Various types of nanoobjects such as metal, semiconductor, and polymer particles, various types of hydrogels and polymer micelles have been incorporated into living cells. [1] Many of these experiments were directed to cell imaging or drug delivery whereas others were intended to introduce optical sensors into the cells.[2]
Many types of nanoparticles need to be surface-modified or encapsulated by inert material to avoid cytotoxicity, and the nature of the modified surface must be appropriate to promote uptake of these particles by cells. We are interested in lanthanide (Ln) NPs,[3] employed as tags and designed for elemental analysis using Inductively Coupled Mass Spectrometer (ICP-MS). These NPs are known for their unique optical properties and have been considered as promising materials for biodiagnostic purposes.[4] In our work, the elemental composition of NPs and signal intensity indicate their type and number, respectively and are used to bar code live cells.
While there are many ways that one might attempt to incorporate Ln NPs into aqueous microgels,[5,6] these types of microgels are often much larger than the often-cited 200 nm limit for non-specific endocytosis by cells.[7] Previous approaches to synthesizing such small aqueous microgels (nanogels) by free radical polymerization employ large amounts of surfactant or ionic comonomers, making the materials obtained incompatible with living cells. Here we show that integration of reactive NPs into the colloidal polymer network during the polymerization step reduces the colloid dimensions into the nanogel range and creates a core-shell structure that isolates the NPs from the surrounding environment. We use monomers that impart thermal sensitivity to the nanogels and maintain colloidal stability in both the swollen and collapsed state. Preliminary experiments show that these particles can be taken up into human leukemia cells, but that the choice of monomers must be modified for effective cell up take both in buffer and in serum.
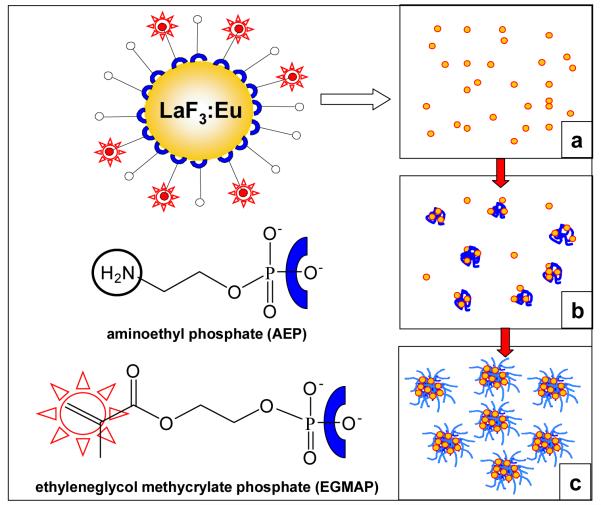
In this study, we employed LaF3:Eu NPs (diameter d = 4 nm by transmission electron microscopy, TEM) bearing surface ligands with a reactive double bond. This functionality was introduced during NP synthesis by employing a combination of two ligands: aminoethyl phosphate (AEP) and ethyleneglycol methacrylate phosphate (EGMAP) (AEP:EGMAP molar ratio 3:1) (see Figure 1). AEP and EGMAP bind to the NP surface via the phosphate group, and provide colloidal stabilization of the NPs in water. These reactive NPs were added to a reaction mixture containing monomers (N-vinylcaprolactam (VCL) (626 mg) and acetoacetoxyethyl methacrylate (AAEM) (14 mg)), initiator 2,2′-azobis(2-methylpropionamidine) dihydrochloride (AMPA) (16.6 mg), crosslinker (methylene bisacrylamide) (20 mg) and water (51 g). The NP concentration in the reaction system was varied from 0.2 g/ml to 0.8 g/ml. In one set of reactions, we modified the monomer content for the nanogel synthesis to include 45 mg of HO(CH2CH2O)n-methacrylate (Mn = 526 g/mol) in the monomer mixture. These modified nanogels contain 4.0 wt % LaF3:Eu. Polymerizations were carried out at 70°C in a stirred reactor under N2. The polymerization mechanism is presented schematically in Figure 1a-c. At the beginning of the reaction, NPs and other ingredients are homogeneously distributed in the aqueous phase (Figure 1a). As the polymerization begins, the NPs participate in the nucleation process and become captured by growing polymer chains (Figure 1b), and the reactive double bonds on their surface act as cross-link sites for the polymer. The polymerization process yields nanogels with a dense AAEM-rich core containing the NPs surrounded by a water-swollen VCL-rich corona (Figure 1c).[8] We will refer to the two samples used in cell uptake experiments as NG-3.8 and NG-4-PEG, containing 3.8 and 4.0 wt % LaF3:Eu, respectively.
