Abstract
Cyclooxygenase (COX) plays an important role in the induction of pain and inflammation as well as the analgesic actions of NSAIDs and coxibs. This study evaluates the expression of the two isoforms COX-1 and COX-2 in a clinical model in which the surgical removal of impacted third molars is used to evaluate the analgesic activity of anti-inflammatory drugs. A 3 mm punch biopsy was performed on the oral mucosa overlying one impacted third molar immediately before extraction of two impacted lower third molars. After the second tooth was extracted, a second biopsy was performed adjacent to the surgical site either immediately after surgery, 30, 60 or 120 minutes after surgery. RNA was extracted from the biopsies and RT-PCR was performed to assess mRNA levels of COX-1, COX-2 and glyceraldehyde-3-phosphate dehydrogenase (G3PDH). The RT-PCR products in the biopsies were normalized to G3PDH and compared to baseline. COX-2 mRNA was progressively increased at 30, 60, and 120 minutes after surgery (P<0.05); COX-1 mRNA was transiently decreased at 60 minutes during the post-surgical period (P<0.05). The results demonstrate peripheral elevation of COX-2 following tissue injury, which may contribute to increased prostaglandin E2 at the site of injury, pain onset and the analgesic activity of both non-selective NSAIDs and selective COX-2 inhibitors.
Perspective
This clinical study uses a physiologically relevant model to determine the time course of expression of COX -1 and -2 in acute inflammation of the human oral mucosa. This study furthers our understanding of the contribution of the COX isoforms to acute pain.
Introduction
Cyclooxygenase (COX), also known as prostaglandin H synthase, is the key enzyme in the synthesis of prostaglandins (PGs). Elucidation of the two COX isoforms gave rise to the concept that the constitutive enzyme COX-1 was responsible for the production of the PGs with homeostatic functions in tissues such as the stomach, kidney, and platelets, while COX-2, the inducible enzyme, was responsible for the production of the proinflamatory PGs.16, 27, 33
There is extensive evidence based on animal, as well as human studies supporting the role of COX-2 in the development of inflammation.27,32,34 Animal models of inflammation have demonstrated that COX-2 mRNA and protein as well as PGs increase in a time-dependent manner that parallels the inflammatory process.1 Inflammatory cytokines and endotoxins can induce a 10 to 80-fold increase in the level of COX-2 expression in monocytes, macrophages, chondrocytes, fibroblasts, and endothelial cells.1,2 The contribution of COX-2 to inflammation is further supported by demonstration that the expression of COX-2 and production of PGs can be inhibited by anti-inflammatory cytokines and glucocorticoids.6, 25
The concept that COX-2 is the only COX isoform involved in inflammation has been challenged by a number of studies.10,21,35 It is now believed that COX-1 is responsible for the initial prostanoid response to inflammatory stimuli, while COX-2 becomes the major contributor to prostanoid synthesis as inflammation progresses.9,16,31 PGE2 can be produced by PGE synthase from many different cell types including neurons, endothelial cells, and neutrophils. PGE2 released in inflamed tissue sensitizes the terminals of afferent nerve fibers thereby enhancing nocicpetive processing within the spinal cord and brain to evoke hyperalgesia.29 COX-1 mRNA has a half-life of about 12-15 hours while COX-2 has a shorter half-life of less than 3.5 hours,19 suggesting a close temporal link between tissue injury, COX-2 expression and elevated PGE2 in comparison to constitutively expressed COX-1.
A previous study in the oral surgery model demonstrated differential production of products of COX-1 (thromboxane B2, the stable metabolite of thromboxane A2) and PGE2 production mediated by both COX-1 and COX-2. The selective COX-2 inhibitor celecoxib did not have any detectable effect on thromboxane B2 levels and only suppressed PGE2 levels from 120-240 minutes following oral surgery.14 A similar time course of action was also demonstrated for rofecoxib in the oral surgery model with the effects of the selective COX-2 inhibitor being seen at 60-240 minutes post-surgery.15 These data suggest that the expression of COX-2 following tissue injury takes 1-2 hours to produce increased prostanoid levels that contribute to pain and the acute inflammatory process. We conducted a study to examine the in vivo expression of COX-1 and -2 in the human oral mucosa prior to and following post-surgical trauma and the onset of inflammation. Our results demonstrate that COX-2 mRNA rapidly increases in a time-dependent manner following surgery while the level of COX-1 mRNA transiently decreases, but otherwise shows no sustained alteration, during the post-surgical period.
