Abstract
We assessed age-specific CD4 T-cell counts and their determinants among Tanzanian children born to HIV-infected mothers to address a major research gap. A total of 474 HIV-uninfected and 69 HIV-infected children were followed until age of 12 months. Maternal predictors were measured during pregnancy and child predictors at birth and throughout the follow up. Child CD4 T-cell counts were evaluated at the age of 3 months and subsequent 3-month intervals; they decreased linearly among HIV-infected (β = –8 cells per week; 95% CI –12 to –4; P = 0.0003) and increased linearly among HIV-uninfected children (β = 4 cells/week; 95% CI 2–7; P = 0.0008). Decreased child counts were predicted by low child anthropometry, maternal HIV stage ≥2, and maternal mid-upper arm circumference <27 cm among HIV-infected children; and by weight-for-height <–2 z-score, maternal HIV stage ≥2, maternal erythrocyte sedimentation rate <81 mm/h and maternal haemoglobin <8.5 g/dl among HIV-uninfected children. The maternal and child predictors described may serve as intervention targets among HIV-exposed children.
Introduction
About 2 million children currently live with HIV and 370 000 become newly infected every year, largely through mother-to-child transmission (MTCT) of the virus. [1]. Children infected through the in utero or intrapartum routes often present with clinical symptoms in the first year of life, and about one-third die during infancy [2, 3]. As a result, all children under 12 months of age with confirmed HIV infection are recommended to receive highly active antiretroviral therapy (HAART), regardless of clinical or immunological stage [4].
There are substantial barriers to scaling up access to pediatric antiretroviral treatment and as a result, <5% of children have access to HAART [5, 6]. While those barriers are broken down, the prevention of MTCT of HIV should receive continued attention. Yet low access to pediatric HAART and sometimes prevention of MTCT of HIV also mean that other interventions are needed to improve the health of children born to HIV-infected mothers. Recent studies indicate that maternal HIV disease stage may predict mortality and morbidity risks among children born to HIV-infected mothers, even if children remain HIV-uninfected [3, 7, 8]. Furthermore, child undernutrition has been identified as a strong risk factor for mortality among HIV-exposed children [7].
However, the mechanisms underlying these associations are largely unknown. It is possible that pediatric immune system responses may play a role, such as measured by child CD4 T-cell counts. CD4 T cells activate and direct other immune cells [9–11]. Among HIV-infected children, the CD4 T-cell depletion rate is predictive of mortality and poor clinical outcome [12]. Among HIV-uninfected children, CD4 T-cell counts are of relevance as low counts may increase the severity of common childhood infections [13].
There is some evidence that CD4 T-cell counts are lower among children from sub-Saharan Africa compared to children from industrialized countries [14–20]. However, there is a scarcity of longitudinal datasets containing CD4 T-cell counts from sub-Saharan Africa, the region with the highest global prevalence of HIV infection, to allow for comparison with datasets from industrialized countries.
To address these outstanding research questions, we used data from a Tanzanian cohort of HIV-infected women and their children to identify levels of pediatric CD4 T-cell counts as well as their maternal and pediatric predictors.
Methods
Between 1995 and 1997, 1078 HIV-infected pregnant women from Dar es Salaam, Tanzania were enrolled into a trial to examine the effect of maternal vitamin supplementation on maternal and child health outcomes. Details of the trial have been published elsewhere [21, 22]. In brief, eligible participants were pregnant between 12 and 27 weeks of gestation and intended to stay in the city through delivery and ≥1 year thereafter. At baseline, participants were randomly assigned to receive, from enrollment and throughout the pregnancy and lactation periods, a daily oral dose of (i) vitamin A + β-carotene (30 mg of β-carotene + 5000 IU of preformed vitamin A); (ii) vitamins B, C and E (20 mg of vitamin B1, 20 mg of vitamin B2, 25 mg of vitamin B6, 100 mg of niacin, 50 μg of vitamin B12, 500 mg of vitamin C, 30 mg of vitamin E and 0.8 mg of folic acid); (iii) vitamins B, C and E + vitamin A + β-carotene; or (iv) placebo. Women and children, regardless of experimental group, received standard prenatal and child care services. All women received ferrous sulphate and folate daily, and prophylactic chloroquine phosphate weekly during the antenatal period. Children received 100 000 IU of vitamin A at 6 months of age and twice that amount every 6 months thereafter. At the time of the study, antiretroviral therapy was not available to the majority of women in Tanzania, including those who participated in the study.
