Abstract
Pneumonia associated with Iegionnaires's disease is initiated in humans after inhalation of contaminated aerosols. In the environment, Legionella pneumophila is thought to survive and multiply as an intracellular parasite within free-living amoeba. In the genome of L. pneumophila Lens, we identified a unique gene, tolC, encoding a protein that is highly homologous to the outer membrane protein TolC of Escherichia coli. Deletion of tolC by allelic exchange in L. pneumophila caused increased sensitivity to various drugs. The complementation of the tolC mutation in trans restored drug resistance, indicating that TolC is involved in multi-drug efflux machinery. In addition, deletion of tolC caused a significant attenuation of virulence towards both amoebae and macrophages. Thus, the TolC protein appears to play a crucial role in virulence which could be mediated by its involvement in efflux pump mechanisms. These findings will be helpful in unraveling the pathogenic mechanisms of L. pneumophila as well as in developing new therapeutic agents affecting the efflux of toxic compounds.
Introduction
Legionella pneumophila (L. pneumophila), the main causative agent of the Legionnaire's disease in humans is commonly found in freshwater environments where it can replicate within protozoa [1]. Development of air-conditioning systems, cooling towers and other water aerosols has created conditions for the direct access of this opportunistic bacterium to human lungs, where it can multiply within alveolar macrophages [2]. During the intracellular infectious cycle in amoeba and macrophages, L. pneumophila evades the phagolysosome fusion and establishes a replicative vacuole studded with ribosomes [1], [3], [4]. The subsequent modified vacuole offers a nutrient rich environment, which allows the bacteria to replicate. When nutrients become scarce, the bacteria undergo a phenotypic switch leading to the expression of virulent traits including flagella expression and an increased resistance to antibiotics and stress. Finally, Legionellae lyse the vacuole and the membrane host, probably by secreting pore-forming toxins [5].
Up to date, prevention of legionellosis outbreaks has been based on the control of the legionellae population in cooling towers by using chemical treatments. Little is known about the resistance of L. pneumophila to these biocides. One universal mechanism underlying drug resistance to various toxic compounds, namely multi-drug resistance (MDR; [6]), is the expression of efflux pumps that drive drugs outside the target cell. Five families of efflux pumps have been described on the basis of the inner membrane protein structure: the major facilitator (MF) superfamily, the ATP-binding cassette (ABC) family, the resistance-nodulation-division (RND) family, the small multi-drug resistance (SMR) family and the multi-drug and toxic compound extrusion (MATE) family [7], [8]. The ABC-efflux pump system is ATP-dependent whereas MF, RND and SMR systems use the proton motive force. MATE-family transporters are mainly Na+/substrate antiporters, but few members have been described as H+/drug antiporters.
SMR and MATE systems have been described to transport drugs from the cytoplasm to the periplasm, then releasing them to the extracellular medium via porins of the outer membrane. Tripartite systems (MFS, ABC and RND) have also been described, such as the AcrA-like/AcrB-like/TolC efflux pump belonging to the RND family [9], [10]. TolC is a protein found in all Gram-negative bacteria, forming a channel through the outer membrane and interacting with the AcrA periplasmic protein. This AcrA lipoprotein anchored to the inner membrane via a lipid motif also interacts with AcrB, an integral inner membrane translocase acting as a proton/drug antiporter [11].
Besides its role in the efflux of various molecules, TolC has been recently reported to play a key role in bacterial virulence in the Gram negative bacteria such as Francisella tularensis [12], Brucella suis [13], Salmonella enterica serovar Typhimurium [14] and Salmonella enteritidis [15]. Here we report that L. pneumophila Lens encodes a protein homologous to Escherichia coli TolC. We demonstrate that the TolC protein is involved in multidrug-resistance of L.pneumophila and that it plays an essential role in virulence.
Results
L.pneumophila Encodes a TolC Ortholog
Using BLAST and PSI-BLAST analysis of the L. pneumophila Lens genome (http://genolist.pasteur.fr/LegioList/) [16], 116 ORFs were identified by significant homology with known proteins involved in efflux pump machineries of many bacteria species: 12 outer membrane factors (OMF), 16 periplasmic membrane fusion proteins (MFP) and 88 inner membrane proteins (IMP) belonging to four classes of efflux pumps (MF, ABC, RND and SMR) [8]. No member of the multi-drug and toxic compound extrusion (MATE) family was identified. Among the 12 OMF proteins identified, one shares a significant homology (36% identity and 55% similarity) with the Escherichia coli TolC protein and will be referred to here as L. pneumophila TolC.
L. pneumophila TolC contains a predicted leader sequence with the cleavage site located between residues 20 and 21 (VFA↓TD) and,, as a consequence is predicted to be localized in the outer membrane. TolC contains duplicate pfam02321 domains forming trimeric channels that allow export of a variety of substrates in Gram-negative bacteria [17]. The trimeric channel is composed of a 12-stranded beta sheet barrel that spans the outer membrane, and a long helical barrel that spans the periplasm. These domains are part of the TolC family domain (COG1538; [17]).
Inactivation of the tolC Gene Has No Effect on Growth of L. pneumophila Lens
In order to identify the role of the TolC protein in L. pneumophila, a tolC mutant was constructed as described in Materials and Methods. This mutant, called MF201, is partially deleted for tolC by a unique insertion of a kanamycine cassette (tolC::kan), verified by PCR and Southern hybridization (data not shown).
A pUC18 derivative vector, namely pML005, was constructed by site-directed mutagenesis to obtain a plasmid that displays a previously described mutation which enhances its stability in L. pneumophila [18] and allows complementation experiments in derivative strains. A preliminary bioluminescence assay indeed confirmed the stability of a lux cassette expressing pML005 during 30 generations without selection pressure (data not shown). The strain MF201 was transformed by plasmid pML005-tolC, which efficiently expressed the L. pneumophila Lens tolC gene, to obtain the MF213 strain.
Because the tolC mutation has been shown to affect bacterial cell division in E. coli [19], the growth kinetics of the tolC mutant (MF201) was compared to that of the parental (Lp01) and complemented strains (MF213). MF201, MF213 and MF214 exhibited no growth defects either in liquid media (Figure S1) or on solid media (data not shown). Thus, L. pneumophila behaved as Francisella tularensis for which the deletion of tolC had no effect on bacterial growth [12].