Materials and Methods
Subjects and Study Design
The study was approved by the Institutional Review Board, National Institute of Dental and Craniofacial Research, National Institutes of Health. Informed consent was obtained from all subjects. Subjects, 16 years or older, were enrolled as outpatients and underwent surgical removal of impacted mandibular third molars. Inclusion criteria were the presence of two mandibular third molars, classified as partial or full bony impaction by clinical and radiographic examination. Exclusion criteria included the presence of infection or inflammation at either of the two extraction sites as determined by clinical examination. Subjects who were pregnant or nursing were excluded from the study as were those taking anti-depressants, diuretics, aspirin, coumadin or any other anticoagulants and any drugs such as steroids which might influence pain report or the synthesis and activity of COX.
Pre- and post-operative punch biopsies were obtained from each subject. The pre-operative biopsy was performed from the oral mucosa overlying one impacted third molar, immediately before the surgical extraction. Following this, both mandibular third molars were removed and the postoperative biopsy was obtained from the other extraction site. Subjects (n ≥ 10 per group) were randomly allocated with respect to the second biopsy into one of four groups: (1) time 0, immediately after surgery (n=10); (2) 30 min post-surgery (n=13); (3) 60 min post-surgery (n=10); or (4) 120 min post-surgery (n=10).
RNA extraction and RT-PCR Analysis
The samples were immediately frozen and maintained at −80° C. Total RNA was extracted using the RNeasy RNA extraction kit (Qiagen, CA, USA). RNA yield was quantified using the RiboGreen RNA Quantitation Kit (Molecular Probes, Oregon, USA). The RNA yield was 246 ± 42 ng/mg tissue weight. RT-PCR was carried out using the Access RT-PCR system (Promega, Madison, WI, USA). Primer sequences and assay parameters are given in Table 1.
Table 1. Sequence of primers used and their product length.
| Gene | Sequence of primers (5′ to 3′) | Product length (bp) | RNA Template (ng) | Number of cycles for PCR |
|---|---|---|---|---|
| COX-1 | Forward-CAGACGACCCGCCTCATCCTCATAG Reverse-GCCTCAACCCCATAGTCCACCAACA |
275 | 4 | 40 |
| COX-2 | Forward–TGGGAAGCCTTCTCTAACCTCTCCT Reverse-CTTTGACTGTGGGAGGATACATCTC |
388 | 8 | 40 |
| G3DPH | Forward-GACCCCTTCATTGACCTCAACTAC Reverse- CATCGCCCCACTTGATTTTG |
167 | 2 | 26 |
The program used for RT-PCR on a thermocycler (Robocycler Gradient 96, Stratagene) consisted of 48 °C for 45 minutes and 94 °C for 2 minutes for the synthesis of first strand cDNA by reverse transcriptase; followed by 40 cycles of a denaturation step at 94 °C for 30 seconds, an annealing step at 55 °C for 1 minute, and an extension step at 68 °C for 2 minutes for amplification of cDNA reverse transcribed from COX-2 mRNA. The final extension was done at 72 °C for 7 minutes. The program for COX-1 was similar except that the extension was done at 72 °C. For G3DPH, the extension and the final extension were done at 68 °C. The amount of RNA template and number of cycles that would yield relative values in the linear range of amplification for reach target transcript in the RT-PCR were determined by preliminary experiments. Each group of RT-PCR experiments included a negative control in which the extracted RNA was replaced by RNase-free water.
The PCR products were detected by electrophoresis in 2% agarose gels visualized with ethidium bromide staining. A fluorescence imaging system (AlphaImager, Alpha Innotech Corp., San Leandro, CA) was used to acquire the image of the fluorescent bands. Analysis of band intensity was performed on a Macintosh computer using the public domain NIH Image program (developed at the U.S. National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image). The RT-PCR products were normalized to G3PDH and the results of post-surgical biopsies were compared to those of pre-surgical biopsies.
Statistical Analysis
Changes in the ratio of COX-1/G3PDH and COX-2/G3PDH over time were compared to ratios in the pre-surgical biopsies. A permutation test for two related samples tested for change over time in levels of each COX isoforms was performed with post hoc testing to determine if any time points differed from baseline. Statistical analyses were performed with SPSS 6.1 (SPSS, Chicago, IL).
Results
Subjects
The study sample consisted of 43 usable subjects distributed among the treatment groups (Table 2). The mean age, 20 years, is characteristic of the young adult population undergoing the removal of impacted third molars. Subjects in the four groups did not differ in terms of demographic and surgical factors such as extraction difficulty, the dose of midazolam or the amount of lidocaine administered, that could affect the outcome of the study.
