Abstract
Background
Gamma-hydroxybutyrate (GHB) is a common drug of abuse that can produce serious toxicity, particularly when used with other sedatives. We examined the individual and combined effects of GHB and ethanol in human volunteers.
Methods
Sixteen healthy adults (7 men) were given 50 mg/kg GHB (Xyrem), 0.6 g/kg ethanol in 2 doses, alone and combined in a double-blind, placebo-controlled, crossover study. Plasma concentrations, heart rate (HR), blood pressure (BP), and oxygen saturation (O2sat) were serially monitored for 24 hours.
Results
Adverse events included 2 instances of hypotension and 6 episodes of vomiting with GHB-plus-ethanol ingestion. Oxygen saturation was decreased by GHB and ethanol individually, and maximally decreased by the drugs combined (max −2.1% ± 0.3%, P < 0.0001 vs placebo). Compared with baseline, systolic and diastolic BP were significantly decreased, and HR was increased by ethanol but not affected by GHB alone (maximum systolic BP change −15.7 ± 3.0 mm Hg, P = 0.0006; maximum HR change 13.5 ± 2.3 beats per minute, P = 0.006). Ethanol coingestion resulted in 16% higher GHB maximal plasma concentration and 29% longer elimination half-life, indicating possible enhanced bioavailability or reduced clearance of GHB caused by ethanol, however, these effects were not statistically significant.
Conclusions
Modest doses of GHB do not affect hemodynamic function, but O2sat was decreased. Gamma-hydroxybutyrate-plus-ethanol resulted in more adverse effects, including gastrointestinal disturbances, hypotension, and decreased O2sat, but only minimal pharmacokinetic interactions were observed.
Gamma-hydroxybutyrate (GHB) has become a common drug of abuse in the United States and Europe in recent years. GHB was used in the late 1980s by body builders as a nonanabolic steroid performance enhancer and became widely available for sale in health food stores, gymnasiums, and via the Internet. Later, as the reputation for its euphoric effects grew, GHB became popular as a drug of abuse at dance clubs and "rave" parties. More recently, GHB has been used as a drug to facilitate sexual assault. Because of its abuse potential, GHB was classified as a Schedule I controlled substance in 2000, with dual scheduling instituted 2 years later when GHB became Food and Drug Administration approved as the pharmaceutical product Xyrem.
In recent years, GHB has been a major cause of emergency department visits for drug-induced central nervous system depression.1–5 In overdose, GHB produces coma, often associated with hypothermia, bradycardia, hypotonia, myoclonus, and seizure-like activity.1,2 In very high doses, respiratory depression and death can occur.3–5 At lower doses, the primary effects of GHB are drowsiness, alcohol-like inebriation, dizziness, and induction of sleep.6,7
Gamma-hydroxybutyrate was previously studied as an anesthetic agent, and has also been studied as a medication for the treatment of cataplexy, a symptom of narcolepsy. Some effectiveness for this indication has been demonstrated, and GHB is currently Food and Drug Administration approved for use as a Schedule III drug for the treatment of narcolepsy.8 In addition, GHB has been marketed in Italy for the treatment of alcoholism since 1994. Psychomotor performance has been studied in healthy volunteers receiving GHB 12.5 and 25 mg/kg, and no significant effects of GHB were observed.6
Gamma-hydroxybutyrate is found naturally in the human body as a degradation product of the neurotransmitter, gamma-aminobutyric acid. GHB is extensively metabolized, in part to succinic acid, then through the tricarboxylic acid pathway, and in part by beta oxidation.9,10 Alcohol dehydrogenase has been postulated to contribute to GHB metabolism in animal and human liver.11 There is evidence of dose-dependent metabolism. The disposition kinetics of GHB in humans have been described in 3 published studies using doses of 12.5 to 50 mg/kg orally.12–14 The average elimination half-life after single doses ranged from 30 to 50 minutes, and the time to peak concentration averaged 30 to 40 minutes, consistent with rapid gastrointestinal absorption.