TolC Is Involved in MDR of L. pneumophila Lens
The sensitivity of strain MF201 compared to the wild type was tested towards 17 toxic compounds. The drugs were chosen from a wide range of toxic compounds which have been identified to be expelled by efflux [20]: detergents (SDS, CTAB), antibiotics (erythromycin, novobiocin, nalidixic acid, norfloxacin and tetracyclin), dyes (methylene blue, acridine orange and rhodamine), quaternary ammonium or benzalkonium chloride, intercalating agents (ethidium bromide), bile salts (sodium deoxycholate) and heavy metals (nickel, zinc and manganese). Interestingly, strain MF201 (tolC::kan) showed an increased sensitivity towards 11 compounds over the 17 tested, with a reduced MIC ranging from 2 to 16-fold (erythromycin) compared to that of the wild-type strain Lp01 (Table 1). No difference in MIC was observed in the presence of cobalt, tetracycline, nalidixic acid, acridine orange, zinc and manganese (data not shown). These results ruled out the possibility of a bacterial envelope defect leading to an non-specific release of all tested drugs, but favored an alteration of the drug efflux mechanism of the tolC mutant. As expected, the complemented strain MF213 displayed similar drug sensitivity compared to the parental strain. Taking together, these results support that MF201 phenotype was the result of tolC inactivation.
Table 1. Drugs susceptibility of L. pneumophila Lens grown on BCYE Agar.
| MIC 100 (µg/mL)b | ||||||||||||
| Straina | Genotype | SDS | CTAB | ERY | BENZ | NOV | DEO | NOR | ETB | MB | R6G | Ni |
| Lp01 | WT | 0,125 | 50 | 0,5 | 100 | 6,25 | 100 | 12 | 100 | 100 | 50 | 800 |
| MF201 | tolC :: kan | 0,031 | 25 | 0,031 | 25 | 3,125 | 12,5 | 6 | 25 | 50 | 25 | 400 |
| MF213 | tolC::kan/pML005-tolC | 0,125 | 50 | 0,5 | 100 | 6,25 | 100 | 12 | 100 | 100 | 50 | 800 |
| MF214 | tolC :: kan /pML005 | 0,031 | 25 | 0,031 | 25 | 3,125 | 12,5 | 6 | 25 | 50 | 25 | 400 |
| Lpl701 | dotA:: kan | 0,125 | 50 | 0,5 | 100 | 6,25 | 100 | 12 | 100 | 100 | 50 | 800 |
Strains used are described in Materials and Methods.
MIC 100 were determined as the minimal inhibitory concentration leading to a complete inhibition of bacterial growth using agar dilution method (see Materials and Methods). Results were reproduced three times.
Abbreviations: SDS: Sodium dodecyl sulfate, CTAB: hexadecyltrimethylammonium bromide, ERY: Erythromycin, BENZ: Benzalkonium chloride, NOV: Novobiocin, DEO: Sodium Deoxycholate, NOR: Norfloxacin, ETB: Ethidium bromide, MB: Methylene Blue, R6G: Rhodamine 6G, Ni: nickel sulfate.WT: wild type.
It is commonly assumed that ethidium bromide is a substrate of TolC-dependent efflux pumps [13]. Therefore, the accumulation of ethidium bromide in bacteria was measured in L. pneumophila Lens and derivatives (Figure 1). A stable level of ethidium bromide (close to 4 units of fluorescence) was observed in L. pneumophila Lens over a period of 120 min. The addition of carbonyl cyanide m-chlorophenylhydrazone (CCCP; 2.5 mg/L), known to disrupt the proton motive force, resulted in a rapid increase of ethidium bromide concentration inside bacterial cells. High accumulation of ethidium bromide was observed in the TolC defective mutant (MF201), which was fully reversed by the tolC complementation (strain MF213) (Fig. 1). This accumulation was even higher than that observed in the wild type in presence of CCCP. However, the concentration of CCCP used was low (2.5 mg) in order to limit cellular death [21] and might result in partial inhibition of efflux pump systems, which may explain the mild level of inhibition.
Figure 1. Ethidium bromide accumulation in L. pneumophila Lens derivatives.
Bacteria were grown in liquid medium (BYE medium) at 30°C until stationary phase. The accumulation assay was done as described in Materials and Methods in presence of 0.5 mg/l of ethidium bromide. When indicated, carbonyl cyanide m-chlorophenylhydrazone (CCCP) was added (2.5 mg/l).
All these results confirm that TolC of L. pneumophila Lens is a component part of a functional efflux pump with wide substrate specificity and driven by the proton motive force.
TolC Contributes to Stress Resistance in L. pneumophila Lens
Legionellae were exposed to chemical compounds (ethidium bromide, H2O2, cooling tower biocides) or high temperature (50°C) for 1 hour in liquid medium. After ethidium bromide stress, the viability of MF201 was 18-fold lower (p-value = 0.0014) than that of the parental strain (Fig. 2). The sensitivity of MF201 increased as a function of the hydrogen peroxide concentration (53- (p-value = 0.003) and 102-fold (p-value = 9×10−6) decrease with 177 and 221 mM H202, respectively). In contrast, strain MF201 displayed similar resistance as the parental strain towards thermal stress and biocides used as disinfectants in cooling towers. Therefore, TolC contributes to oxidative stress resistance. Besides, these results also confirmed that the deficiency in TolC protein is not correlated with a bacterial envelope alteration inducing a general sensitivity of bacterial cells.
Figure 2. Cultivable surviving bacteria after exposition to different types of stress.
Strains Lens (WT; back bars) and MF201 (tolC::kan; grey bars) were subjected to oxidative stress (H202), to thermal stress, and to the effect of a cooling tower biocide (CT) for 1 hour. Bacteria were then washed and diluted for colony enumeration on BCYE agar.
The tolC Gene Is Essential for Virulence of Legionella towards Protozoa and Macrophages
The microscopic observations infections conducted in Acanthamoeba castellanii and Dictyosteliun discoideum showed that strain MF201 failed to “stress” the protozoan cells, which remained adherent even after 72 hours post-infection (Fig. 3). At this time, the wild type strain was able to lyse almost all amoebae present in the monolayer (the remaining amoebae were round) and many highly motile bacteria (virulent phenotype) were present in the media. Interestingly, the defect in virulence observed with strain MF201 was similar to that observed with strain lpl701 (avirulent control strain) known to be defective for virulence secretion factors [5]. The restoration of the virulent phenotype after complementation (strain MF213) and the fact that the empty plasmid could not restore this phenotype ruled out the possibility that the defective virulence observed with strain MF201 was due to spontaneous or secondary mutations.
