Abstract
Triatoma infestans Klug is dimorphic for flight muscles. This dimorphism may affect flight dispersal, reinfestation patterns, and transmission risk. To understand the contributions of genes and environment to morph determination, it is first necessary to characterize the temporal dynamics of flight muscle development. Field-collected T. infestans adults were dissected 20 or 100 d after collection, and those collected as nymphs were dissected at ≈4, 15, or 44 d after the imaginal molt. The occurrence of flight muscles was additionally assessed by minimally invasive, repeated observations through the scutum of live bugs. Overall, 33.5% of 179 males and 7.8% of 179 females had no flight muscles. Neither dissections nor repeated observations evidenced changes in morph type during adult life, suggesting that the occurrence of flight muscles is mostly irreversible within the time span of observations and is determined before or during final ecdysis. Flight muscles were detectable at 4 d after emergence and achieved complete development within 4–15 d after emergence. The repeated observation of thoracic contents through the scutum showed very high sensitivity and specificity and apparently had minor effects on mortality. In another bug population located 360 km away, 16.4% of 177 males and 6.7% of 149 females had no flight muscles. Current results show that the sex-biased flight muscle dimorphism is a regionally widespread character in T. infestans. This character should be considered in Field population studies that seek to measure reinfestation risk and dispersal in T. infestans and other Triatominae.
Keywords: flight muscle development, dispersal polymorphism, Triatominae, Chagas disease
Flight is one of the most distinctive characteristics of insects. It is an important means of dispersal and may exert crucial effects on population dynamics, distribution, and gene flow. Heterogeneity in flight capacity has been reported for several species, mostly as wing polymorphisms, but also as flight muscle dimorphisms (Harrison 1980, Zera and Denno 1997). Such heterogeneity is caused by genetic variance (as in some beetles), environmental variance (as in aphids), or most frequently both, as in waterstriders, planthoppers, and crickets (Zera and Denno 1997, Zera 2004).
Triatoma infestans Klug, the main vector of Chagas disease in South America, is dimorphic for flight muscles in natural populations; 22% of males and 9% of females did not have flight muscles (Gurevitz et al. 2007). This heterogeneity in flight capacity may be important for reinfestation patterns and the spatio-temporal distribution of transmission risk (Cecere et al. 2004, Gurevitz et al. 2006). To inquire about the contributions of genes and the environment to morph determination, it is first necessary to determine whether these morphs are stable throughout the adult stage, because the absence of developed flight muscles could be caused by degeneration of developed muscles, arrested growth of muscles, or both (Zera and Denno 1997, Marden 2000). Here we report the temporal dynamics of flight muscle development in T. infestans adults, its implications for how flight muscle dimorphism arises, and compare the frequency of dimorphism between two distant bug populations.
Materials and Methods
Insects
Approximately 360 adults and 600 nymphs of T. infestans were collected manually using 10 person-hours of capture effort in two storerooms of the same house in the rural village of Santo Solano (27°15′ S, 63°19′ W) in the Department of Figueroa, Province of Santiago del Estero, Argentina, in April 2006. Additional bug collections were conducted in a storeroom and corrals of a house located 400 m from the former. Adult T. infestans collected as such were fed on chickens 40 d after collection. Fourty-eight fifth-instar nymphs molted in the absence of blood feeding after field collection. The remainder fourth- and fifth-instar nymphs were fed on chickens until they molted to the adult stage. The age of the adult bugs that emerged in the laboratory was recorded with 1-d precision. All bugs were kept at 24–26°C. Newly emerged adults were separated by sex to keep them virgin.
An additional sample of T. infestans adults was collected from 51 houses in Pampa del Indio (25°54′ S, 60°00′ W), Department San Martín, Province of Chaco, Argentina, in November and December 2007. All of these bugs were kept alive at 22–25°C and frozen at −20°C within 30 d after collection.
