Abstract
Long QT syndrome, either inherited or acquired from drug treatments, can result in ventricular arrhythmia (torsade de pointes) and sudden death. Human ether-a-go-go-related gene (hERG) channel inhibition by drugs is now recognized as a common reason for the acquired form of long QT syndrome. It has been reported that more than 100 known drugs inhibit the activity of the hERG channel. Since 1997, several drugs have been withdrawn from the market due to the long QT syndrome caused by hERG inhibition. Food and Drug Administration regulations now require safety data on hERG channels for investigative new drug (IND) applications. The assessment of compound activity on the hERG channel has now become an important part of the safety evaluation in the process of drug discovery. During the past decade, several in vitro assay methods have been developed and significant resources have been used to characterize hERG channel activities. However, evaluation of compound activities on hERG have not been performed for large compound collections due to technical difficulty, lack of throughput, and/or lack of biological relevance to function. Here we report a modified form of the FluxOR thallium flux assay, capable of measuring hERG activity in a homogeneous 1536-well plate format. To validate the assay, we screened a 7-point dilution series of the LOPAC 1280 library collection and reported rank order potencies of ten common hERG inhibitors. A correlation was also observed for the hERG channel activities of 10 known hERG inhibitors determined in this thallium flux assay and in the patch clamp experiment. Our findings indicate that this thallium flux assay can be used as an alternative method to profile large-volume compound libraries for compound activity on the hERG channel.
Keywords: Human ether-a-go-go-related gene (hERG), Quantitative high-throughput screening, (qHTS), FluxOR, Thallium flux, Baculovirus, BacMam
During the 1960s, Kaplan and Trout reported a new mutant phenotype in the fruit fly Drosophila melanogaster called ether-ago-go (Eag). It was named due to the observation that the legs of the flies started to shake following etherization [1], similar to the once popular dancing at the Whisky A Go-Go nightclub in West Hollywood, California. The Eag gene was functionally linked to a potassium channel [2,3] and led to the cloning of the Eag potassium channel in Drosophila [4]. The human ether-a-go-go-related gene (hERG)1 was cloned in 1994 [5] and characterized as a voltage-gated, inwardly rectifying potassium channel [6]. The hERG potassium channel has an α-subunit with six membrane spanning domains and a putative β-subunit [7] and belongs to the Kv11.1 (KCNH2) family of voltage-gated potassium channels. Physiologically, the hERG potassium channel is responsible for the repolarization of the cardiac action potential, described as the Ikr current in electrophysiology experiments.
The relationship of hERG with certain forms of inherited and acquired long QT syndrome (LQTS) was discovered in 1995 [8,9]. LQTS is a heart condition measured specifically by an electrocardiogram (ECG). It is caused by prolonged repolarization (recovery) following depolarization (excitation) of the cardiac myocytes. LQTS may lead to torsade de pointes (TdP), a rare ventricular arrhythmia that can result in ventricular fibrillation and sudden death. The TdP ventricular arrhythmia associated with inherited and acquired LQTS often occurs during exercise or excitement. Among eight genes related to the hereditary form of LQTS, hERG is most often linked to drug inhibition that results in acquired LQTS [7]. More than 100 drugs reportedly have the potential to induce LQTS by blocking the hERG channel [10]. The risk of sudden death makes these drugs unacceptable for the treatment of non-life-threatening diseases, although the incidence of TdP in patients with drug treatment usually is rather low [11]. During the 10-year span between 1997 and 2006, the Food and Drug Administration announced the withdrawal of 23 drugs from the U.S. market due to safety issues. Of these, 10 drugs caused LQTS [12] and have been linked to hERG inhibition.
Unlike other ion channels that interact only with ligands of specific structural classes, the hERG potassium ion can accept molecules of many different chemotypes that block the channel function [13,14]. Therefore, functional screening methods are needed to identify and remove potential hERG blockers at the early stages of drug development in order to reduce costly attrition in the late development [15–17].
Screening assays for the assessment of compound activities on hERG channels in vitro have been greatly improved over the last 10 years. Patch clamp electrophysiology remains the “gold standard” for in vitro hERG measurement, whereas the ECG (or EKG) is the main in vivo approach. However, the traditional manual patch clamp assay has very limited throughput—usually one compound per day per individual researcher. The automated patch clamp (APC) technology developed recently has increased the throughput of the hERG channel assay by one to two orders of magnitude [18,19], but the comparatively low throughput and high cost still limit its use to secondary screens for compound follow-up experiments and dedicated cardiac liability testing of identified drug candidates. Radioligand binding and displacement assays have been used extensively as a first-line screening method for the hERG activity in drug development [20–23]. Recently, a binding assay using fluorescence polarization has also been applied to hERG channels [24,25]. However, the use of binding assays is limited by the structure of labeled ligand because there may be multiple binding sites on hERG and it is a nonfunctional assay, rendering potential allosteric modulators and activators invisible to the methodology. Fluorescent membrane potential indicators such as DiBAC4(3) have also been used to measure hERG activity [26,27]. This method indirectly measures the function of the hERG channel by detecting the change in membrane potential. This assay suffers from interference by the fluorescent/quenching properties of certain compounds. Furthermore, the activities of compounds can be significantly right shifted because the assay uses an indirect measurement of channel activity, and can be affected by off-target effects like inhibition of electrogenic transporters or other ion channels which are ubiquitous in mammalian cells and function to keep the membrane potential.
Ion flux assays represent another type of functional assay that measures movement of radioisotopic or surrogate ion species through hERG channels. A radioactive Rb86 flux assay was initially reported for the hERG [28], but the intense radioactivity of this isotope has limited its use in large compound screens. More recently, a nonradioactive Rb+ flux assay that measures the efflux of Rb+ ion through hERG channels with atomic absorbance detection has been developed [29,30]. However, the screening throughput of this method is still quite limited due to the nature of the atomic absorbance method. In addition, Rb+ flux is heterogeneous and requires several cell wash steps. Thus, none of the assays described so far are suitable for a functional screen of hERG activity with large compound collections.
Recently, a fluorescent assay for the measurement of thallium ions through potassium channels was reported [31,32], and a new version of this thallium flux assay is now commercially available (FluxOR™). In combination with the hERG–BacMam expression system, this thallium flux assay has improved the assay window and throughput for functional measurement of hERG activity. Here, we describe a significant modification of the FluxOR™ assay, enabling screening in a homogenous 1536-well plate format for quantitative, high throughput screening. By using an extracellular quencher dye, we were able to eliminate wash steps from the protocol. This assay is robust and suitable for high throughput screening, and its results are comparable to patch clamp data.