Figure 3. Infected monolayers of D. discoideum at 72 hours post-infection (X20).
Monolayers were observed with a Nikon inverted microscope coupled with an Olympus camera (DP120).
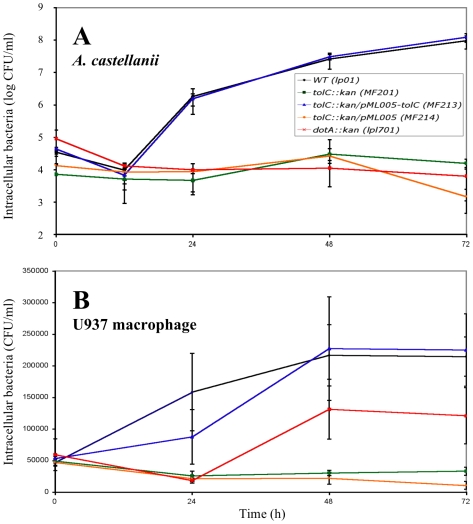
The amoebae A. castellanii and D. discoideum as well as U937 macrophages were infected with the parental strain Lp01, the TolC defective strain MF201, the complemented strain MF213, the corresponding plasmid-control strain MF214, or the avirulent dotA mutant. Extracellular Legionellae were counted at 24, 48 and 72 h (Fig. 4). As expected, infections of the three eukaryotic cell monolayers with the parental strain Lp01 showed an increase of extracellular Legionellae between 0 and 24 h post-infection (580-, 2.9- and 4-fold increase, respectively). Between 24 and 48 hours after infection, there was a significant increase of bacterial egress from the amoeba A. castellanii and to a lesser extent from D. discoideum (10 and 3 log increase, respectively). This burst in bacterial egress can be correlated with the switch to a virulent phenotype after multiplication within a eukaryotic host. It must be underlined that beyond 48 h of post-infection the level of bacterial egress from amoebae and macrophages remains stable.
Figure 4. Legionella cells released from protozoa and macrophages.
A. castellanii (A), D. discoideum (B) and U937 human monocyte-derived macrophages (C) were infected at an MOI of 10 for 1 h with L. pneumophila strains Lp01 (WT), MF201 (tolC::kan), MF213 (tolC::kan/pML005-tolC), MF214 (tolC::kan/pML005) and lpl701 (dotA::kan). At different times post-infection, the bacteria released in the supernatants were diluted in the appropriate medium (see material and methods) and spread on BCYE agar plates for colony enumeration. The initial time points (t = 0) represent the number of extracellular bacteria after 1 hour of infection. The data are representative of two independent experiments performed in triplicate and error bars represent standard deviations.
Compared to the parental strain, there was a severe defect of egress of MF201 cells at 24 h (400-fold less bacteria from A. castellanii (p-value = 0.015) and 4-fold difference from U937 (p-value = 9×10−4)) and at 48 h (12 log less bacteria from A. castellanii (p-value = 0.015), 2 log less bacteria from D. discoideum (p-value = 2.8×10−4) and 5.6 fold less bacteria from U937 (p-value = 3×10−4)). This defect of MF201 cell egress was similar to the one observed with the avirulent dotA strain at 72 h (p-value>0.05) in A. castellanii and in U937 cells. The tolC complementation restored the Legionellae egress from the three tested eukaryotic cells (Fig. 4) and no significant difference was observed between the toxicity of parental strain (Lp01) and complemented strain MF213 (p-value>0.05) after 72 h of A. castellanii or U937 infections.
In addition, Alamar blue dye was used to quantify the viability of eukaryotic cells present in the infected monolayers. The cytotoxicity of the parental strain towards A. castellanii, D. discoideum and U937 macrophages was respectively estimated at 95%, 64% and 67%, (Fig. 5). Compared to the wild type strain, the cytotoxicity of the tolC mutant was significantly lower: 23% (p-value = 1.2×10−6), 29% (p-value = 1.2×10−7) or 28% (p-value = 0.017) towards A. castellanii, D. discoideum or U937 macrophages, respectively. As expected, the cytotoxicity level was fully restored in the complemented strain MF213 (p-value>0.05) in the three hosts.
Figure 5. Cytotoxity of L. pneumophila Lens derivatives towards different hosts.
A. castellanii (A), D. discoideum (B) and U937 human monocyte-derived macrophages (C) cells were infected at an MOI of 10 for 1 h with L. pneumophila strains: Lp01 (WT), MF201 (tolC::kan), MF213 (tolC::kan/pML005-tolC), MF214 (tolC::kan/pML005) and lpl701 (dotA::kan). After 48 h (D. discoideum) or 72 h (A. castellanii and U937) of infection, the monolayers were washed and the reduction of the Alamar blue dye was measured and compared to non-infected cells (100%). These data are representative of two independent experiments done in triplicate (error bars represents standard deviations).
Taken together, these results confirm a severe defect of virulence and cytotoxicity correlated with the absence of the TolC protein in L. pneumophila.
TolC Is Required for Multiplication of L. pneumophila at the Onset of Early Steps of the Intracellular Infectious Cycle
Intracellular Legionellae were followed during 72 h of infection to differentiate between two possible consequences of the TolC deficiency: a defect in intracellular multiplication or in the capacity to lyse eukaryotic cells. The intracellular concentration of the L. pneumophila Lens cells within A. castellanii was increased 52- and 14-fold during the first 24 h and the 24–48 h period, respectively (Fig. 6A). In contrast, the level of MF201 cells did not significantly increase over the 72 h of post-infection within A. castellanii and U937 macrophages (Fig. 6). Therefore, at 72 h post-infection, the number of MF201 intracellular bacteria was 6000 fold lower (p-value = 2.5×10−3) in A. castellanii and 6.5 fold lower (p-value = 5.2×10−5) in U937 macrophages compared to the parental strain Lp01. Similar results were observed using D. discoideum as host (data not shown). This low level of intracellular MF201 Legionellae could not be explained by a defect in host adherence because no difference was observed between the numbers of non-adherent parental or mutant cells (data not shown).
