Abstract
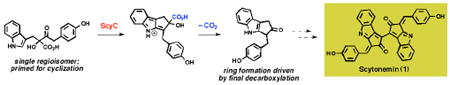
Previous studies of the biosynthetic enzymes involved in the assembly of scytonemin (1), a cyanobacterial sunscreen, have identified β-ketoacid 2 as an important intermediate that is produced by ThDP-dependent enzyme ScyA. We now report that ScyC, previously annotated as a hypothetical protein, catalyzes cyclization and decarboxylation of 2 to generate ketone 5. Assembly of the cyclopentyl[b]indole structure in this manner has little precedent in the chemical literature. Additional mechanistic experiments have revealed that cyclization likely precedes decarboxylation and that the latter event may provide a driving force for cyclopentane formation.
The dimeric alkaloid scytonemin (1) is synthesized by numerous strains of cyanobacteria.1 It is one of the few bacterial secondary metabolites to have a confirmed role as a sunscreen; expression of the scytonemin biosynthetic gene cluster is triggered by exposure to UV-A, resulting in extracellular pigment accumulation and blockage of further incident radiation.1,2 In addition to this important function, scytonemin also possesses kinase inhibitory activity3 and an unusual chemical structure.4
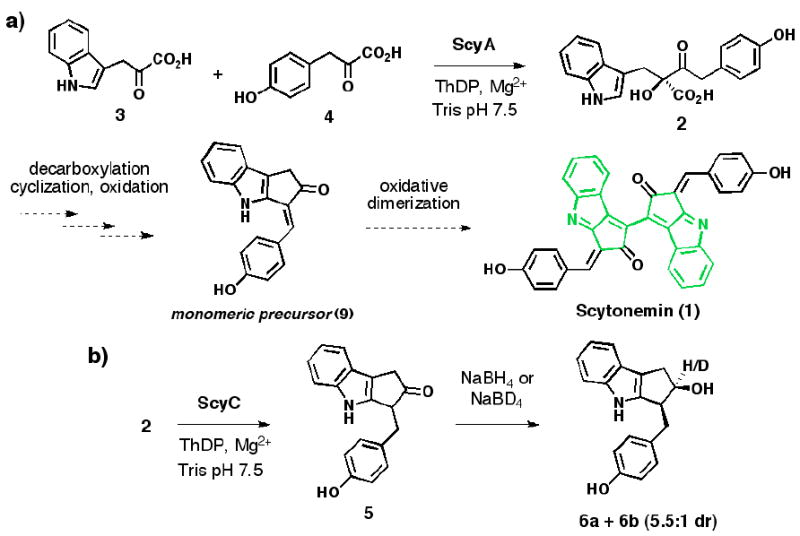
We have recently proposed a possible biosynthetic route to scytonemin (Scheme 1a) and performed in vitro characterization of two enzymes encoded in the biosynthetic gene cluster,5 ScyA (NpR1276) and ScyB (NpR1275).6 ScyB, a leucine dehydrogenase homolog, converts L-tryptophan to indole-3 pyruvic acid (3). ScyA is a thiamin (ThDP)-dependent enzyme responsible for the regioselective acyloin coupling of 3 and p-hydroxyphenylpyruvic acid (4) to afford a single, labile β-ketoacid product (2). Our previous work revealed the biosynthetic logic involved in assembling the linear carbon backbone of the scytonemin skeleton.7 However, many important questions concerning pigment assembly remained unaddressed, including the enzyme(s) responsible for cyclopentane formation and the mechanism of this unusual transformation. In this report we disclose the discovery that ScyC (NpR1274), an enzyme encoded by the scytonemin cluster, catalyzes cyclization and decarboxylation of ScyA product 2 to form ketone 5, thereby constructing the indole-fused cyclopentane moiety of the natural product.
Scheme 1.
The cyclopentyl[b]indole ring system is found in several other secondary metabolites, including the bruceollines8 and the indole-diterpene mycotoxins.9 To date no enzymes involved in the assembly of this structure have been characterized in vitro. Consequently, identifying the scytonemin biosynthetic enzymes responsible for cyclization was a significant challenge.
Of the uncharacterized genes in the scytonemin cluster, scyC (NpR1274), encoding a hypothetical protein, was deemed the best initial candidate as its lack of an N-terminal signal peptide sequence might simplify overexpression and could indicate involvement early in the scytonemin pathway, before pigment export from the cyanobacterial cells. ScyC was amplified from N. punctiforme ATCC 29133 genomic DNA, subcloned into pET-28b and -29b vectors, and overexpressed in E. coli as N- and C-His6-tagged fusions. Preliminary screens for enzymatic activity were performed using unpurified cell-free extracts as cofactor requirements were uncertain. Assays were performed in tandem with ThDP-dependent enzyme ScyA, with ScyC extract added to the reaction mixture after conversion to β-ketoacid 2 from pyruvic acid derivatives 3 and 4 was complete as judged by HPLC analysis. After the introduction of ScyC, appearance of a new peak was accompanied by the disappearance of 2 (see Supporting Information for all HPLC data). The qualitative rate of product formation was greatly reduced with extracts from uninduced cultures. Additionally, extracts generated from E. coli strains lacking a scyC construct did not exhibit any conversion.
