, foveolar hyperplasia and dysplasia. (g–i) Equivalent gastric inflammation developed in all groups at 32 WPI, although gulo−/− mice with low vitamin C supplementation (g) had less severe epithelial defects, mucous metaplasia and foveolar hyperplasia.
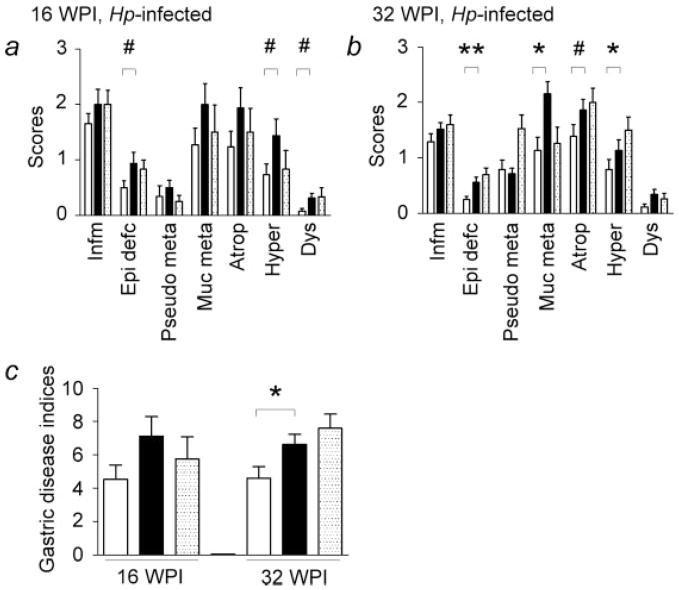
Figure 3.
Histological scores and gastric disease indices in H. pylori (Hp)-infected mice. Gulo−/− mice supplemented with high vitamin C had similar gastric lesions and gastric disease indices compared to C57BL/6 (WT) (dotted bars) mice at 16 and 32 WPI. (a) At 16 WPI, there was a trend for gulo−/− mice supplemented with low vitamin C (white bars) compared to those supplemented with high vitamin C (black bars) for less severe epithelial defects, foveolar hyperplasia, and dysplasia (#, 0.05 < p < 0.10). (b) At 32 WPI, H. pylori-infected gulo−/− mice supplemented with low vitamin C had significantly lower degrees of epithelial defects, mucous metaplasia, and foveolar hyperplasia relative to those supplemented with high vitamin C (*, p < 0.05; **, p < 0.01). There was a trend toward less severe oxyntic atrophy in gulo−/− mice supplemented with low vitamin C compared to those mice supplemented with high vitamin C (#, 0.05 < p < 0.10). (c) At 32 WPI, gastric disease indices were significantly lower in gulo−/− mice that received low vitamin C than those mice receiving high vitamin C (*, p < 0.05). Infm, inflammation; Epi defc, epithelial defects; Pseudo meta, psuedopyloric metaplasia; Muc Meta, mucous metaplasia; Atroph, oxyntic atrophy; Hyper, foveolar hyperplasia; Dys, dysplasia.
At 16 and 32 WPI, H. pylori-infected gulo−/− mice supplemented with high vitamin C developed gastric lesions of comparable severity relative to infected wt mice. At 16 WPI, there was a trend for H. pylori-infected gulo−/− mice supplemented with low vitamin C to have fewer epithelial defects (p = 0.094), foveolar hyperplasia (p = 0.069), pseudopyloric metaplasia (p = 0.15), oxyntic atrophy (p = 0.15), and dysplasia (p = 0.078) relative to infected gulo−/− mice supplemented with high vitamin C. These trends were also reflected in slightly lower gastric disease indices at 16 WPI in H. pylori-infected gulo−/− mice supplemented with low vitamin C (p = 0.095).
Compared to H. pylori-infected gulo−/− mice supplemented with high vitamin C, by 32 WPI H. pylori-infected gulo−/− mice supplemented with low vitamin C demonstrated trends for less severe gastritis (p = 0.11), oxyntic atrophy (p = 0.06), and dysplasia (p = 0.12) and had significantly less extensive epithelial defects (p < 0.01), mucous metaplasia (p < 0.05), and foveolar hyperplasia (p < 0.05). When comparing gastric disease indices between low and high vitamin C supplementation levels, infected gulo−/− mice supplemented with low vitamin C had statistically significant lower gastric disease indices compared to those mice supplemented with high vitamin C (p < 0.05). On the basis of differences identified in cytokine gene expression (below), one-way trend analysis of H. pylori-infected gulo−/− mice with low vitamin C compared to gulo−/− mice with high vitamin C followed by comparison with WT mice demonstrated a difference in oxyntic atrophy (p = 0.053) and significant differences in epithelial defects (p < 0.001), foveolar hyperplasia (p < 0.05), mucous metaplasia (p < 0.05), and gastric disease indices (p < 0.05).
Less severe gastritis in H. pylori-infected gulo−/− mice supplemented with low vitamin C was associated with lower gastric mRNA levels of IFN-γ and TNF-α
Control gulo−/− mice supplemented with low or high vitamin C and uninfected WT mice had similar background gastric mRNA levels of IFN-γ and TNF-α at 32 WPI. H. pylori infection significantly upregulated the mRNA levels of IFN-γ and TNF-α in the stomachs of gulo−/− and WT mice at 32 WPI. IFN-γ and TNF-α expression levels were elevated by H. pylori infection to a greater extent in gulo−/− mice supplemented with high vitamin C (p < 0.001) and WT mice (p < 0.001) compared to gulo−/− mice given low vitamin C supplementation (IFN-γ p < 0.01, TNF-α p =0.088) (Fig. 4). Among H. pylori-infected mice, gulo−/− mice supplemented with low vitamin C had lower gastric mRNA levels of IFN-γ and TNF-α compared to gulo−/− mice supplemented with high vitamin C or WT mice (p < 0.05 or lower). There were no significant differences in gastric mRNA levels of IFN-γ and TNF-α between H. pylori-infected gulo−/− mice supplemented with high vitamin C and infected WT mice (p = 0.31, p = 0.23, respectively).