Enrollment and follow-up
During the enrollment visit, trained research nurses obtained a blood sample from participants and collected socio-demographic and anthropometric data. Participants were followed up through monthly research visits during pregnancy. Immediately after delivery, a research midwife measured the weight of the baby. The duration of pregnancy was calculated based on the difference between the date of last menstrual period and the date of delivery. Postnatal follow-up of mothers and children occurred from the 6-week postnatal visit through monthly visits. If a woman missed a scheduled visit with her child, a home visit was conducted to determine their vital status. For the present analysis, child follow-up was restricted to the duration from age 6 weeks to 12 months.
For the diagnosis of child HIV infection, a whole-blood sample was obtained at birth, 6 weeks postpartum and at 3-month intervals thereafter. Child CD4 T-cell counts were measured 3 months postpartum and subsequently in 3-month intervals. Blood samples obtained at 6 weeks and 6 months postpartum were used to obtain plasma levels of selenium, albumin, ferritin, vitamin A, vitamin B12 and vitamin E (adjusted for cholesterol).
The study was approved by the institutional review boards of the Harvard School of Public Health and the Muhimbili University College of Health Sciences, as well as the Ethical Committee of the National AIDS Control Program of the Tanzanian Ministry of Health and Social Welfare.
Laboratory analyses
Absolute T lymphocyte counts of CD4 and CD8 T-cell counts among adults and children were measured using the FACSCount system (Becton-Dickinson, San Jose, CA). When child CD4 T-cell counts were ≥2000 cells/µl, the FACSCan system (Becton-Dickinson, San Jose, CA) was used. A child was considered to be HIV-infected if a peripheral blood mononuclear cell specimen tested positive in a HIV DNA polymerase chain reaction (PCR) or in an Amplicor HIV DNA assay (Roche Diagnostics, Indianapolis, IN) at any point in time.
Statistical analyses
Child CD4 T-cell counts were stratified by child HIV status at age 6 weeks. Children who were HIV-uninfected at 6 weeks but who were subsequently infected were censored at the date of HIV diagnosis. The Wilcoxon rank-sum test was used to test for differences by HIV status and grouped follow-up time.
General linear models (PROC MIXED; SAS Institute, Cary, NC) with an empirical variance estimator were used to model mean CD4 T-cell counts and 95% confidence intervals (CI) [23]. In these models, restricted cubic splines were used to allow for flexible non-linear shapes. To assess the shape of CD4 T-cell curves over time, we chose a model with four knots and employed an automatic knot selection procedure using p < 0.10 for time variables to enter and remain in final statistical model. CD4 T-cell counts for both HIV-infected and HIV-uninfected children followed a linear trend over time. Therefore, spline variables were eliminated from statistical models.
Continuous predictors among mothers and children were dichotomized (Table 1). Standards developed by the United States Centers for Disease Control and Prevention were used to calculate the indexes weight-for-height, height-for-age and weight-for-age and to calculate z-scores [24]. Predictors with p < 0.2 in univariate models were entered into multivariate models and predictors with p < 0.05 were retained in final multivariate models. We created interaction terms between predictors and the term for linear time to assess whether effects varied over time. All univariate and multivariate models were adjusted for the trial regimen groups.
Table 1.
Distribution of child predictors (evaluated at child age 6 weeks) and maternal predictors (evaluated at 12–27 weeks’ gestation)
| Risk factor | N | n (%) |
|---|---|---|
| Child characteristics | ||
| Presence of HIV infection | 543 | 69 (14.3) |
| Male gender | 543 | 278 (50.1) |
| Birth weight <2500 g | 501 | 41 (8.2) |
| Gestational age <37 weeks at birth | 543 | 115 (21.2) |
| Weight-for-height z-score <–2 | 511 | 8 (1.6) |
| Height-for-age z-score <–2 | 529 | 37 (7.0) |
| Weight-for-age z-score <–2 | 532 | 27 (5.1) |
| Breastfeeding | 539 | 522 (96.8) |
| Plasma vitamin A <10 µg/dl | 438 | 82 (18.7) |
| Plasma vitamin E < 5 µmol/dl | 438 | 76 (17.4) |
| Plasma selenium <57.3 µg/l | 430 | 100 (23.3) |
| Plasma vitamin B12 <200 pg/ml | 392 | 26 (6.6) |
| Plasma albumin <3.5 g/dl | 439 | 110 (25.1) |
| Plasma ferritin <200 µg/l | 410 | 183 (44.6) |
| Maternal characteristics | ||
| Age >25 years | 543 | 278 (51.2) |
| Money spent on food per day <500 TSha | 487 | 197 (40.5) |
| ≤ 4 years of primary schooling | 543 | 67 (12.3) |
| Mid-upper arm circumference <27 cm | 537 | 352 (65.6) |
| CD4 T-cell count <350 cells/µl | 504 | 172 (34.1) |
| CD8 T-cell count <565 cells/µl | 508 | 178 (35.0) |
| Erythrocyte sedimentation rate <81 mm/h | 489 | 376 (76.9) |
| Total lymphocyte count <1340/µl | 538 | 146 (27.1) |
| WHO HIV disease stage ≥ 2 | 543 | 71 (13.1) |
| Viral Load > 50 000 copies/ml | 230 | 90 (39.1) |
| Haemoglobin <11 g/dl | 537 | 398 (74.1) |
| Plasma vitamin A <20 µg/dl | 374 | 122 (32.6) |
| Plasma vitamin E <9.7 µmol/dl | 374 | 173 (46.3) |
| Plasma selenium <104 µg/l | 475 | 68 (14.3) |
aTSh: Tanzanian Shillings. At the time of the study, 1 US$ equalled ∼600 TSh. Reflects money spent per household member.
