Abstract
The aim of this study was to examine the associations of Apolipoprotein E (APOE) genotype, metabolic changes in the posterior cingulate detected by 1H magnetic resonance spectroscopy (MRS), and neuropsychologic measures of memory and cognition both in normally aging elderly, and in patients with mild cognitive impairment (MCI) and AD. We studied 67 controls, 18 MCI and 33 AD patients. We used the Dementia Rating Scale total score (DRSTOT) as a measure of general cognitive function and the total learning from the Auditory Verbal Learning Test (AVTOT) as a measure of memory performance. No differences were noted on 1H MRS metabolite ratios or cognitive measures across APOE genotype within control and patient groups.. In controls, age was a significant predictor of both cognitive test scores, and NAA/Cr was a univariate associate of DRSTOT. All three 1H MRS metabolite ratios, N-acetylaspartate (NAA)/Creatine (Cr), myoinositol (MI)/Cr, and NAA/MI, were univariate associates of AVTOT and DRSTOT scores in the combined MCI and AD group. In stepwise regression analyses in the combined patient group only NAA/MI entered the model. These data suggest NAA/Cr could be a modest predictor of general cognitive function in both healthy elderly and impaired patients, while MI/Cr is a more specific marker for neuropsychologic dysfunction associated with neurodegenerative disease. Among 1H MRS measurements, the NAA/MI ratio maybe the most efficient predictor of memory and cognitive function in patients with MCI and AD.
Keywords: 1HMRS, Cognition, Aging, Mild Cognitive Impairment, Alzheimer’s Disease
INTRODUCTION
Recognition of Apolipoprotein E (APOE) as a susceptibility gene for Alzheimer’s disease (AD) (Strittmatter et al., 1993), along with the development of medications, which may serve to slow or delay the clinical course of AD (Rogers et al., 1998) have only served to intensify efforts at the early, even pre-clinical, detection of AD. APOE genotype is the best established susceptibility gene and has been shown to influence age of onset (Mayeux et al., 1993; Tsai et al., 1994) and the underlying histopathology of AD (Rebeck, et al., 1993; Strittmatter et al., 1994). Yet knowledge of APOE genotype has limited utility for early detection of disease (Relkin, et al., 1996) or as an indictor of disease progression (Waring et al., 1996). To date cognitive measures continue to serve as perhaps the best tools for early or pre-clinical detection of dementia, but the association of APOE genotype and cognitive measures remains controversial (Bondi, et al., 1999; Mayeux, et al., 2001; Smith et al., 1998).
An important clinically defined group for early diagnosis and treatment of AD are people with amnestic mild cognitive impairment (MCI) (Petersen et al., 2001; Smith et al., 1996). These individuals have progressive loss of memory efficiency, and the majority of them develop AD within 5 years of receiving a diagnosis of MCI (Petersen et al., 1995; Petersen et al., 1999). While patients with amnestic MCI do not meet the clinical criteria for dementia, many of them have early AD pathology (Grober et al., 1999; Kordower et al., 2001; Schmitt et al., 2000). Neuroimaging measurements that can predict disease severity in patients with MCI and AD may be used as a marker for monitoring therapeutic efficacy in drug trials.
1H MRS has identified increased myoinositol (MI)/Creatine (Cr) levels in patients with MCI and AD, and a regional reduction of N-acetylaspartate (NAA)/Cr in people with AD in line with the spatial distribution of neurofibrillary pathology (Catani et al., 2001; Kantarci et al., 2000). NAA is a marker for neuronal integrity, and NAA levels positively correlate with Mini-Mental State Examination scores (MMSE; Folstein, et al., 1975) in AD; both at baseline, and when followed longitudinally (Doraiswamy, et al., 1998; Huang et al., 2001; Jessen et al., 2001; Kwo-On-Yuen et al., 1994; Rose et al., 1999; Schuff et al., 1998). On the other hand, MI levels were found to be associated with MMSE in one study (Rose et al., 1999), and not associated with MMSE in another (Huang et al., 2001). Although the reason for elevation of MI levels in AD is not clear, in the brain, highest concentration of MI is present in glial cells (Ross et al., 1998). It is possible that elevated MI level is an indicator of glial proliferation in AD (Huang et al., 2001; Kantarci et al., 2000; Valenzuela and Sachdev, 2001).
Alteration of NAA and MI levels in AD, and recently in patients with MCI have been confirmed by several investigators (Catani et al., 2001; Kantarci et al., 2000; Klunk, et al., 1992; Miller et al., 1993; Schuff et al., 1997). The relationship of these biochemical changes to the progressive functional decline in MCI and AD, however, is not clear. The aim of this study was to extend our prior study of 1H MRS variables in normally aging elderly and in patients with MCI and AD (Kantarci et al., 2000) by including both APOE and cognitive data.
Methods
Recruitment and Characterization of Participants
All of the participants were recruited from the Alzheimer’s Disease Research Center (ADRC)/Alzheimer’s Disease Patient Registry (ADPR) at the Mayo Clinic, Rochester, Minnesota. These are Institutional Review Board (IRB) approved prospective studies of aging and dementia (Petersen et al., 1990). Informed consent for participation was obtained from every subject and/or an appropriate surrogate. Individuals participating in these ADRC/ADPR projects were evaluated by a behavioral neurologist and a neuropsychologist. An extensive battery of neuropsychological tests was performed on all participants. From this battery, we were primarily interested in measures of global cognitive function and memory that would minimize ceiling and floor effects across groups. We elected to use the Dementia Rating Scale total score (DRSTOT) (Mattis, 1998) as the measure of general cognitive function. DRSTOT has a strong association with disease progression (Smith et al., 1994; Smith et al., 2001) and is less susceptible to ceiling effects in normals than traditional mental status measures like the MMSE. For memory we considered using delayed recall measures but were concerned about floor effects in the cognitively impaired sample. Thus total learning (sum of trials 1–5) from the Auditory Verbal Learning Test (AVTOT) (Rey, 1964) was selected as the measure of memory performance.