Materials and methods
Materials
BacMam hERG, FluxOR thallium assay kit, and cell culture reagents (Dulbecco’s modified Eagle’s medium [DMEM], Opti-MEM, penicillin/streptomycin, and TrypLE) were purchased from Invitrogen (Carlsbad, CA, USA). U-2 OS, CKO-K1, HEK293, and HeLa cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Fetal bovine serum (FBS) was purchased from HyClone (Logan, UT, USA). The LOPAC 1280 library collection was purchased from Sigma–Aldrich (St. Louis, MO, USA). hERG inhibitors were purchased from Sigma–Aldrich or Tocris (Ellisville, MO, USA).
For the no-wash assay, loading buffer consisted of 100 μl of water-soluble probenecid, 1 ml of 100 mM Red 40 (Spectrum Chemicals, Gardena, CA, USA), 10 μl of thallium dye, and 9 ml of phosphate-buffered saline (PBS, pH 7.4) containing 20 mM Hepes. Stimulation buffer consisted of 100 ml of H2O, 20 ml of 50 mM Tl2SO4 (component G), 40 ml of 125 mM K2SO4 (component F), and 40 ml of 5× chloride-free assay buffer (component E).
Cell culture and viral transduction of U-2 OS cells
U-2 OS cells (a human osteosarcoma cell line, ATCC no. HTB-96) were maintained in DMEM medium containing 10% FBS and 1× penicillin/streptomycin and passaged when they reached 80–90% confluency. All experiments used cells with a passage number of 30 or less. For viral transduction, cells were grown to approximately 70–80% confluence in T175 flasks, medium was removed, and 2 ml of hERG–BacMam virus plus 13 ml of PBS (corresponding roughly to a multiplicity of infection ratio of 100 virus particles/cell) was added to the cells for a 4-h incubation at room temperature in the dark. After transduction, virus was removed and 35 ml of complete medium was added to the flasks and then cultured at 37 °C overnight. The next day, cells were rinsed in PBS, trypsinized (with a mixture of 2.5 ml Try-pLE + 5 ml PBS), resuspended in complete medium, and spun at 900 rpm for 5 min to pellet cells. The cells were resuspended in Opti-MEM medium containing 2% fetal calf serum at a density of 667 cells/μl. Using a Multidrop Combi 8 channel dispenser (Thermo Fisher, Waltham, MA, USA), cells were dispensed into 1536-well, black high-base plates at 3 μl/well and allowed to adhere at 37 °C for 4 h.
FluxOR reagents and assay
After 4 h of recovery, the cells were loaded with loading buffer (1 μl/well) and incubated at room temperature in the dark for 60 min. Compounds were dissolved in 100% dimethyl sulfoxide (DMSO) at concentrations ranging from 10 mM to 600 nM and were plated in 1536-well compound plates. A Pintool (Kalypsys, San Diego, CA, USA) transferred 23 nl of compound to plates containing 3 μl of cells in medium + 1 μl of loading buffer (final DMSO concentration of 0.58%). Controls were present in columns 1 through 4. Plates were incubated at room temperature for 10 min in the presence of compounds, and then hERG channels were stimulated by the addition of 1 μl of stimulation buffer (Table 1). Plates were measured with an FDSS 7000 kinetic plate reader (Hamamatsu, Hamamatsu City, Japan) in 1-s intervals for 180 s using a standard Fluo-4 480-nm excitation and 530-nm emission filter set. A baseline recording of 10 cycles was recorded prior to stimulation.
Table 1.
Protocol for thallium flux assay in 1536-well plates for screening of hERG channel activity.
| Sequence | Parameter | Value | Description |
|---|---|---|---|
| 1 | Cells | 2000 cells (4 μl/well) | U-2 OS cells transduced with hERG channels and loaded with buffer seeded in 1536-well plates |
| 2 | Compounds | 23 nl/well | Compounds in DMSO solution |
| 3 | Incubation | 10 min | At room temperature |
| 4 | Detection | 10 reads | FDSS 7000 kinetic plate reader at 1 Hz (Ex = 480 nm and Em = 530 nm) |
| 5 | Reagent | 1 μl/well | Stimulation buffer |
| 6 | Detection | 180 reads | FDSS 7000 kinetic plate reader at 1 Hz (Ex = 480 nm and Em = 530 nm) |
Automated patch clamp experiments
Cells and cell culture for automated patch clamp
CHO-K1 cells (from ATCC) stably transfected with hERG complementary DNA (cDNA) (GenBank sequence NM_000238) were constructed at Cerep (Redmond, WA, USA) and used in the automated patch clamp experiments. The cells were cultured in F-12 Kaighn’s Nutrient Mixture medium (Invitrogen,) +10% FBS at 37 °C for 1–3 days. The cells were harvested by trypsinization and kept in serum-free medium (Invitrogen) for up to 4 h at room temperature before recording. The cells were washed and resuspended in extracellular solution before being applied to the patch clamp sites.
Automated patch clamp
QPatch 16 (Sophion Biosciences, Denmark) was used for automated patch clamp. After whole cell configuration was achieved, the cell was held at −80 mV. A 50-ms pulse to −40 mV was delivered to measure the leak current, which was subtracted from the tail current online. Then the cell was depolarized to +20 mV for 2 s, followed by a 1-s pulse to −40 mV to reveal hERG tail current. This paradigm was delivered once every 5 s to monitor the tail current amplitude. Intracellular solution: 130 mM KCl, 10 mM NaCl, 1 mM MgCl2, 10 mM ethyleneglycoltetraacetic acid (EGTA), 5 mM Mg-ATP, and 10 mM Hepes (pH adjusted to 7.2 with KOH). Extracellular solution: 137 mM NaCl, 4 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 10 mM D(+)-glucose, and 10 mM Hepes (pH adjusted to 7.4 with NaOH).
Test compounds
The compounds were obtained from Sigma–Aldrich except for E-4031, which was obtained from Wako (Richmond, VA, USA), All compounds were prepared as 10-mM stock solution in DMSO. The test solutions were prepared in the extracellular solution from the 10-mM stock solution on the day of patch clamp assays. After the whole cell configuration was achieved, the extracellular solution (control) was applied first and the cell was stabilized in extracellular solution for 5 min. Then the test compound was applied from low concentrations to high concentrations cumulatively. The cell was incubated with each test concentration for 5 min. During the incubation, the cell was stimulated repetitively using the voltage protocol described above and the tail current amplitude was monitored continuously.
Data analysis
Data analysis for thallium flux assay
The slope of fluorescence intensities versus the time of first 30 s after compound addition was calculated from the kinetic results. The signal-to-basal ratio was calculated as the slope of DMSO solvent controls in the stimulated group divided by the slope of an unstimulated group. The concentration responses of compound inhibition from the experiments were analyzed with Prism software (GraphPad, San Diego, CA, USA).