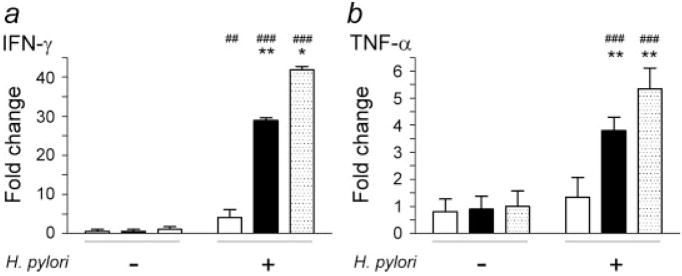
Figure 4.
Gastric mRNA levels of IFN-γ (a) and TNF-α (b) in gulo−/− and C57BL/6 (WT) mice at 32 WPI. Fold changes in expression levels were normalized using data from uninfected WT mice. There were no differences in IFN-γ and TNF-α mRNA levels among uninfected WT or gulo−/− mice that received low or high vitamin C. H. pylori infection upregulated IFN-γ mRNA levels in gulo−/− mice supplemented with low vitamin C (white bar, p < 0.01) and to the greatest extent in gulo−/− mice supplemented with high vitamin C (black bar) and WT mice (dotted bar) (p < 0.001). H. pylori-infected gulo−/− mice supplemented with low vitamin C had significantly lower IFN-γ mRNA levels compared to gulo−/− mice supplemented with high vitamin C or WT mice (p < 0.01 and 0.05, respectively). H. pylori infection significantly upregulated TNF-α mRNA levels in gulo−/− mice supplemented with high vitamin C and WT mice (p < 0.001), but not in gulo−/− mice supplemented with low vitamin C. Therefore, TNF-α mRNA levels in gulo−/− mice supplemented with low vitamin C were significantly lower than the other two groups (p < 0.01). (Compared to uninfected wt mice, ##, p < 0.01; ###, p < 0.001. Compared to H. pylori-infected gulo−/− mice supplemented with low vitamin C, *, p < 0.05; **, p < 0.01).
Gulo−/− mice supplemented with low vitamin C had lower H. pylori-specific IgG2c responses
H. pylori infection resulted in a Th1-predominant IgG2c response in WT and gulo−/− mice as previously reported33,36 (Fig. 5). At 32 WPI, H. pylori-specific IgG2c levels were significantly higher in H. pylori-infected WT mice than in infected gulo−/− mice supplemented with low vitamin C (p < 0.05) but IgG2c responses were similar between gulo−/− mice given low or high vitamin C (p = 0.44). There was a trend for infected WT mice to have higher H. pylori-specific IgG2c levels than gulo−/− mice supplemented with high vitamin C (p = 0.051). However, in the 6 H. pylori-infected WT mice given vitamin C supplementation, there was no difference in H. pylori-specific IgG2c responses compared to the 10 infected WT mice that did not receive oral vitamin C supplementation (p = 0.51, data not shown). H. pylori infection induced a low IgG1 response in all infected groups.
Figure 5.
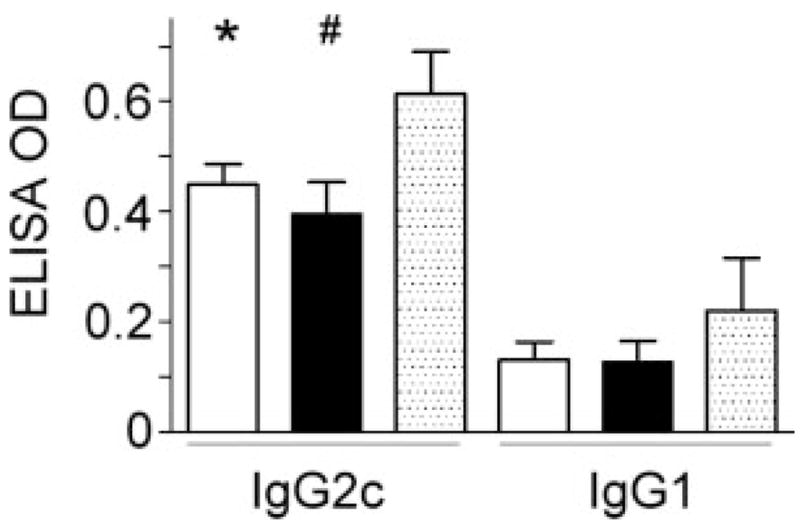
H. pylori-specific IgG2c and IgG1 levels in H. pylori-infected mice. At 32 WPI, there was no difference in IgG2c levels among gulo−/− mice supplemented with low vitamin C (white bar) and high vitamin C (black bar). Gulo−/− mice supplemented with low or high vitamin C had lower IgG2c levels than did C57BL/6 (WT) mice (dotted bar) but differences were significant only for gulo−/− mice supplemented with low vitamin C (*, p < 0.05). IgG1 levels among these 3 groups of mice were low and similar at 32 WPI (p = 0.44).
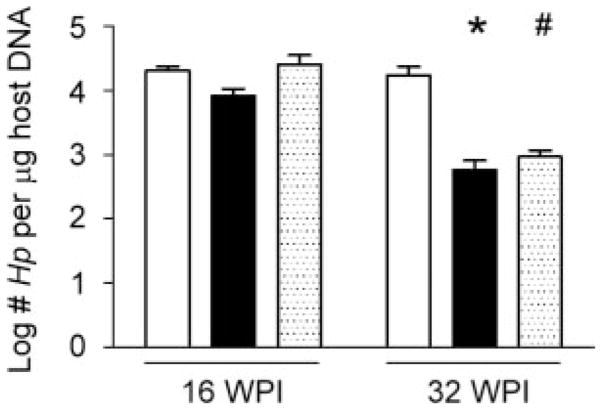
H. pylori colonization levels were higher in gulo−/− mice supplemented with low vitamin C at 32 WPI
H. pylori colonization levels in the stomach were comparable among infected gulo−/− mice supplemented with low or high vitamin C and WT mice at 16 WPI (Fig. 6). By 32 WPI, infected gulo−/− mice supplemented with low vitamin C maintained higher H. pylori colonization levels than gulo−/− mice supplemented with high vitamin C (p < 0.05) or WT mice (p = 0.082) in which H. pylori colonization levels decreased over time in concert with development of more severe gastritis.
