For several decades methylphenidate (MPH, Ritalin) has been the primary treatment for children with attention-deficit disorder (ADD), a disorder affecting 3–5% of U.S. children. The therapeutic effects of MPH are mediated through its action at the dopamine transporter (DAT, Cheon et al., 2003; Krause et al., 2000; Vles et al., 2003). Currently, positron emission tomography (PET) is being used to study the DAT effects of MPH and other psychomotor stimulants that can be related to their behavioral or therapeutic effects (Volkow et al., 1999; Volkow et al., 1998; Wilcox et al., 2002). For instance, Volkow et al. (1998) reported that therapeutic doses of oral MPH produced at least 50% DAT occupancy in adults without ADD.
Macaques are used in common preclinical models of the effects of psychomotor stimulants on the central nervous system (CNS); however, few studies have investigated the bioavailability and CNS penetration of oral MPH in macaques. One study in adult rhesus monkeys found that blood plasma concentration reached 16 ng/ml after oral administration of 3.0 mg/kg MPH (Doerge et al., 2000). In adult humans with ADD therapeutic doses of oral MPH range from 0.15 to 0.3 mg/kg and produce blood plasma levels of 3.5–7.8 ng/ml (Volkow et al., 1998; Wargin et al., 1983). In children with ADD, MPH is prescribed in the range of 10–60 mg/day (0.4–2.4 mg/kg/day, (Patrick and Markowitz, 1997), and a dose of 20mg in children (0.8 mg/kg) results in a blood plasma concentration of 20 ng/ml two hours after dosing (Swanson and Volkow, 2003). These studies suggest that oral MPH is less bioavailable in macaques than in humans. Preadolescent macaques are being increasingly used in preclinical studies of drugs of abuse, including stimulants used to treat ADD; therefore, the present study evaluated the relationship between DAT occupancy in the striatum, and blood plasma concentration for several doses of oral MPH in preadolescent macaques.
Two preadolescent male rhesus monkeys (2–2.5 yrs old) served as the subjects for the present study. Anesthesia was maintained with saffan in all studies with one subject (04-207; derived from (Guilarte et al., 2006). However, in the middle of the study saffan became unavailable and some scans for one monkey (04-185) were performed using propofol (adapted from: (Hartvig et al., 1997). Monkey 04-185 received an additional baseline PET scan when the anesthesia was changed to propofol. There were no apparent differences in binding potential due to the change in anesthesia. Monkeys received the MPH orally after induction of anesthesia with MPH dissolved in 5 ml/kg water 2 hrs prior to the scans.
Dynamic PET scans were performed on a high resolution research tomography (HRRT) PET scanner (Horti et al., 2006). A bolus of [11C]MPH [injected dose 6.2 mCi (0.1 sem); specific activity 6115 mCi/hmol (481 sem)] was injected i.v. and dynamic images of 30 frames were reconstructed for 90 min data acquisition in each study. Tracer binding potential (BP) was estimated by fitting a simplified reference tissue model to the measured striatum time activity curve (Lammertsma and Hume, 1996; Zhou et al., 2007), where cerebellum was used a reference tissue devoid of specific binding. The occupancy of MPH was calculated as percent changes of BP from baseline as 100(BP(baseline)-BP(MPH))/BP(baseline). Blood was drawn every 30 minutes during the 90 minute scan to determine MPH plasma levels.