Data analysis for automated patch clamp
The degree of inhibition (%) of hERG tail current was calculated by
where Control is the mean hERG tail current amplitude from the data collected in 24 s in the presence of control and Test Compound is the mean hERG tail current amplitude from the data collected in 24 s in the presence of test compound at each concentration.
A concentration–response curve for the test compound was constructed from the mean inhibition (%) of tail current amplitudes by QPatch software. The following equation was used:
where y is the inhibition (%) of hERG tail current, n is Hill slope, C is the test compound concentration, and IC50 is the test compound concentration producing 50% inhibition of hERG tail current.
Results
Assay principle
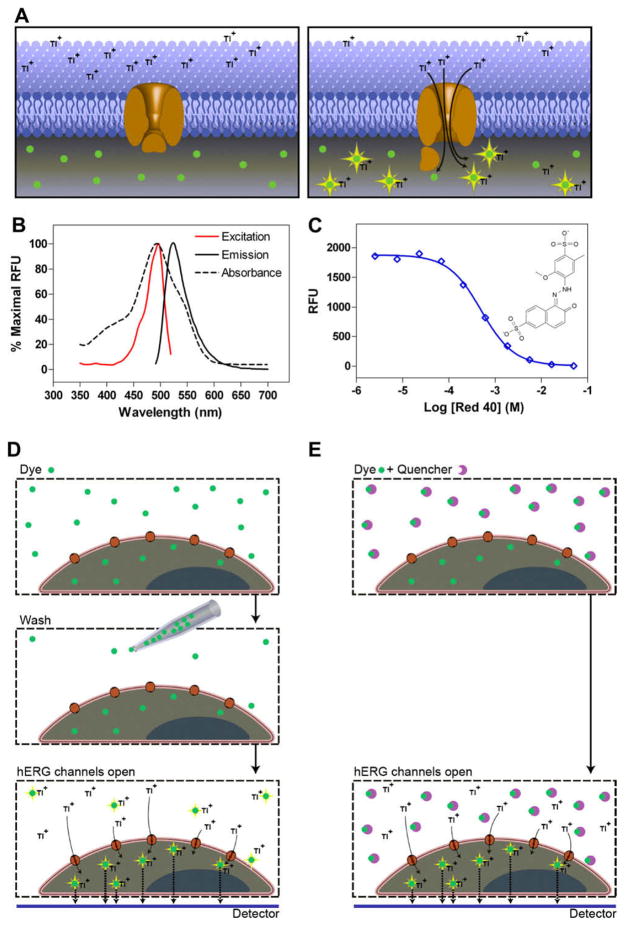
Thallium ions are used as surrogate ions in this assay to measure activity of the hERG potassium channel. This is possible because of the selective permeability of all potassium ion channels for thallium and the strong driving force for thallium entry into the cells when the channels are opened. In brief, FluxOR dye is loaded into the cells prior to the experiment (Fig. 1A). FluxOR dye contains aminomethyl (AM) ester groups that render the molecule membrane permeant from the extracellular medium. Once in the cytosol, the AM ester groups are cleaved by intracellular esterases, resulting in a charged dye species that stays trapped inside the cells for the duration of the experiment. Thallium-bound dye is capable of fluorescing only when deesterified and excited with green light. The FluxOR dye–Tl+ complex has an excitation peak at 495 nm and an emission peak at 525 nm (Fig. 1B). The increase in the intracellular fluorescence signal grows in proportion to the number of open channels, therefore quantitating channel activity is made possible by the kinetic measurement of fluorescence intensity. The intracellular fluorescence signal is inhibited in the presence of a hERG channel blocker, and activators or agonists can be observed with increases in signal above basal activation.
Fig. 1.
(A) Schematic diagram of the FluxOR thallium assay for the measurement of hERG channel activity. Cells that express hERG channels are loaded with dye from the FluxOR thallium assay kit and then washed to remove the dye from medium. When the cells are stimulated, thallium ions enter the cells through open hERG channels and bind to the dye, yielding an increase in green fluorescence (530 nm) on excitation at 480 nm. (B) Excitation and emission spectra of FluxOR dye (red and black solid lines, respectively) and Red 40 absorbance (black dotted line). FluxOR dye was deesterified by treating with PBS (pH 10.0) for 3 days, followed by the addition of stimulation buffer. Measurements were taken on a Tecan monochrometer. RFU, relative fluorescence units. (C) Titration curve of Red 40 absorbance as measured on a Tecan monochrometer. Molecular structure of Red 40 presented on right. (D) Schematic diagram of traditional wash assay. Cells are loaded with FluxOR dye. As the dye crosses the cell membrane, intracellular esterases cleave the ester group, trapping the dye within the cell. Extracellular deesterified dye is present due to efflux as well as extracellular esterase activity. After loading, the dye is removed by an aspiration step and cells are loaded with assay buffer. Stimulation of channel opening and subsequent signaling are accomplished by the addition of stimulation buffer containing thallium. False signal is generated by extracellular deesterified dye. (E) Schematic diagram of quenching effect of Red 40. The cell-permeable FluxOR dye enters cells and becomes deesterified. Thallium ion binds to the dye that forms an excitable complex emitting at 530 nm. Cell-impermeable Red 40 quenches the fluorescence caused by extracellular dye that had leaked out of cells or had become deesterified by extracellular esterases. A bottom reading plate reader will collect the fluorescence signal inside cells that is generated only by the cells attached to the bottom of a plate.
The presence of hydrolyzed FluxOR dye in the medium can result in high background fluorescence and a lower signal window, and for most applications a cell wash step is needed to remove the extracellular dye after loading. Because it is known that wash steps can cause well-to-well variation in the 1536-well plate format with kinetic measurements, we applied Red 40, a fluorescence quenching agent, to absorb the extracellular fluorescence from FluxOR dye and limit the source of the signal to the cytosol. We found that Red 40 effectively absorbed light emission between 400 and 570 nm (Fig. 1B), making it a reasonable candidate for the suppression of FluxOR dye outside of the cells. Fluorescence quenching by Red 40 concentration-dependently reduced the signal emitted from FluxOR–thallium ion complex and almost completely inhibited the fluorescence emission at and above a concentration of 2.5 mM (Fig. 1C). In addition, because Red 40 is charged and does not enter cells, it does not affect the intracellular fluorescence emission signal when the detection mode comes from the bottom of assay plates (Fig. 1D and 1E) [33]. Thus, the addition of this fluorescence quenching agent enabled us to eliminate the cell wash step without sacrificing the assay window, resulting in a no-wash assay format ready for high-throughput screening (HTS).