All of the participants underwent laboratory tests including a chest radiograph, ECG, chemistry profile, CBC count, thyroid function tests, vitamin B-12 level, folic acid level, syphilis serology, and structural brain MRI. Apolipoprotein E genotype was determined by established polymerase chain reaction techniques (Hixon & Vernier, 1990). Participants with genotype 2\2, 2\3, and 3\3 were labeled E4 −. Genotypes 3\4 and 4\4 were labeled E4 +. Since the impact of genotype 2\4 on AD risk remains unclear, data for this genotype was suppressed (n=2 controls, 3 AD and 1 MCI). APOE genotype data was missing for 1 MCI and 4 controls. At the completion of the evaluation, a consensus committee meeting was held involving the behavioral neurologists, neuropsychologists, nurses, and the geriatrician who evaluated the participants. Participants with structural abnormalities that could produce dementia other than AD, e.g., cortical infarction, tumor, subdural hematoma or who had concurrent illnesses or treatments interfering with cognitive function were excluded. Participants were not excluded for the presence of leukoariosis.
The diagnosis of probable AD was made according to the Diagnostic and Statistical Manual for Mental Disorders 3rd edition – revised (DSM-III-R; American Psychiatric Association, 1987)criteria for dementia, and the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorder’s Association (NINCDS/ADRDA; McKhann et al., 1984) criteria for AD.
The clinical criteria for MCI were: (1) memory complaint, preferably corroborated by an informant, (2) objective memory impairment, (3) normal general cognitive function, (4) intact activities of daily living, (5) not demented (Petersen et al., 2001). Controls were defined as individuals who: (1) were independently functioning community dwellers, (2) did not have active neurological or psychiatric conditions, (3) had no cognitive complaints, (4) had a normal neurological exam, and (5) were not taking any psychoactive medications in doses that would impact cognition (Ivnik et al., 1992). Because the controls’ cognitive data is used in normative neuropsychological studies, clinicians were not permitted to use the cognitive data in determining control status.
One hundred eighteen participants were included in this study. One hundred four of these participants were included in our prior report on group differences in 1H MRS (Kantarci et al., 2000). At the time of imaging, 72 of these participants were deemed controls, 25 met criteria for MCI and 21 met criteria for AD. All ADPR and ADRC participants received longitudinal follow-up including reassessment with neuropsychological measures and review of consensus diagnoses. For the participants in this study, the average follow-up interval was 17.1 (± 10.3) months. At last evaluation, five of the 72 normals had developed cognitive impairment (4 met MCI criteria, 1 met probable AD criteria). Eleven of the twenty-five MCI patients had progressed to a dementia diagnosis. Since final diagnosis incorporates the most comprehensive clinical data on the patient, this diagnosis used to classify participants for the analyses. Thus the final groups included 67 controls, 18 MCI patients and 33 AD patients.
MRI and 1H-MRS
MR imaging and single voxel (SV) 1H MRS studies were performed on a 1.5 T (Signa; General Electric Medical Systems, Milwaukee, WI) scanner within six months of the neuropsychological assessment. After an axial scout, T1 weighted images in sagittal plane was obtained for localizing the 1H MRS voxel. 1H MRS studies were performed with the LX system automated single voxel MRS package: Proton Brain Examination/Single Voxel (PROBE/SV) (Webb et al., 1994) (General Electric Medical Systems, Milwaukee, WI). Point resolved spectroscopy (PRESS) pulse sequence with TR/TE = 2000/30 ms, 2048 data points and 128 excitations were used for the examinations. The prescan algorithm of PROBE automatically adjusts the transmitter and receiver gains and center frequency. The local magnetic field homogeneity is optimized with the three-plane, auto-shim procedure, and the flip angle of the third water suppression pulse is adjusted for chemical-shift-water suppression (CHESS) prior to PRESS acquisition.
We have previously studied regional variations of 1H MRS findings in patients with MCI and AD. Of the neocortical (superior temporal and occipital) and limbic cortical regions (posterior cingulate gyrus) studied, posterior cingulate gyrus NAA/Cr, MI/Cr and NAA/MI ratios best distinguished among controls, MCI, and AD patients (Kantarci et al., 2000). We therefore studied the posterior cingulate gyri for this project. An 8 cm3 (2×2×2cm) volume of interest (VOI) was prescribed on a mid-sagittal T1 weighted image, placed below the cingulate sulci and above the parieto-occipital sulci, covering the posterior cingulate gyri and inferior precunei bilaterally (Figure 1). We analyzed the metabolites NAA/Cr and MI/Cr intensity ratios, which were automatically calculated at the end of each PROBE/SV acquisition, with respect to the metabolite creatine (Cr). We did not include diabetic participants in the study group because MI/Cr ratios increase in the brains of patients with diabetes mellitus (Kreis and Ross, 1992).
Figure 1.
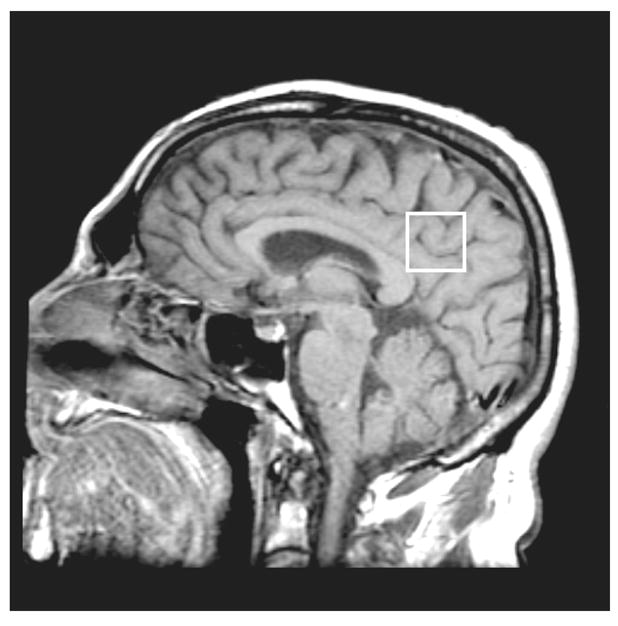
Placement of the 1H MRS volume of interest (VOI) on a mid-sagittal T1-weighted image. The VOI is placed below the cingulate sulci and above the parieto-occipital sulci covering both of the posterior cingulate gyri and inferior precunei.
Statistical Analyses
One way ANOVAs, with post-hoc pairwise comparisons (utilizing Tukey’s Honestly Significant Difference), were used to compare the clinical groups on mean age, years of education, neuropsychological test measurements and 1H MRS variables. Differences on cognitive and 1H MRS variables based on APOE status were examined within clinical groups using standard t-tests. To analyze the association between the neuropsychological test measurements and 1H MRS variables, stepwise linear regression models were constructed for each test measure (DRSTOT and AVTOT). Since predictor and dependent variables displayed differences across groups, regression analyses were completed on separate groups: (1) controls, (2) MCI patients alone, (3) AD patients alone and (4) a combined patient sample of both MCI and AD. First univariate correlations were examined in the control and combined patient sample. Then stepwise multiple regression analyses followed. Default p values for entry in the stepwise models were <0.10. Models were constructed for each neuropsychologic test measurement with the variables: age, education, NAA/Cr, and MI/Cr and NAA/MI..