Figure 6.
H. pylori(Hp) colonization levels (log CFU/μg host DNA). At 16 WPI, there were no differences in H. pylori colonization levels across groups. At 32 WPI, gulo−/− mice supplemented with low vitamin C (white bars) had higher H. pylori colonization levels than gulo−/− mice supplemented with high vitamin C (black bars) and C57BL/6 (WT) mice (dotted bars). (*, p < 0.05; #, 0.05 < p < 0.10).
Gastrin amidation was not impaired in vitamin C-supplemented gulo−/− mice
Amidation of the intermediate glycine-extended gastrin (G-gly) is vitamin C dependent11,37 and amidated gastrin levels are elevated in H. pylori infection in C57BL/6 mice.8 At 32 WPI, levels of the intermediate G-gly in plasma from most WT and gulo−/− mice supplemented with low or high vitamin C were below the assay detection limit (data not shown). Uninfected WT mice and gulo−/− mice supplemented with low or high vitamin C had similar amidated gastrin levels (p = 0.13) (Fig. 7). Uninfected and H. pylori-infected gulo−/− mice supplemented with low vitamin C had comparable plasma amidated gastrin levels (p = 0.82). There was a trend toward higher plasma amidated gastrin levels in H. pylori-infected gulo−/− mice supplemented with high vitamin C compared to uninfected counterparts (p = 0.21). In contrast, H. pylori infection was associated with a significant increase in plasma amidated gastrin levels in WT mice compared to uninfected WT controls (p < 0.01). Among the H. pylori-infected gulo−/− and WT mice, gulo−/− mice supplemented with low vitamin C had lower amidated gastrin levels compared to infected gulo−/− mice supplemented with high vitamin C and WT mice (p = 0.065 and <0.05, respectively).
Figure 7.
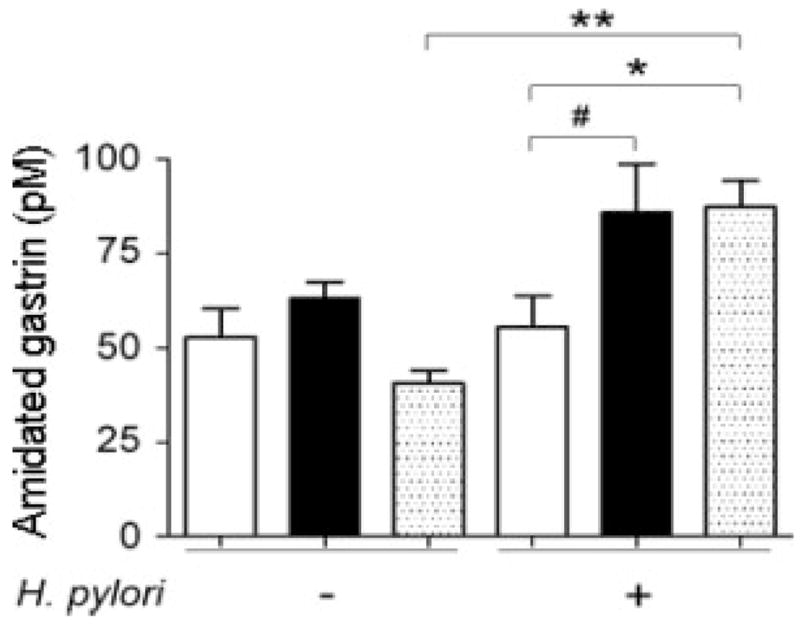
Plasma levels of amidated gastrin at 32 WPI. Among uninfected mice, C57BL/6 (WT) mice (dotted bar) and gulo−/− mice supplemented with low (white bar) or high (black bar) vitamin C had similar levels of amidated gastrin (p = 0.13) H. pylori infection significantly upregulated amidated gastrin levels in wt mice (p < 0.01), but not in gulo−/− mice that received low or high vitamin C. Among H. pylori-infected mice, gulo−/− mice supplemented with low vitamin C had amidated gastrin levels lower than gulo−/− mice supplemented with high vitamin C and wt mice (p = 0.065 and <0.05, respectively). (*, p < 0.05; **, p < 0.01; #, p = 0.065).
Male gulo−/− mice supplemented with low vitamin C developed less severe pathology, lower gastric vitamin C and lower IFN-γ levels compared to female mice
We and others have previously reported on male- or female-predominant helicobacter-associated gastric disease in select strains of mice.38,39. In the current study, gender effect was not analyzed for 16 WPI because there were only 4 male and 2 female WT mice that were included as positive controls for confirming the pathogenicity of the H. pylori SS1 inoculum. Statistical analysis of the infected WT mice (5 males, 10 females) at 32 WPI revealed no gender effect on total gastric lesion indices. Only 1 subfeature, intestinal metaplasia, was less severe in infected female WT mice (p < 0.05). Consistent with the results from infected WT mice, analysis of pathology lesions in infected gulo−/− mice supplemented with high vitamin C, which were comparable to WT mice in vitamin C status, revealed no gender effect on gastric disease indices at 16 weeks (4 males, 4 females) or at 32 WPI (15 males, 11 females) (data not shown). Only one feature, mucous metaplasia, was less severe in males (p < 0.05).