Assay optimization
Cell line selection
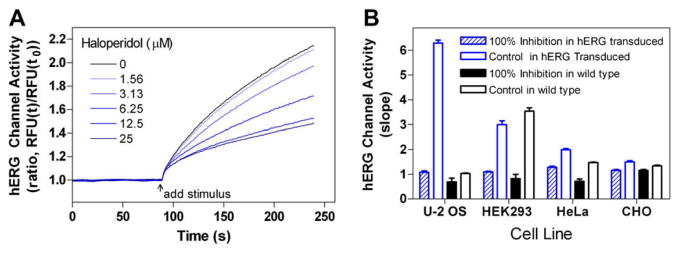
Four commonly used cell lines were tested in this assay: CHO-K1, HEK293, HeLa, and U-2 OS cells. The hERG–BacMam vector, provided in the assay kit, was used to transduce these cell lines in order to obtain hERG channel-expressing cells. BacMam is a modified version of the baculovirus vector that has been widely used for expression of G protein-coupled receptors (GPCRs) and ion channels in mammalian cells for drug screening [34,35]. The cells were seeded into 1536-well assay plates after transduction and cultured at 37 °C for 4 h. FluxOR dye was then loaded to cells in the assay plates, followed by the addition of compounds to the cells. When hERG channels were opened with stimulation buffer during the assay, thallium ions flowed down their concentration gradient into cells through the open channels and activated the FluxOR dye. The resulting increase in the fluorescence emission was rapid, and inhibition of maximal signal by a known hERG channel blocker, haloperidol, occurred in a dose-dependent fashion (Fig. 2A). We found that the rate of increase in the fluorescence intensity was linear during the first 30 s and that the slope of increase in fluorescence intensity at this period was a better parameter for calculating the compound activities compared with that of maximal signal divided by minimal signal (data not shown).
Fig. 2.
(A) Time course of hERG channel stimulation in U-2 OS cells in the presence of haloperidol dilutions. The max/min ratio was calculated from time of stimulation over 1-s intervals. The linear range of response within 30 s of stimulation was used to calculate slope. (B) Comparison of transduced and nontransduced cells stimulated in the presence and absence of 50 μM haloperidol. All transduced cells were treated with a 100:1 multiplicity of infection virus-to-cell ratio. Each data point represents the mean + S.E.M. (n = 4).
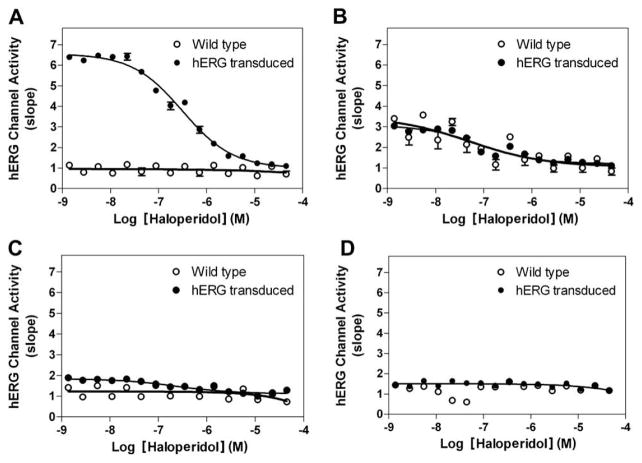
Among the four cell lines tested, we found that the U-2 OS cells transduced by the hERG–BacMam vector had a better signal-to-basal ratio and higher assay window. The fluorescence signal increased three- to five-fold in the hERG channel-expressing cells compared with the parental cells. The total signal was very low in the untransduced parental cells, indicating the low level of endogenous potassium channels, including the hERG channel (Fig. 2B). The fluorescence signal in the hERG-expressing U-2 OS cells was concentration-dependently inhibited by haloperidol (Fig. 3A). HEK cells showed significant endogenous activity of ion channels that were inhibited by haloperidol (Figs. 2B and 3B). The expression of the BacMam–hERG channel in HeLa cells was very poor, and the hERG channel activity was very low, although the endogenous potassium channel activity was low in this cell line (Figs. 2B and 3C). Previous literature has shown that CHO and other cell types often require higher doses of BacMam vector to achieve expression [36,37], and based on these results, the U-2 OS cell line was selected for further experiments (Fig. 3D).
Fig. 3.
Concentration-dependent inhibition of haloperidol on hERG channel activity in the transduced cells in comparison with that in untransduced cells. (A) U-2 OS cells. The IC50 value of haloperidol was 257 nM in the transduced cells, whereas haloperidol had no effect on the untransduced U-2 OS cells. hERG channel activity is measured as slope. (B–D) HEK293 (B), HeLa (C), and CHO-K1 (D) exhibited relatively low levels of stimulation and blocking by haloperidol. Cells were transduced with a multiplicity of infection of 100:1. Each data point represents the mean + S.E.M. (n = 4).
Cell density
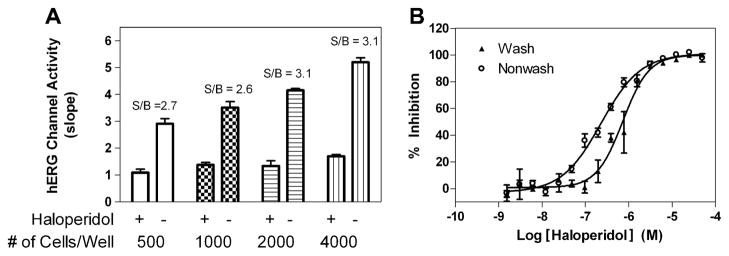
The effect of cell density was examined by varying cell numbers in the assay plate using U-2 OS cells expressing hERG channels. We used a saturating concentration (10 μM) of haloperidol to define the basal signal level and ran the assay in several conditions to find the largest signal-to-basal ratio. We found that the signal-to-basal ratio increased between 500 and 2000 cells/well but did not increase after 2000 cells/well (Fig. 4A). The signal-to-basal ratios were 2.6-, 2.7-, 3.1-, and 3.1-fold for the cell densities of 500, 1000, 2000, and 4000 cells/well, respectively, in these trials. Thus, 2000 cells/well was selected as an optimal number for further experiments.
Fig. 4.