Figure 1.
Schematic representation of a LaF3:Eu nanoparticle and different stages of the heterophase polymerization process: a) homogeneous distribution of NPs in the reaction mixture; b) early stages of the reaction indicating incorporation of NPs into the growing polymer particles; c) final NP-containing microgel particles.
One important result of these experiments is that the amount of reactive NPs in the reaction mixture has a profound effect on the number and size of the microgels formed. The results of dynamic and static light scattering measurements (Figure S2, Supporting Information) indicate that both hydrodynamic radii (Rh) and radii of gyration (Rg) of the microgels in water at 23 °C decrease considerably with an increase of the NP concentration in the reaction mixture. This change is accompanied by an increase of the Rg/Rh ratio from 0.52 for microgels without NPs[9] to 0.75, characteristic of a more compact structure, for microgels containing 6.1 wt % NPs. The particle size distribution remained narrow, and no free NPs were detected in the aqueous phase.
The decrease in microgel size with increasing NP content is confirmed by the TEM images in Figure 2, where the core diameter decreased from 280 nm with no NPs to 100 nm with 6.1 wt % NPs. By comparison with the corresponding Rh values in solution, we learn that these smaller particles also have thinner coronae, consistent with the more compact structures inferred from the evolution in Rg/Rh ratios. The images in Figure 2b and c, and the magnified image of a single microgel particle shown in Figure 2d suggest that nearly all of the NPs are confined to the AAEM-rich microgel core. Thus the presence of reactive LaF3:Eu NPs in the reaction mixture has several important consequences. It increases the number of nuclei formed in the reaction, leading to formation of a larger number of smaller particles. It ensures localization of the NPs in the microgel interior, and it leads to essentially quantitative capture of the NPs introduced into the reaction This is an advantage over methods based on NP diffusion, where incorporation efficiencies of <50% are common.
Figure 2.
Dark-field TEM images of hybrid microgels: a) no NPs; b) 3.8 wt %; c) 6.1 wt %; d) higher magnification image of one microgel with 6.1 wt % NPs. The light gray circles are the AAEM-rich microgel cores with collapsed PVCL chains at the surface; the intense white spots are due to the NPs.
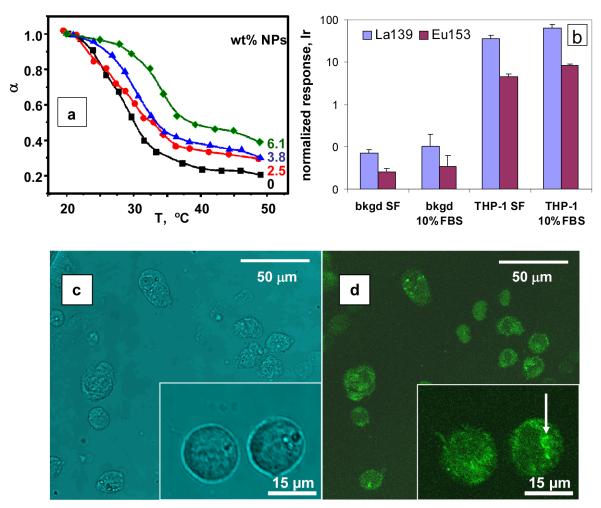
The microgel hybrids retain their thermo-sensitive properties. Figure 3a shows the variation of the normalized microgel volume ratio (α = Rh3/ Rh,swollen3) with temperature. Similar microgels prepared without NPs exhibit a volume phase transition temperature at ca. 30 °C.[8] The PVCL-rich shell of the microgels collapses upon heating due to the destruction of hydrogen bonds to water and enhanced hydrophobic interactions between polymer segments. Here the increase of in NP content in the microgel induces a decrease in the magnitude of α and an increase in the volume phase-transition temperature. The decrease in α is a reflection of the smaller size and more compact structure of the microgels with large NP content. The shift of volume phase transition temperature to higher values is more subtle. We believe that this shift is related to changes in the overall composition of the VCL-rich corona chains caused by preferential reaction of the methacrylate groups of AAEM with those of AMPA during the microgel synthesis. Corona chains richer in VCL would require higher temperatures to undergo collapse. The shrinkage of the microgels upon heating is fully reversible and no particle aggregation could be detected.