Results
Of the 1078 women enrolled in the multivitamin supplementation trial, there were 1017 singleton pregnancies with known pregnancy outcomes that resulted in 939 liveborn infants. Of those, 543 were alive at 6 weeks with known HIV status and with data on CD4 T-cell counts in the first 12 months of life. A total of 69 (12.7%) children were HIV-infected at 6 weeks and had on average 2.1 (SD 1.0) CD4 T-cell count samples available. The 474 (87.3%) children negative at 6 weeks had on average 2.3 (SD 1.0) samples available. Among the initially HIV-negative children, 15 (3.2%) were infected with HIV at a later stage and thus censored from follow up at the time of HIV diagnosis.
Mothers entered the study at a mean gestational age of 20.3 weeks (SD 3.3). A total of 172 (34.1%) mothers had a CD4 T-cell count < 350 cells/µl, 71 (13.1%) were at WHO HIV disease stage ≥2, and 398 (74.1%) had haemoglobin <11 g/dl (Table 1).
CD4 T-cell counts were significantly lower among HIV-infected children compared to HIV-uninfected children at all time points (p ≤ 0.02) (Table 2).
Table 2.
CD4 T-cell counts (per µl) among children aged 6 weeks to 24 months, stratified by child HIV status
| Child age (weeks) | HIV-infected |
HIV-uninfected |
pa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | Median | Fifth percentile | Ninety-fifth percentile | N | Mean | SD | Median | Fifth percentile | Ninety-fifth percentile | ||
| 14 | 41 | 1355 | 561 | 1350 | 560 | 2370 | 291 | 1635 | 575 | 1597 | 834 | 2610 | 0.002 |
| 26 | 47 | 1345 | 687 | 1203 | 500 | 2540 | 312 | 1801 | 625 | 1736 | 1034 | 3060 | <.0001 |
| 38 | 19 | 1324 | 609 | 1030 | 490 | 2640 | 201 | 1654 | 511 | 1640 | 875 | 2460 | 0.02 |
| 50 | 36 | 1179 | 478 | 1172 | 210 | 1877 | 294 | 1864 | 787 | 1752 | 850 | 3340 | <.0001 |
aP-values calculated with Wilcoxon-rank sum test.