Figure 6. Intracellular growth of L. pneumophila Lens derivatives.
Intracellular growth kinetics of strains Lp01 (WT), MF201 (tolC::kan), MF213 (tolC::kan/pML005-tolC), MF214 (tolC::kan/pML005) and lpl701 (dotA::kan) in A. castellanii (A) and U937 human monocyte-derived macrophages (B). These cells were infected at an MOI of 10 for 1 h with L. pneumophila. At different times postinfection, A. catellanii or U937 monolayers were lysed either hypotonically (sterile water for U937 cells) or with a mild detergent (0,04% Triton X-100 for A. castellanii). Aliquots were diluted immediately and plated on BCYE agar plates for enumeration of intracellular bacteria. Each experiment was performed twice in triplicate. Error bars represent standard deviations.
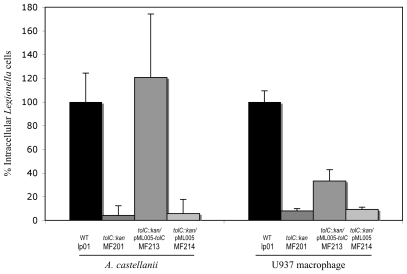
Thus, our results reveal that Legionellae could not efficiently initiate an infectious cycle without the TolC protein. This statement was confirmed by the results obtained after a treatment of extracellular Legionellae with gentamicin at the onset of host infection: the intracellular level of the mutant MF201 corresponded to less than 10% of the parental strain amount within A. castellanii (p-value = 3.1×10−6) or U937 macrophages (p-value = 0.039) (Fig. 7). This result points out the major role of TolC in the early steps of eukaryotic cell infection. However, the reference level of bacteria (Lp01) was fully recovered by the complemented strain MF213 in A. castellanii infection (p-value>0.05), but only partially recovered during macrophage invasion. In that case, the number of Legionella MF213 cells is still significantly different from the control level (Lp01) (p-value = 0.019). This latter result might be due to the genetic system we used: tolC gene expression in trans was under the control of a constitutive promoter, therefore expression may have been at a lower level than the parental tolC gene, whose expression may be fully induced by host stress signals at some steps of the infectious cycle. As a consequence, a lower level of TolC in the complemented strain compared to the wild type during macrophage infection cannot be ruled out.
Figure 7. Invasion ability of L. pneumophila Lens derivatives strains.
Host cells were infected at an MOI of 10 with strains Lp01 (WT), MF201 (tolC::kan), MF213 (tolC::kan/pML005-tolC), MF214 (tolC::kan/pML005) and lpl701 (dotA::kan). After 60 minutes of contact with L. pneumophila, the monolayers were treated 1 hour with gentamycin to kill adherent bacteria and disrupted with either distilled water (for U937 cells) or Triton 0,04% (for A. castellanii). Intracellular bacteria were diluted and plated on BCYE agar plates for colony enumeration. Results are expressed as the relative value (%) compared to control invasion experiment with wild-type strain Lp01. These data are representative of two independent experiments done in triplicate for which error bars represents standard deviations.
Discussion
Our analysis of the L. pneumophila Lens genome revealed a unique ORF sharing significant homology with the TolC protein from Escherichia coli, which is the prototypical outer membrane channel component involved in MDR and type I secretion [22], [23], [24]. The L. pneumophila Lens tolC::kan mutant was sensitive to a variety of compounds including antibiotics and detergents, which supports the involvement of the TolC protein in a functional MDR machinery with wide substrate specificity. The TolC pump activity was clearly demonstrated by the increase of ethidium bromide accumulation in the TolC deficient strain compared to the parental strain. The trans-complementation of tolC restored the pumping efficiency suggesting that the drug sensitivities were specifically due to the tolC knockout. Thus, our work confirms the role of L. pneumophila TolC in drug efflux mechanisms.
Moreover, the tolC mutant of L. pneumophila was clearly more sensitive to H2O2 than the wild type, which shows that TolC protects L. pneumophila against oxidative stress. This result is in agreement with the newly identified role of TolC protein in S. meliloti and S. enterica [25], [26]. Actually, oxidative stress occurs when organisms encounter reactive oxygen species (ROS) such as superoxide anion, hydrogen peroxide and hydroxyl radical. ROS are known to be produced during the oxidative burst of professional phagocytes to kill intracellular bacteria during an infection [27], [28]. Therefore, in phagocytic cells, bacteria have to cop with a stressful and hostile environment (ROS, antimicrobial peptides, degradative enzyme for example) in which multi-drug and stress resistance mechanisms can confer a selective advantage for intracellular survival in the host. The exact role of TolC in response to oxidative stress is still unclear, but it could participate in the efflux of toxic ROS in addition to ROS degradation by the bacterial periplasmic and cytoplasmic catalases and dismutases [29], [30], [31]. Moreover, in E. coli, tolC belongs to the marA/soxS/rob regulon including over 40 genes that promote resistance to multiple antibiotics and to superoxides [32]. Four tolC promoters have been described in E. coli, suggesting the involvement of multiple transcriptional regulatory elements in response to different environments [33].
In addition to increased drug sensitivity, the L. pneumophila Lens TolC defective mutant was highly attenuated for virulence in amoebae and macrophages. Actually, the TolC defective mutant was unable to multiply within host cells from the early steps of host invasion. It exhibits the same defect of virulence as that which was described for the model avirulent dotA mutant. The DotA protein belongs to the Dot/Icm type IVB secretion system which is required for replication in amoebae and macrophages and today is thought to secrete more than one hundred substrates [34], [35]. This result demonstrates for the first time the key role of the TolC protein in virulence of L. pneumophila. The attenuated virulence phenotype associated with TolC absence has recently been reported in other Gram-negative bacteria [12], [14], [36], [37].
In conclusion, we have demonstrated the major role of the TolC protein in L. pneumophila MDR as a component in efflux pump systems. Moreover, TolC has to be considered as a new virulence factor in L. pneumophila by its essential role during the early steps of invasion of both amoebae and macrophages. This role could be due to TolC involvement in type I secretion mechanisms. Further work will focus on the determination of TolC partners and substrates of TolC-dependent systems in L. pneumophila. These findings may contribute to understanding the molecular mechanisms involved in the export of molecules in L. pneumophila. and represent a step towards the development of novel therapeutic agents, especially for affecting efflux or secretion systems [38].