RESULTS
Demographic, genotype, cognitive, and 1H MRS aspects of the study groups of 67 controls, 18 MCI patients, and 33 AD patients are presented in Table 1.
Table 1.
Demographic, cognitive and 1H magnetic resonance spectroscopy variable by group
| Control | MCI | AD | |
|---|---|---|---|
| N | 67 | 18 | 33 |
| Age (mean ± SD) | 80.8 ± 7.1 | 82.0 ± 5.4 | 79.8 ± 6.0 |
| Male/Female | 26/41 | 10/8 | 18/15 |
| % E4+ | 13a | 56b | 40b |
| Education (mean ± SD) | 13.5 ± 2.9 | 13.3 ± 3.6 | 13.3 ± 3.1 |
| AVTOT (mean ± SD) | 39.3 a ± 8.0 | 28.1 b ± 6.9 | 19.8 c ± 6.2 |
| DRSTOT (mean ± SD) | 138.0a ± 4.6 | 132.6b ± 6.4 | 117.9c ± 14.1 |
| NAA/Cr (mean ± SD) | 1.52a ± .10 | 1.52a ± .08 | 1.42b ± .11 |
| MI/Cr (mean ± SD) | 0.63a ± .08 | 0.67 a,b ± .08 | 0.70 b ± .09 |
| NAA/MI (mean ± SD) | 2.47a ± .36 | 2.30a,b ± .33 | 2.08b ± .30 |
Note; AVTOT=Auditory Verbal Learning Test Sum of Trials 1–5, DRSTOT=Dementia Rating Scale Total Score, NA= N-acetylaspartate, MI=myoinositol,. Cr=Creatine. Means in the same row that have different subscripts differ at p <.05 level on Tukey’s honestly significant difference comparison.
Age (F(2,115=0.70, p=.50) and years of education (F(2,115=0.11, p=.90) were not different across groups. There was no difference in the male/female ratios (χ2 (2, N=118)=3.027, p=0.22). As expected, the controls had a lower frequency of E4+ than the clinical groups (χ2(2, N=107)=15.45, p= 0.001). Groups differed on AVTOT, (F(2,115)=81.34, p=0.0001),) with AD<MCI<Controls. Also as expected, DRSTOT scores differed across groups (F(2,115)=60.77, p=0.0001), with AD<MCI<Controls. Group differences were detected for NAA/Cr (F(2,115)= 10.27, p=0.0001) with only the AD<MCI=control. MI/Cr (F(2,115)=8.89, p=0.0003) and NAA/MI (F(2,115)=14.29, p=.0001) also differed across groups with AD>Control and no pairwise differences for MCI for both variables.
Table 2 presents mean comparisons on cognitive and 1H MRS data by APOE group (E4+ versus E4−) separately for the control and patient samples. There were no significant differences observed for across DRSTOT, AVTOT, NAA/Cr, MI/Cr or NAA/MI for either sample.
Table 2.
Mean and standard deviation for cognitive and 1H magnetic resonance spectroscopy variables by apolipoprotein E-4 genotype status and group.
| Controls (N = 61) | Patients (N = 46) | |||
|---|---|---|---|---|
| E4+ (N=8) | E4−(N=53) | E4+ (N=21) | E4−(N=25) | |
| AVLT Total | 39.1 ± 9.8 | 39.3 ± 8.1 | 24.0 ± 8.2 | 21.6 ± 7.5 |
| DRS Total | 135.4 ± 6.0 | 138.4 ± 4.3 | 123.2 ± 12.1 | 123.1 ± 16.1 |
| NAA/Cr | 1.54 ± 0.08 | 1.51 ± 0.10 | 1.47 ± 0.10 | 1.46 ± 0.11 |
| MI/Cr | 0.65 ± 0.09 | 0.63 ± 0.08 | 0.68 ± 0.10 | 0.69 ± 0.09 |
| NAA/MI | 2.39 ± 0.28 | 2.46 ± 0.37 | 2.19 ± 0.33 | 2.16 ± 0.34 |
Note: AVLT Total =Auditory Verbal Learning Test Sum of Trials 1–5, DRS=Dementia Rating Scale, NAA= N-acetylaspartate, MI=myoinositol,. Cr=Creatine. All values are means ± standard deviations. Two controls, 3 AD patients and 1 MCI patient with genotype 2\4 were excluded. APOE genotype data was missing for 1 MCI and 4 controls. All within group comparisons, p>0.05.
Table 3 lists the univariate Pearson correlations by group for variables subsequently included in stepwise multivariate modeling. Within controls both cognitive variables were significantly associated with age. DRSTOT was modestly associated with education and with NAA/CR as well. AVTOT was not associated with any of the 1HMRS metabolite ratios. In the patients, both cognitive variables were strongly associated with all of the 1HMRS metabolite ratios. In addition DRSTOT was marginally associated with education.
Table 3.
Univariate Pearson correlations among demographic, cognitive and 1H magnetic resonance spectroscopy variables by group.
| Controls | Age | Education | DRS Total | AVLT Total | NAA/CR | MI/CR | NAA/MI |
|---|---|---|---|---|---|---|---|
| Patients | |||||||
| Age | −.04 | −.32*** | −.35*** | −.21* | −.04 | −.03 | |
| Education | .13 | .22* | .18 | .15 | −.19 | .23** | |
| DRS Total | .08 | .26* | .41*** | .26** | .09 | .06 | |
| AVLT Total | .04 | .19 | .73*** | .11 | −.03 | .03 | |
| NAA/Cr | .22 | .12 | .41*** | .34** | .04 | .43*** | |
| MI/CR | −.23 | −.18 | −.44*** | −.31** | −.09 | −.85*** | |
| NAA/MI | .33** | .22 | .53*** | .46*** | .50*** | −.86*** |
Note: AVLT Total =Auditory Verbal Learning Test Sum of Trials 1–5, DRS=Dementia Rating Scale, NAA= N-acetylaspartate, MI=myoinositol,. Cr=Creatine.
p<0.10,
p<0.05,
p<0.01.