Analysis for gender effects in the gulo−/− mice supplemented with low vitamin C revealed that at 16 WPI, gastric disease indices were equivalent between 5 males and 8 females. In contrast, the features of mucous metaplasia, and of more significance to helicobacter-associated disease in humans, oxyntic atrophy, were less severe in male mice (p < 0.05). At 32 WPI, the additional features of intestinal metaplasia and the gastric disease indices were also less severe in male gulo−/− mice on the low vitamin C intake compared to females (p < 0.05). Notably, at 16 WPI, lower vitamin C levels in gastric tissues were observed in gulo−/− males on low vitamin C compared to females (p < 0.05) with a similar trend in the high vitamin C gulo−/− mice (p = 0.06) (data not shown). This correlation between gender effects and vitamin C content of gastric tissues was not observed at 32 WPI in the low vitamin C supplemented gulo−/− mice. The high vitamin C supplemented male gulo−/− mice had lower gastric vitamin C content than females, but this was in the absence of a corresponding gender effect on pathology, as previously stated. Additionally, plasma levels of vitamin C were not impacted by gender in any experimental group.
In support of potential female-predominant disease in H. pylori-infected gulo−/− mice on low vitamin C supplementation, IFN-γ expression levels in gastric tissues were lower in males at 32 WPI (p < 0.05). IFN-γ levels were also lower in the male gulo−/− mice supplemented with high vitamin C (p < 0.05) despite an equivalent severity of pathology to female mice. However, there were no differences in H. pylori-specific IgG2c and IgG1 levels between male and female mice in any experimental group (data not shown).
Discussion
Using the gulo−/− mouse model, we were able to accurately assess and correlate low and high vitamin C supplementation with corresponding levels in plasma and gastric tissue, thus enabling in vivo evaluation of the role of dietary vitamin C in H. pylori-associated gastric disease. We observed that plasma and gastric tissue vitamin C levels in gulo−/− mice directly correlated with vitamin C intake, consistent with human studies.19,40 Gulo−/− mice supplemented with high vitamin C and chronically infected with H. pylori over a period of 32 weeks developed chronic gastritis and oxyntic atrophy comparable to WT mice, indicating high vitamin C supplementation did not protect infected gulo−/− mice from gastritis nor the development of premalignant lesions.
Plasma vitamin C levels in gulo−/− mice directly correlated with the level of dietary vitamin C and were not affected by H. pylori infection. Gastric tissue vitamin C levels also correlated with dietary vitamin C intake but in contrast to plasma levels, gastric tissue vitamin C levels were reduced in H. pylori-infected gulo−/− mice at 16 WPI which is consistent with reduced vitamin C levels in gastric juice during acute H. pylori infection in humans.41 However, gastric tissue vitamin C levels were increased in H. pylori-infected gulo−/− mice at 32 WPI. The increased gastric tissue vitamin C levels may have been associated with enhanced infiltration of lymphocytes and granulocytes, which have high intracellular vitamin C levels.42 Notably, gastric tissue vitamin C levels were not impacted by H. pylori infection in WT mice which may be confounded by endogenous synthesis of vitamin C in normal mice. In humans, intravenous dosing with vitamin C was shown to block further secretion of vitamin C into gastric juice during acute H. pylori infection,41 but it has not been conclusively shown that naturally acquired H. pylori infection affects vitamin C levels in human gastric mucosa.20,43 Therefore, additional studies are necessary to evaluate the impact of H. pylori gastritis on vitamin C levels in the gastric tissues of humans.
Several clinical trials have examined nutritional interventions using vitamin C alone or in combination with H. pylori eradication or other nutrients in preventing gastric carcinogenesis in humans; none of these studies demonstrated a reproducible benefit of vitamin C supplementation.18,21,44,45 However, the results of these studies are difficult to interpret given the probability of preexisting H. pylori-associated premalignant lesions in the target populations when vitamin C intervention or H. pylori eradication therapies were initiated.18,21,44–46 To obviate these variables, in the gulo−/− mouse model vitamin C supplementation was administered orally at lower and higher levels than the recommended level for maintenance, allowing us to establish dietary levels of vitamin C prior to development of H. pylori-associated gastric lesions.
Despite the limitation of using rodents that endogenously synthesize vitamin C, rodent studies have reported that 7–10 days of vitamin C supplementation reduced gastric inflammation, H. pylori colonization levels, and lipid peroxidation in BALB/c mice given 400 mg vitamin C/kg/day25 and decreased H. pylori colonization levels in Mongolian gerbils given 10 mg vitamin C/day.24 However, long-term vitamin C supplementation at 50 mg/kg/day over a period of 52 weeks had no protective effect on severity of gastritis, bacterial colonization levels, and mucosal levels of 8-hydroxydeoxyguanosine, a marker of oxidative DNA damage, in H. pylori-infected gerbils.26 Supplementation with vitamin C in the diet at 10-fold higher levels than a maintenance dose (3,102 mg/kg) for 6 weeks in guinea pigs, which lack L-gulono-γ-lactone oxidase like humans and gulo−/− mice, did not impact H. pylori colonization levels nor severity of gastritis.47 Similar to the guinea pig study, H. pylori-infected gulo−/− mice supplemented with high vitamin C had comparable gastric inflammation and premalignant lesions compared to the infected WT mice. Thus, high level supplementation with vitamin C in the gulo−/− mouse model as well as in guinea pigs did not prevent the development of H. pylori gastric disease or impact disease progression.