Experimental Design
To assess when flight muscles were detectable during the adult stage, when they completed development, and whether they eventually degenerated, adults that emerged in the laboratory were dissected at an average adult age of four (range, 3–6), 15 (14–16), or 44 (38–53) d after emergence. To further assess flight muscle degeneration, bugs collected as adults were dissected 20 or 100 d after Field collection. In all cases, insects were chosen and assigned to each observation group at random. To reduce heterogeneity between age groups (arising mainly from differences in time spent in the laboratory), each newly emerged adult was randomly assigned to a particular age class of dissection. Before dissection, bugs were weighed on an electronic balance (AEG-220; Shimadzu Libror, Duisburg, Germany) (precision ± 0.1 mg). The occurrence of flight muscles was first assessed by observation through the scutum. This consisted of slightly lifting the posterior part of the pronotum using a scalpel to expose a minimal portion (≈ 1 mm) of the scutum, which is thin and translucid, and recording the color of thoracic contents. A light-brown color indicated the presence of flight muscles, whereas a dull whitish color indicated their absence. Subsequently, the pronotum and scutum were completely removed, the occurrence of flight muscles was directly observed, and the thoracic contents were completely removed of cuticle and weighed on an electronic balance. Adult bugs collected in Pampa del Indio were dissected likewise.
To assess flight muscle degeneration of individually identified bugs over several months, 34 males and 51 females that emerged in the laboratory were followed up by repeated observations through the scutum every 2 wk for 2–4 mo starting on day 15 after emergence. On average, bugs were observed individually five times (range, three to seven times). Some of these bugs were fed on chickens once during the follow-up.
To evaluate the effects on mortality of repeated observations through the scutum, 38 males field collected as adults were observed through the scutum twice a week during an average of 40 d (range, 7–54 d), resulting in an average of 12 observations (range, 3–15 observations) per individual. The mortality of this group was compared with that of the remainder (62) of field-collected males not manipulated during the same period of follow-up.
Data Analysis
All comparisons between proportions were done using χ2 tests. Bugs were pooled together according to adult age (days after emergence) at dissection. One-way analyses of variance were performed to assess differences in flight muscle mass between groups within each sex. The Scheffé method was used for post hoc comparisons. The relationship between total body mass and flight muscle mass was assessed using Pearson’s correlation coefficient. The effects on mortality of assessing the occurrence of flight muscles by observation through the scutum twice a week were tested using the log-rank test. All these analyses were run on Stata 9.0 (Stata-Corp 2005). Mortality rates and their variation along time were estimated on a daily basis using the Gompertz curve (Carey 1993) with Matlab 7.3 (The Math-works 2006). The sensitivity and specificity (Fleiss 1981) of the technique of observation through the scutum to detect the occurrence of flight muscles used the subsequent results of individual dissections as gold standards.
Results
Santo Solano Population
Overall, 33.5% of 179 males and 7.8% of 179 females had no flight muscles when dissected (P < 0.0001). For neither sex did the proportion of bugs with no flight muscles differ significantly between collection sites (P > 0.08), age classes (P > 0.2), stage at field collection (P > 0.10), or days after being field-collected as adults (P > 0.79) (Table 1). None of the 123 bugs observed through the scutum during 1.5–4.5 mo showed a clear change from having to not having flight muscles. Overall, the technique of observing through the scutum detected the occurrence of flight muscles in 86% (243/284) of the bugs that actually had flight muscles on subsequent dissection (i.e., sensitivity) and was able to show its absence in 91% (67/74) of the bugs that actually did not have flight muscles on dissection (i.e., specificity). Excluding adults <7 d old, sensitivity and specificity were 97 (238/246) and 90% (57/63), respectively. Observation through the scutum and the concomitant manipulation significantly increased mortality when performed twice a week (χ2 = 90.1, df =1, P <0.0001).
Table 1.