Table 2. Demographic and surgical features of the patient sample.
| Time of biopsy from end of surgery (min) | N | Age (years) | Gender M/F | Extraction Difficulty* | Midazolam Dose (mg) | Lidocaine Dose (mg) |
|---|---|---|---|---|---|---|
| 0 | 10 | 19.0 ± 3.9 | 6/4 | 6.4 ± 1.6 | 4.5 ± 0.7 | 155.6 ± 13 |
| 30 | 13 | 18.9 ± 3.5 | 7/6 | 6.5 ± 1.1 | 4.3 ± 0.7 | 144.8 ± 30 |
| 60 | 10 | 23.1 ± 5.9 | 3/7 | 7.0 ± 1.2 | 4.7 ± 0.5 | 164.0 ± 25 |
| 120 | 10 | 19.0 ± 3.3 | 4/6 | 7.0 ± 1.2 | 4.6 ± 0.6 | 162.0 ± 26 |
Extraction difficulty is the sum calculated by assigning a score of (2) for soft tissue impactions, (3) for partial bony impactions and (4) for full bony impactions.
COX-2 mRNA
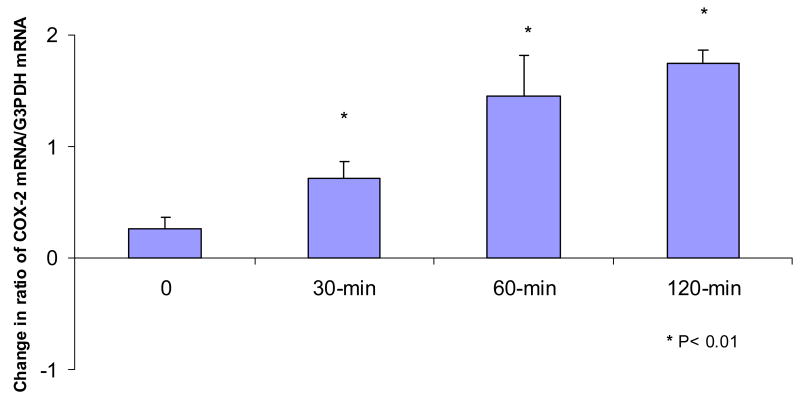
Under the conditions used COX-2 expression in the preoperative biopsies was either not detectable or was seen as a faint band (Figure 1). Low levels of COX-2 message were detected in 51% of the pre-surgical biopsies. The ratio of COX-2 mRNA/G3PDH mRNA in biopsies obtained at time 0 immediately after surgery did not differ from that in the pre-surgical biopsies. Biopsies collected at 30, 60 and 120 minutes after surgery demonstrated a progressive significant increases (P<0.01) in the level of COX-2 message over time as compared to preoperative levels (Figures 1 and 2). The presence of additional bands in Figure 1 is likely due to the large number of PCR cycles needed to detect COX-2.
Figure 1.
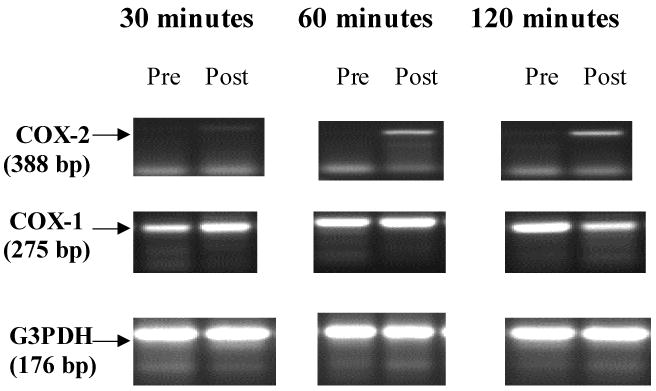
Representative products of the RT-PCR for COX-2, COX-1 and G3PDH. Notice that COX-2 is either not detectable in the pre-surgical biopsies or is barely detected and is induced at 30, 60 and 120 minutes after surgery in these samples. In contrast, COX-1 was detected at all time points examined.
Figure 2.
Change in the ratio of COX-2 mRNA/G3PDHmRNA in the postoperative biopsies as compared to the preoperative biopsies. “0” represents biopsies which were obtained immediately after surgery. A significant increase was detected in the biopsies obtained at 30, 60 and 120 minutes after surgery. *P<0.05
COX-1 mRNA
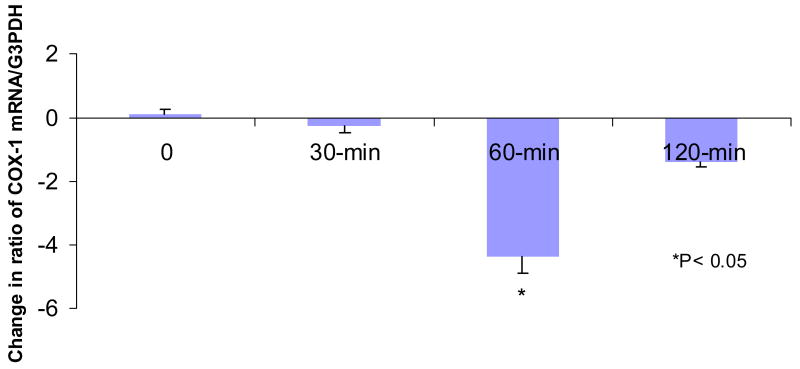
The COX-1 transcript was detected in all the pre-surgical and post-surgical biopsies (Figures 1 and 3). The ratio of COX-1 mRNA/G3PDH mRNA in the biopsies collected immediately after surgery did not differ significantly from that of the pre-operative biopsies (Figure 3). We detected a significant decrease in the ratio of COX-1 mRNA/G3PDH mRNA in samples collected at 60 min after surgery as compared to baseline (P< 0.05). The level of COX-1 mRNA was numerically lower at 30 and 120 minutes after surgery as compared to baseline but was not statistically significant. No consistent change was observed with G3PDH.