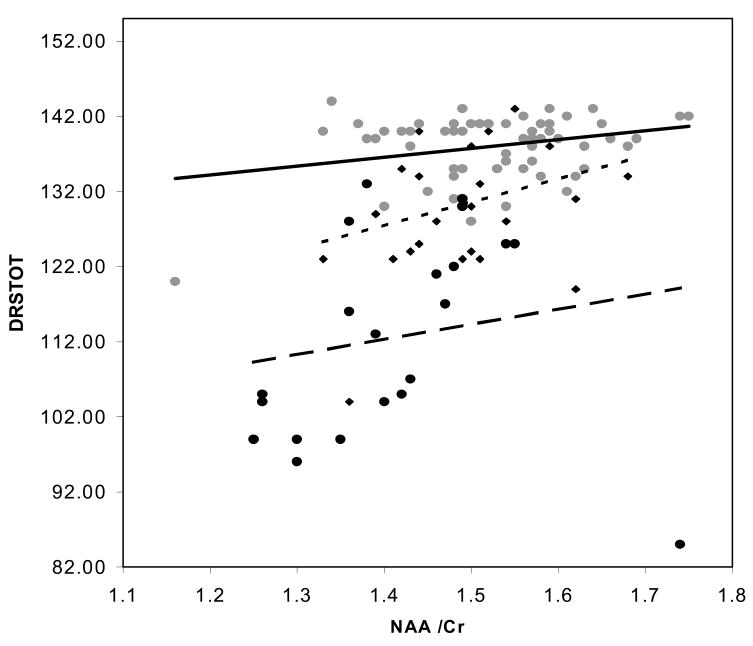
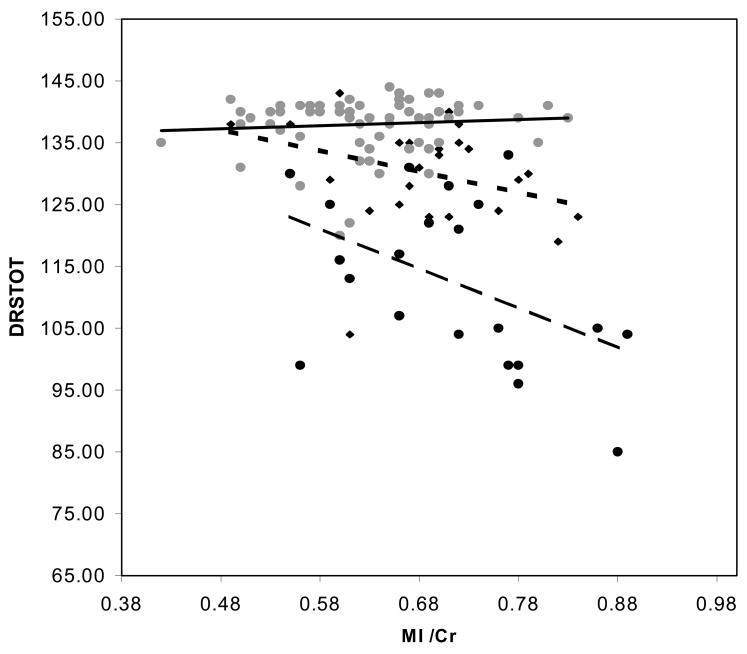
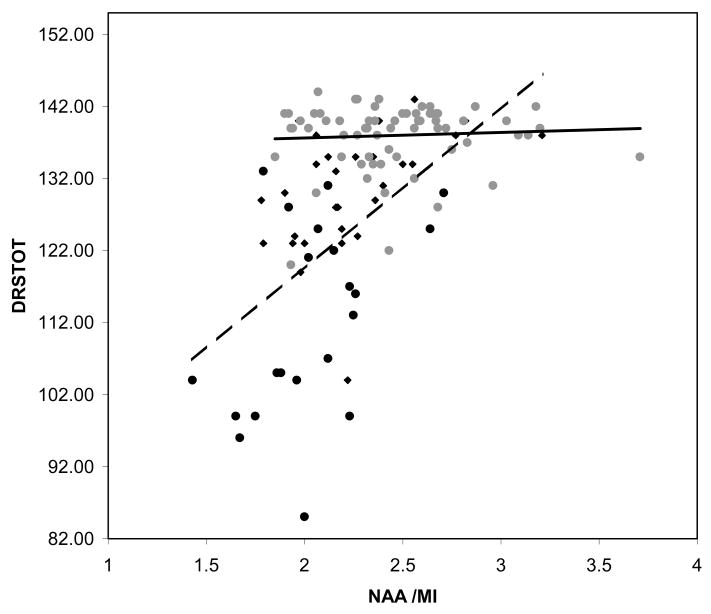
Since, age, education and all three metabolite ratios variously associated with the cognitive variables in univariate analyses, all 5 were included in stepwise multiple regression analyses. Table 4 lists predictors of AVTOT scores in the controls, MCI patients, AD patients and combined patient sample. For controls, age was a significant predictor of AVTOT scores. MI/Cr was a significant predictor of AVTOT for the MCI patients and no predictor reached significance in the AD group. In the combined sample NAA/MI served as the sole significant AVTOT predictor. Table 5 lists the regression models for DRSTOT. Education was a marginal predictor, of DRSTOT in controls along with age. MI/Cr again proved a significant predictor of DRSTOT in the MCI group. NAA/MI was the sole term to enter the model for both AD alone and the combined patient sample. Thus, none of 1HMRS metabolite rations were predictors of AVTOT and DRSTOT scores in controls after age had entered the model. NAA/MI was the sole predictor of both AVTOT and DRSTOT in the combined patients models. Contrary to findings in the controls, age was not a predictor of test scores in the patient sample. The relationship between DRSTOT scores and NAA/Cr, MI/Cr, in the controls, and in MCI and AD patient samples are displayed in Figure 2. The relationship between DRSTOT scores and NAA/MI in controls and in the combined patient sample is displayed in Figure 3.
Table 4.
Predictors of Auditory Verbal Learning Test total learning by final diagnostic group.
| Test | Predictor variables | Step | Model R2 | P |
|---|---|---|---|---|
| Controls (n=67)l | Age | 1 | 0.12 | 0.004 |
| MCI (n=18) | .MI/CR | 1 | 0.23 | .04 |
| AD (n=33) | - | - | - | - |
| MCI+AD (n=51) | NAA/MI | 1 | 0.21 | 0.0007 |
Note: NAA= N-acetylaspartate, MI=myoinositol,. Cr=Creatine. Age, education, NAA/Cr, MI/Cr, and NAA/MI were candidates to enter the stepwise regression model.