It is interesting, in the present study, that low vitamin C-supplemented gulo−/− mice tended to have lower degrees of inflammation, oxyntic atrophy and dysplasia and significantly less severe epithelial defects, pseudopyloric metaplasia and foveolar hyperplasia relative to high vitamin C-supplemented gulo−/− and WT mice. Helicobacter-induced gastric disease in humans and mice is mediated by a Th1-predominant host immune response.48–50 Gulo−/− mice supplemented with high vitamin C had a similar Th1-promoted inflammatory response to H. pylori as infected WT mice. We observed that low vitamin C-supplemented, H. pylori-infected gulo−/− mice had less severe gastric lesions associated with suppressed Th1 responses as evidenced by reduced levels of plasma IgG2c, reduced expression of gastric IFN-γ and TNF-α mRNA, and increased H. pylori colonization levels. These data suggest that low dietary vitamin C impairs the host’s ability to sustain an inflammatory response and conversely, that adequate vitamin C levels in the infected host may be important for maintaining a robust Th1 immune response to chronic H. pylori infection. Consistent with our data, others have reported attenuated Th1 immune responses in vitamin C-deprived gulo−/− mice. This was manifested by less severe pneumonia and suppressed pulmonary expression of proinflammatory IL-1β and TNF-α mRNA in vitamin C-deprived gulo−/− mice during the first few days of acute viral influenza.51 In hosts with low or no vitamin C intake, attenuated Th1 responses to other types of infection, such as tuberculosis in humans or Klebsiella pneumoniae sepsis in gulo−/− mice, may also be important in predicting survival.52,53
Using a mouse model of intestinal parasitic infection that causes a Th2 immune response, Fox et al. examined the effect of modulating the Th1-associated response to H. felis infection.54 In C57BL/6 mice coinfected with helminths and H. felis, reduced systemic Th1 immune responses and lower levels of Th1-mediated gastric cytokines were associated with increased H. felis colonization levels and less severe premalignant lesions.54 These results may in part explain the “African enigma” where the incidence of gastric cancer is low in some African countries, where parasitic infections are common, despite a high prevalence of H. pylori infection.55 Our findings using the H. pylori-infected, low vitamin C-supplemented gulo−/− mouse model may offer another possible explanation to the African enigma. In Gambia, due to the impact of drought on the food supply, mean daily intake of vitamin C approaches zero for 7 months of the year, and is accompanied by low plasma vitamin C levels.56 It is tempting to speculate based on our animal studies, that minimal vitamin C intake during H. pylori infection may be one of the factors contributing to a lower incidence of gastric cancer in some populations in Africa because of attenuated gastric immune responses or inflammation. Although this hypothesis is consistent with a report of diminished mitogen responses of peripheral blood mononuclear cells from pigs affected by heritable vitamin C deficiency,57 the biological significance of these responses is unclear.
Plasma amidated gastrin levels are increased in H. pylori-infected C57BL/6 mice.8 Overexpression of amidated gastrin promotes the progression of H. pylori-associated gastritis and gastric cancer.39 In a guinea pig model, vitamin C-deprivation impaired the amidation of gastrin and levels of intermediate G-gly were 30-fold higher than normal in the gastric antra.37 In our study, uninfected gulo−/− mice supplemented with low vitamin C had undetectable G-gly but comparable levels of amidated gastrin relative to uninfected gulo−/− mice supplemented with high vitamin C. Consistent with a previous report,8 H. pylori infection significantly increased plasma amidated gastrin levels in wt mice (p < 0.01). However, H. pylori infection only slightly increased amidated gastrin levels in gulo−/− mice supplemented with high vitamin C, with no increase in those supplemented with low vitamin C. These results suggest that the dose used for low vitamin C supplementation was sufficient for gastrin amidation in uninfected animals but not sufficient to sustain increased amidated gastrin levels during H. pylori infection. Higher levels of admidated gastrin in H. pylori-infected gulo−/− mice supplemented with high vitamin C, compared to those with low vitamin C, may in part explain the higher degree of premalignant lesions in the high vitamin C supplemented gulo−/− mice.
In the current study, H. pylori-infected gulo−/− mice developed marked mucous metaplasia in the corpus, which was more prominent in high vitamin C-supplemented mice that developed more severe gastric pathology. In humans, H. pylori-associated gastric carcinogenesis has been associated with atrophy and intestinal and mucous metaplasia.18 Helicobacter-associated mucous metaplasia has been associated with increased expression of trefoil factor 2 (TFF2) by gastric mucous neck cells in mice58,59 and has been suggested to be a precursor lesion of gastric cancer in mice60 and humans.61 In contrast, TFF2 has been reported to be a negative regulator of helicobacter-associated gastritis.62,63 Additionally, mucous metaplasia has been observed to develop spontaneously in some strains of mice64 as well as in T cell-reconstituted Rag2 −/− mice irrespective of H. pylori infection status. The significance of mucous metaplasia is unclear and requires further study.
In summary, our study indicates that gulo−/− mice, unlike other rodent models, provide a reliable model to study the role of dietary vitamin C in H. pylori-associated gastric disease. High vitamin C supplementation in this model, similar to previous epidemiological studies in humans,18–21 did not prevent progression of H. pylori-induced gastritis and development of premalignant gastric lesions. In contrast, low dietary vitamin C resulted in less severe gastric disease by downregulating gastric and systemic Th1 immune responses to H. pylori infection. We and others have previously reported on male- or female-predominant helicobacter-associated gastric disease in select strains of mice38,39 and the data presented here also support gender effects on gastric disease when vitamin C status is low. Additional studies are needed to confirm these observations in concert with determining a mechanism for suppressed inflammatory or immune responses when vitamin C supplementation is low.
Acknowledgments
NIH; Grant numbers: R01AI37750, P01CA26731, P30ES02109.
Authors thank Ms. Liz Horrigan for animal care; Ms. Sandy Xu and Ms. Nancy Taylor for culturing H. pylori; Ms. Kristen Clapp and Ms. Juri Miyamae for performing necropsies; Ms. Kathy Cormier for providing histology expertise and Ms. Gayle Petty and Ms. Bella Gindelsky for expert assistance with vitamin C measurements.