Figure 3.
(a) Values of α as a function of the temperature. The response of NG-4-PEG is similar to that of NG-3.8. (b) Metal atom signals obtained for suspensions of THP-1 cells in serum-free (SF) media and 10% FBS media after treatment at 37 °C with NG-4-PEG. These signals were normalized to an internal control (1 ppb Ir) and to constant cell numbers. The corresponding signals from cell-free wells are labelled bkgd SF and bkgd 10% FBS. (c) Optical and (d) laser confocal fluorescence microscope (LCFM) images of THP-1 cells following exposure to NG-4-PEG:FITC microgels.
Samples NG-3.8 and NG-4-PEG were tested for cell uptake, monitored by an assay based upon inductively coupled mass spectrometry (ICP-MS) as described previously for polystyrene nanoparticles.[10] In this analysis, samples were digested with con.HCl prior to injection into the ICP-MS instrument. Nanogel suspensions were diluted 1:10 with sterile PBS to 0.019 wt % solids. (For the nanogels themselves, La and Eu content were measured after further 1/104 dilutions in water.) Non-adherent growing human monocytic leukemia cells (THP-1) were seeded into wells of 24-well plates (106 cells/mL media/well) in triplicate. Two conditions were tested: serum-free media (SF) and media supplemented with 10% FBS (fetal bovine serum). Nanogel samples were also added to wells without cells to determine adsorption to plastic. 5 μL of each nanogel type was added per well and plates were incubated for 5 hours at 37°C in 5% CO2. For NG-3.8, two problems were encountered. The nanogels were not stable in 10% FBS; and while cells in PBS readily endocytosed these nanogels, the assay was complicated by nanogel adhesion to the substrate.
In contrast, NG-4-PEG was colloidally stable in 10% FBS, and had negligible adsorption to the plastic substrate. Cells treated with these nanogel particles were incubated as described above, and then were washed with PBS (phosphate-buffered saline) three times by low speed centrifugation. Wells without cells were treated identically. Finally, 2% formaldehyde was added, followed by a Rh-containing metal intercalator for cell DNA labelling.[11] Organic material was dissolved in con.HCl overnight; 1.0 ppb Ir was added as an internal standard, and samples were analyzed by ICP-MS. Signals were normalized initially to Ir to determine absolute metal content, and then to Rh, which for each cell type is a measure of cell number. Results for THP-1 are shown in Figure 3b and compared to metal ion detected from the plastic substrate. These results show that in both serum-free (SF) media and FBS-containing media, NG-4-PEG is effectively taken up by cells, yielding a normalized ICP-MS signal more than 20x larger than background. The average Eu/La ratio corresponds 8.5% Eu, consistent with the 10% Eu content of the NPs. We note that endocytosis is more effective in serum than in SF, which is likely the reflection of the physiological cell state in normal growth conditions (10% FBS).
To visualize the endocytosis process, the nanogel sample NG-4-PEG was labeled with 1% fluorescent dye fluorescein 5(6)-isothiocyanate (FITC) (sample NG-4-PEG:FITC). THP-1 cells were incubated for 3h with the dye-labeled nanogel sample. The fluorescence images recorded after cell washing indicate that nanogels are effectively incorporated into cells. The small fluorescent dots at low magnification of the cells in the upper right corner of the confocal microscopy image (Figure 3d) indicate the presence of microgels on the cell surface. At higher magnification (see inset in Figure 3d), fluorescence is seen from microgels inside the cells, which appear to be incorporated into endosomes (as indicated in Figure 3d by the arrow in the inset).
Cell viability was determined by the methylthiazolyldiphenyl-tetrazolium bromide (MTT) colorimetric assay based on the ability of live cell mitochondria to reduce the MTT substrate.[12] The experimental results show that the cytotoxicity of both NG-3.8 and NG-4-PEG is low even at high concentrations of microgels in the cell media (Supporting Information, Figure S9). Moreover, the biocompatibility of NG-4-PEG is greater than NG-3.8 since the macrophage-like differentiated THP-1 cells display much less cytotoxicity in the presence of PEG-modified nanogels.