In recreational use, GHB is frequently ingested with other drugs, including most commonly ethanol, which has sedative effects similar to those of GHB. There is a concern that these drugs may interact synergistically in producing sedation. However, this has not been well studied in doses of GHB that are relevant to therapeutic dosing or to recreational use. One study of the combination of GHB and low-dose ethanol showed significant impairment of reaction time with the combination.15 There have been no human studies conducted on the pharmacokinetic interactions between GHB and ethanol.
We examined the effects of ethanol on the pharmacokinetics of GHB, and the pharmacodynamic interactions between ethanol and GHB in healthy human volunteers. We used a 50-mg/kg dose of GHB, which is comparable to doses used in the treatment of narcolepsy. We administered 0.6-g/kg dose of ethanol, which was projected to produce blood alcohol concentrations of about 50 mg/dL. Our objectives were to (1) identify any significant pharmacokinetic interactions that exist between these 2 sedative-hypnotic drugs; (2) determine if coingestion of ethanol and GHB produces greater cognitive, hemodynamic, and subjective mood effects than either GHB or ethanol alone; and (3) explore differences in response to GHB and ethanol between men and women, and people of different races. In this article, we report our findings on the pharmacokinetics and the hemodynamic effects of GHB and ethanol. Subjective drug effects, effects of race and sex, and cognitive testing will be reported separately.
METHODS
Study Design and Procedures
Twenty-two healthy subjects between the ages of 21 and 45 years were recruited. Potential subjects were not selected on the basis of race or ethnicity. The subjects were deemed healthy based on medical history, physical examination, and screening laboratory tests. Exclusion criteria included any significant medical history, and history of pregnancy or obesity. Also excluded were illicit drug users, prescription drug users (other than oral contraceptives), and more than light users of alcohol (defined as greater than 3 drinks/week) or of GHB and its derivatives (defined as greater than 2 times in last 6 months). Volunteers provided written informed consent before enrollment. The Committee on Human Research at the University of California San Francisco approved the study.
The study used a randomized, double-blinded, 4-arm, crossover design. Subjects were given placebo, ethanol, GHB, or ethanol-plus-GHB in random order. Randomization was established by use of a 4 × 4 Latin square. All subjects were admitted to the General Clinical Research Center at San Francisco General Hospital for four 24-hour visits after an overnight fast from food, caffeine, and tobacco. Subjects were asked not to drink alcoholic beverages for 3 days before each study day. The washout period between treatments was a minimum of 2 days. At approximately 8:00 am on each study day, subjects received a dose of ethanol 0.3 g/kg (in the form of vodka) or placebo in orange or cranberry juice. Fifteen minutes later, the subjects received GHB 50 mg/kg (Xyrem, Orphan Medical Co., Minnetonka, MN) or placebo, and a second dose of ethanol 0.3 g/kg or placebo in juice.
Two predose recordings of heart rate (HR), blood pressure (BP), respiratory rate, and skin temperature were made 30 and 15 minutes before dosing, and again at the time of dosing, and then every 15 minutes for the first 2 hours, and every 30 minutes for the subsequent 2 hours. A Critikon Dinamap automated BP instrument (GE Medical Systems Information Technologies, Waukesha, WI) was used to monitor vital signs. Oxygen saturation (O2sat) was monitored by continuous pulse oximetry for 4 hours. Subjects were asked to remain in a supine position for 3 hours after ingesting the doses. They were then allowed caffeine-free meals and beverages.
Blood samples were collected from a forearm vein through an intravenous catheter at the time of each dose and at 15, 30, 45, 60, and 90 minutes, and 2, 3, 4, 5, 6, 12, and 24 hours after GHB dosing. Urine was collected in intervals of 0 to 3, 3 to 6, 6 to 12, 12 to 24, and post-24 hours after dosing. Plasma and urine samples were stored at −20°C until the time of analysis for GHB and ethanol concentrations.