References
- 1.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–15. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 2.Houghton J, Fox JG, Wang TC. Gastric cancer: laboratory bench to clinic. J Gastroenterol Hepatol. 2002;17:495–502. doi: 10.1046/j.1440-1746.2002.02770.x. [DOI] [PubMed] [Google Scholar]
- 3.International Agency for Research on Cancer. Schistosomes liver flukes and Helicobacter pylori. 1. Lyon: IARC; 1994. [Google Scholar]
- 4.Howson CP, Hiyama T, Wynder EL. The decline in gastric cancer: epidemiology of an unplanned triumph. Epidemiol Rev. 1986;8:1–27. doi: 10.1093/oxfordjournals.epirev.a036288. [DOI] [PubMed] [Google Scholar]
- 5.World Cancer Research Fund and American Institute for Cancer Research. Food, nutrition and the prevention of cancer: a global perspective. 1. Washington, DC: The Institute; 1997. [DOI] [PubMed] [Google Scholar]
- 6.Harrison LE, Zhang ZF, Karpeh MS, Sun M, Kurtz RC. The role of dietary factors in the intestinal and diffuse histologic subtypes of gastric adenocarcinoma: a case-control study in the U. S Cancer. 1997;80:1021–8. [PubMed] [Google Scholar]
- 7.Su L, Fontham E, Ruiz B, Schmidt S, Correa P, Bravo L. Association of dietary antioxidants on the severity of gastritis in a high risk population. Ann Epidemiol. 2000;10:468. doi: 10.1016/s1047-2797(00)00135-6. [DOI] [PubMed] [Google Scholar]
- 8.Fox JG, Dangler CA, Taylor NS, King A, Koh TJ, Wang TC. High-salt diet induces gastric epithelial hyperplasia and parietal cell loss, and enhances Helicobacter pylori colonization in C57BL/6 mice. Cancer Res. 1999;59:4823–8. [PubMed] [Google Scholar]
- 9.Mannick EE, Bravo LE, Zarama G, Realpe JL, Zhang XJ, Ruiz B, Fontham ET, Mera R, Miller MJ, Correa P. Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res. 1996;56:3238–43. [PubMed] [Google Scholar]
- 10.Zhang ZW, Patchett SE, Perrett D, Katelaris PH, Domizio P, Farthing MJ. The relation between gastric vitamin C concentrations, mucosal histology, and CagA seropositivity in the human stomach. Gut. 1998;43:322–6. doi: 10.1136/gut.43.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stepan V, Sugano K, Yamada T, Park J, Dickinson CJ. Gastrin biosynthesis in canine G cells. Am J Physiol Gastrointest Liver Physiol. 2002;282:G766–G775. doi: 10.1152/ajpgi.00167.2001. [DOI] [PubMed] [Google Scholar]
- 12.Pinnell SR. Regulation of collagen biosynthesis by ascorbic acid: a review. Yale J Biol Med. 1985;58:553–9. [PMC free article] [PubMed] [Google Scholar]
- 13.Correa P, Malcom G, Schmidt B, Fontham E, Ruiz B, Bravo JC, Bravo LE, Zarama G, Realpe JL. Review article: antioxidant micronutrients and gastric cancer. Aliment Pharmacol Ther. 1998;12(Suppl 1):73–82. doi: 10.1111/j.1365-2036.1998.00006.x. [DOI] [PubMed] [Google Scholar]
- 14.Dabrowska-Ufniarz E, Dzieniszewski J, Jarosz M, Wartanowicz M. Vitamin C concentration in gastric juice in patients with precancerous lesions of the stomach and gastric cancer. Med Sci Monit. 2002;8:CR96–CR103. [PubMed] [Google Scholar]
- 15.Jenab M, Riboli E, Ferrari P, Sabate J, Slimani N, Norat T, Friesen M, Tjonneland A, Olsen A, Overvad K, Boutron-Ruault MC, Clavel-Chapelon F, et al. Plasma and dietary vitamin C levels and risk of gastric cancer in the European prospective investigation into cancer and nutrition (EPIC-EURGAST) Carcinogenesis. 2006;27:2250–7. doi: 10.1093/carcin/bgl096. [DOI] [PubMed] [Google Scholar]
- 16.Ekstrom AM, Serafini M, Nyren O, Hansson LE, Ye W, Wolk A. Dietary antioxidant intake and the risk of cardia cancer and noncardia cancer of the intestinal and diffuse types: a population-based case-control study in Sweden. Int J Cancer. 2000;87:133–40. [PubMed] [Google Scholar]
- 17.Correa P, Fontham ET, Bravo JC, Bravo LE, Ruiz B, Zarama G, Realpe JL, Malcom GT, Li D, Johnson WD, Mera R. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-Helicobacter pylori therapy. J Natl Cancer Inst. 2000;92:1881–8. doi: 10.1093/jnci/92.23.1881. [DOI] [PubMed] [Google Scholar]
- 18.Mera R, Fontham ET, Bravo LE, Bravo JC, Piazuelo MB, Camargo MC, Correa P. Long term follow up of patients treated for Helicobacter pylori infection. Gut. 2005;54:1536–40. doi: 10.1136/gut.2005.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waring AJ, Drake IM, Schorah CJ, White KL, Lynch DA, Axon AT, Dixon MF. Ascorbic acid and total vitamin C concentrations in plasma, gastric juice, and gastrointestinal mucosa: effects of gastritis and oral supplementation. Gut. 1996;38:171–6. doi: 10.1136/gut.38.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phull PS, Price AB, White KL, Schorah CJ, Jacyna MR. Gastroduodenal mucosal vitamin-C levels in Helicobacter pylori infection. Scand J Gastroenterol. 1999;34:361–6. doi: 10.1080/003655299750026362. [DOI] [PubMed] [Google Scholar]
- 21.Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet. 2004;364:1219–28. doi: 10.1016/S0140-6736(04)17138-9. [DOI] [PubMed] [Google Scholar]
- 22.Lee A, Fox JG, Otto G, Murphy J. A small animal model of human Helicobacter pylori active chronic gastritis. Gastroenterology. 1990;99:1315–23. doi: 10.1016/0016-5085(90)91156-z. [DOI] [PubMed] [Google Scholar]