(A) Cell number titration of U-2 OS hERG cells treated with or without haloperidol. The signal-to-basal (S/B) ratios defined by with (+) and without (−) haloperidol in the thallium flux assay for 500, 1000, 2000, and 4000 cells/well were 2.7-, 2.6-, 3.1-, and 3.1-fold, respectively. (B) Comparison of wash and no-wash protocols. For the wash protocol, cells in suspension were loaded in Opti-MEM medium containing 2% serum, FluxOR dye, and probenecid for 60 min at room temperature. Cells were then pelleted, washed, and plated at a density of 2000 cells/3 μl in assay buffer containing probenecid. Immediately after plating, cells were treated with 50 μM haloperidol or DMSO. For the no-wash protocol, cells were plated at a density of 2000 cells/3 μl in Opti-MEM containing 2% serum and allowed to adhere to the bottom of the plate for 4 h at 37 °C. Cells were then loaded with 1 μl of dye mixture and incubated at room temperature prior to being assayed. The S/B ratios were 3.1 and 4.2 for wash and no-wash, respectively. Each data point represents the mean + S.E.M. (n = 4).
Cell wash versus nonwash
The addition of Red 40 effectively removed the need for a cell wash step in the assay that was included in the original guidelines of the manufacturer. To be certain that the suppression dye did not significantly alter the pharmacology of the hERG potassium channel, we then tested the effect of Red 40 on the activities of known hERG inhibitors in the FluxOR assay. The IC50 value of haloperidol was 0.23 μM in the nonwash assay, similar to 0.73 μM in the cell wash assay (Fig. 4B). The IC50 values of several other known hERG inhibitors were tested and were found to be similar in both non-wash and cell wash assays (data not shown). This result indicated that the Red 40 dye did not affect the compound activity in this thallium flux assay. In addition, Red 40 exhibited no significant inhibition in the patch clamp experiments when tested at concentrations up to 2.5 mM (data not shown).
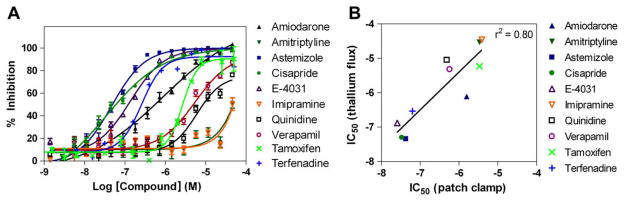
Results from known hERG inhibitors
To validate this hERG channel assay in the new thallium ion flux assay format, we tested 10 known hERG inhibitors for their activity on the hERG channel in thallium flux and whole cell patch clamp. These compounds exhibited concentration-dependent inhibition of the hERG channel activity as measured by the thallium ion flux in cells (Fig. 5A). The IC50 values of these compounds ranged from 0.13 to 5.60 μM. We then compared the IC50 values of these 10 compounds with those from automated patch clamp experiments carried out in whole cell mode using the QPatch APC platform. The IC50 values of these known hERG inhibitors showed an R2 of 0.80 for the thallium flux assay compared with the patch clamp experiment (Fig. 5B), indicating that the activities of hERG channel inhibitors determined in this thallium flux assay correlated well with those from the patch clamp assay and that IC50 values were in fact predictive of the gold standard measurements obtained in whole cell voltage clamp.
Fig. 5.
(A) Concentration responses of 10 known hERG inhibitors determined in the thallium flux assay using the hERG channel-transduced U-2 OS cells. Each data point represents the mean + S.E.M. (n = 6). (B) Correlation of IC50 values from thallium flux assay with those determined in manual patch clamp data from Cerep. Goodness-of-fit between patch clamp and HTS had an R2 value of 0.80. Each data point represents the mean + S.E.M. (n = 5).
Screen validation
DMSO plate test
This thallium flux assay was then tested using a DMSO plate in a 1536-well plate format. The DMSO plate test is commonly used to obtain the assay parameters for evaluating a screening assay. The signal-to-basal ratio determined from this DMSO plate test was 3.3-fold, the coefficient of variation (CV) was 5.5%, and the Z′ was 0.68, indicating that our modifications to the FluxOR flux assay in the homogeneous format in 1536-well plates was robust and suitable for HTS [14].
LOPAC library screening test
The LOPAC library collection contains 1280 compounds with known pharmacological activities and is commonly used to develop and test screening assays. We carried out quantitative HTS (qHTS) of this library using the modified thallium flux assay with the hERG channel-expressed U-2 OS cells as described previously. All of the compounds were screened in a titration series as reported previously, and the potency and efficacy of each of the active compounds were immediately available after the primary screening [38]. Using the selection criteria of IC50 value less than 0.3 μM and inhibition greater than 60%, 12 active compounds were identified, correlating to a hit rate of 0.9% (Table 2). In a counter-screen with the untransduced U-2 OS cells in the same assay format, none of the 12 compounds exhibited notable activity. Because of the exceptional “druggable” nature of the hERG channel and the fact that most compounds in the LOPAC library have known pharmacological properties, this higher hit rate from this test screen was expected. Of the active compounds identified, many of them were the known hERG inhibitors. The hit rate from the primary screen can be varied by the cutoff threshold. The hit rates (and hit numbers) with cutoffs of 5, 10, and 30 μM in IC50 value were 8.4% (108), 11.9% (152), and 16.1% (206), respectively.
Table 2.
Top 12 compounds identified in the LOPAC library screen (based on IC50 < 0.3 μM).
| Sample name | IC50 (μM) | Maximum inhibition (%) | Mechanism of action |
|---|---|---|---|
| Eliprodil | 0.02 | −104 | NMDA receptor antagonist |
| Pimozide | 0.06 | −105 | Antipsychotic D2 antagonist |
| Amperozide hydrochloride | 0.06 | −112 | Antipsychotic 5HT antagonist |
| Trifluperidol hydrochloride | 0.06 | −105 | Antipsychotic, D1/2 antagonist |
| Ritanserin | 0.07 | −109 | Antipsychotic 5HT2a antagonist |
| Amiodarone hydrochloride | 0.08 | −97 | nonselective ion channel blocker |
| JHW 007 hydrochloride | 0.09 | −98 | Dopamine uptake inhibitor |
| Vinpocetine | 0.14 | −103 | PDE1 inhibitor |
| Domperidone | 0.22 | −108 | D2 antagonist |
| GBR-12909 dihydrochloride | 0.22 | −98 | Dopamine uptake inhibitor |
| Haloperidol hydrochloride | 0.25 | −100 | Antipsychotic D1/2 antagonist |
| Phentolamine mesylate | 0.28 | −124 | Alpha adrenoreceptor antagonist |
Discussion
Unlike other ion channels that interact only with ligands of specific structural classes, the hERG potassium ion channel can be blocked or modulated by a broad spectrum of structurally diverse compounds. Thus, the ligand binding assay has clear limitations on the assessment of compound activity at hERG channels because competition with a labeled ligand is limited to structurally similar compounds. This means that binding/displacement assays that pick up compounds occupying the same binding site as the labeled ligand may show false-negative results for hERG inhibitors with different binding sites and that negative results in a binding assay cannot exclude the possibility of an allosteric or modulatory hERG inhibitory effect of the compounds. Therefore, a functional assay of hERG channels is the ultimate methodology for examining the hERG activity of compounds. We found that the activities of hERG channel inhibitors in this thallium flux assay are well correlated with those obtained in automated patch clamp experiments. Thus, this thallium flux assay was validated as an effective and alternative method for large-scale compound screens to assess compound activity on the hERG channel in vitro.