Study participants completed a visual analog scale to evaluate subjective drug effects and a computerized cognitive battery. These findings will be reported separately.
Pharmacokinetic Analysis
Plasma concentration-versus-time data for GHB were evaluated by noncompartmental analysis using WinNonlin (version 4.0, Pharsight Corporation, Mountain View, CA). The pharmacokinetic parameters time at maximum concentration (Tmax) and maximum drug concentration (Cmax) were obtained directly from the plasma concentration-versus-time data. The area under the plasma concentration-time curve (AUC) was calculated using the trapezoidal rule and extrapolated from the last measured concentration to infinity. The elimination rate constant (k) was estimated from the slope of the linear portion of the log plasma concentration-versus-time curve. The elimination half-life (t1/2) was calculated by dividing 0.693 by k. The clearance of each drug was calculated by dividing the oral dose by the AUC. Renal clearance was calculated by dividing the total amount recovered in urine by the plasma AUC from 0 to 24 hours. For ethanol, which undergoes zero-order elimination, a rate of blood alcohol decline was calculated from the slope of the arithmetic curve of the concentration-versus-time plot.
Analytical Methods
Plasma and urine samples obtained from human volunteers administered GHB were stored frozen at −20°C until the time of analysis. A novel gas chromatography-mass spectrometry method for analysis of GHB in plasma and urine was developed and validated, which is described in detail separately.16 Limits of detection for GHB were found to be 0.5 μg/mL in plasma and 0.25 μg/mL in urine. For these analyses, we established a limit of quantitation of 5 μg/mL because concentrations below this threshold are in the range of endogenous GHB levels.17 Blood ethanol concentrations were determined using an enzymatic DRI ethanol assay (Microgenics Inc., Fremont, CA) on a Bayer ADVIA 1650 chemistry analyzer.
Statistical and Data Analysis
The primary aim of the study was to investigate the pharmacokinetic and pharmacodynamic interactions between GHB and ethanol. The study was powered to detect a 25% difference in the clearance of GHB caused by the presence of ethanol. A minimum sample size of 16 was needed for 80% power and a 2-tailed test of significance of 0.05. Pharmacokinetic parameters were analyzed by paired students t tests. For hemodynamic variables, baseline values were the average of measurements taken at time 0, 15, and 30 minutes before dosing. For all pharmacodynamic data, means, standard deviations, and 95% confidence intervals were calculated for each treatment, followed by pair-wise comparisons. The AUC for change from baseline over time was estimated using the trapezoidal rule from baseline until the end of the measurement period for O2sat (0–4 hours) and from 0 to 6 hours for HR and BP, at which time GHB plasma levels were nondetectable. These data were analyzed with a mixed effects regression model with fixed effects of treatment, and all the 2-way interactions and a random subject effect. The data were analyzed using SAS (Version 8.2., SAS Institute, Cary, NC). Statistical significance was defined a priori as a 2-sided [alpha] < 0.05.
RESULTS
Subject Characteristics
Twenty-two healthy adults were enrolled, with 16 subjects (7 men and 9 women) aged 22 to 34 years completing the study. One male subject withdrew from the study because of disinterest after his first visit. Five female subjects withdrew from the study (1 because of disinterest, 2 because of poor intravenous access, and 2 because of protocol violations). No adverse events that necessitated subject withdrawal or medical intervention occurred during the study. Subjects ranged in weight from 47.9 to 83.0 kg (mean, 66.2 kg) and in height from 146 to 183 cm (mean, 169 cm). Of the subjects completing the study, 7 (44%) were white, 6 (38%) were Asian/Pacific Islander, 1 (6%) was Latino, and 2 (12%) reported multiple ethnicities.
Adverse Events
Vomiting was the most common adverse event, which occurred during 8 separate study days. Six of these visits involved combined GHB and ethanol treatment, one involved GHB alone and one involved ethanol alone. Two episodes of brief asymptomatic hypotension (systolic BP [SBP], 71–73 mm Hg) lasting less than 15 minutes were also observed after GHB-plus-ethanol. None of the adverse events necessitated therapeutic intervention, and all resolved spontaneously.