To investigate whether active endocytosis was involved in nanogel accumulation within these human leukaemia cells, we used chemical and physical methods of actin disruption — a fungal toxin, Cytochalasin B, and cold treatment. Recent literature reports indicate that an intact actin microfilament network in the cells is necessary for endocytosis to occur.[13] The experimental results (Supporting Information, Figure S10) indicate that treatment of cells with Cytochalasin B alone or in combination with low temperature led to a decrease in the amount of microgel particles taken up by cells. Thus the nanogels are actively endocytosed and not only adsorbed on the surface of the cells.
In summary, we report a simple method for the preparation of hybrid nanogels with excellent colloidal and temperature-sensitive properties that are easily taken up by living cells, presumably by non-specific endocytosis. With different inorganic NPs incorporated into the nanogels, a wide range of different materials for bioassays and in vivo imaging become available.
Experimental Section
Characterization techniques
Light scattering measurements were performed using a commercial laser light scattering spectrometer (ALV/DLS/SLS-5000) equipped with an ALV-5000/EPP multiple digital time correlator and laser goniometer system ALV/CGS-8F S/N 025 with a helium-neon laser (Uniphase 1145P, output power of 22 mW and wavelength of 632.8 nm) as light source. The DLS experiments were carried out in the range of scattering angles θ = 30-90°. The SLS experiments were carried our in the range of scattering angles θ =18 – 130° (step 10°). All solutions were filtered using a 5.0 μm cellulose membrane filter before the measurements.
Electron microscopy (TEM) images were obtained with a Hitachi HD 2000 instrument operating at 200 kV. Diluted microgel dispersions were placed onto carbon-coated copper grids and dried at room temperature. Fluorescence images were recorded on a Leica Confocal Laser Scanning microscope, using a 53X-water objective and a 488 nm laser for excitation of the fluorescent dye (FITC). Fluorescence measurements were performed with a SPEX Fluorolog 3 Spectrometer (scans from 530 nm to 730 nm; λex = 393 nm). NMR experiments were made with a Varian Unity 500 instrument (solvent D2O). TGA measurements were performed with a Q-700 (TA Instruments) (temperature range 20 – 700 °C; heating rate 10 K/min).
Electrophoretic mobility was measured at 20°C by using a Zeta-sizer Nano ZS (Malvern Instruments). The pH was adjusted with an autotitration unit using (0.25 M) NaOH and (0.25 M) HCl solutions. Sedimentation velocities at room temperature of nanogels samples in different media were measured with an analytical centrifuge (LUM) (LUM GmbH, Berlin) using a rotation speed of 3000 rpm.
ICP-MS measurements
ICP-MS measurements were made on a commercial ICP-MS instrument ELAN DRCPlusTM (PerkinElmer SCIEX) operated under normal plasma conditions. The sample uptake rate was adjusted depending on the particular experiment and sample size, typically 100 μl/min. A MicroFlow PFA-ST concentric nebulizer (Elemental Scientific, Inc) was used in all instances. Experiments were performed using an autosampler (Perkin Elmer AS 91) modified for operation with Eppendorf 1.5 ml tubes. Sample size varied from 150 to 300 μl. Standards were prepared from 1000 μg/mL PE Pure single-element standard solutions (PerkinElmer, Shelton, CT) by sequential dilution with high-purity deionized water (DIW) produced using a Elix/Gradient (Millipore, Bedford, MA) water purification system. Analyte signals were normalized to the signal of an internal standard of 1 ppb Ir added during sample preparation.