Table 5.
Predictors of Dementia Rating Scale total scores by final diagnostic group.
| Test | Predictor variables | Step | Model R2 | P |
|---|---|---|---|---|
| Controls (n=67) | Age | 1 | 0.10 | 0.009 |
| Education | 2 | 0.14 | 0.072 | |
| MCI (n=18) | MI/CR | 1 | .35 | .01 |
| AD (n=33) | NAA/MI | 1 | .21 | .007 |
| MCI+AD (n=51) | NAA/MI | 1 | 0.28 | 0.0001 |
Note: NAA= N-acetylaspartate, MI=myoinositol,. Cr=Creatine. Age, education, NAA/Cr, MI/Cr, and NAA/MI were candidates to enter the stepwise regression model
Figure 2.
Plot of Dementia Rating Scale Total scores and (a) = N-acetylaspartate, to creatine ratio, (b) myoinositol to creatine ratio, and (c) N-acetylaspartate to myoinositol ratio. Controls represented by ( ) marker and solid regression line, Patients represented by (◆) and dotted line for MCI and (●) and dashed trend line for AD.
) marker and solid regression line, Patients represented by (◆) and dotted line for MCI and (●) and dashed trend line for AD.
Figure 3.
Plot of Dementia Rating Scale Total scores and N-acetylaspartate to myoinositol ratio. Controls represented by ( ) marker and solid regression line, Patients represented by (ms;) for MCI, and (l;) and for AD. The regression line for the combined AD and MCI sample is dashed.
) marker and solid regression line, Patients represented by (ms;) for MCI, and (l;) and for AD. The regression line for the combined AD and MCI sample is dashed.
DISCUSSION
Group Assignment and Region of Interest
The participants of this study span the spectrum of cognitive performance from normal aging through undetected cognitive impairment, to MCI, and AD. The neuropsychologic measures clearly demonstrate this progressive decline of memory efficiency and cognition from controls to AD. The present analyses encompass a dilemma that arises in studies of this continuum. This dilemma involves how to categorize participants with dynamic status. Our approach was to use all clinical data available to us at the time of the analyses to assign participants to groups. This includes clinical evaluations collected after the time of scanning and cognitive testing. Use of all available longitudinal data at the time of the analyses enabled us to identify five initial controls who had progressed to have cognitive impairment, as well as to recognize that just under 50% of our MCI patients had progressed, as expected, to AD.
Use of this approach is justified since the histopathology underlying AD (and most degenerative dementias) is evolving for years prior to clinical manifestation (Braak and Braak, 1991). This histopathology probably influences brain-behavior relationships, such as the association of MRS metabolites and cognition, prior to clinical detection. The most accurate assignment of persons to groups would involve waiting for autopsy to determine the presence or absence of AD histopathology (i.e. Definite AD diagnosis) and then retrospectively assign groups. However this delay can be prohibitive to research progress. Use of longitudinal data provides the next best alternative for accurate classification of patients. If clinical diagnosis at the time of data collection is used to assign groups, even when longitudinal information shows this diagnosis to have changed, then error is knowingly being introduced into within group data analyses. While clinical diagnosis is always inaccurate to a degree in dementia research, and may be even molre so for “preclinical disease”, use of longitudinal data improves diagnostic accuracy
We chose to study the posterior cingulate gyri because it is a limbic cortical region involved with the neurofibrillary pathology of AD fairly early in the disease course (Braak and Braak, 1991). Besides, our previous study in an overlaping sample showed that 1H MRS of the posterior cingulate gyri was more sensitive to the biochemical changes in patients with MCI and AD than 1H MRS of other neocortical regions in the brain (Kantarci et al., 2000). Studies with PET have also identified the posterior cingulate gyri as being the most significantly affected region of the brain in both patients with early AD, and asymptomatic carriers of the APOE ε4 allele (Minoshima et al., 1997, Reiman et al., 1996) An alternative region to study would have been the entorhinal cortex and hippocampus. However this was not possible due to the technical difficulties of achieving an adequately homogenous magnetic field close to the susceptibility effects by the tissue-air interface near the petrous bone. Moreover, obtaining spectra from a voxel small enough to sample the anteromedial temporal lobe structures without partial voluming could not be achieved. Thus, the most technically robust and functionally appropriate region to study was the posterior cingulate gyri.
APOE Effects
In line with prior research, APOE 4 is disproportionately represented in our patient sample (46% of the patient sample was E4+ compared to 13% of the control sample). These findings are consistent with the well know association of APOE 4+ status and risk for AD.
These findings also reinforce the absence of APOE 4+ effects on cognitive performance (Smith et al., 1998), especially once control samples are “cleaned” of undetected cases via longitudinal follow-up (Bondi et al., 1999). [To further illustrate this point we conducted an incidental analysis wherein the five converts were included with the control sample as would have occurred if the longitudinal data were not incorporated into clinical classification. Using this group assignment, there was a significant difference across APOE groups for DRSTOT (E4+ mean = 134.9, sd = 6.8; E4− mean = 138.4, sd = 4.2; T = −2.2, df = 64, p = .02]. The present findings of no association in the patient sample between APOE 4 status and cross-sectionally measured cognitive function are also generally consistent with recent research (Mayeux et al., 2001; Small et al., 2000). However, these results appear at odds with our own prior findings (Smith et al., 1998), specifically of APOE differences on the Mayo Cognitive Factor Scores (MCFS) (Smith et al., 1994) Learning Factor. Statistical power or methodological differences (e.g., AVTOT as used here was age unadjusted, while the MCFS Learning Factor is age adjusted) may account for these discrepant findings, but further investigation of this issue is warranted.
The finding of no association between APOE 4 status and 1HMRS metabolite ratios have previously been reported in a largely overlapping sample (Kantarci et al., 2000). This dissociation between APOE genotype and 1HMRS changes is also apparent in the relationship of these variables with the neuropsychological test scores in our data. While all 1HMRS metabolite ratios (NAA/Cr, MI/Cr, NAA/MI) were associated with neuropsychologic measures of memory and cognitive function in patients with MCI and AD, APOE 4 status was not. In AD, the degree of cognitive impairment correlates with neurofibrillary tangle but not with senile plaque burden (Arriagata et al., 1992). On the contrary, APOE 4 status correlates with senile plaque burden but not with the number of neurofibrillary tangles (Gomes-Isla et al., 1996). It is possible that APOE 4 status and 1H MRS measurements relate to different pathologic features of AD. For example, as APOE 4 status correlates with senile plaque burden, 1H MRS measurements may be associated with the number of neurofibrillary tangles. Investigating histopathologic correlates of 1H MRS findings may clarify this dissociation.