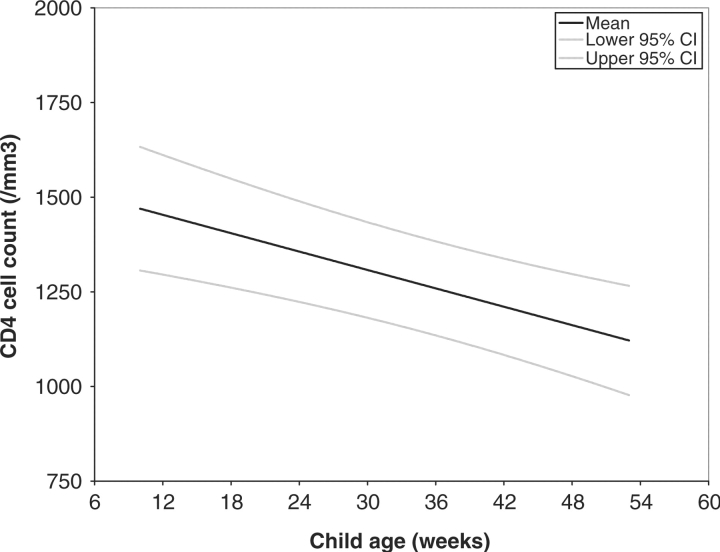
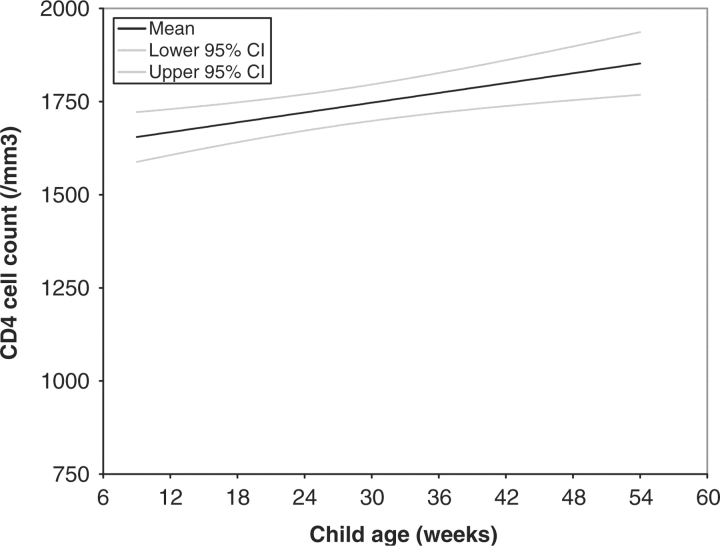
Using general linear models, it was determined that among HIV-infected children, CD4 T-cell counts declined linearly with child age (β = –8 cells/week; 95% CI –12 to –4; P = 0.0003) (Fig. 1), whereas CD4 T-cell counts increased linearly among HIV-uninfected children (β = 4 cells/week; 95% CI 2–7; p = 0.0008) (Fig. 2).
Fig. 1.
Predicted child CD4 T-cell counts among 69 children who were HIV-infected at 6 weeks of age.
Fig. 2.
Predicted CD4 T-cell counts among 474 children who were HIV-uninfected at 6 weeks of age.
In multivariate models among HIV-infected children, those with <–2 z-scores for weight-for-height, height-for-age and weight-for-age had CD4 T-cell counts that were ∼300 cells/µl lower compared with counts of those with z-scores ≥–2 (Table 3). HIV-infected children born to mothers with mid-upper arm circumference (MUAC) <27 cm or WHO HIV disease stage ≥2 had mean CD4 T-cell deficits of similar magnitude.
Table 3.
Multivariate predictors of CD4 T-cell counts in the first 24 months of life, stratified by child HIV statusa
| HIV-positive at 6 weeks |
HIV-negative at 6 weeks |
|||||
|---|---|---|---|---|---|---|
| Risk factor | Mean lower CD4 T-cell count | 95% CI | p | Mean lower CD4 T-cell count | 95% CI | p |
| Child characteristics | ||||||
| Weight-for-height (z-score) | ||||||
| <–2 | 319 | (55–582) | 0.02 | 264 | (62–465) | 0.01 |
| ≥–2 | Reference | Reference | ||||
| Height-for-age (z-score) | ||||||
| <–2 | 268 | (61–475) | 0.01 | NSb | ||
| ≥–2 | Reference | |||||
| Weight-for-age (z-score) | ||||||
| <–2 | 321 | (108–533) | 0.004 | NS | ||
| ≥–2 | Reference | |||||
| Maternal characteristics | ||||||
| Mid-upper arm circumference (cm) | ||||||
| < 27 | 308 | (63–552) | 0.02 | |||
| ≥ 27 | Reference | |||||
| HIV Disease Stage | ||||||
| 1 | Reference | Reference | ||||
| ≥2 | 280 | (37–524) | 0.03 | 128 | (13–244) | 0.03 |
| Erythrocyte sedimentation rate (mm/h) | ||||||
| <81 | NS | 135 | (12–259) | 0.03 | ||
| ≥81 | Reference | |||||
| Haemoglobin (g/dl) | ||||||
| <8.5 | NS | 148 | (40–255) | 0.007 | ||
| ≥8.5 | Reference | |||||
aFinal models were run separately for the child anthropometric indexes weight for height, height for age and weight for age. Results presented for variables other than the anthropometric indexes were derived from models containing weight for height as the only child anthropometric index.
bNot significant. If noted, these variables were not included in the final multivariate model.