Preliminary attempts to isolate and characterize the new product from large-scale enzymatic reactions were complicated by its facile decomposition. Ultimately, a sodium borohydride quench afforded two stable, separable products, diastereomeric cyclopentanols 6a and 6b, in a 5.5:1 ratio (Scheme 1b).10 The formation of the cyclopentyl[b]indole skeleton was confirmed by NMR analysis (1H, 13C, HSQC, HMBC) and the relative stereochemistry assigned from 1D-NOE experiments. These data clearly indicate that 6a and 6b derive from reduction of a ketone precursor (5), the immediate product of the ScyC reaction. The ratio and configuration of the diastereomeric alcohol products are consistent with hydride delivery from the less sterically hindered face of 5. Additionally, quenching experiments with sodium borodeuteride show deuterium incorporation only at the carbon atom bearing the alcohol (see Supporting Information).
We next focused on further characterization of the ScyC enzyme and its mechanism. Activity was retained after large-scale overexpression and purification of both ScyC fusions, indicating minimal additional cofactor requirements; this considerably narrows the potential mechanisms of the ScyC-mediated transformation.11 We initially considered the two possibilities outlined in Figure 2a. In pathway A, initial intramolecular attack by the indole ring onto the ketone at the C2 position could be followed by dehydration to generate an electophilic iminium intermediate that would undergo facile decarboxylation. Tautomerization of the resulting enol would afford ketone 5. Alternatively, an initial decarboxylation step might occur, providing one of two possible α-hydroxyketones (7 or 8). Either intermediate could potentially be processed to 5 via substitution or carbonyl addition mechanisms (Pathways B and C).
To distinguish between these possibilities, we evaluated the ability of ScyC to accept decarboxylated intermediates. Treatment of a mixture of α-hydroxyketones regioisomers (7:8 = 1.4:1)12 with ScyC in the presence of both ThDP and Mg2+ resulted in no product formation (Scheme 2b). The possibility of ScyC inhibition by 7 or 8 was ruled out by doping tandem ScyA/ScyC assay mixtures; no change in the qualitative rate of formation of ketone 5 was observed in the presence of 7 and 8 in comparison to a DMSO control. Based on these experiments, we favor pathway A as a reasonable mechanism for the action of ScyC.
Scheme 2.

One of the most interesting aspects of the ScyC-catalyzed cyclization is the lack of analogous reactions in the chemical literature. Synthetic and medicinal chemists have developed various strategies to access the cyclopentyl[b]indole motif due to its potential biological activity.13 However, none of these methods have employed an intramolecular attack of an unfunctionalized indole onto a pendant ketone; this is perhaps surprising given the widespread use of intramolecular indole cyclizations to form β-carboline derivatives and may reflect the difficulty of such transformations.14 In contrast, intermolecular additions of indoles to ketones have been described.15 We hypothesize that intramolecular reactions may involve reversible indole attack and that a thermodynamic driving force is needed for successful cyclopentane formation. This proposal is supported by reports of cyclizations onto carboxylic acid derivatives, which may be driven by the departure of the carbonyl substituent.9f,g ScyC may overcome the problem of reversibility by coupling ring formation to decarboxylation. This logic is akin to Nature’s use of malonyl thioesters in decarboxylative Claisen condensations to drive iterative C–C bond formations in fatty acid and polyketide chain elongations.16
Overall, we have discovered that enzymes ScyA and ScyC act in tandem to construct the tricyclic cyclopentyl[b]indole framework needed for scytonemin biosythesis. ScyA produces a single β-ketoacid isomer (2), which undergos a facile, non-enzymatic decarboxylation. However, in the presence of ScyC, this off-pathway reaction is suppressed in favor of an intramolecular cyclization, and the resultant cyclopentyl intermediate is primed for decarboxylation to trap out the annular cyclopentyl[b]indole as an irreversible event. At the time of our initial experiments we could not readily explain the preferential formation of a single isomer by ScyA; the discovery of the ScyC provides that missing insight, as only the observed β-ketoacid regioisomer (2) could have a ketone appropriately positioned for cyclization. The ScyC product 5 is only one oxidation state away from a potential dimerization substrate (9) that could be progressed to the complete scytonemin skeleton.