The whole cell patch clamp experiment is traditionally used as a gold standard method for the functional screen assay for voltage-gated ion channels, including the hERG channel. However, the screening throughput using manual instrumentation and devices is very low and not practically approachable for compound screens. The automated patch clamp instruments developed during recent years have significantly increased compound screening throughput and become more routinely used in the compound follow-up stage for drug development. However, the costs of supplies and the instrument both are still high, and the throughput is limited to small-scale screens, with extensive optimization needed for each new cell type and ion channel target class. The other functional assays for potassium channels available include membrane potential dye and ion flux assay using radioisotope or atomic absorbance measurement with rubidium. However, these methods suffer from nonhomogeneous format and/or poor correlation of the IC50 values with the patch clamp data. The concentrations of ions such as Na+, K+, Ca2+, and Cl− in cytosol are markedly different from those in the extracellular medium, and ion concentration gradients are important for maintaining membrane potential, cell function, and cell viability. In the resting state the concentration of cytosolic Ca2+ is 0.0002 mM inside cell compared with 1.8 mM outside cell, whereas in the stimulated state the cytosolic Ca2+ increases dramatically to 0.1 mM. The low concentration in the resting state and huge increase in the stimulated state of cytosol Ca2+ enables the measurement of the change in cytosol Ca2+ using fluorescent dyes preloaded inside cells. Based on this mechanism, Fluo-4-based Ca2+ assays have become important compound screening methods for GPCRs and calcium channels. However, the difference in K+ concentrations, 139 mM inside cell and 4 mM outside cell, does not allow the use of a K+ fluorescence dye to detect K+ flux through the potassium channels. Thus, thallium, a surrogate ion that is not present in cells physiologically, is used in this ion flux assay to measure potassium channel function with a fluorescence dye.
Thallium ions are permeable to potassium channels and have been used as a research tool for many years [39]. Fluorescence dye-based thallium assays have been reported for KCNQ2, GIRKs, and calcium-activated potassium channels [34,32,31]. These assays used a fluorescent dye, BTC–AM (benzothiazole coumarin acetoxymethyl ester, Invitrogen), to detect thallium flux into cells. However, the wash steps, high thallium concentrations, limited signal window, and Cl−-free buffer requirement limited the application of the assay to a 1536-well format. Here we optimized a new fluorescent thallium dye, FluxOR, with better sensitivity and physiological medium/buffer than was used previously. In addition, we applied a quenching agent, enabling a homogeneous assay format without a cell wash step. The thallium flux assay is an equilibrium assay that reports the relative activity of ion channel populations as they cycle among open, closed, and inactivated states over a time course in the range of tens of seconds. Lacking the kinetic information and exquisite voltage control allowed in patch clamp electrophysiology, the flux-based ion assay relies on the steady accumulation of a surrogate ion following the change in state that accompanies the modest depolarization in the stimulus. Despite these differences, our results indicated that the IC50 values of 10 known hERG channel inhibitors from thallium assay were in good correlation with those obtained from patch clamp measurements. Thus, this thallium assay can be used as an alternative screening method for large-scale compound profiling or as a library screen to which the patch clamp assay could not be applied.
Mammalian transduction of the hERG baculovirus is straightforward and quite tolerant of changes in protocol parameters. For example, we recently transduced hERG–BacMam by adding virus directly to adherent cells in complete medium and incubating overnight at 37 °C. There was no appreciable difference in signal-to-basal ratio or EC50 between 4 h room temperature transduction in PBS and overnight transduction in complete medium at 37 °C (data not shown). In addition, the manufacturer’s protocol recommends trying BacMam enhancer to see whether it helps with expression of the gene of interest. In the case of U-2 OS cells expressing hERG, enhancer had no benefit on expression (data not shown). Recently there have been reports that expression of hERG and clinically relevant mutant constructs of hERG exhibit increased expression (via translocation to the plasma membrane) when grown at decreased temperatures [35,40]. We have not observed increased expression when cells were transduced and grown overnight at 32 °C (data not shown). A limitation in this study was that two different cell types were used in the thallium flux assay and patch clamp experiment. Although a good IC50 correlation of the 10 known hERG inhibitors was observed, it would be of interest to test these compounds using the same cell lines in the thallium flux assay and patch clamp experiment.
The application of fluorescence quenching agents has been reported for reducing the fluorescence background in other cell-based assays. Crystal violet and trypan blue were originally used as quenching dyes to reduce the extracellular fluorescence background while measuring the intracellular internalized vesicles [41,42]. This quenching method was also used in flow cytometry studies to reduce the cellular autofluorescence background [43]. Recently, the quenching method was used in conjunction with fluorescent calcium dye assays to mask the extracellular fluorescence while the intracellular fluorescence signal is recorded [44]. We applied Red 40 as a quenching agent in the FluxOR dye-based thallium flux assay to suppress the extracellular fluorescence while the intracellular fluorescence signal is measured for hERG channel activity. We found that Red 40 has broad absorbance spectra for green and blue fluorescence and can completely inhibit the fluorescence intensity emitted from extracellular FluxOR dye. Thus, the addition of the fluorescence quenching agent Red 40 allows the homogeneous measurement of the thallium flux into cells without a cell wash step. This new homogeneous thallium flux assay is robust and has been miniaturized to a 1536-well plate format for HTS.
In summary, we have developed a homogeneous thallium flux assay in a 1536-well plate format for assessment of compound activity on the hERG channel based on the commercially available FluxOR assay and the addition of a fluorescence quenching dye, Red 40, in assay buffer. The IC50 values of ten known hERG inhibitors from this assay correlated well with those determined in the patch clamp assay. Therefore, this thallium flux assay can be used as a valid functional assay to profile the hERG channel activity of large compound collections. In addition, this assay can be used for other potassium channels in the HTS campaign to identify the lead compounds and generate structure–activity relationship profiles for compounds in safety screens.
Acknowledgments
This research was supported by the Molecular Libraries Initiative of the NIH Roadmap for Medical Research and the Intramural Research Programs of the National Human Genome Research Institute. The authors thank Paul Shinn for assistance with compound management. The authors also thank Shouming Du of Hamamatsu for technical support with the FDSS 7000 kinetic plate reader; Michael O’Grady, Matt Robers, and George Hanson of Invitrogen/Life Technologies and Blake Anson of Cellular Dynamics for technical assistance; and Darryl Leja for help with artwork.