Among HIV-uninfected children, child weight-for-height z-score <–2 was predictive of lower mean CD4 T-cell counts over follow-up. HIV-uninfected children born to mothers with WHO HIV disease stage ≥2 had a 128 cell/µl (95% CI 13–244) mean decrease in CD4 T-cell counts, and those born to women with haemoglobin <8.5 g/dl or erythrocyte sedimentation rate <81 mm/h had decreases of similar magnitude.
We assessed whether the association between predictors and mean CD4 T-cell counts varied over time. However, there was no evidence for a time-dependent effect among HIV-infected (p ≥ 0.45) or HIV-uninfected children (p ≥ 0.13).
Discussion
In this cohort of HIV-exposed infants, mean CD4 T-cell counts were significantly lower among the HIV-infected as compared with HIV-uninfected group. Among HIV-infected children, gradual decreases in CD4 T-cell counts are well documented [9, 10], but little is known on rates of decline among infants from sub-Saharan Africa who are positive at age 6 weeks. We estimate that these infants experience an average decline of eight cells per microliter per month in the first year. The declines may be due to constant viral activity and ensuing lymphocyte apoptosis and impairments of CD4 T-cell homoeostasis [25].
HIV-uninfected infants experienced a linear CD4 T-cell increase, which may reflect the active nature of the developing immune system in the early months of life [26]. Among HIV-exposed but uninfected Kenyan children [16] and Zambian children who were presumably HIV-unexposed [17], CD4 T-cell counts increased throughout the first year.
Based on the age dependency of CD4 T-cell counts among children, age-specific CD4 T-cell count thresholds have been defined to classify paediatric immunodeficiency [2]. When compared with CD4 T-cell counts, CD4 T-cell percentages vary less with age and are thus preferred among children younger than 5 years of age; however, absolute CD4 T-cell count measurements are known to predict adverse outcomes among children [26–29]. Therefore, in case CD4 T-cell percentages cannot be determined, absolute CD4 T-cell counts may be a viable alternative to identify immunodeficiency even among children aged 5 years or less [2].
In geographical comparisons, HIV-uninfected children from sub-Saharan Africa [14–18] generally have lower CD4 T-cell counts than their counterparts from the United States or Europe [14, 19, 20]. The causes of these regional differences are poorly understood, but children from sub-Saharan Africa may have lower counts due to genetic reasons [19, 26] or due to a high burden of childhood infections [14]. Regional comparisons should take into account the HIV-exposure status among HIV-uninfected children, as the maternal HIV challenge may lower child CD4 T-cell counts even if the child escapes HIV infection [16, 30].
Childhood undernutrition, as described by low anthropometry, predicted lower CD4 T-cell counts among both HIV-infected and HIV-uninfected children. This finding is in line with the association of undernutrition as an underlying cause of child deaths associated with common childhood diseases [31], mediated by the interaction of malnutrition with infection and immunity [32]. In line with this, growth faltering (defined as low weight for age) predicted mortality among HIV-infected Kenyan [33] and Ugandan children [34], while wasting was highly predictive of mortality among HIV-infected Tanzania children [35]. Undernutrition is firmly linked to thymic atrophy, which induces a loss of immature CD4+CD8+ T-cells, decreases thymocyte proliferation, and may therefore provide a mechanistic basis for lower CD4 T-counts among undernourished children in this cohort [36].
Advanced maternal HIV disease stage was related to lower CD4 T-cell counts among both HIV-infected and HIV uninfected children. In a study from Europe, advanced maternal HIV disease stage, as evidenced by low maternal CD4 T-cell counts, was associated with reduced child CD4 T-cell counts [26]. In a study from Zambia, HIV-exposed but uninfected children were more likely to die or be hospitalized if they were born to mothers with low CD4 T-cell counts [8]. In the present study cohort, low maternal CD4 cell counts and high viral load during pregnancy were related to increased mortality risks among both HIV-infected and HIV-uninfected children [7]. It has been hypothesized that children born to mothers with advanced HIV disease may acquire less passive immunity in the form of antibodies through the transplacental and possibly breastfeeding routes [37]. Neonatal exposure to maternal HIV or other maternal pathogens, suboptimal breast milk quality and poor caring practices have also been proposed as mechanisms for adverse outcomes among HIV-uninfected children born to HIV-infected mothers [3, 8]. Our findings indicate that some of the increased mortality and morbidity risks among HIV-exposed children may be mediated by decreases in CD4 cell counts.