- 23.Lee A, O’Rourke J, De Ungria MC, Robertson B, Daskalopoulos G, Dixon MF. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology. 1997;112:1386–97. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 24.Zhang HM, Wakisaka N, Maeda O, Yamamoto T. Vitamin C inhibits the growth of a bacterial risk factor for gastric carcinoma: Helicobacter pylori. Cancer. 1997;80:1897–903. [PubMed] [Google Scholar]
- 25.Wang X, Willen R, Wadstrom T. Astaxanthin-rich algal meal and vitamin C inhibit Helicobacter pylori infection in BALB/cA mice. Antimicrob Agents Chemother. 2000;44:2452–7. doi: 10.1128/aac.44.9.2452-2457.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun YQ, Girgensone I, Leanderson P, Petersson F, Borch K. Effects of antioxidant vitamin supplements on Helicobacter pylori-induced gastritis in Mongolian gerbils. Helicobacter. 2005;10:33–42. doi: 10.1111/j.1523-5378.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- 27.Maeda N, Hagihara H, Nakata Y, Hiller S, Wilder J, Reddick R. Aortic wall damage in mice unable to synthesize ascorbic acid. Proc Natl Acad Sci USA. 2000;97:841–6. doi: 10.1073/pnas.97.2.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bergin IL, Sheppard BJ, Fox JG. Helicobacter pylori infection and high dietary salt independently induce atrophic gastritis and intestinal metaplasia in commercially available outbred Mongolian gerbils. Dig Dis Sci. 2003;48:475–85. doi: 10.1023/a:1022524313355. [DOI] [PubMed] [Google Scholar]
- 29.Nakata Y, Maeda N. Vulnerable atherosclerotic plaque morphology in apolipoprotein E-deficient mice unable to make ascorbic acid. Circulation. 2002;105:1485–90. doi: 10.1161/01.cir.0000012142.69612.25. [DOI] [PubMed] [Google Scholar]
- 30.Lee CW, Rao VP, Rogers AB, Ge Z, Erdman SE, Whary MT, Fox JG. Wild-type and interleukin-10-deficient regulatory T cells reduce effector T-cell-mediated gastroduodenitis in Rag2 −/− mice, but only wild-type regulatory T cells suppress Helicobacter pylori gastritis. Infect Immun. 2007;75:2699–707. doi: 10.1128/IAI.01788-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKay DL, Perrone G, Rasmussen H, Dallal G, Hartman W, Cao G, Prior RL, Roubenoff R, Blumberg JB. The effects of a multivitamin/mineral supplement on micronutrient status, antioxidant capacity and cytokine production in healthy older adults consuming a fortified diet. J Am Coll Nutr. 2000;19:613–21. doi: 10.1080/07315724.2000.10718959. [DOI] [PubMed] [Google Scholar]
- 32.Rogers AB, Taylor NS, Whary MT, Stefanich ED, Wang TC, Fox JG. Helicobacter pylori but not high salt induces gastric intraepithelial neoplasia in B6129 mice. Cancer Res. 2005;65:10709–15. doi: 10.1158/0008-5472.CAN-05-1846. [DOI] [PubMed] [Google Scholar]
- 33.Ihrig M, Whary MT, Dangler CA, Fox JG. Gastric helicobacter infection induces a Th2 phenotype but does not elevate serum cholesterol in mice lacking inducible nitric oxide synthase. Infect Immun. 2005;73:1664–70. doi: 10.1128/IAI.73.3.1664-1670.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maurer KJ, Rogers AB, Ge Z, Wiese AJ, Carey MC, Fox JG. Helicobacter pylori and cholesterol gallstone formation in C57L/J mice: a prospective study. Am J Physiol Gastrointest Liver Physiol. 2006;290:G175–82. doi: 10.1152/ajpgi.00272.2005. [DOI] [PubMed] [Google Scholar]
- 35.Varro A, Voronina S, Dockray GJ. Pathways of processing of the gastrin precursor in rat antral mucosa. J Clin Invest. 1995;95:1642–9. doi: 10.1172/JCI117839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin RM, Brady JL, Lew AM. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J Immunol Methods. 1998;212:187–92. doi: 10.1016/s0022-1759(98)00015-5. [DOI] [PubMed] [Google Scholar]
- 37.Hilsted L, Rehfeld JF, Schwartz TW. Impaired α-carboxyamidation of gastrin in vitamin C-deficient guinea pigs. FEBS Lett. 1986;196:151–4. doi: 10.1016/0014-5793(86)80231-9. [DOI] [PubMed] [Google Scholar]
- 38.Court M, Robinson PA, Dixon MF, Jeremy AH, Crabtree JE. The effect of gender on Helicobacter felis-mediated gastritis, epithelial cell proliferation, and apoptosis in the mouse model. J Pathol. 2003;201:303–11. doi: 10.1002/path.1422. [DOI] [PubMed] [Google Scholar]
- 39.Fox JG, Rogers AB, Ihrig M, Taylor NS, Whary MT, Dockray G, Varro A, Wang TC. Helicobacter pylori-associated gastric cancer in INS-GAS mice is gender specific. Cancer Res. 2003;63:942–50. [PubMed] [Google Scholar]
- 40.Levine M, Wang Y, Padayatty SJ, Morrow J. A new recommended dietary allowance of vitamin C for healthy young women. Proc Natl Acad Sci USA. 2001;98:9842–6. doi: 10.1073/pnas.171318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sobala GM, Crabtree JE, Dixon MF, Schorah CJ, Taylor JD, Rathbone BJ, Heatley RV, Axon AT. Acute Helicobacter pylori infection: clinical features, local and systemic immune response, gastric mucosal histology, and gastric juice ascorbic acid concentrations. Gut. 1991;32:1415–8. doi: 10.1136/gut.32.11.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J, Cantilena LR. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci USA. 1996;93:3704–9. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Drake IM, Mapstone NP, Schorah CJ, White KL, Chalmers DM, Dixon MF, Axon AT. Reactive oxygen species activity and lipid peroxidation in Helicobacter pylori associated gastritis: relation to gastric mucosal ascorbic acid concentrations and effect of H. pylori eradication. Gut. 1998;42:768–71. doi: 10.1136/gut.42.6.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.You WC, Brown LM, Zhang L, Li JY, Jin ML, Chang YS, Ma JL, Pan KF, Liu WD, Hu Y, Crystal-Mansour S, Pee D, et al. Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. J Natl Cancer Inst. 2006;98:974–83. doi: 10.1093/jnci/djj264. [DOI] [PubMed] [Google Scholar]