Synthesis of LaF3:Eu NPs
To synthesize LaF3:Eu NPs stabilized with 3:1 AEP/EGMAP, a solution of the ligands (AEP, 0.102 g, 0.746 mmol; + EGMAP, 0.076 g, 0.362 mmol) in 10 mL water was neutralized to pH = 6.5 with 1.5 M NH3·H2O. To this solution a mixture of La(NO3)3·6H2O (0.50 g, 1.17 mmol) and Eu(NO3)3·5H2O (0.07 g, 0.16 mmol) in 10 mL of water was added to form the La/Eu-AEP/EGMAP complex at room temperature and then stirred at 1000 rpm for 10 minutes. To the clear solution, NaF (0.13 g, 3.00 mmol) in 13 mL was added drop-wise via a feed pump at 0.2 mL/min. The mixed solution was further stirred for 16 hours (at 1000 rpm), to yield a clear solution. The NPs solution was purified by dialysis at room temperature for 2-3 days using a dialysis bag (1 K Dalton Spectra/Pro 7 Dialysis Membranes). The synthesis of LaF3:Eu NPs without double bonds was carried out in the same way but without EGMAP. NMR analysis: a) LaF3:Eu NPs with AEP ligands on the surface: 1H NMR: δ (D2O): 3.7 (bs, 2H, POCH2CH2NH2), 3.0 (bs, 2H, POCH2CH2NH2); b) AEP ligand: 1H NMR: δ (D2O): 3.9 (bs, 2H, POCH2CH2NH2), 3.1 (bs, 2H, POCH2CH2NH2); c) LaF3:Eu NPs with both AEP and EGMAP ligands on the surface: 1H NMR: δ (D2O): 6.0 (dd, 1H, C=CHb,c), 5.6 (dd, 1H, C=CHc,b), 1.8 (s, 3H, CCH3), 4.0 (bs, 2H, POCH2CH2), 3.1 (bs, 2H, POCH2CH2)
Synthesis of microgels
Microgels were synthesized by placing appropriate amounts of VCL, AAEM, BIS, and PEGMA (for sample NG-4-PEG) (see Table S1, Supporting Information) into a 100 ml 3-neck round-bottom flask equipped with a mechanical stirrer. The ingredients dissolved after addition of deionized water under stirring and with a N2 flow. The LaF3:Eu NP aqueous solution was then added slowly to the mixture, followed by stirring at 70°C for 1 h under a nitrogen flow. Then the aqueous initiator solution was added drop wise into the reaction vessel, and the polymerization was carried out for 8 hours. Stable microgel dispersions were obtained and were purified by dialysis over 6 days.
Fluorescent nanogels were prepared by adding fluorescein 5(6)-isothiocyanate (FITC, Fluka. O.1 wt % with respect to the nanogel mass) directly to a solution of NP-containing nanogels in water, and stirring overnight. FITC reacts with pendant amino groups on the NP surface. Unreacted dye was then removed by dialysis.
Endocytosis experiments
Endocytosis experiments were carried out as follows. A suspension of nanogel particles was diluted 1:10 in sterile PBS (Phosphate-buffered saline) to a solids content of. 0.019%. The La and Eu content of this solution was measured after a further 1:104 dilution in water. Non-adherent growing human monocytic leukemia cells (THP-1, purchased from ATCC) were seeded in triplicate into wells of 24-well plates (1×10 cells/ml media/well). Two conditions were tested: serum free alpha-MEM media and media supplemented with 10% FBS (fetal bovine serum). Also, particles were added to wells without cells to determine adsorption to plastic. 5 μL of nanogel suspension in PBS was added per 1 ml media in each well (final s.c. 1.0×10−4 wt%), and plates were incubated for 5 hours at 37°C in 5% CO2. Cells were washed with PBS three times by low speed centrifugation (300 × g, 5 min). Wells without cells were treated identically to wells with cells. Finally, 500 μl 2% formaldehyde/PBS was added to each cell pellet or samples collected from wells without cells, followed by 1 μM Rh-containing metal intercalator (bis(phenanthrenequinone diimine)(bipyridyl)-Rhodium (III))). Organic material was dissolved in con.HCl overnight, combined with equal volumes of 1ppb Ir/10%HCl internal standard solution and samples were analyzed by ICP-MS.
Supplementary Material
Footnotes
We thank NIH (grant R01 GM076127) (M.A.W., V.B., O.O.) NSERC Canada (M.A.W.), Deutsche Forschungsgemeinschaft (DFG) (A.P.), and Genome Canada (O.O. and V.B.) for financial support.
Supporting Information is available on the WWW under http://www.small-journal.com or from the author.