Several mechanisms may explain the relation between maternal anaemia and lower CD4 T-cell counts among HIV-infected children. In this study cohort, we documented that a high proportion of maternal anaemia is related to iron deficiency [38]. Low maternal iron status may depress infant iron status and therefore impair child immunity [39]. Maternal anaemia may also act as a marker for HIV disease progression [40, 41], which predicted lower child CD4 T-cell counts in this cohort.
Maternal MUAC was related to CD4 T-cell counts among HIV-infected children. MUAC is a measure of lean and fat body mass and low values are a marker of malnutrition [42, 43]. Therefore, the relation between low maternal MUAC and child CD4 T-cell counts link maternal undernutrition during pregnancy with impaired child immunity.
Unexpectedly, low levels of maternal erythrocyte sedimentation rate (ESR) were associated with reduced CD4 T-cell counts. Elevated ESR is a sign of inflammation and immune activation, and was associated with lower maternal CD4 T-cell count in this cohort (10). However, maternal inflammation may have induced a general leucocytosis in the developing fetus, which may have augmented the number of CD4 T-cells while actually decreasing their proportion as part of white blood cells.
Our study has limitations inherent in many cohort designs. First, associations reported may be due to unmeasured or residual confounding. Levels of CD4 T-cell counts in later follow up may not be representative of the entire starting cohort, as it is possible that children who died or were lost to follow up had lower counts. Loss to follow-up may have biased associations observed [44]. Lastly, findings should not be generalized to mother–child pairs receiving HAART.
In conclusion, the progressive decline in CD4 T-cell counts among HIV-infected children underscores the need for clinical interventions that stabilize immune function, such as HAART, to prevent mortality and poor clinical outcome [12]. Improving child nutritional status and preventing maternal HIV disease progression during pregnancy may improve CD4 T-cell counts among HIV-exposed children regardless of their infection status. Improving maternal nutritional status during pregnancy may also confer benefits for HIV-infected children, while preventing anaemia may benefit HIV-exposed children who escape HIV infection. The proposed infant and maternal interventions may be valuable in complementing standard programs to provide HAART and to prevent mother-to-child transmission of HIV.
Funding
National Institute of Child Health and Human Development (NICHD R01 32257); Fogarty International Center (NIH D43 TW00004).
Disclaimer
The opinions and statements in this article are those of the author, and may not reflect official UNICEF policies.
References
- 1.UNAIDS. AIDS Epidemic Update. Geneva, Switzerland: UNAIDS; 2007. [Google Scholar]
- 2.World Health Organization. Geneva: World Health Organization; 2006. Antiretroviral therapy of HIV infection in infants and children: towards universal access. Recommendations for a Public Health Approach. [PubMed] [Google Scholar]
- 3.Newell ML, Coovadia H, Cortina-Borja M, et al. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–43. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Geneva, Switzerland: World Health Organization; 2008. Report of the WHO Technical Reference Group, Paediatric HIV/ART Care Guideline Group Meeting. [Google Scholar]
- 5.World Health Organization. Geneva, Switzerland: World Health Organization; 2008. Preferred Antiretroviral Medicines for Treating and Preventing HIV Infection in Younger Children: Report of the WHO Paediatric Antiretroviral Working Group. [Google Scholar]
- 6.United Nations Children's Fund. Children. New York: United Nations Children's Fund; 2005. The missing face of AIDS. [Google Scholar]
- 7.Chatterjee A, Bosch RJ, Hunter DJ, et al. Maternal disease stage and child undernutrition in relation to mortality among children born to HIV-infected women in Tanzania. J Acquir Immune Defic Syndr. 2007;46:599–606. doi: 10.1097/QAI.0b013e31815a5703. [DOI] [PubMed] [Google Scholar]