Footnotes
Abbreviations used: hERG, human ether-a-go-go related gene; LQTS, long QT syndrome; ECG (or EKG), electrocardiogram; TdP, torsade de pointes; APC, automated patch clamp; DMEM, Dulbecco’s modified Eagle’s medium; ATCC, American Type Culture Collection; FBS, fetal bovine serum; PBS, phosphate-buffered saline; DMSO, dimethyl sulfoxide; cDNA, complementary DNA; EGTA, ethyleneglycoltetraacetic acid; AM, acetoxymethyl; HTS, high-throughput screening; GPCR, G protein-coupled receptor; CV, coefficient of variation; qHTS, quantitative HTS; BTC–AM, benzothiazole coumarin acetoxymethyl ester.
References
- 1.Kaplan WD, Trout WE., 3rd The behavior of four neurological mutants of Drosophila. Genetics. 1969;61:399–409. doi: 10.1093/genetics/61.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganetzky B, Wu CF. Neurogenetic analysis of potassium currents in Drosophila: synergistic effects on neuromuscular transmission in double mutants. J Neurogenet. 1983;1:17–28. doi: 10.3109/01677068309107069. [DOI] [PubMed] [Google Scholar]
- 3.Wu CF, Ganetzky B, Haugland FN, Liu AX. Potassium currents in Drosophila: different components affected by mutations of two genes. Science. 1983;220:1076–1078. doi: 10.1126/science.6302847. [DOI] [PubMed] [Google Scholar]
- 4.Warmke J, Drysdale R, Ganetzky B. A distinct potassium channel polypeptide encoded by the Drosophila eag locus. Science. 1991;252:1560–1562. doi: 10.1126/science.1840699. [DOI] [PubMed] [Google Scholar]
- 5.Warmke JW, Ganetzky B. A family of potassium channel genes related to eag in Drosophila and mammals. Proc Natl Acad Sci USA. 1994;91:3438–3442. doi: 10.1073/pnas.91.8.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trudeau MC, Warmke JW, Ganetzky B, Robertson GA. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science. 1995;269:92–95. doi: 10.1126/science.7604285. [DOI] [PubMed] [Google Scholar]
- 7.Zünkler BJ. Human ether-a-go-go-related (HERG) gene and ATP-sensitive potassium channels as targets for adverse drug effects. Pharmacol Therapeut. 2006;112:12–37. doi: 10.1016/j.pharmthera.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 9.Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 10.De Ponti F, Poluzzi E, Cavalli A, Recanatini M, Montanaro N. Safety of non-antiarrhythmic drugs that prolong the QT interval or induce torsade de pointes: an overview. Drug Safety. 2002;25:263–286. doi: 10.2165/00002018-200225040-00004. [DOI] [PubMed] [Google Scholar]
- 11.Yap YG, Camm AJ. Drug induced QT prolongation and torsades de pointes. Heart. 2003;89:1363–1372. doi: 10.1136/heart.89.11.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah RR. Can pharmacogenetics help rescue drugs withdrawn from the market? Pharmacogenomics. 2006;7:889–908. doi: 10.2217/14622416.7.6.889. [DOI] [PubMed] [Google Scholar]
- 13.Aronov AM. Tuning out of hERG. Curr Opin Drug Discov Dev. 2008;11:128–140. [PubMed] [Google Scholar]
- 14.Smith PL, Baukrowitz T, Yellen G. The inward rectification mechanism of the HERG cardiac potassium channel. Nature. 1996;379:833–836. doi: 10.1038/379833a0. [DOI] [PubMed] [Google Scholar]
- 15.Crumb W, Cavero II. QT interval prolongation by non-cardiovascular drugs: issues and solutions for novel drug development. Pharm Sci Technol Today. 1999;2:270–280. doi: 10.1016/s1461-5347(99)00172-8. [DOI] [PubMed] [Google Scholar]
- 16.Recanatini M, Cavalli A, Masetti M. Modeling HERG and its interactions with drugs: recent advances in light of current potassium channel simulations. ChemMedChem. 2008;3:523–535. doi: 10.1002/cmdc.200700264. [DOI] [PubMed] [Google Scholar]
- 17.Recanatini M, Poluzzi E, Masetti M, Cavalli A, De Ponti F. QT prolongation through hERG K+ channel blockade: current knowledge and strategies for the early prediction during drug development. Med Res Rev. 2005;25:133–166. doi: 10.1002/med.20019. [DOI] [PubMed] [Google Scholar]
- 18.Kiss L, Bennett PB, Uebele VN, Koblan KS, Kane SA, Neagle B, Schroeder K. High throughput ion-channel pharmacology: planar-array-based voltage clamp. Assay Drug Dev Technol. 2003;1:127–135. doi: 10.1089/154065803321537845. [DOI] [PubMed] [Google Scholar]
- 19.Tao H, Santa Ana D, Guia A, Huang M, Ligutti J, Walker G, et al. Automated tight seal electrophysiology for assessing the potential hERG liability of pharmaceutical compounds. Assay Drug Dev Technol. 2004;2:497–506. doi: 10.1089/adt.2004.2.497. [DOI] [PubMed] [Google Scholar]
- 20.Chiu PJ, Marcoe KF, Bounds SE, Lin CH, Feng JJ, Lin A, Cheng FC, Crumb WJ, Mitchell R. Validation of a [3H]astemizole binding assay in HEK293 cells expressing HERG K+ channels. J Pharmacol Sci. 2004;95:311–319. doi: 10.1254/jphs.fpe0040101. [DOI] [PubMed] [Google Scholar]
- 21.Diaz GJ, Daniell K, Leitza ST, Martin RL, Su Z, McDermott JS, Cox BF, Gintant GA. The [3H]dofetilide binding assay is a predictive screening tool for hERG blockade and proarrhythmia: comparison of intact cell and membrane preparations and effects of altering [K+]o. J Pharmacol Toxicol Methods. 2004;50:187–199. doi: 10.1016/j.vascn.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Kongsamut S, Kang J, Chen XL, Roehr J, Rampe D. A comparison of the receptor binding and HERG channel affinities for a series of antipsychotic drugs. Eur J Pharmacol. 2002;450:37–41. doi: 10.1016/s0014-2999(02)02074-5. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Della Penna K, Wang H, Karczewski J, Connolly TM, Koblan KS, Bennett PB, Salata JJ. Functional and pharmacological properties of canine ERG potassium channels. Am J Physiol Heart Circ Physiol. 2003;284:H256–H267. doi: 10.1152/ajpheart.00220.2002. [DOI] [PubMed] [Google Scholar]