Frequency of T. infestans males and females with no flight muscles on dissection according to source population, adult age class, and stage when field collected
| Source population | Adult age class (d) | % bugs with no flight muscles (no. bugs dissected) |
|
|---|---|---|---|
| Males | Females | ||
| Santo Solano | |||
| Collected as nymphs | |||
| 3–6 | 37.5 (24) | 8.0 (25) | |
| 14–16 | 15.4 (26) | 18.2 (33) | |
| 38–53 | 29.0 (31) | 4.7 (43) | |
| Collected as adults | |||
| 320a | 38.1 (42) | 4.5 (44) | |
| 3100a | 39.3 (56) | 5.9 (34) | |
| Total | 33.5 (179) | 7.8 (179) | |
| Pampa del Indio | |||
| Collected as adults | |||
| 16.4 (177) | 6.7 (149) | ||
Categories >20 and >100 refer to bugs collected as adults and dissected 20 and 100 d after field collection, respectively.
According to the Gompertz curve, the initial mortality rate, A0, was 0.0022 (95% confidence interval [CI]: ±0.0005) and the rate of senescence, G, was 0.084 (95% CI: ±0.007) for the manipulated group, whereas for the unmanipulated group, A0 = 0.004 (95% CI: ±0.003) and G = −0.02 (95% CI: ±0.03). The 34 males and 51 females observed through the scutum every 2 wk lived an average of 89.5 d and were all dead 4.5 mo after emergence. For this group, A0 = 0.0053 (95% CI:±0.0006) and G = 0.023 (95% CI: ±0.002).
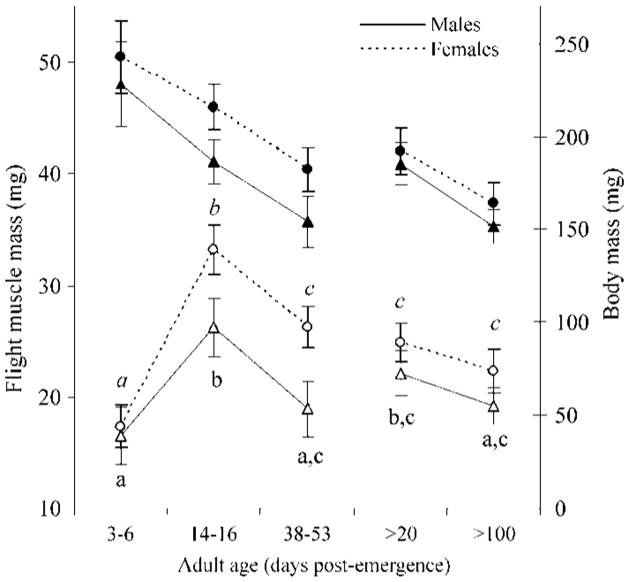
Both flight muscle morphs were clearly distinct on dissection at 4 d after emergence; bugs either had their thoracic cavity practically empty (except for some whitish, almost liquid contents), or had flight muscles that did not differ in their mass from older adults, except for 15-d-old bugs (Fig. 1; although not as firm in consistency and with a lighter color). Flight muscle mass varied significantly with adult age in both sexes (males: F = 9.5; df = 4,114; P < 0.0001; and females: F = 26.2; df = 4,160; P < 0.0001); it peaked at 15 d after emergence and decreased at 44 d after emergence (Scheffé comparisons, P <0.001; Fig. 1). Flight muscle mass at 44 d after emergence did not differ significantly from that of bugs collected as adults (Scheffé comparisons, P > 0.2). Flight muscle mass decreased slightly in bugs collected as adults and dissected 100 d after field collection compared with bugs dissected 20 d after collection. In both study groups, decreases in flight muscle mass correlated significantly with decreases in total body mass (r = 0.36, P < 0.001).
Fig. 1.
Sex-specific flight muscle mass (open symbols) and total body mass (closed symbols) according to adult age class of T. infestans from Santo Solano. Categories >20 and >100 refer to bugs collected as adults and dissected 20 and 100 d after field collection, respectively. Bars indicate 95% confidence interval around the mean. Shared letters indicate no significant differences (P > 0.05). Italized letters correspond to females, whereas normal letters correspond to males.