Pharmacokinetics
Mean plasma concentration-over-time curves for GHB and ethanol, alone and in combination, are shown in Figures 1A, B. The mean pharmacokinetic parameters for GHB and ethanol are listed in Table 1 and Table 2, respectively. Although differences between treatments in pharmacokinetics of GHB and ethanol were not statistically significant, Cmax for GHB was 16% greater and t1/2 was 29% longer in the presence of ethanol, indicating possible enhanced bioavailability or reduced clearance of GHB because of ethanol coingestion.
FIGURE 1.
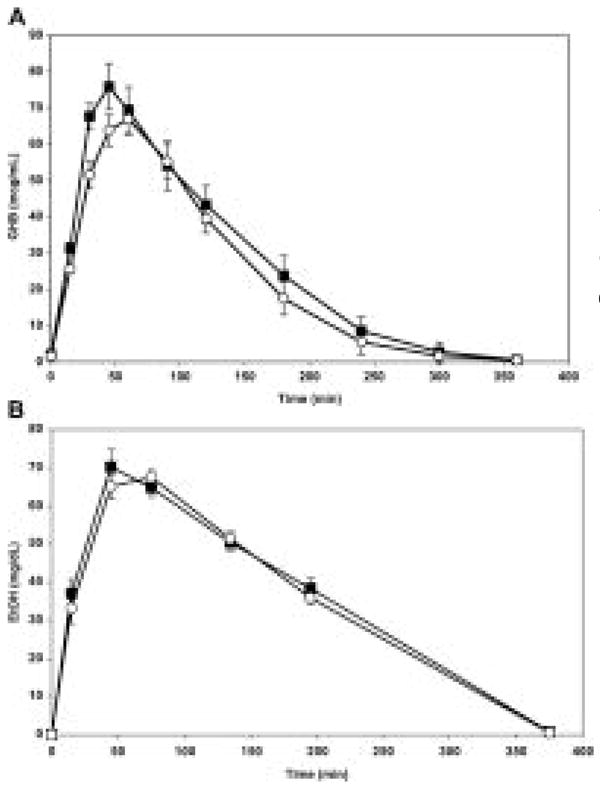
Mean plasma concentrations over time for GHB (A) and ethanol (B) after oral doses of the drugs were administered alone (open circles) and in combination (solid squares) (n = 16). Note that ethanol was dosed 15 minutes before GHB.
TABLE 1.
Mean Pharmacokinetic Parameters of 50 mg/kg GHB Given Alone and in Combination With Ethanol
| Cmax (μg/mL) | Tmax (min) | t1/2 (min) | AUC (μg· min/mL) | Clearance (mL/min· kg) | Renal Clearance (mL/min) | % Dose Recovered in Urine in 24 Hours | |
|---|---|---|---|---|---|---|---|
| GHB alone | 72.6 ± 16.2 | 57.2 ± 12.5 | 51.8 ± 21.3 | 9330 ± 3390 | 6.2 ± 2.7 | 17.8 ± 13.1 | 4.8 ± 3.1 |
| GHB with ethanol | 84.0 ± 25.8 | 54.0 ± 27.9 | 67.0 ± 48.4 | 11,360 ± 4796 | 5.0 ± 2.4 | 19.3 ± 17.4 | 5.0 ± 4.1 |
Values are expressed as means (n = 16) ± standard deviations. All comparisons were not statistically significant (P > 0.05).
TABLE 2.