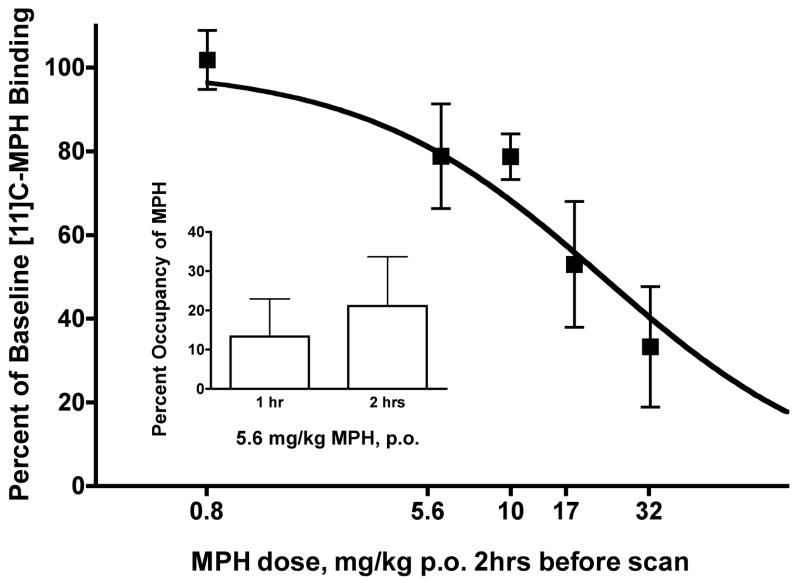
Figure 1 shows percent DAT occupancy for various doses of MPH (0.8–32 mg/kg) in each monkey. The dose of MPH was increased until DAT occupancy was at least 50%, which required the administration of 17 mg/kg (04-185) or 32 mg/kg (04-207). The EC50 for DAT occupancy was 21.5 mg/kg with a 95% confidence of 13.00 to 35.64. Average peak plasma levels ranged from 3.0 ng/ml (SEM 0.15) for 5.6 mg/kg to 61 ng/ml (SEM 19) for 32 mg/kg (Table 1). Each monkey also received 5.6 mg/kg MPH 1 hour prior to a scan, and those data suggest that DAT occupancy was similar at 1 and 2 hrs (Figure 1, inset). There was a positive relationship between plasma concentration and DAT occupancy for MPH, consistent with a previous report (Volkow et al., 1998).
Figure 1.
Reductions in DAT binding following oral MPH administration. Main panel: Y axis represents percent of baseline [11C]-MPH binding. X axis represents oral dose of MPH (mg/kg) administered 2 hour prior to PET scan. Inset: X axis represents oral dose of 5.6 mg/kg MPH administered 1 or 2 hours prior to PET scan.
Table 1.
Peak plasma concentration for oral MPH (0.80–32 mg/kg) in two male juvenile rhesus monkeys.
| Dose (mg/kg) | Peak Plasma Concentration (ng/ml) | |
|---|---|---|
| 04-207 | 04-185 | |
| 0.8 | - | - |
| 5.6 | 2.9 | 3.2 |
| 10.0 | 5.7 | 11.0 |
| 17.0 | 9.1 | 25.0 |
| 32.0 | 42.0 | 80.0 |
Oral MPH was much less potent to occupy the DAT in juvenile macaques than human adults. The EC50 for DAT occupancy was 21.5 mg/kg in macaques (present study), compared to 0.25 mg/kg in humans (Volkow et al., 1998). Blood plasma concentrations support the finding that oral MPH is less bioavailable in preadolescent macaques. A dose of 0.8 mg/kg in children results in a blood plasma concentration of 20 ng/ml two hours after dosing (Swanson and Volkow, 2003). Blood plasma concentrations in the juvenile monkeys did not reach 20 ng/ml until 17 or 32 mg/kg MPH were administered. Interestingly, MPH was also less bioavailable in juvenile than adult macaques with juveniles requiring doses approximately 5 to 10-fold higher than required for therapeutic blood levels in adult rhesus macaques (Doerge et al., 2000; Volkow et al., 1998; Wargin et al., 1983). In the present study, blood concentrations are not likely to be affected by oral administration after anesthesia as an additional 8 juvenile macaques have demonstrated similar blood levels after drinking MPH in a Tang solution (data not shown).
In summary, oral MPH is less bioavailable in juvenile macaques than humans; however, once sufficient MPH enters the blood, occupancy of striatal DATs occurs at similar blood levels between juvenile macaques, and humans. Therefore, once the dose is titrated to the appropriate blood levels, the juvenile macaque is a good model for investigating the CNS effects of oral MPH administration.
Acknowledgments
We would like to acknowledge the expert technical assistance of Debbie Van Kempen, Stacey Perry, Lani Swarthout, and Virginia Bogdan. We would like to acknowledge Drs. Nora D. Volkow, Nancy A. Ator, and Mark A. Riddle for helpful discussion during the planning of the experiments.