- 8.Kuhn L, Kasonde P, Sinkala M, et al. Does severity of HIV disease in HIV-infected mothers affect mortality and morbidity among their uninfected infants? Clin Infect Dis. 2005;41:1654–61. doi: 10.1086/498029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang W, Perkins H, Anderson RE, et al. Patterns of T lymphocyte changes with human immunodeficiency virus infection: from seroconversion to the development of AIDS. J Acquir Immune Defic Syndr. 1989;2:63–9. [PubMed] [Google Scholar]
- 10.Levy JA. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993;57:183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abbas AK, Lichtman AH, Pober JS. Cellular and molecular immunology. 4th ed. Philadelphia: W.B. Saunders Company; 2000. [Google Scholar]
- 12.Chattopadhya D, Baveja UK, Bose M, et al. Disease progression markers during asymptomatic phase of HIV-1 infected children with unimpaired CD4+ cell values: evaluation of repeat CD4+ cell evaluation vs. other immunological parameters. J Trop Pediatr. 2002;48:340–7. doi: 10.1093/tropej/48.6.340. [DOI] [PubMed] [Google Scholar]
- 13.Kiepiela P, Coovadia HM, Coward P. T helper cell defect related to severity in measles. Scand J Infect Dis. 1987;19:185–92. doi: 10.3109/00365548709032397. [DOI] [PubMed] [Google Scholar]
- 14.Lisse IM, Aaby P, Whittle H, et al. T-lymphocyte subsets in West African children: impact of age, sex, and season. J Pediatr. 1997;130:77–85. doi: 10.1016/s0022-3476(97)70313-5. [DOI] [PubMed] [Google Scholar]
- 15.Moodley D, Bobat RA, Coovadia HM, et al. Lymphocyte subset changes between 3 and 15 months of age in infants born to HIV-seropositive women in South Africa. Trop Med Int Health. 1997;2:415–21. [PubMed] [Google Scholar]
- 16.Embree J, Bwayo J, Nagelkerke N, et al. Lymphocyte subsets in human immunodeficiency virus type 1-infected and uninfected children in Nairobi. Pediatr Infect Dis J. 2001;20:397–403. doi: 10.1097/00006454-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Ndhlovu Z, Ryon JJ, Griffin DE, et al. CD4+ and CD8+ T-lymphocyte subsets in Zambian children. J Trop Pediatr. 2004;50:94–7. doi: 10.1093/tropej/50.2.94. [DOI] [PubMed] [Google Scholar]
- 18.Lugada ES, Mermin J, Kaharuza F, et al. Population-based hematologic and immunologic reference values for a healthy Ugandan population. Clin Diagn Lab Immunol. 2004;11:29–34. doi: 10.1128/CDLI.11.1.29-34.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bunders M, Cortina-Borja M, Newell ML. Age-related standards for total lymphocyte, CD4+ and CD8+ T cell counts in children born in Europe. Pediatr Infect Dis J. 2005;24:595–600. doi: 10.1097/01.inf.0000168835.01233.64. [DOI] [PubMed] [Google Scholar]
- 20.Erkeller-Yuksel FM, Deneys V, Yuksel B, et al. Age-related changes in human blood lymphocyte subpopulations. J Pediatr. 1992;120:216–22. doi: 10.1016/s0022-3476(05)80430-5. [DOI] [PubMed] [Google Scholar]
- 21.Fawzi WW, Msamanga GI, Spiegelman D, et al. Randomised trial of effects of vitamin supplements on pregnancy outcomes and T cell counts in HIV-1-infected women in Tanzania. Lancet. 1998;351:1477–82. doi: 10.1016/s0140-6736(98)04197-x. [DOI] [PubMed] [Google Scholar]
- 22.Fawzi WW, Msamanga GI, Spiegelman D, et al. Rationale and design of the Tanzania Vitamin and HIV Infection Trial. Control Clin Trials. 1999;20:75–90. doi: 10.1016/s0197-2456(98)00045-2. [DOI] [PubMed] [Google Scholar]
- 23.Diggle P, Liang K, Zeger S. Analysis of Longitudinal Data. London, UK: Oxford University Press; 1994. [Google Scholar]
- 24.Hamill PV, Drizd TA, Johnson CL, et al. 1977. NCHS growth curves for children birth-18 years. Hyattsville (MD): National Center for Health Statistics. (DHEW publication 78-1650) [PubMed] [Google Scholar]
- 25.Douek DC. Disrupting T-cell homeostasis: how HIV-1 infection causes disease. AIDS Rev. 2003;5:172–7. [PubMed] [Google Scholar]
- 26.European Collaborative Study. Are there gender and race differences in cellular immunity patterns over age in infected and uninfected children born to HIV-infected women? J Acquir Immune Defic Syndr. 2003;33:635–41. doi: 10.1097/00126334-200308150-00013. [DOI] [PubMed] [Google Scholar]