Mean Pharmacokinetic Parameters of 600 mg/kg Ethanol Given Alone and in Combination With GHB
| Cmax (mg/dL) | Tmax (min) | AUC (mg· min/dL) | Rate of Blood Ethanol Decline (mg/dL·min) | |
|---|---|---|---|---|
| Elhanol alone | 70.7 ± 11.6 | 46.9 ± 15.4 | 19,810 ± 5342 | 0.24 ± 0.07 |
| Elhanol with GHB | 76.6 ± 13.3 | 39.4 ± 14.4 | 18,840 ± 6450 | 0.26 ± 0.08 |
Values are expressed as means (n = 16) ± standard deviations. All comparisons were not statistically significant (P > 0.05).
Pharmacodynamics
Blood Pressure
Ethanol significantly decreased mean SBP for 5 hours after dosing. The peak decrease from baseline SBP occurred at 105 minutes with a mean change of −15.7 ± 3.0 mm Hg (P = 0.0006 vs GHB alone). Gamma-hydroxybutyrate alone had no significant effect on BP, and the 2 drugs combined did not have a greater effect on SBP than ethanol alone.
Ethanol also decreased diastolic BP (DBP) compared with placebo and GHB alone. There was no significant difference between the DBP-lowering effects of ethanol administered alone or in combination with GHB. The peak change in DBP from baseline observed 2 hours after dosing with ethanol + GHB was −16.9 ± 2.5 mm Hg (P = 0.012 vs placebo).
Heart Rate
Heart rate was significantly increased by ethanol taken alone. The maximum HR change observed 45 minutes after dosing with ethanol was 13.5 ± 2.3 beats per minute (P = 0.006 vs placebo). Gamma-hydroxybutyrate alone did not affect HR, and there was no influence of GHB coadministration on the HR-accelerating effects of ethanol.
Oxygen Saturation
Oxygen saturation was significantly decreased by all 3 active treatments relative to placebo (Fig. 2). Ethanol + GHB produced the greatest decrease in O2sat, with a maximal effect at 90 minutes of −2.12% ± 0.34%, which was significantly greater than the effects of placebo (P < 0.0001), GHB (P = 0.027), or ethanol alone (P = 0.013). Oxygen saturation remained significantly decreased from baseline with all 3 active treatments throughout the 4-hour period of pulse oximetry monitoring.
FIGURE 2.
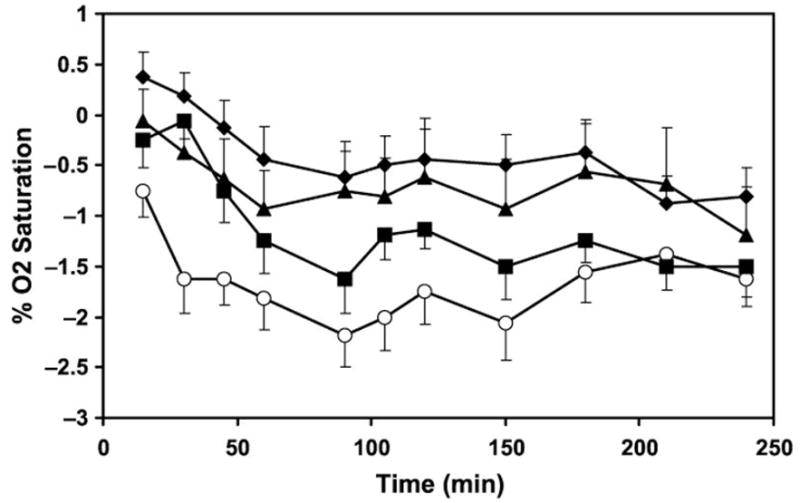
Mean change in percent O2sat relative to baseline after a single oral dose of GHB plus ethanol (open circles), GHB (squares), ethanol (triangles), and placebo (solid diamonds). Data are presented as means ± 95% confidence interval.
Skin Temperature
Skin temperature was increased by all 3 active treatments relative to placebo in the first post-dosing hour only. The maximal effect on skin temperature was observed with ethanol + GHB at 45 minutes. The mean maximum increase was 2.9 ± 0.7°F, which was significantly higher than placebo (P < 0.001) or ethanol alone (P = 0.028).