Materials and Methods
Bacterial Strains, Plasmids, Media and Growth Conditions
Bacterial strains and plasmids used in this study are summarized in Table S1. L. pneumophila serogroup 1 strain Lens (Lp01) was isolated from a patient and is a kind gift of Jérome Etienne and Sophie Jarraud from the CNRL (Centre National de Référence des Légionelles, Lyon, France). L. pneumophila strains were grown at 30°C either on buffered charcoal yeast extract (BCYE) agar (Difco) or in BYE liquid medium; each media supplemented with chloramphenicol (Cm; 5 µg/ml), kanamycine (Km; 10 µg/ml) or sucrose (5%) where appropriate. Escherichia coli strains were grown at 37°C in LB medium supplemented with chloramphenicol (Cm; 5 µg/ml) or kanamycin (Km; 50 µg/ml).
Cells Culture
Axenic Acanthamoeba castellanii cells were grown on PYG medium (Proteose yeast extract glucose medium) at 30°C and split once or twice a week. Dyctiostelium discoideum axenic strain DH1 (ax3; DBS0266325 identification on dictybase http://dictybase.org/) was obtained from François Letourneur (Laboratoire de Transport et Compartimentation Intracellulaire, Institut de Biologie et Chimie des Protéines, UMR 5086 CNRS, IFR 128 BioSciences Lyon-Gerland 7, passage du Vercors, 69367, Lyon, France) and were grown at 25°C in HL5 medium. Macrophage-like U937 cells obtained from Maëlle Molmeret (INSERM E230 Faculté de médecine RTH Laennec 7–11 rue Guillaume Paradin 69372 Lyon Cedex 08) were maintained at 37°C and 5% CO2 in rpmi 1640 tissue culture medium supplemented with 10% heat-inactivated fetal calf serum. Prior to infection, the cells were differentiated in 96 well tissue culture plates for 48 h, using phorbol 12-myristate 13-acetate. Differentiated cells are non replicative, adherent, macrophage-like cells.
Electroporation of L. pneumophila and Screening of Transformants
To prepare competent cells, L. pneumophila grown on BCYE agar plates were resuspended in 200 ml of sterile water to an OD600 between 0,5–1. The suspension was divided in four 50 ml tubes (falcon) that were subjected to centrifugation at 4500 rpm during 10 min at 4°C. The bacterial pellet was washed twice with 30 ml of sterile water and the cells were resuspended in glycerol 30%. Competent cells obtained were immediately used or conserved at −80°C as 100 µl samples for long term storage. For electroporation, 3 µl of a plasmid preparation was added to an aliquot of competent cells and submitted to 2,5 kV 600 Ohms and 25 µF using a Biorad electroporation apparatus. Then cells were inoculated in 900 ml BYE liquid medium and incubated at 30°C for 60 minutes before plating on BCYE agar containing the appropriate antibiotic or sucrose for selection.
Construction of Plasmids
To obtain a L. pneumophila Lens mutant defective for tolC, a homologous recombination strategy was choosen. A derivative plasmid of pCDP05 was constructed in the laboratory. pCDP05 plasmid is a suicide vector which was used in a previous study to obtain random insertions of a kanamycine resistance cassette on the chromosome of Legionella pneumophila [39]. This plasmid bears the sacB gene of Bacillus subtilis which expression in the presence of sucrose leads to the accumulation of levans at noxious concentrations to Gram-negative bacterial cells when accumulated in periplasmic space. The derivative plasmid was obtained by the deletion of a 4,3 kb NotI fragment containing the kanamycine cassette flanked with the two IS10 sequences and ats1ats2 sequences (alteration of target site recognition). The resulting plasmid, p695, with a unique NotI restriction site, confers chloramphenicol resistance and is counter-selectable in presence of sucrose. Two fragments in 5′ and 3′ region of tolC were amplified using the primers pairs P1/P2 (amplification of the 5′ region) and primers P3/P4 (amplification of the 3′ region) (Table S2). Primers were flanked with NotI restriction site for primers P1 and P4 or SalI restriction site for P2 and P3. The two fragments generated by couple of primers P1/P2 and P3/P4 were digested with SalI enzyme and ligated. PCR was made on the product of ligation using primers P1 and P4. The resulting fragment of approximatively 1 kb was then digested with NotI for subcloning in p695. The plasmid p695 with the insertion of P1/P4 fragment was digested with SalI enzyme to insert a kanamycine resistance cassette between the two fragments corresponding to 5′ and 3′ region of tolC. The resulted plasmid was named pMF1.
A complementation plasmid was also constructed. The plasmid pML005, derived from PUC18 with Cm cassette in exchange of bla gene and a mutation in the ColE1-type replication promoter (Table S1) which was previously shown to confer a stability to the plasmid in L. pneumophila [18], was used to clone tolC gene under the control of a constitutive promoter (Pkan, constitutive promoter of kan gene from plasmid pCDP05).
Construction of L. pneumophila Lens tolC::kan Strain
The plasmid pMF1 was electroporated in L. pneumophila. Kanamycine resistant clones of Legionella were plated on BCYE agar containing kanamycine and sucrose for selection of recombinants. The recombinants obtained were controlled by PCR on chromosome, by sequencing and by southern blot analysis. PCR with primers P1/P4 allowed the amplification of a 2 kb fragment which, after SalI restriction, gave two type of fragments: a 1 kb fragment corresponding to the kanamycine cassette and fragments at 500 pb corresponding to the two 5′ and 3′ region of the tolC gene. Southern blot on the chromosome digested by BamHI and BglII restriction enzymes was use to reveal the insertion of the kanamycine cassette.