- 24.Piper DR, Duff SR, Eliason HC, Frazee WJ, Frey EA, Fuerstenau-Sharp M, et al. Development of the predictor HERG fluorescence polarization assay using a membrane protein enrichment approach. Assay Drug Dev Technol. 2008;6:213–223. doi: 10.1089/adt.2008.137. [DOI] [PubMed] [Google Scholar]
- 25.Singleton DH, Boyd H, Steidl-Nichols JV, Deacon M, Groot MJ, Price D, et al. Fluorescently labeled analogues of dofetilide as high-affinity fluorescence polarization ligands for the human ether-a-go-go-related gene (hERG) channel. J Med Chem. 2007;50:2931–2941. doi: 10.1021/jm0700565. [DOI] [PubMed] [Google Scholar]
- 26.Baxter DF, Kirk M, Garcia AF, Raimondi A, Holmqvist MH, Flint KK, et al. A novel membrane potential-sensitive fluorescent dye improves cell-based assays for ion channels. J Biomol Screen. 2002;7:79–85. doi: 10.1177/108705710200700110. [DOI] [PubMed] [Google Scholar]
- 27.Dorn A, Hermann F, Ebneth A, Bothmann H, Trube G, Christensen K, Apfel C. Evaluation of a high-throughput fluorescence assay method for HERG potassium channel inhibition. J Biomol Screen. 2005;10:339–347. doi: 10.1177/1087057104272045. [DOI] [PubMed] [Google Scholar]
- 28.Cheng CS, Alderman D, Kwash J, Dessaint J, Patel R, Lescoe MK, Kinrade MB, Yu W. A high-throughput HERG potassium channel function assay: an old assay with a new look. Drug Dev Ind Pharm. 2002;28:177–191. doi: 10.1081/ddc-120002451. [DOI] [PubMed] [Google Scholar]
- 29.Rezazadeh S, Hesketh JC, Fedida D. Rb+ flux through hERG channels affects the potency of channel blocking drugs: correlation with data obtained using a high-throughput Rb+ efflux assay. J Biomol Screen. 2004;9:588–597. doi: 10.1177/1087057104264798. [DOI] [PubMed] [Google Scholar]
- 30.Tang W, Kang J, Wu X, Rampe D, Wang L, Shen H, et al. Development and evaluation of high throughput functional assay methods for HERG potassium channel. J Biomol Screen. 2001;6:325–331. doi: 10.1177/108705710100600506. [DOI] [PubMed] [Google Scholar]
- 31.Niswender CM, Johnson KA, Luo Q, Ayala JE, Kim C, Conn PJ, Weaver CD. A novel assay of Gi/o-linked G protein-coupled receptor coupling to potassium channels provides new insights into the pharmacology of the group III metabotropic glutamate receptors. Mol Pharmacol. 2008;73:1213–1224. doi: 10.1124/mol.107.041053. [DOI] [PubMed] [Google Scholar]
- 32.Weaver CD, Harden D, Dworetzky SI, Robertson B, Knox RJ. A thallium-sensitive, fluorescence-based assay for detecting and characterizing potassium channel modulators in mammalian cells. J Biomol Screen. 2004;9:671–677. doi: 10.1177/1087057104268749. [DOI] [PubMed] [Google Scholar]
- 33.Weber M, Ferrer M, Zheng W, Strulovici B, Kunapuli P. A 1536-well cAMP assay for Gs- and Gi-coupled receptors using enzyme fragmentation complementation. Assay Drug Dev Technol. 2004;2:39–49. doi: 10.1089/154065804322966306. [DOI] [PubMed] [Google Scholar]
- 34.Jorgensen S, Johansen TH, Dyhring T. Fluorescence-based Tl+-influx assays as a novel approach for characterization of small-conductance Ca2+-activated K+ channel modulators. Methods Mol Biol. 2008;491:257–266. doi: 10.1007/978-1-59745-526-8_20. [DOI] [PubMed] [Google Scholar]
- 35.Anderson CL, Delisle BP, Anson BD, Kilby JA, Will ML, Tester DJ, et al. Most LQT2 mutations reduce Kv11.1 (hERG) current by a class 2 (trafficking-deficient) mechanism. Circulation. 2006;113:365–373. doi: 10.1161/CIRCULATIONAHA.105.570200. [DOI] [PubMed] [Google Scholar]
- 36.Ames R, Fornwald J, Nuthulaganti P, Trill J, Foley J, Buckley P, Kost T, Wu Z, Romanos M. BacMam recombinant baculoviruses in G protein-coupled receptor drug discovery. Receptors Channels. 2004;10:99–107. doi: 10.1080/10606820490514969. [DOI] [PubMed] [Google Scholar]
- 37.Kost TA, Condreay JP, Ames RS, Rees S, Romanos MA. Implementation of BacMam virus gene delivery technology in a drug discovery setting. Drug Discov Today. 2007;12:396–403. doi: 10.1016/j.drudis.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 38.Inglese J, Auld DS, Jadhav A, Johnson RL, Simeonov A, Yasgar A, Zheng W, Austin CP. Quantitative high-throughput screening: a titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc Natl Acad Sci USA. 2006;103:11473–11478. doi: 10.1073/pnas.0604348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hille B. Potassium channels in myelinated nerve: selective permeability to small cations. J Gen Physiol. 1973;61:669–686. doi: 10.1085/jgp.61.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen MX, Sandow SL, Doceul V, Chen YH, Harper H, Hamilton B, Meadows HJ, Trezise DJ, Clare JJ. Improved functional expression of recombinant human ether-a-go-go (hERG) K+ channels by cultivation at reduced temperature. BMC Biotechnol. 2007;7:93. doi: 10.1186/1472-6750-7-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hed J. The extinction of fluorescence by crystal violet and its use to differentiate between attached and ingested microorganisms in phagocytosis. FEMS Microbiol Lett. 1977;1:357–361. [Google Scholar]
- 42.Loike JD, Silverstein SC. A fluorescence quenching technique using trypan blue to differentiate between attached and ingested glutaraldehyde-fixed red blood cells in phagocytosing murine macrophages. J Immunol Methods. 1983;57:373–379. doi: 10.1016/0022-1759(83)90097-2. [DOI] [PubMed] [Google Scholar]
- 43.Bjerknes R, Bassoe CF. Phagocyte C3-mediated attachment and internalization: flow cytometric studies using a fluorescence quenching technique. Blut. 1984;49:315–323. doi: 10.1007/BF00320205. [DOI] [PubMed] [Google Scholar]
- 44.Zheng W, Spencer RH, Kiss L. High throughput assay technologies for ion channel drug discovery. Assay Drug Dev Technol. 2004;2:543–552. doi: 10.1089/adt.2004.2.543. [DOI] [PubMed] [Google Scholar]