Supported by NIH grants MH075378, DA00412, MH78175, AA12839
References
- Cheon KA, Ryu YH, Kim YK, Namkoong K, Kim CH, Lee JD. Dopamine transporter density in the basal ganglia assessed with [123I]IPT SPET in children with attention deficit hyperactivity disorder. Eur J Nucl Med Mol Imaging. 2003;30(2):306–311. doi: 10.1007/s00259-002-1047-3. [DOI] [PubMed] [Google Scholar]
- Doerge DR, Fogle CM, Paule MG, McCullagh M, Bajic S. Analysis of methylphenidate and its metabolite ritalinic acid in monkey plasma by liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom. 2000;14(8):619–623. doi: 10.1002/(SICI)1097-0231(20000430)14:8<619::AID-RCM916>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Chen MK, McGlothan JL, Verina T, Wong DF, Zhou Y, Alexander M, Rohde CA, Syversen T, Decamp E, Koser AJ, Fritz S, Gonczi H, Anderson DW, Schneider JS. Nigrostriatal dopamine system dysfunction and subtle motor deficits in manganese-exposed non-human primates. Exp Neurol. 2006;202(2):381–390. doi: 10.1016/j.expneurol.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Hartvig P, Torstenson R, Tedroff J, Watanabe Y, Fasth KJ, Bjurling P, Langstrom B. Amphetamine effects on dopamine release and synthesis rate studied in the Rhesus monkey brain by positron emission tomography. J Neural Transm. 1997;104(4–5):329–339. doi: 10.1007/BF01277655. [DOI] [PubMed] [Google Scholar]
- Horti AG, Fan H, Kuwabara H, Hilton J, Ravert HT, Holt DP, Alexander M, Kumar A, Rahmim A, Scheffel U, Wong DF, Dannals RF. 11C-JHU75528: a radiotracer for PET imaging of CB1 cannabinoid receptors. J Nucl Med. 2006;47(10):1689–1696. [PubMed] [Google Scholar]
- Krause KH, Dresel SH, Krause J, Kung HF, Tatsch K. Increased striatal dopamine transporter in adult patients with attention deficit hyperactivity disorder: effects of methylphenidate as measured by single photon emission computed tomography. Neurosci Lett. 2000;285(2):107–110. doi: 10.1016/s0304-3940(00)01040-5. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4(3 Pt 1):153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Patrick KS, Markowitz JS. Pharmacology of Methlyphenidate, Amphetamine Enantiomers and Pemoline in Attention-Deficit Hyperactivity Disorder. Human Psychopharmacology. 1997;12:527–546. [Google Scholar]
- Swanson JM, Volkow ND. Serum and brain concentrations of methylphenidate: implications for use and abuse. Neurosci Biobehav Rev. 2003;27(7):615–621. doi: 10.1016/j.neubiorev.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Vles JS, Feron FJ, Hendriksen JG, Jolles J, van Kroonenburgh MJ, Weber WE. Methylphenidate down-regulates the dopamine receptor and transporter system in children with attention deficit hyperkinetic disorder (ADHD) Neuropediatrics. 2003;34(2):77–80. doi: 10.1055/s-2003-39602. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Gatley SJ, Dewey SL, Wang GJ, Logan J, Ding YS, Franceschi D, Gifford A, Morgan A, Pappas N, King P. Comparable changes in synaptic dopamine induced by methylphenidate and by cocaine in the baboon brain. Synapse. 1999;31(1):59–66. doi: 10.1002/(SICI)1098-2396(199901)31:1<59::AID-SYN8>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, Hitzemann R, Pappas N. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry. 1998;155(10):1325–1331. doi: 10.1176/ajp.155.10.1325. [DOI] [PubMed] [Google Scholar]
- Wargin W, Patrick K, Kilts C, Gualtieri CT, Ellington K, Mueller RA, Kraemer G, Breese GR. Pharmacokinetics of methylphenidate in man, rat and monkey. J Pharmacol Exp Ther. 1983;226(2):382–386. [PubMed] [Google Scholar]
- Wilcox KM, Lindsey KP, Votaw JR, Goodman MM, Martarello L, Carroll FI, Howell LL. Self-administration of cocaine and the cocaine analog RTI-113: relationship to dopamine transporter occupancy determined by PET neuroimaging in rhesus monkeys. Synapse. 2002;43(1):78–85. doi: 10.1002/syn.10018. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Weed MR, Chen M-K, Rahmim A, Ye W, Brašik JR, Alexander M, Crabb AH, McGlothan JL, Ali F, Guilarte TR, Wong DF. Quantitative dopamine transporter imaging studies in nonhuman primates with a GE Advance and high resolution research tomography (HRRT) PET scanners. J Nucl Med. 2007;48(Suppl 2):158P. [Google Scholar]