Multidrug Sensitivity
Sensitivity to different drugs as detergent, dyes, antibiotics and metals was tested by an agar dilution method. Briefly, solution of drug were made in water or ethanol: sodium dodecyl sulfate (SDS; Euromedex), hexadecyltrimethylammonium bromide (CTAB; Sigma-Aldrich), Polymixin B (Sigma-Aldrich), Benzalkonium chlorid (Sigma-Aldrich), Tetracyclin (Sigma-Aldrich), Erythromycin (Sigma-Aldrich), Sodium Déoxycholate (Sigma-Aldrich), Nalidixic acid (Serva), Norfloxacin(Sigma-Aldrich), Ethidium Bromid (Sigma-Aldrich), Acridin Orange (Sigma-Aldrich), Methylene Blue (Prolabo), Rhodamine 6G (Sigma-Aldrich), zinc sulfate heptahydrate (Prolabo), nickel sulfate (Sigma-Aldrich), manganese sulfate (Sigma-Aldrich), Cobalt (Co(II) chloride hexahydrate) (Acros organics). Test drugs were then diluted by twofold in sterile water in a 24 multiwell plate to obtain a final volume of 500 µl of each dilution. Then 500 µl of BCYE agar in surfusion (60°C) was added to each drug dilution and immediately mixed.
The day of the test, L. pneumophila cells from fresh BCYE agar plates (4 days of growth at 30°C) were resuspended in sterile water and adjusted to a final suspension with an OD600nm of 5. 10 µl of the bacterial suspension was added into each well of the plate with different dilutions of the drugs. After 5 days at 30°C, the growth into each dilution well was visualized. The well corresponding to the lowest concentration of the drug with no visible sign of growth was reported as MIC 100 for Minimal Inhibitory Concentration of the drug leading to 100% of lethality of bacterial cells.
Ethidium Bromide Accumulation Assay
This method was based on the method already described to study active efflux in Salmonella enterica strains [40]. Bacteria were grown in liquid medium (BYE) at 30°C until stationnary phase corresponding to an optical density of 3.6–3.9. The cells where centrifuged for 5 min at 13 000 rpm and the pellet was washed twice with sterile water. The OD600nm of the final cellular supension was adjusted to 0.3 in sterile water and the cells were incubated or not with carbonyl cyanide m-chlorophenylhydrazone (CCCP; 2,5 mg/L) for 30 minutes. After incubation, ethidium bromide was added to the suspension at a final concentration of 0,005 mg/mL and the bacterial suspension was distributed by aliquots of 100 µL in a 96 multiplate well. The change in fluorescence was recorded every 2 minutes on a « Xenius » (Safas) spectrofluorimeter (excitation 518 nm; emission 605 nm).
Measurement of Bacterial Release and Intracellular Growth
Intracellular growth of L. pneumophila strains was assayed using three eukarytotic hosts: two protozoan cells A. castellanii and D. discoideum and one mammalian cell: U937 macrophages. These hosts were choosed for there implication in environmental spreading of L. pneumophila (protozoa, natural host) or for there role in a clinical infection (macrophages, defective host). L. pneumophila were grown on BCYE agar for five days at 30°C prior to infection of protozoan cells and three days at 37°C before the infection of U937 macrophages. A. castellanii cells, D. discoideum cells and U937 macrophages were first seeded in plates of 96 multiwell plate to a final concentration of 1×105 cells.ml−1 in PY, MB and RPMI medium respectively. After a two-hour period of adhesion (except for U937 macrophages with a 2 day differenciation prior to infection) cells were washed four times and L. pneumophila was added to an MOI of 10 (in triplicate). The plates were spun at 2000×g for 10 min followed by an incubation of 1 hour (30°C for A. castellanii, 37°C for macrophages and 25°C for D. discoideum). At the end of this infection period, monolayers were washed four times with tissue culture medium to remove non-adherent bacteria. The time point at the end of the final wash was the initial time point (To). After several times post-infection (0, 12, 24, 48 and 72 h) aliquots of the supernatant were diluted on BCYE agar plates for enumeration of extracellular bacteria. For enumeration of intracellular bacteria the monolayers were washed at different times post-infection and were disrupted either hypotonically (serile water for U937 cells) or with a mild detergent (0,04% Triton X100 for A. castellanii and D. discoideum). Bacteria were then diluted in sterile water and plated on BCYE agar for enumeration. We verified that our mutant (strain MF201) exhibited the same sensitivity to detergent at the concentration we used. The experiment was repeated at least twice for each infection.
Cytotoxicity to U937 Cells, A. castellanii and D. discoideum
For measurement of the number of viable cells remaining, the monolayers were treated with 10% Alamar blue (Invitrogen) as recommended by the manufacturer. Briefly, at the time point indicated monolayers were washed four times with the appropriate medium and then 100 µl of the medium containing 10% (v/v) of Alamar blue was added in each well. After an incubation of several hours (4 h for macrophages, 9 h for A. castellanii and approximatively 48 h for D. discoideum), measurements of the optical density were performed at a wavelength of 570 nm and corrected for background at 600 nm with a µquant microplate reader. The relative degree of macrophage or amoeba cytotoxicity was expressed as the ratio of the optical density value of an infected monolayer to that of uninfected one with the formula {1-(mean OD value of infected/mean OD value of uninfected} x 100%.
Study of Invasion of U937 Cells, A. castellanii and D. discoideum
The study of levels of invasion were performed using a gentamycin protection assay. Briefly after two times of incubation of bacteria with eukaryotic cells (30 and 60 min), monolayers were washed and treated with gentamycin (50 µg/ml) for 1 hour. Then, monolayers were washed to remove gentamycin and were disrupted (either hypotonically for U937 macrophages or with Triton 0,04% for protozoa) to collect intracellular bacteria. Bacteria were diluted in sterile water and plated on BCYE agar for colony enumeration.
Statistical Analysis
All the results of statitical analysis were obtained using a student's t-test. All the t-test results mentioned correspond to the comparison with the parental strain value in the same conditions. R software (http://www.R-project.org) was used.
Acknowledgments
We thank Maëlle Molmeret and Christophe Ginevra (INSERM E230 - Faculté de médecine RTH Laennec 7–11 rue Guillaume Paradin 69372 Lyon Cedex 08) for the help with the macrophages infection experiments.
Supporting Information
Growth kinetics of L. pneumophila Lens derivatives. Strains lp01 (WT), MF201 (tolC::kan), MF213 (pML005-tolC), MF214 (pML005) and lpl701 (dotA::kan) were inoculated to an OD600 = 0.2 into BYE medium and grown at 30°C. OD600 at the time points were read using a microplate reader. The results are the mean of two experiments performed in triplicate. Error bars represent standard deviation.