Pampa del Indio Population
Bugs with no flight muscles represented 16.4% of the 177 males and 6.7% of the 149 females dissected (P = 0.007; Table 1). Compared with Santo Solano bugs collected as adults, males from Pampa del Indio showed a significantly higher frequency of bugs with no flight muscles (P < 0.0001), whereas females showed no significant differences (P = 0.64).
Discussion
The occurrence of flight muscles in the Santo Solano population was mostly irreversible within the time span of observations and was determined before or during adult emergence. This is supported by the stable proportions of each morph recorded during adult lifespan and by the repeated observations through the translucid scutum on 123 individuals during 1.5–4.5 mo. These results do not support the hypothesis of flight muscle degeneration proposed previously (based on limited evidence) as a mechanism for explaining an increase in the frequency of male T. infestans with no flight muscles during adult lifespan (Gurevitz et al. 2007).
The Santo Solano adult T. infestans were collected from the same populations either as such or as fourth-or fifth-instar nymphs. These nymphs developed to adults under different laboratory conditions (temperature, photoperiod) and feeding frequency than bugs collected as adults. Regardless of these differences, both groups of bugs exhibited similar frequencies of flight muscle morphs. This suggests that differences in temperature, photoperiod, and feeding frequency were not crucial for morph determination during the fourth or fifth nymphal stage of T. infestans.
The occurrence of flight muscles was detectable in T. infestans adults as early as 4 d after emergence. However, such flight muscles were not yet fully developed because their mass increased until 15 d after emergence, and their consistency was apparently not as firm as in all adults with developed flight muscles and >15 d old. Complete development was achieved within 4–15 d after emergence. This developmental pattern in T. infestans is consistent with data collected from 17 adults with tethered flights of short duration (Ward et al. 1982) and with the finding that adult T. infestans rarely initiated flight during the first 10 d after emergence (Ward and Baker 1982, Lehane and Schofield 1982). The significant decrease in flight muscle mass observed between 15 and 44 d after emergence was correlated with a simultaneous decrease in total body mass; these bugs had been last blood-fed at least 15–20 d before final ecdysis. A similar, although not statistically significant, decrease was also observed in bugs dissected 100 d after field collection in comparison to those dissected 20 d after collection; the former group was fed once in the laboratory 40 d after field collection and then fasted for 60 d. This points to dehydration or to some kind of degradation of flight muscles, related to extended fasting, as possible causes of such decrease in flight muscle mass.
The observation of the occurrence of flight muscles through the scutum allowed us to assess degeneration of flight muscles without relying on variations in the proportions of each morph type over time determined from different samples of insects. The sensitivity and specificity of the technique were fairly high, especially for adults with fully developed flight muscles. Semi-weekly observations significantly increased mortality, whereas bugs observed every 2 wk had a longevity similar to previous estimates (Canale and Carcavallo 1988). Thus, this minimally invasive technique allows repeated observations of an internal structure on live T. infestans for several months with minimal effects on mortality. Its use in other Triatominae needs to be assessed.
The Pampa del Indio population, 360 km northeast of Santo Solano, also showed flight muscle dimorphism, demonstrating that this character is present regionally. This is further emphasized by the occurrence of flight muscle dimorphism in T. infestans populations sampled from peridomestic sites some 30 km away from the current source sites, 2 yr earlier during the same season (Gurevitz et al. 2007), and in a laboratory-reared second generation of T. infestans collected in the Province of San Luis, Argentina (J.M.G., unpublished data). It is noteworthy the higher frequency of males than females with no flight muscles appears in all populations examined thus far, although the frequency may vary within each sex. These results do not allow to distinguish the basis of this systematic bias; straightforward possibilities could be a sex-linked genetic basis or a sex-morph–specific selection, resulting from differential mortality, a differential capacity of feeding or reproducing, or differential dispersal.
The source sites in Santo Solano that provided the study bugs had very high densities of bugs of all stages, many of which had recently engorged, and numerous hosts. These characteristics jointly indicate a well-established population under stable conditions. Flight muscle dimorphism and sex bias in morph frequencies were present in adults collected as such in the field and in those that emerged later from nymphs collected from the same populations. These findings, together with the regional presence of the dimorphism as well as its sex-biased distribution, suggest that such phenomena are not a response to transient conditions. They seem to derive from rather permanent characteristics, such as the genetic base of the character or some general conditions under which these populations usually develop. The regionally widespread occurrence of flight muscle dimorphism in T. infestans populations underscores the need to consider this character in studies focusing on reinfestation risk and dispersal of this and other Triatominae.