We have commented above that direct cyclizations to form cyclopentyl[b]indole scaffolds have been elusive, indicating that additional study of the structure and mechanism of ScyC is warranted. Efforts toward further characterization of this enzyme, as well as investigation of the remaining steps in scytonemin biosynthesis, are currently underway.
Supplementary Material
Acknowledgments
This work is supported by the NIH (GM20011). E.P.B. is the recipient of an NIH postdoctoral fellowship.
Footnotes
Supporting Information Available: Experimental details and characterization data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Garcia-Pichel F, Castenholz RW. J Phycol. 1991;27:395–409. [Google Scholar]
- 2.a) Soule T, Garcia-Pichel F, Stout V. J Bacteriol. 2009;191:4639–4646. doi: 10.1128/JB.00134-09. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Sorrels CM, Proteau PJ, Gerwick WH. Appl Environ Microbiol. 2009;75:4861–4869. doi: 10.1128/AEM.02508-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Stevenson CS, Capper EA, Roshak AK, Marquez B, Grace K, Gerwick WH, Marshall LA. Inflamm Res. 2002;51:112–114. doi: 10.1007/BF02684014. [DOI] [PubMed] [Google Scholar]; (b) Stevenson CS, Capper EA, Roshak AK, Marquez B, Eichman C, Jackson JR, Mattern M, Gerwick WH, Jacobs RS, Marshall LA. J Pharmacol Exp Ther. 2002;303:858–866. doi: 10.1124/jpet.102.036350. [DOI] [PubMed] [Google Scholar]
- 4.Proteau PJ, Gerwick WH, Garcia-Pichel F, Castenholz R. Experientia. 1993;49:825–829. doi: 10.1007/BF01923559. [DOI] [PubMed] [Google Scholar]
- 5.Soule T, Stout V, Swingley WD, Meeks JC, Garcia-Pichel F. J Bacteriol. 2007;189:4465–4472. doi: 10.1128/JB.01816-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balskus EP, Walsh CT. J Am Chem Soc. 2008;130:15260–15261. doi: 10.1021/ja807192u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.We note that the order of steps in the scytonemin biosynthetic pathway has not been clearly delineated in vivo.
- 8.Ouyang Y, Koike K, Ohmoto T. Phytochemistry. 1994;37:575–578. doi: 10.1016/s0031-9422(00)89758-7. [DOI] [PubMed] [Google Scholar]
- 9.Saikia S, Nicholson MJ, Young C, Parker EJ, Scott B. Mycological Res. 2008;112:184–199. doi: 10.1016/j.mycres.2007.06.015. and references therein.
- 10.The absolute stereochemistry and enantiomeric ratios of 6a and b have not yet been assigned.
- 11.Assays were not affected by the addition of stoichiometric amounts of EDTA relative to Mg2+ prior to the introduction of ScyC, indicating that a diffusible metal cofactor is not required (see Supporting Information for details).
- 12.α-Hydroxyketones 7 and 8 were isolated from a large-scale ScyA reaction as described previously in reference 6.
- 13.For synthetic routes to cyclopentyl[b]indoles, see: Wender PA, White AW. Tetrahedron. 1983;39:3767–3776.Hillier MC, Marcoux J-F, Zhao D, Grabowski EJJ, McKeown AE, Tillyer RD. J Org Chem. 2005;70:8385–8394. doi: 10.1021/jo051146p.Franceschetti L, Garzon-Aburbeh A, Mahmoud MR, Natalini B, Pellicciari R. Tetrahedron Lett. 1993;34:3185–3188.Salim M, Capretta A. Tetrahedron. 2000;56:8063–8069.Cuevas-Yañez E, Muchowski JM, Cruz-Almanza R. Tetrahedron. 2004;60:1505–1511.Cui D-M, Zhang C, Kawamura M, Shimada S. Tetrahedron Lett. 2004;45:1741–1745.Katritzky AR, Jiang R, Suzuki K. J Org Chem. 2005;70:4993–5000. doi: 10.1021/jo050226q.
- 14.Cox ED, Cook JM. Chem Rev. 1995;95:1797–1842. [Google Scholar]
- 15.a) Ryang H-S, Sakurai H. J Chem Soc Chem Commun. 1972:77. [Google Scholar]; b) Garbe TR, Kobayashi M, Shimizu N, Takesue N, Ozawa M, Yukawa H. J Nat Prod. 2000;63:596–598. doi: 10.1021/np990517s. [DOI] [PubMed] [Google Scholar]
- 16.For reviews, see: Hill AM. Nat Prod Rep. 2006;23:256–320. doi: 10.1039/b301028g.Staunton J, Weissman KJ. Nat Prod Rep. 2001;18:380–416. doi: 10.1039/a909079g.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