(0.72 MB RTF)
Bacterial strains and plasmids
(0.02 MB RTF)
List of primers used in this study
(0.02 MB RTF)
Footnotes
Competing Interests: One team in the authors' Laboratory is working in association with a research team belonging to Bayer CropScience. However, the team does not have any research program in association with Bayer CropScience. Dr. Gilbert confirms that it does not alter his adherence to all the PLoS ONE policies on sharing data and materials, as detailed online in the guide for authors.
Funding: Université Lyon 1, Lyon, F-69003, France, http://www.univ-lyon1.fr/ Centre National de la Recherche Scientifique (CNRS), France, http://www.cnrs.fr/ Cluster Infectiologie region Rhone-Alpes 2007, France, http://www.cluster-infectiologie.fr/ The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Shin S, Roy CR. Host cell processes that influence the intracellular survival of Legionella pneumophila. Cellular Microbiology. 2008;10:1209–1220. doi: 10.1111/j.1462-5822.2008.01145.x. [DOI] [PubMed] [Google Scholar]
- 2.Swanson MS, Isberg RR. Association of Legionella pneumophila with the macrophage endoplasmic-reticulum. Infection and Immunity. 1995;63:3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tilney LG, Harb OS, Connelly PS, Robinson CG, Roy CR. How the parasitic bacterium Legionella pneumophila modifies its phagosome and transforms it into rough ER: implications for conversion of plasma membrane to the ER membrane. Journal of Cell Science. 2001;114:4637–4650. doi: 10.1242/jcs.114.24.4637. [DOI] [PubMed] [Google Scholar]
- 4.Isberg RR, O'Connor TJ, Heidtman M. The Legionella pneumophila replication vacuole: making a cosy niche inside host cells. Nature Reviews Microbiology. 2009;7:12–24. doi: 10.1038/nrmicro1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molmeret M, Bitar DM, Han LH, Abu Kwaik Y. Cell biology of the intracellular infection by Legionella pneumophila. Microbes and Infection. 2004;6:129–139. doi: 10.1016/j.micinf.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Poole K. Overcoming multidrug resistance in Gram-negative bacteria. Curr Opin Investig Drugs. 2003;4:128–139. [PubMed] [Google Scholar]
- 7.Li XZ, Nikaido H. Efflux-mediated drug resistance in bacteria. Drugs. 2004;64:159–204. doi: 10.2165/00003495-200464020-00004. [DOI] [PubMed] [Google Scholar]
- 8.Poole K. Efflux-mediated multiresistance in Gram-negative bacteria. Clinical Microbiology and Infection. 2004;10:12–26. doi: 10.1111/j.1469-0691.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- 9.Baucheron S, Tyler S, Boyd D, Mulvey MR, Chaslus-Dancla E, et al. AcrAB-TolC directs efflux-mediated multidrug resistance in Salmonella enterica serovar Typhimurium DT104. Antimicrobial Agents and Chemotherapy. 2004;48:3729–3735. doi: 10.1128/AAC.48.10.3729-3735.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishino K, Yamada J, Hirakawa H, Hirata T, Yamaguchi A. Roles of TolC-dependent multidrug transporters of Escherichia coli in resistance to beta-lactams. Antimicrobial Agents and Chemotherapy. 2003;47:3030–3033. doi: 10.1128/AAC.47.9.3030-3033.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seeger MA, Diederichs K, Eicher T, Brandstatter L, Schiefner A, et al. The AcrB Efflux Pump: Conformational Cycling and Peristalsis Lead to Multidrug Resistance. Current Drug Targets. 2008;9:729–749. doi: 10.2174/138945008785747789. [DOI] [PubMed] [Google Scholar]
- 12.Gil H, Platz GJ, Forestal CA, Monfett M, Bakshi CS, et al. Deletion of TolC orthologs in Francisella tularensis identifies roles in multidrug resistance and virulence. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12897–12902. doi: 10.1073/pnas.0602582103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Posadas DM, Martin FA, Garcia J, Spera JM, Delpino MV, et al. The TolC homologue of Brucella suis is involved in resistance to antimicrobial compounds and virulence. Infection and Immunity. 2007;75:379–389. doi: 10.1128/IAI.01349-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckley AM, Webber MA, Cooles S, Randall LP, La Ragione RM, et al. The AcrAB-TolC efflux system of Salmonella enterica serovar Typhimurium plays a role in pathogenesis. Cellular Microbiology. 2006;8:847–856. doi: 10.1111/j.1462-5822.2005.00671.x. [DOI] [PubMed] [Google Scholar]
- 15.Stone BJ, Miller VL. Salmonella enteritidis has a homolog of TolC that is required for virulence in balb/c mice. Molecular Microbiology. 1995;17:701–712. doi: 10.1111/j.1365-2958.1995.mmi_17040701.x. [DOI] [PubMed] [Google Scholar]
- 16.Cazalet C, Rusniok C, Bruggemann H, Zidane N, Magnier A, et al. Evidence in the Legionella pneumophila genome for exploitation of host cell functions and high genome plasticity. Nature Genetics. 2004;36:1165–1173. doi: 10.1038/ng1447. [DOI] [PubMed] [Google Scholar]
- 17.Marchler-Bauer A, Anderson JB, Derbyshire MK, DeWeese-Scott C, Gonzales NR, et al. CDD: a conserved domain database for interactive domain family analysis. Nucleic Acids Research. 2007;35:D237–D240. doi: 10.1093/nar/gkl951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen DQ, Zheng XC, Lu YJ. Identification and characterization of novel ColE1-type, high-copy number plasmid mutants in Legionella pneumophila. Plasmid. 2006;56:167–178. doi: 10.1016/j.plasmid.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Lau SY, Zgurskaya HI. Cell division defects in Escherichia coli deficient in the multidrug efflux transporter AcrEF-TolC. Journal of Bacteriology. 2005;187:7815–7825. doi: 10.1128/JB.187.22.7815-7825.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sulavik MC, Houseweart C, Cramer C, Jiwani N, Murgolo N, et al. Antibiotic susceptibility profiles of Escherichia coli strains lacking multidrug efflux pump genes. Antimicrobial Agents and Chemotherapy. 2001;45:1126–1136. doi: 10.1128/AAC.45.4.1126-1136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viveiros M, Martins A, Paixao L, Rodrigues L, Martins M, et al. Demonstration of intrinsic efflux activity of Escherichia coli K-12 AG100 by an automated ethidium bromide method. International Journal of Antimicrobial Agents. 2008;31:458–462. doi: 10.1016/j.ijantimicag.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 22.Bokma E, Lobedanz S, Symmons MF, Koronakis E, Brandstatter H, et al. Assembly and mechanism of the Type I multi-drug efflux pump AcrAB-TolC from Escherichia coli. Amino Acids. 2007;33:VIII–IX. [Google Scholar]