DISCUSSION
This study provides novel clinical data on the effects and interactions of GHB and ethanol, a combination that is frequently coingested in the setting of recreational drug use. Significant pharmacokinetic interactions between GHB and ethanol were not observed; however, the 2 drugs taken together resulted in more adverse effects, including gastrointestinal disturbances, hypotension, and decreased O2sat.
In overdose, GHB is well described to produce coma, bradycardia, agitation, hypotension, hypothermia, and respiratory depression.1–5 Ethanol is a frequent coingestant in many instances of GHB intoxication, which is believed to enhance the adverse sedative effects of GHB.1,15,18 Previous research involving rats showed that ethanol administration increases endogenous GHB concentrations in the liver and brain.19,20 Thus, it was postulated that ethanol potentiates the sedative effects of GHB through pharmacokinetic interactions.18 However, a recent study in rats found no differences in GHB plasma concentrations or AUCs with ethanol coadministration, although significant pharmacodynamic interactions, including prolonged sleeping time were seen.21 These findings are consistent with the results of our study in which we found minimal pharmacokinetic interactions between GHB and ethanol after single oral doses, but significant adverse effects were observed with the 2-drug combination.
A small effect of ethanol on the clearance and/or bioavailability of GHB was observed, resulting in greater Cmax and longer t1/2 of GHB when the 2 drugs were coadministered. Although this finding did not reach statistical significance, this drug interaction could have a more pronounced clinical effect at higher GHB doses, such as unintentional overdoses. We also describe GHB pharmacokinetic parameters that are slightly different than previously reported. In prior published studies using single doses of GHB ranging from 12.5 to 50 mg/kg orally, the elimination half-life averaged 30 to 50 minutes, and the Tmax averaged approximately 30 to 40 minutes.12–14 In the present study, Tmax and the elimination half-life averaged around 1 hour. However, these differences are unlikely to be of clinical significance.
In the modest GHB dose used in this study, significant mean changes in vital signs were not observed with GHB alone. In contrast, ethanol administration caused a pronounced decrease in mean systolic and diastolic BP and increased HR. These findings confirm previous reports that acute ethanol consumption causes short-term lowering of BP through peripheral vasodilation, with a reflex increase in HR.22,23 Increased skin temperature observed in this study could also be attributable to peripheral vasodilation. A new finding is that GHB, in the modest dose administered, did not exacerbate the hypotensive or HR-accelerating actions of ethanol.
Gamma-hydroxybutyrate intoxication is well described to result in respiratory depression, in some cases, requiring mechanical ventilation.1–5 In 1 study of 88 patients presenting to the emergency department with self-reported GHB intoxication, 11 were intubated and 21 had documented arterial pCO2 of greater than 45 mm Hg.1 Other reports have documented bradypnea, significant reductions in oxygen blood levels, and respiratory arrest associated with GHB ingestion.3–5 In this study, we demonstrated that relatively small doses of GHB can significantly decrease O2sat. The combination of GHB and ethanol was found to have increased respiratory depressant effects compared with either drug alone. Although the 2% change in O2sat was statistically significant, this is a modest difference in absolute terms. However, even such a small decrement could be clinically important if a person has taken other respiratory depressant drugs or has underlying pulmonary disease. Ethanol and sedative-hypnotic drugs produce hypoventilation by central suppression of respiratory drive.24 In low doses, GHB has been shown to slow respiratory rate, but ventilation depth is increased, so that minute ventilation is maintained. Our results indicate that concomitant use of GHB and ethanol intensifies respiratory depression, which may explain some of the severe toxicity and deaths reported with recreational use of GHB.1–5
In summary, we found that coadministration of modest doses of GHB and ethanol resulted in increased episodes of vomiting and hypotension, and a greater decrease in O2sat, but only minimal pharmacokinetic interactions were observed.