References
- [1] a).Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambir SS, Weiss S. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Bailey RC, Nam JM, Mirkin CA, Hupp JT. J. Am. Chem. Soc. 2003;125:13541–13547. doi: 10.1021/ja035479k. [DOI] [PubMed] [Google Scholar]; c) Parak WJ, Gerion D, Pellegrino T, Znachet D, Micheel G, Williams SC, Boudreu R, LeGros MA, Larabell CA, Alivisatos AP. Nanotechnology. 2003;14:15–27. [Google Scholar]; d) Zhang L, Nguyen T. L. Uyen, Bernard J, Davis TP, Barner-Kowollik C, Stenzel MH. Biomacromolecules. 2007;8:2890–2901. doi: 10.1021/bm070370g. [DOI] [PubMed] [Google Scholar]
- [2] a).Gao D, Xu H, Philbert MA, Kopelman R. Angew. Chem. 2007;46:2224–2227. doi: 10.1002/anie.200603927. [DOI] [PubMed] [Google Scholar]; b) Gao D, Agayan RR, Xu H, Philbert MA, Kopelman R. Nano Lett. 2006;6:2383–2386. doi: 10.1021/nl0617179. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Lee Koo Y-E, Cao Y, Kopelman R, Koo AM, Brausel M, Philbert MA. Anal. Chem. 2004;76:2498–2505. doi: 10.1021/ac035493f. [DOI] [PubMed] [Google Scholar]; d) Toita S, Hasegawa U, Koga H, Sekiya I, Muneta T, Akiyoshi K. J. Nanosci. Nanotech. 2008;8:2279–2285. doi: 10.1166/jnn.2008.240. [DOI] [PubMed] [Google Scholar]
- [3] a).Stouwdam JW, van Veggel FCJM. Nano Lett. 2002;2:733–737. [Google Scholar]; b) Stouwdam JW, van Veggel FCJM. Langmuir. 2004;20:11763–11771. doi: 10.1021/la048379g. [DOI] [PubMed] [Google Scholar]; c) Sudarsan V, van Veggel FCJM, Herring RA, Raudsepp M. J Mater Chem. 2005;15:1332–1342. [Google Scholar]; d) Vetrone F, Boyer JC, Capobianco JA, Speghini A, Bettinelli AM. J Phys Chem B. 2003;107:1107–1112. doi: 10.1021/jp052192w. [DOI] [PubMed] [Google Scholar]
- [4] a).Sivakumar S, Diamente PR, van Veggel FCJM. Chem Eur J. 2006;12:5878–5884. doi: 10.1002/chem.200600224. [DOI] [PubMed] [Google Scholar]; b) Diamente PR, van Veggel FCJM. J. Fluoresc. 2005;15:543–551. doi: 10.1007/s10895-005-2827-5. [DOI] [PubMed] [Google Scholar]
- [5] a).Pelton R. Adv. Colloid Interface Sci. 2000;85:1–33. doi: 10.1016/s0001-8686(99)00023-8. [DOI] [PubMed] [Google Scholar]; b) Nayak S, Lyon LA. Angew. Chem. Int. Ed. 2005;44:7686–7708. doi: 10.1002/anie.200501321. [DOI] [PubMed] [Google Scholar]; c) Das M, Zhang H, Kumacheva E. Annu. Rev. Mater. Res. 2006;36:117–142. [Google Scholar]
- [6] a).Zhang J, Xu S, Kumacheva E. J. Am. Chem. Soc. 2004;126:7908–7914. doi: 10.1021/ja031523k. [DOI] [PubMed] [Google Scholar]; b) Antonietti M, Grohn F, Hartmann J, Bronstein L. Angew. Chem. Int. Ed. 1997;36:2080–2083. [Google Scholar]; c) Kuang M, Yang D, Bao H, Gao M, Möhwald H, Jiang M. Adv. Mater. 2005;17:267–270. [Google Scholar]
- [7].Chithrani BD, Ghazani AA, Chan WC. Nano Lett. 2006;6:662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- [8].Boyko V, Pich A, Lu Y, Richter S, Arndt K-F, Adler H-J. Polymer. 2003;44:7821–7827. [Google Scholar]
- [9] a).Burchard W. Adv. Polym. Sci. 1983;48:1–123. [Google Scholar]; b) Burchard W, Schmidt M, Nerger D. Polymer. 1979;20:582–588. [Google Scholar]; c) Senff H, Richtering W. Coll. Polym. Sci. 2000;278:830–840. [Google Scholar]
- [10].Vancaeyzeele C, Ornatsky O, Baranov V, Shen L, Abdelrahman A, Winnik MA. J. Am. Chem. Soc. 2007;129:13653–13660. doi: 10.1021/ja073970w. [DOI] [PubMed] [Google Scholar]
- [11].Ornatsky OI, Lou X, Nitz M, Schafer S, Sheldrick WS, Baranov VI, Bandura DR, Tanner SD. Anal. Chem. 2008;80:2539–2547. doi: 10.1021/ac702128m. [DOI] [PubMed] [Google Scholar]
- [12].Mosmann T. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- [13].Samaj J, Baluska F, Voigt B, Schlicht M, Volkmann D, Menzel D. Plant. Physiol. 2004;135:1150–1161. doi: 10.1104/pp.104.040683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.