- 27.Shearer WT, Easley KA, Goldfarb J, et al. Evaluation of immune survival factors in pediatric HIV-1 infection. Ann NY Acad Sci. 2000;918:298–312. doi: 10.1111/j.1749-6632.2000.tb05499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindsey JC, Hughes MD, McKinney RE, et al. Treatment-mediated changes in human immunodeficiency virus (HIV) type 1 RNA and CD4 cell counts as predictors of weight growth failure, cognitive decline, and survival in HIV-infected children. J Infect Dis. 2000;182:1385–93. doi: 10.1086/315865. [DOI] [PubMed] [Google Scholar]
- 29.Palumbo PE, Raskino C, Fiscus S, et al. Predictive value of quantitative plasma HIV RNA and CD4+ lymphocyte count in HIV-infected infants and children. JAMA. 1998;279:756–61. doi: 10.1001/jama.279.10.756. [DOI] [PubMed] [Google Scholar]
- 30.Gesner M, Papaevangelou V, Kim M, et al. Alteration in the proportion of CD4 T lymphocytes in a subgroup of human immunodeficiency virus-exposed-uninfected children. Pediatrics. 1994;93:624–30. [PubMed] [Google Scholar]
- 31.Caulfield LE, de Onis M, Blossner M, et al. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr. 2004;80:193–8. doi: 10.1093/ajcn/80.1.193. [DOI] [PubMed] [Google Scholar]
- 32.Scrimshaw NS, SanGiovanni JP. Synergism of nutrition, infection, and immunity: an overview. Am J Clin Nutr. 1997;66:464S–77S. doi: 10.1093/ajcn/66.2.464S. [DOI] [PubMed] [Google Scholar]
- 33.Obimbo EM, Mbori-Ngacha DA, Ochieng JO, et al. Predictors of early mortality in a cohort of human immunodeficiency virus type 1-infected african children. Pediatr Infect Dis J. 2004;23:536–43. doi: 10.1097/01.inf.0000129692.42964.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berhane R, Bagenda D, Marum L, et al. Growth failure as a prognostic indicator of mortality in pediatric HIV infection. Pediatrics. 1997;100:E7. doi: 10.1542/peds.100.1.e7. [DOI] [PubMed] [Google Scholar]
- 35.Villamor E, Misegades L, Fataki MR, et al. Child mortality in relation to HIV infection, nutritional status, and socio-economic background. Int J Epidemiol. 2005;34:61–8. doi: 10.1093/ije/dyh378. [DOI] [PubMed] [Google Scholar]
- 36.Savino W. The thymus gland is a target in malnutrition. Eur J Clin Nutr. 2002;56 (Suppl 3):S46–9. doi: 10.1038/sj.ejcn.1601485. [DOI] [PubMed] [Google Scholar]
- 37.Pitt J, Henrard D, FitzGerald G, et al. Human immunodeficiency virus (HIV) type 1 antibodies in perinatal HIV-1 infection: association with human HIV-1 transmission, infection, and disease progression. For the Women and Infants Transmission Study. J Infect Dis. 2000;182:1243–6. doi: 10.1086/315809. [DOI] [PubMed] [Google Scholar]
- 38.Kupka R, Msamanga GI, Mugusi F, et al. Iron status is an important cause of anemia in HIV-infected Tanzanian women but is not related to accelerated HIV disease progression. J Nutr. 2007;137:2317–23. doi: 10.1093/jn/137.10.2317. [DOI] [PubMed] [Google Scholar]
- 39.Allen LH. Pregnancy and iron deficiency: unresolved issues. Nutr Rev. 1997;55:91–101. doi: 10.1111/j.1753-4887.1997.tb06460.x. [DOI] [PubMed] [Google Scholar]
- 40.Moyle G. Anaemia in persons with HIV infection: prognostic marker and contributor to morbidity. AIDS Rev. 2002;4:13–20. [PubMed] [Google Scholar]
- 41.O’Brien ME, Kupka R, Msamanga GI, et al. Anemia is an independent predictor of mortality and immunologic progression of disease among women with HIV in Tanzania. J Acquir Immune Defic Syndr. 2005;40:219–25. doi: 10.1097/01.qai.0000166374.16222.a2. [DOI] [PubMed] [Google Scholar]
- 42.Kotler DP, Thea DM, Heo M, et al. Relative influences of sex, race, environment, and HIV infection on body composition in adults. Am J Clin Nutr. 1999;69:432–9. doi: 10.1093/ajcn/69.3.432. [DOI] [PubMed] [Google Scholar]
- 43.Grinspoon S, Corcoran C, Miller K, et al. Body composition and endocrine function in women with acquired immunodeficiency syndrome wasting. J Clin Endocrinol Metab. 1997;82:1332–7. doi: 10.1210/jcem.82.5.3907. [DOI] [PubMed] [Google Scholar]
- 44.Greenland S. Response and follow-up bias in cohort studies. Am J Epidemiol. 1977;106:184–7. doi: 10.1093/oxfordjournals.aje.a112451. [DOI] [PubMed] [Google Scholar]