Acknowledgments
The authors thank Gina Lowry for subject recruitment and assistance with the study, Faith Allen for protocol and data management, Dr. Peter Bacchetti and Alan Bostrom for statistical analysis, and the San Francisco General Hospital General Clinical Research Center for care of the research subjects. Xyrem used in this study was generously provided by Orphan Medical Company (Minnetonka, Minn). The authors also thank Pharsight Corporation (Mountain View, Calif) for donation of a WinNonlin license.
This work was supported by Public Health Service grants DA 14935 and DA12393 from the National Institute on Drug Abuse (NIDA), a clinical pharmacology training grant award (T32 GM007546-28) and a General Clinical Research Center Award (M01RR00083-41) from the National Institutes of Health.
Footnotes
Conflict of Interest Statement
Dr. Dyer and Dr. Haller have provided paid medicolegal consultation in cases involving GHB. Drs Thai, and Benowitz have no potential conflicts of interest to disclose.
Disclosure Statement: Dr. Dyer and Dr. Haller have provided paid medicolegal consultation in cases involving GHB. Dr. Thai and Dr. Benowitz have no potential conflicts of interest to disclose.
References
- 1.Chin RL, Sporer KA, Cullison B, et al. Clinical course of gamma-hydroxybutyrate overdose. Ann Emerg Med. 1998;31(6):716–722. [PubMed] [Google Scholar]
- 2.Zvosec DL, Smith SW, McCutcheon JR, et al. Adverse events, including death, associated with the use of 1,4-butanediol. N Engl J Med. 2001;344(2):87–94. doi: 10.1056/NEJM200101113440202. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Stokes SA, Woechener A. A tale of novel intoxication: seven cases of [gamma]-hydroxybutyric acid overdose. Ann Emerg Med. 1998;31:723–728. doi: 10.1016/s0196-0644(98)70231-8. [DOI] [PubMed] [Google Scholar]
- 4.Couper FJ, Thatcher JE, Logan BK. Suspected GHB overdoses in the emergency department. J Anal Toxicol. 2004;28(6):481–484. doi: 10.1093/jat/28.6.481. [DOI] [PubMed] [Google Scholar]
- 5.Ingels M, Rangan C, Bellezzo J, et al. Coma and respiratory depression following the ingestion of GHB and its precursors: three cases. J Emerg Med. 2000;19(1):47–50. doi: 10.1016/s0736-4679(00)00188-8. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara SD, Giorgetti R, Zancaner S, et al. Effects of single dose of gamma-hydroxybutyric acid and lorazepam on psychomotor performance and subjective feelings in healthy volunteers. Eur J Clin Pharmacol. 1999;45:821–827. doi: 10.1007/s002280050560. [DOI] [PubMed] [Google Scholar]
- 7.Tunnicliff G. Sites of action of gamma-hydroxybutyrate (GHB)-a neuroactive drug with abuse potential. J Toxicol Clin Toxicol. 1997;35(6):581–590. doi: 10.3109/15563659709001236. [DOI] [PubMed] [Google Scholar]
- 8.Fuller DE, Hornfeldt CS. From club drug to orphan drug: sodium oxybate (Xyrem) for the treatment of cataplexy. Pharmacotherapy. 2003;23(9):1205–1209. doi: 10.1592/phco.23.10.1205.32756. [DOI] [PubMed] [Google Scholar]
- 9.Palatini P, Tedeschi L, Frison G, et al. Dose-dependent absorption and elimination of gamma-hydroxybutyric acid in healthy volunteers. Eur J Clin Pharmacol. 1993;45(4):353–356. doi: 10.1007/BF00265954. [DOI] [PubMed] [Google Scholar]