1HMRS Metabolites and Cognition
In normal elderly, age was associated with both AVTOT and DRSTOT scores. Education showed a trend towards association with DRSTOT. The only 1H MRS variable and cognitive measure to show a univariate association was NAA/CR with DRSTOT. NAA is found in neurons, and it decreases with neuron loss and neuronal function (Barker, 2001; Hugg et al., 1996; Tsai and Coyle, 1995). The trend of positive correlation between DRSTOT and NAA/Cr may be explained in three ways: one explanation is that brain Cr levels increase with aging (Chang, et al., 1996; Pfefferbaum, et al, 1999). Although there are conflicting results in the literature, many have shown that NAA levels are fairly stable with aging (Chang et al., 1996; Charles et al., 1994; Pfefferbaum et al., 1999). Results of a recent study (Valenzuela et al., 2000) that showed that the relation between NAA/Cr ratios and fluid intellectual ability in healthy elderly were independent of age, argue against this interpretation. An alternate and more viable explanation is that, NAA is a marker for neuronal integrity, and thus cognitive functional ability in normal participants. This interpretation is consistent with data reported in younger adults, indicating a positive correlation between cognitive performance and NAA levels (Jung et al., 1999). A third interpretation of the association between NAA/Cr and DRSTOT scores in controls is that individuals with lower NAA/Cr and lower DRSTOT scores were actually pre-symptomatic patients within the control group who did not convert to MCI nor AD during the follow-up period, producing this association. If this were the case however, one would expect to see a similar effect with MI/Cr or NAA/MI ratios, a finding not present in our data. In any event, once age enters the multivariate model NAA/Cr does not explain significant residual variance in cognitive function.
Age was not a predictor of test scores for patients with MCI and AD, presumably because the disease process itself has a far greater effect on memory and cognition in these patients than aging. Both NAA/Cr and MI/Cr were associated with AVTOT and DRSTOT scores in univariate analysis. Naturally, by extending the ranges of both the cognitive and metabolite scores, these associations were clearest in the combined patient sample. These findings are in-line with previous studies (Huang et al., 2001; Kwo-On-Yuen et al., 1994; Rose et al., 1999; Schuff et al., 1998) that showed a positive correlation between NAA levels, and a negative correlation between MI levels and MMSE. For patients, the ratio NAA/MI appeared to capitalized on the joint associations of NAA/Cr and MI/Cr with neuropsychologic test scores in order to be the sole significant term in stepwise multiple regression modeling of DRSTOT than AVTOT.
Conclusion
Overall, our data suggest that NAA/Cr may be an age-related correlate of general cognitive function in healthy elderly individuals. In neuropsychologically impaired patients (with MCI and\or AD), NAA/Cr may also be a non-specific marker for neuropsychologic functioning. On the other hand, MI/Cr is associated with cognition only in MCI and AD patients. Therefore, MI/Cr might be a specific marker for neurodegenerative disease. Because MI is predominantly present in astrocytes, a possible relationship between the elevation of MI levels, and glial activation in AD is an important research question. Combining NAA/Cr and MI/Cr ratios into NAA/MI, seems to maximize the association of 1H MRS metabolites and cognitive performance in patients with MCI and AD. Hence, among the 1H MRS metabolites, posterior cingulate gyri measured NAA/MI may be particularly be a useful marker for depicting therapeutic effects in AD clinical trials. Moreover, dissociations between NAA/MI measures and cognitive performance may identify people whose cognitive dysfunction is not attributable to glial-activating disease processes. For MCI patients, such dissociations may have implications for likelihood and rate of subsequent decline. Inquiry into this possibility is currently underway.
Acknowledgments
This study supported by the National Institute on Aging grants AG11378, AG06786, and AG16574.
This research supported by National Institute on Aging, grants AG11378 AG08031, AG06786, AG16574, and by the Dana Foundation.
References
- American Psychiatric Association. DSM-III-R Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association Press; 1987. (Revised 3rd ed.) [Google Scholar]
- Arriagata P, Growdon J, Hedley-Whyte E, Hyman B. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- Barker P. N-acetyl aspartate--a neuronal marker? Annals of Neurology. 2001;49:423–424. [PubMed] [Google Scholar]
- Bondi M, Salmon D, Galasko D, Thomas R, Thal L. Neuropsychological function and apolipoprotein E genotype in the preclinical detection of Alzheimer’s disease. Psychology & Aging. 1999;14:295–303. doi: 10.1037//0882-7974.14.2.295. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer’s disease. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Catani M, Cherubin IA, Howard R, Tarducci R, Pelliccioli G, Piccirilli M, Gobbi G, Senin U, Mecocci P. 1H-MR spectroscopy differentiates mild cognitive impairment from normal brain aging. Neuroreport. 2001;12:2315–2317. doi: 10.1097/00001756-200108080-00007. [DOI] [PubMed] [Google Scholar]
- Chang L, Ernst T, Poland R, Jenden D. In vivo proton magnetic resonance spectroscopy of the normal aging human brain. Life Sciences. 1996;58:2049–2056. doi: 10.1016/0024-3205(96)00197-x. [DOI] [PubMed] [Google Scholar]
- Charles H, Lazeyras F, Krishnan K, Boyko O, Patterson L, Doraiswamy P, McDonald W. Proton spectroscopy of human brain: effects of age and sex. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 1994;18:995–1004. doi: 10.1016/0278-5846(94)90125-2. [DOI] [PubMed] [Google Scholar]