- 45.Plummer M, Vivas J, Lopez G, Bravo JC, Peraza S, Carillo E, Cano E, Castro D, Andrade O, Sanchez V, Garcia R, Buiatti E, et al. Chemoprevention of precancerous gastric lesions with antioxidant vitamin supplementation: a randomized trial in a high-risk population. J Natl Cancer Inst. 2007;99:137–46. doi: 10.1093/jnci/djk017. [DOI] [PubMed] [Google Scholar]
- 46.Taylor PR. Prevention of gastric cancer: a miss. J Natl Cancer Inst. 2007;99:101–3. doi: 10.1093/jnci/djk026. [DOI] [PubMed] [Google Scholar]
- 47.Sjunnesson H, Sturegard E, Willen R, Wadstrom T. High intake of selenium, β-carotene, and vitamins A, C, and E reduces growth of Helicobacter pylori in the guinea pig. Comp Med. 2001;51:418–23. [PubMed] [Google Scholar]
- 48.Mohammadi M, Nedrud J, Redline R, Lycke N, Czinn SJ. Murine CD4 T-cell response to Helicobacter infection: TH1 cells enhance gastritis and TH2 cells reduce bacterial load. Gastroenterology. 1997;113:1848–57. doi: 10.1016/s0016-5085(97)70004-0. [DOI] [PubMed] [Google Scholar]
- 49.Fox JG, Wang TC. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117:60–9. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.D’Elios MM, Manghetti M, De Carli M, Costa F, Baldari CT, Burroni D, Telford JL, Romagnani S, Del Prete G. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J Immunol. 1997;158:962–7. [PubMed] [Google Scholar]
- 51.Li W, Maeda N, Beck MA. Vitamin C deficiency increases the lung pathology of influenza virus-infected gulo−/− mice. J Nutr. 2006;136:2611–16. doi: 10.1093/jn/136.10.2611. [DOI] [PubMed] [Google Scholar]
- 52.Gaut JP, Belaaouaj A, Byun J, Roberts LJ, IInd Maeda N, Frei B, Heinecke JW. Vitamin C fails to protect amino acids and lipids from oxidation during acute inflammation. Free Radic Biol Med. 2006;40:1494–501. doi: 10.1016/j.freeradbiomed.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 53.Hemila H, Kaprio J, Pietinen P, Albanes D, Heinonen OP. Vitamin C and other compounds in vitamin C rich food in relation to risk of tuberculosis in male smokers. Am J Epidemiol. 1999;150:632–41. doi: 10.1093/oxfordjournals.aje.a010062. [DOI] [PubMed] [Google Scholar]
- 54.Fox JG, Beck P, Dangler CA, Whary MT, Wang TC, Shi HN, Nagler-Anderson C. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat Med. 2000;6:536–42. doi: 10.1038/75015. [DOI] [PubMed] [Google Scholar]
- 55.Holcombe C. Helicobacter pylori: the African enigma. Gut. 1992;33:429–31. doi: 10.1136/gut.33.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bates CJ, Prentice AM, Paul AA. Seasonal variations in vitamins A, C, riboflavin and folate intakes and status of pregnant and lactating women in a rural Gambian community: some possible implications. Eur J Clin Nutr. 1994;48:660–8. [PubMed] [Google Scholar]
- 57.Schwager J, Schulze J. Modulation of interleukin production by ascorbic acid. Vet Immunol Immunopathol. 1998;64:45–57. doi: 10.1016/s0165-2427(98)00120-2. [DOI] [PubMed] [Google Scholar]
- 58.Fox JG, Li X, Cahill RJ, Andrutis K, Rustgi AK, Odze R, Wang TC. Hypertrophic gastropathy in Helicobacter felis-infected wild-type C57BL/6 mice and p53 hemizygous transgenic mice. Gastroenterology. 1996;110:155–66. doi: 10.1053/gast.1996.v110.pm8536852. [DOI] [PubMed] [Google Scholar]
- 59.Wang TC, Goldenring JR, Dangler C, Ito S, Mueller A, Jeon WK, Koh TJ, Fox JG. Mice lacking secretory phospholipase A2 show altered apoptosis and differentiation with Helicobacter felis infection. Gastroenterology. 1998;114:675–89. doi: 10.1016/s0016-5085(98)70581-5. [DOI] [PubMed] [Google Scholar]
- 60.Fox JG, Wang TC, Rogers AB, Poutahidis T, Ge Z, Taylor N, Dangler CA, Israel DA, Krishna U, Gaus K, Peek RM., Jr Host and microbial constituents influence Helicobacter pylori-induced cancer in a murine model of hypergastrinemia. Gastroenterology. 2003;124:1879–90. doi: 10.1016/s0016-5085(03)00406-2. [DOI] [PubMed] [Google Scholar]
- 61.Schmidt PH, Lee JR, Joshi V, Playford RJ, Poulsom R, Wright NA, Goldenring JR. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest. 1999;79:639–46. [PubMed] [Google Scholar]
- 62.Fox JG, Rogers AB, Whary MT, Ge Z, Ohtani M, Kurt Jones E, Wang TC. Accelerated progression of gastritis to dysplasia in the pyloric antrum of TFF2 −/− C57BL x Sv129 H. pylori infected mice. Am J Pathol. doi: 10.2353/ajpath.2007.070249. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kurt-Jones EA, Cao L, Sandor F, Rogers AB, Whary MT, Nambiar PR, Cerny A, Bowen G, Yan J, Takaishi S, Chi AL, Reed G, et al. Trefoil family factor 2 is expressed in murine gastric and immune cells and controls both gastrointestinal inflammation and systemic immune responses. Infect Immun. 2007;75:471–80. doi: 10.1128/IAI.02039-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kang W, Rathinavelu S, Samuelson LC, Merchant JL. Interferon gamma induction of gastric mucous neck cell hypertrophy. Lab Invest. 2005;85:702–15. doi: 10.1038/labinvest.3700260. [DOI] [PubMed] [Google Scholar]