Acknowledgments
L. Ceballos, R. Stariolo, and P. Ordóñez Krasnowski provided essential assistance in field and laboratory work. This project was supported by Grant R01 TW05836 to U.K. and R.E.G.; in part by Grant 1 C06 RR 16515 to University of Illinois at Urbana-Champaign College of Veterinary Medicine both from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH); and by the Agencia Nacional de Promoción Científica y Técnica (Argentina) and the University of Buenos Aires to R.E.G. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. R.E.G. is member of Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) of Argentina Researcher’s Career.
References Cited
- Canale DM, Carcavallo RU. Factores biológicos y ecológicos en la Enfermedad de Chagas. Ministerio de Salud y Acción Social de Argentina; Buenos Aires, Argentina: 1988. Triatoma infestans (Klug) [Google Scholar]
- Carey JR. Applied demography for biologists. Oxford University Press; New York: 1993. [Google Scholar]
- Cecere MC, Vazquez-Prokopec GM, Gürtler RE, Kitron U. Spatio-temporal analysis of reinfestation by Triatoma infestans (Hemiptera: Reduviidae) following insecticide spraying in a rural community in northwestern Argentina. Am J Trop Med Hyg. 2004;71:803–810. [PMC free article] [PubMed] [Google Scholar]
- Fleiss JL. Statistical methods for rates and proportions. Wiley; New York: 1981. [Google Scholar]
- Gurevitz JM, Ceballos LA, Kitron U, Gürtler RE. Flight initiation of Triatoma infestans (Hemiptera: Reduviidae) under natural climatic conditions. J Med Entomol. 2006;43:143–150. doi: 10.1603/0022-2585(2006)043[0143:fiotih]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevitz JM, Kitron U, Gürtler RE. Flight muscle dimorphism and heterogeneity in flight initiation of field-collected Triatoma infestans (Hemiptera: Reduviidae) J Med Entomol. 2007;44:186–191. doi: 10.1603/0022-2585(2007)44[186:fmdahi]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RG. Dispersal polymorphisms in insects. Annu Rev Ecol Syst. 1980;11:95–118. [Google Scholar]
- Lehane MJ, Schofield CJ. Flight initiation in Triatoma infestans (Klug) (Hemiptera: Reduviidae) Bull Entomol Res. 1982;72:497–510. [Google Scholar]
- Marden JH. Variability in the size, composition, and function of insect flight muscles. Annu Rev Physiol. 2000;62:157–178. doi: 10.1146/annurev.physiol.62.1.157. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata statistical software: release 9.0. Stata-Corp; College Station, TX: 2005. [Google Scholar]
- The Mathworks. Matlab version 7.3. The Mathworks; Natick, MA: 2006. [Google Scholar]
- Ward JP, Baker PS. The tethered flight performance of a laboratory population of Triatoma infestans Klug (Hemiptera: Reduviidae) Bull Entomol Res. 1982;72:17–28. [Google Scholar]
- Ward JP, Candy DJ, Smith SN. Lipid storage and changes during flight by triatomine bugs (Rhodnius prolixus and Triatoma infestans) J Insect Physiol. 1982;28:527–534. [Google Scholar]
- Zera AJ. The endocrine regulation of wing polymorphism in insects: state of the art, recent surprises, and future directions. Integr Comp Biol. 2004;43:607–616. doi: 10.1093/icb/43.5.607. [DOI] [PubMed] [Google Scholar]
- Zera AJ, Denno RF. Physiology and ecology of dispersal polymorphism in insects. Annu Rev Entomol. 1997;42:207–230. doi: 10.1146/annurev.ento.42.1.207. [DOI] [PubMed] [Google Scholar]