- Doraiswamy P, Charles H, Krishnan K. Prediction of cognitive decline in early Alzheimer’s disease [letter] Lancet. 1998;352:1678. doi: 10.1016/S0140-6736(05)61449-3. [DOI] [PubMed] [Google Scholar]
- Folstein M, Folstein S, McHugh P. "Mini-mental state": A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Grober E, Dickson D, Sliwinski M, Buschke H, Katz M, Crystal H, Lipton R. Memory and mental status correlates of modified Braak staging. Neurobiology of Aging. 1999;20:573–579. doi: 10.1016/s0197-4580(99)00063-9. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, West H, Rebeck G, Harr S, Growdon J, Locascio J, Perls T, Lipsitz L, Hyman B. Clinical and pathological correlates of apolipoprotein E ε4 in Alzheimer’s disease. Annals of Neurology. 1996;39:62–70. doi: 10.1002/ana.410390110. [DOI] [PubMed] [Google Scholar]
- Hixon J, Vernier D. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with Hha I. Journal of Lipid Research. 1990;31:545–548. [PubMed] [Google Scholar]
- Huang W, Alexander G, Chang L, Shetty H, Krasuski J, Rapoport S, Schapiro M. Brain metabolite concentration and dementia severity in Alzheimer’s disease: a (1)H MRS study. Neurology. 2001;57:626–632. doi: 10.1212/wnl.57.4.626. [DOI] [PubMed] [Google Scholar]
- Hugg J, Kuzniecky R, Gilliam F, Morawetz R, Fraught R, Hetherington H. Normalization of contralateral metabolic function following temporal lobectomy demonstrated by 1H magnetic resonance spectroscopic imaging. Annals of Neurology. 1996;40:236–239. doi: 10.1002/ana.410400215. [DOI] [PubMed] [Google Scholar]
- Ivnik R, Malec J, Smith G, Tangalos E, Petersen R, Kokmen E, Kurland L. Mayo’s Older Americans Normative Studies: WAIS-R, WMS-R and AVLT norms for ages 56 through 97. The Clinical Neuropsychologist. 1992;6(supplement):1–104. [Google Scholar]
- Jessen F, Block W, Traber F, Keller E, Flacke S, Lamerichs R, Schild H, Heun R. Decrease of N-acetylaspartate in the MTL correlates with cognitive decline of AD patients. Neurology. 2001;57:930–932. doi: 10.1212/wnl.57.5.930. [DOI] [PubMed] [Google Scholar]
- Jung R, Yeo R, Chiulli S, Sibbitt W, Jr, Weers D, Hart B, Brooks W. Biochemical markers of cognition: a proton MR spectroscopy study of normal human brain. Neuroreport. 1999;10:3327–3331. doi: 10.1097/00001756-199911080-00014. [DOI] [PubMed] [Google Scholar]
- Kantarci K, Jack C, Jr, Xu Y, Campeau N, O’Brien P, Smith G, Ivnik R, Boeve B, Kokmen E, Tangalos E, Petersen R. Regional metabolic patterns in mild cognitive impairment and Alzheimer’s disease: A 1H MRS study. Neurology. 2000;55:210–217. doi: 10.1212/wnl.55.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klunk W, Panchalingam K, Moossy J, McClure R, Pettegrew J. N-acetylL-aspartate and other amino acid metabolites in Alzheimer’s disease brain: a preliminary proton nuclear magnetic resonance study. Neurology. 1992;42:1578–1585. doi: 10.1212/wnl.42.8.1578. [DOI] [PubMed] [Google Scholar]
- Kordower J, Chu Y, Stebbins G, DeKosky S, Cochran E, Bennett D, Mufson E. Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Annals of Neurology. 2001;49:202–213. [PubMed] [Google Scholar]
- Kreis R, Ross B. Cerebral metabolic disturbances in patients with subacute and chronic diabetes mellitus: detection with proton MR spectroscopy. Radiology. 1992;184:123–130. doi: 10.1148/radiology.184.1.1319074. [DOI] [PubMed] [Google Scholar]
- Kwo-On-Yuen P, Newmark R, Budinger T, Kaye J, Ball M, Jagust W. Brain N-acetyl-L-aspartic acid in Alzheimer’s disease: a proton magnetic resonance spectroscopy study. Brain Research. 1994;667:167–174. doi: 10.1016/0006-8993(94)91494-x. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale: Professional Manual. Odessa, FL: Psychological Assessment Resources, Inc; 1998. [Google Scholar]
- Mayeux R, Small S, Tang M, Tycko B, Stern Y. Memory performance in healthy elderly without Alzheimer’s disease: effects of time and apolipoprotein-E. Neurobiology of Aging. 2001;22:683–689. doi: 10.1016/s0197-4580(01)00223-8. [DOI] [PubMed] [Google Scholar]
- Mayeux R, Stern Y, Ottman R, Tatemichi T, Tang M, Maestre G, Ngai C, Tycko B, Ginsberg H. The Apolipoprotein E4 allele in patients with Alzheimer’s disease. Annals of Neurology. 1993;34:752–754. doi: 10.1002/ana.410340527. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer’s disease: Report of the NINDCS-ADRDA work group under the auspices of Department of Health and Human Services task force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Miller B, Moats R, Shonk T, Ernst T, Woolley S, Ross B. Alzheimer disease: depiction of increased cerebral myo-inositol with proton MR spectroscopy. Radiology. 1993;187:433–437. doi: 10.1148/radiology.187.2.8475286. [DOI] [PubMed] [Google Scholar]
- Minoshima S, Giordani B, Berent S, Frey K, Foster N, Kuhl D. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Annals of Neurology. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- Petersen R, Kokmen E, Tangalos E, Ivnik R, Kurland L. Mayo Clinic Alzheimer’s Disease Patient Registry. Aging. 1990;2:408–415. doi: 10.1007/BF03323961. [DOI] [PubMed] [Google Scholar]
- Petersen R, Smith G, Ivnik R, Tangalos E, Schaid D, Thibodeau S, Kokmen E, Waring S, Kurland L. Apolipoprotein E status as a predictor of the development of Alzheimer’s disease in memory-impaired individuals. Journal of the American Medical Association. 1995;273:1274–1278. [PubMed] [Google Scholar]
- Petersen R, Smith G, Waring S, Ivnik R, Tangalos E, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Archives of Neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Petersen R, Stevens J, Ganguli M, Tangalos E, Cummings J, DeKosky S. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Spielman D, Sullivan E, Lim K. In vivo spectroscopic quantification of the N-acetyl moiety, creatine, and choline from large volumes of brain gray and white matter: effects of normal aging. Magnetic Resonance in Medicine. 1999;41:276–284. doi: 10.1002/(sici)1522-2594(199902)41:2<276::aid-mrm10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Rebeck G, Reiter J, Strickland D, Hyman B. Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions. Neuron. 1993;11:575–580. doi: 10.1016/0896-6273(93)90070-8. [DOI] [PubMed] [Google Scholar]