- 23.Lobedanz S, Bokma E, Symmons MF, Koronakis E, Hughes C, et al. A periplasmic coiled-coil interface underlying TolC recruitment and the assembly of bacterial drug efflux pumps. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4612–4617. doi: 10.1073/pnas.0610160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamanaka H, Kobayashi H, Takahashi E, Okamoto K. MacAB Is Involved in the Secretion of Escherichia coli Heat-Stable Enterotoxin II. Journal of Bacteriology. 2008;190:7693–7698. doi: 10.1128/JB.00853-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cosme AM, Becker A, Santos MR, Sharypova LA, Santos PM, et al. The outer membrane protein TolC from Sinorhizobium meliloti affects protein secretion, polysaccharide biosynthesis, antimicrobial resistance, and symbiosis. Molecular Plant-Microbe Interactions. 2008;21:947–957. doi: 10.1094/MPMI-21-7-0947. [DOI] [PubMed] [Google Scholar]
- 26.Karatzas KAG, Randall LP, Webber M, Piddock LJV, Humphrey TJ, et al. Phenotypic and proteomic characterization of multiply antibiotic-resistant variants of Salmonella entetica serovar typhimurium selected following exposure to disinfectants. Applied and Environmental Microbiology. 2008;74:1508–1516. doi: 10.1128/AEM.01931-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeLeo FR, Allen LAH, Apicella M, Nauseef WM. NADPH oxidase activation and assembly during phagocytosis. Journal of Immunology. 1999;163:6732–6740. [PubMed] [Google Scholar]
- 28.Frohner IE, Bourgeois C, Yatsyk K, Majer O, Kuchler K. Candida albicans cell surface superoxide dismutases degrade host-derived reactive oxygen species to escape innate immune surveillance. Molecular Microbiology. 2009;71:240–252. doi: 10.1111/j.1365-2958.2008.06528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bandyopadhyay P, Byrne B, Chan Y, Swanson MS, Steinman HM. Legionella pneumophila catalase-peroxidases are required for proper trafficking and growth in primary macrophages. Infection and Immunity. 2003;71:4526–4535. doi: 10.1128/IAI.71.8.4526-4535.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Golubeva YA, Slauch JM. Salmonella enterica serovar Typhimurium periplasmic superoxide dismutase SodCI is a member of the PhoPQ regulon and is induced in macrophages. Journal of Bacteriology. 2006;188:7853–7861. doi: 10.1128/JB.00706-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keith KE, Valvano MA. Characterization of SodC, a periplasmic superoxide dismutase from Burkholderia cenocepacia. Infection and Immunity. 2007;75:2451–2460. doi: 10.1128/IAI.01556-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin RG, Bartlett ES, Rosner JL, Wall ME. Activation of the Escherichia coli marA/soxS/rob regulon in response to transcriptional activator concentration. Journal of Molecular Biology. 2008;380:278–284. doi: 10.1016/j.jmb.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang A, Rosner JL, Martin RG. Transcriptional activation by MarA, SoxS and Rob of two tolC promoters using one binding site: a complex promoter configuration for tolC in Escherichia coli. Molecular Microbiology. 2008;69:1450–1455. doi: 10.1111/j.1365-2958.2008.06371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berger KH, Merriam JJ, Isberg RR. Altered intracellular targeting properties associated with mutations in the Legionella pneumophila dotA gene. Molecular Microbiology. 1994;14:809–822. doi: 10.1111/j.1365-2958.1994.tb01317.x. [DOI] [PubMed] [Google Scholar]
- 35.Molmeret M, Santic M, Asare R, Carabeo RA, Abu Kwaik Y. Rapid escape of the dot/icm mutants of Legionella pneumophila into the cytosol of mammalian and protozoan cells. Infection and Immunity. 2007;75:3290–3304. doi: 10.1128/IAI.00292-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Virlogeux-Payant I, Baucheron S, Pelet J, Trotereau J, Bottreau E, et al. TolC, but not AcrB, is involved in the invasiveness of multidrug-resistant Salmonella enterica serovar Typhimurium by increasing type III secretion system-1 expression. International Journal of Medical Microbiology. 2008;298:561–569. doi: 10.1016/j.ijmm.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 37.Bunikis I, Denker K, Ostberg Y, Andersen C, Benz R, et al. An RND-type efflux system in Borrelia burgdorferi is involved in virulence and resistance to antimicrobial compounds. Plos Pathogens. 2008;4 doi: 10.1371/journal.ppat.1000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahamoud A, Chevalier J, Alibert-Franco S, Kern WV, Pages JM. Antibiotic efflux pumps in Gram-negative bacteria: the inhibitor response strategy. Journal of Antimicrobial Chemotherapy. 2007;59:1223–1229. doi: 10.1093/jac/dkl493. [DOI] [PubMed] [Google Scholar]
- 39.Pope CD, Dhand L, Cianciotto NP. Random mutagenesis of Legionella pneumophila with mini-Tn10. Fems Microbiology Letters. 1994;124:107–111. doi: 10.1111/j.1574-6968.1994.tb07269.x. [DOI] [PubMed] [Google Scholar]
- 40.Giraud E, Cloeckaert A, Kerboeuf D, Chaslus-Dancla E. Evidence for active efflux as the primary mechanism of resistance to ciprofloxacin in Salmonella enterica serovar typhimurium. Antimicrobial Agents and Chemotherapy. 2000;44:1223–1228. doi: 10.1128/aac.44.5.1223-1228.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Growth kinetics of L. pneumophila Lens derivatives. Strains lp01 (WT), MF201 (tolC::kan), MF213 (pML005-tolC), MF214 (pML005) and lpl701 (dotA::kan) were inoculated to an OD600 = 0.2 into BYE medium and grown at 30°C. OD600 at the time points were read using a microplate reader. The results are the mean of two experiments performed in triplicate. Error bars represent standard deviation.
(0.72 MB RTF)
Bacterial strains and plasmids
(0.02 MB RTF)
List of primers used in this study
(0.02 MB RTF)