- 10.Van Cauter E, Plat L, Scharf M, et al. Simultaneous stimulation of slow-wave sleep and growth hormone secretion by gamma-hydroxybutyrate in normal young men. J Clin Invest. 1997;100:745–753. doi: 10.1172/JCI119587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poldrugo F, Addolorato G. The role of [gamma]-hydroxybutyric acid in the treatment of alcoholism: from animal to clinical studies. Alcohol Alcohol. 1999;34(1):15–24. doi: 10.1093/alcalc/34.1.15. [DOI] [PubMed] [Google Scholar]
- 12.Brenneisen R, Elsohly MA, Murphy TP, et al. Pharmacokinetics and excretion of gamma-hydroxybutyrate (GHB) in healthy subjects. J Anal Toxicol. 2004;28(8):625–630. doi: 10.1093/jat/28.8.625. [DOI] [PubMed] [Google Scholar]
- 13.Borgen LA, Okerholm RA, Lai A, et al. The pharmacokinetics of sodium oxybate oral solution following acute and chronic administration to narcoleptic patients. J Clin Pharmacol. 2004;44(3):253–257. doi: 10.1177/0091270003262795. [DOI] [PubMed] [Google Scholar]
- 14.Scharf M, Lai A, Branigan B, et al. Pharmacokinetics of gammahydroxybutyrate (GHB) in narcoleptic patients. Sleep. 1998;21:507–514. doi: 10.1093/sleep/21.5.507. [DOI] [PubMed] [Google Scholar]
- 15.Mattila MJ, Palva E, Seppala T, et al. Actions and interactions with alcohol of drugs on psychomotor skills: comparison of diazepam and gamma-hydroxybutyric acid. Arch Int Pharmacodyn Ther. 1978;234:236–246. [PubMed] [Google Scholar]
- 16.Thai DL, Haller CA, Benowitz NL, et al. Rapid and efficient method for the simultaneous measurement of gamma-hydroxybutyrate and 1,4-butanediol in human plasma and urine. J Anal Toxicol. In press. [Google Scholar]
- 17.Elliott SP. Gamma hydroxybutyric acid (GHB) concentrations in humans and factors affecting endogeneous production. Forensic Sci Int. 2003;133:9–16. doi: 10.1016/s0379-0738(03)00043-4. [DOI] [PubMed] [Google Scholar]
- 18.Couper FJ, Marinetti LJ. Gamma-hydroxybutyrate (GHB)-effects on human performance and behavior. Forensic Sci Rev. 2002;14:101–121. [PubMed] [Google Scholar]
- 19.Poldrugo F, Snead OCI. Effect of ethanol and acetaldehyde on gamma-hydroxybutyric acid in the rat brain and liver. Subst Alcohol Actions Misuse. 1984;5:263–271. [PubMed] [Google Scholar]
- 20.Roth RH. Formation and regional distribution of gamma-hydroxybutyric acid in mammalian brain. Biochem Pharmacol. 1970;19:3013–3019. doi: 10.1016/0006-2952(70)90087-0. [DOI] [PubMed] [Google Scholar]
- 21.Van Sassenbroeck DK, De Paepe P, Belpaire FM, et al. Characterization of the pharmacokinetic and pharmacodynamic interaction between gamma-hydroxybutyrate and ethanol in the rat. Toxicol Sci. 2003;73:270–278. doi: 10.1093/toxsci/kfg079. [DOI] [PubMed] [Google Scholar]
- 22.Rosito GA, Fuchs FD, Duncan BB. Dose-dependent biphasic effect of ethanol on 24-h blood pressure in normotensive subjects. Am J Hypertens. 1999;12:236–240. doi: 10.1016/s0895-7061(98)00237-4. [DOI] [PubMed] [Google Scholar]
- 23.Kawano Y, Abe H, Kojima S, et al. Acute depressor effect of alcohol in patients with essential hypertension. Hypertension. 1992;20(2):219–226. doi: 10.1161/01.hyp.20.2.219. [DOI] [PubMed] [Google Scholar]
- 24.Goldfrank LR, Flomenbaum NE, Lewin NA, et al., editors. Goldfrank's Toxicologic Emergencies. 6. Stamford, CT: Appleton & Lange; 1998. p. 338. [Google Scholar]