- Reiman E, Caselli R, Yun L, Chen K, Bandy D, Minoshima S, Thibodeau S, Osborne D. Preclinical evidence of Alzheimer’s disease in persons homozygous for the ∈ 4 allele for apolipoprotein E. New England Journal of Medicine. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- Relkin N, Kwon Y, Tsai J, Gandy S. The National Institute on Aging/Alzheimer’s Association recommendations on the application of apolipoprotein E genotyping to Alzheimer’s disease. Annals of the New York Academy of Sciences. 1996;802:149–176. doi: 10.1111/j.1749-6632.1996.tb32608.x. [DOI] [PubMed] [Google Scholar]
- Rey A. The Clinical Examination in Psychology. Paris: Presses Universitaires de France; 1964. L’examen Clinique en Psychologie. [Google Scholar]
- Rogers S, Farlow M, Doody R, Mohs R, Friedhoff L Donepezil Study Group. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Neurology. 1998;50:136–145. doi: 10.1212/wnl.50.1.136. [DOI] [PubMed] [Google Scholar]
- Rose S, de Zubicaray G, Wang D, Galloway G, Chalk J, Eagle S, Semple J, Doddrell D. A 1H MRS study of probable Alzheimer’s disease and normal aging: implications for longitudinal monitoring of dementia progression. Magnetic Resonance Imaging. 1999;17:291–299. doi: 10.1016/s0730-725x(98)00168-4. [DOI] [PubMed] [Google Scholar]
- Ross B, Bluml S, Cowan R, Danielsen E, Farrow N, Tan J. In vivo MR spectroscopy of human dementia. Neuroimaging Clinics of North America. 1998;8:809–822. [PubMed] [Google Scholar]
- Schmitt F, Davis D, Wekstein D, Smith C, Ashford J, Markesbery W. "Preclinical" AD revisited: neuropathology of cognitively normal older adults. Neurology. 2000;55:370–376. doi: 10.1212/wnl.55.3.370. [DOI] [PubMed] [Google Scholar]
- Schuff N, Amend D, Ezekiel F, Steinman S, Tanabe J, Norman D, Jagust W, Kramer J, Mastrianni J, Fein G, Weiner M. Changes of hippocampal N-acetyl aspartate and volume in Alzheimer’s disease. A proton MR spectroscopic imaging and MRI study. Neurology. 1997;49:1513–1521. doi: 10.1212/wnl.49.6.1513. [DOI] [PubMed] [Google Scholar]
- Schuff N, Amend D, Meyerhoff D, Tanabe J, Norman D, Fein G, Weiner M. Alzheimer disease: quantitative H-1 MR spectroscopic imaging of frontoparietal brain. Radiology. 1998;207:91–102. doi: 10.1148/radiology.207.1.9530304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small B, Graves A, McEvoy C, Crawford F, Mullan M, Mortimer J. Is APOE--epsilon4 a risk factor for cognitive impairment in normal aging? Neurology. 2000;54:2082–2088. doi: 10.1212/wnl.54.11.2082. [DOI] [PubMed] [Google Scholar]
- Smith G, Bohac D, Waring S, Kokmen E, Tangalos E, Ivnik R, Petersen R. Apolipoprotein E genotype influences cognitive ‘phenotype’ in patients with Alzheimer’s disease but not in healthy control subjects. Neurology. 1998;50:355–362. doi: 10.1212/wnl.50.2.355. [DOI] [PubMed] [Google Scholar]
- Smith G, Ivnik R, Malec J, Kokmen E, Tangalos E, Petersen R. Psychometric properties of the Mattis Dementia Rating Scale. Assessment. 1994;2:123–131. doi: 10.1177/1073191194001002002. [DOI] [PubMed] [Google Scholar]
- Smith G, Ivnik R, Malec J, Petersen R, Kokmen E, Tangalos E. Mayo cognitive factor scales: derivation of a short battery and norms for factor scores. Neuropsychology. 1994;8:194–202. [Google Scholar]
- Smith G, O’Brien P, Ivnik R, Kokmen E, Tangalos E. Prospective analysis of risk factors for nursing home placement of dementia patients. Neurology. 2001;57:1467–1473. doi: 10.1212/wnl.57.8.1467. [DOI] [PubMed] [Google Scholar]
- Smith G, Petersen R, Parisi J, Ivnik R. Definition, course, and outcome of mild cognitive impairment. Aging, Neuropsychology and Cognition. 1996;3:141–147. [Google Scholar]
- Strittmatter W, Saunders A, Goedert M, Weisgraber K, Dong L, Jakes R, Huang D, Pericak-Vance M, Schmechel D, Roses A. Isoform-specific interactions of apolipoprotein E with microtubule-associated protein tau: Implications for Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:11183–11186. doi: 10.1073/pnas.91.23.11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter W, Saunders A, Schmechel D, Pericak-Vance M, Enghild J, Salvesen G, Roses A. Apolipoprotein E: High-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai G, Coyle J. N-acetylaspartate in neuropsychiatric disorders. Progress in Neurobiology. 1995;46:531–540. doi: 10.1016/0301-0082(95)00014-m. [DOI] [PubMed] [Google Scholar]
- Tsai MS, Tangalos E, Petersen R, Smith G, Schaid D, Kokmen E, Ivnik R, Thibodeau S. Apolipoprotein E: Risk factor for Alzheimer disease. American Journal of Human Genetics. 1994;54:643–649. [PMC free article] [PubMed] [Google Scholar]
- Valenzuela M, Sachdev P. Magnetic resonance spectroscopy in AD. Neurology. 2001;56:592–598. doi: 10.1212/wnl.56.5.592. [DOI] [PubMed] [Google Scholar]
- Valenzuela M, Sachdev P, Wen W, Shnier R, Brodaty H, Gillies D. Dual voxel proton magnetic resonance spectroscopy in the healthy elderly: subcortical-frontal axonal N-acetylaspartate levels are correlated with fluid cognitive abilities independent of structural brain changes. Neuroimage. 2000;12:747–756. doi: 10.1006/nimg.2000.0629. [DOI] [PubMed] [Google Scholar]
- Waring S, Rocca W, Smith G, Tangalos E, Kokmen E, Petersen R. Apolipoprotein E and rate of clinical progression in Alzheimer’s disease (abstract) Neurology. 1996;46:A348. [Google Scholar]
- Webb P, Sailasuta N, Kohler S, Raidy T, Moats R, Hurd R. Automated single-voxel proton MRS: technical development and multisite verification. Magnetic Resonance in Medicine. 1994;31:365–373. doi: 10.1002/mrm.1910310404. [DOI] [PubMed] [Google Scholar]