Figure 3.
Change in the ratio of COX-1 mRNA/G3PDH mRNA in the postsurgical biopsies as compared to the presurgical ones. “0” represents biopsies which were obtained immediately after surgery. A significant decrease in COX-1 mRNA was detected at 60 minutes after surgery.*P<0.05
Discussion
It is widely accepted that COX-1 is responsible for the immediate prostanoid response to inflammatory stimuli while COX-2 becomes the primary contributor to prostanoid synthesis as inflammation progresses. The results of this study are supportive of this concept as we demonstrate COX-1 message at baseline and throughout the post-operative period in comparison to negligible COX-2 message prior to surgery with an increase in the level of COX-2 message during the post-surgical period.
This study demonstrates a significant decrease in the level of COX-1 mRNA in biopsies obtained 60 minutes after surgery (P< 0.05) as compared to the level of COX-1 in preoperative biopsies. Prior studies have reported that the level of COX-1 mRNA and protein in the peripheral tissues do not change during acute inflammation.27,32 It is conceivable that the discrepancy between these reports and our data is due to differences in the types of stimuli used and in the tissue examined. Liu et al. report a 2 to 5-fold decrease in the level of COX-1 mRNA in the myocardial and pleural tissues of rats following systemic administration of lipopolysaccharides (LPS).18 A similar study reported a decrease in COX-1 mRNA in the rodent renal medulla one hour after injection of LPS.13 While these data support our observations, the systemic administration of LPS is a model of sepsis and may not be reflective of the physiological changes of post-surgical trauma and acute inflammation. A subsequent study in the oral surgery model17 using quantitative real time PCR in a larger sample replicated the observed decrease in COX-1 mRNA in the immediate postoperative period suggesting that acute tissue injury and inflammation in humans not only stimulates increased COX-2 mRNA but also transiently inhibits COX-1 mRNA transcription similar to immune stimuli such as LPS.13,18
The results from this study clearly demonstrate increased expression of COX-2 following the induction of inflammation. Previous studies examining the levels of PGE2 in the extraction sites following oral surgery demonstrate a decrease in PGE2 levels in the immediate post-operative period10,14 followed by an increase which is coincident with report of moderate to severe pain.10,14,26 While the relative contributions of COX-1 and COX-2 to PGE2 production are not known, it is likely that COX-2 is primarily responsible for the increased levels of PGE2 but with continued production of PGE2 by COX-1.
COX-2 is induced in cultured gingival fibroblasts following application of proinflamatory agents such as interleukin-1β (IL-1β), LPS and bradykinin.20,22,23,38 The increased expression of COX-2 in fibroblasts results in enhanced synthesis of PGE2.23,36,37,38 The same phenomenon can be observed in endothelial cells upon induction with IL-α.11 Polymorphonuclear cells also up-regulate COX-2 expression in experimental systems following LPS stimulation.20 Taken together with our results it appears that after tissue trauma, mainly three types of cells contribute to COX-2 induction and subsequently to PG synthesis: the resident fibroblasts and endothelial cells as well as the invading PMNs. The increased levels of PGs may in turn, influence the maintenance of nociceptive processes in post-operative pain.
The oral surgery model is a useful and reproducible model of acute pain, widely used in analgesic research.3,4 Adapting microdialysis to the oral surgery model has facilitated examination of the relationship between mediators of inflammation, pain report and analgesic activity in humans.5,12,26,30 The results of previous studies using microdialysis and the oral surgery model demonstrated increased PGE2 production at later time points during the postoperative period (120-240 minutes) suggestive of increased COX activity. The present study supports these observations by demonstrating increased COX-2 expression with negligible changes in COX-1 expression at the same time points. This increase in COX-2 expression also suggests a role of this isoform in the inflammatory response and subsequent resolution of tissue injury and repair, although the exact role needs clarification.7,28 Taken together with previous demonstrations of a PGE2 time course following surgery,10 differential effects of coxibs on biomarkers for COX-1 and COX-214,15 and the ability to simultaneously measure pain and analgesia in humans, supports the utility and clinical relevance of the oral surgery model for mechanistic studies of inflammation.
To conclude, this study demonstrated the induction of COX-2 mRNA in acute inflammation in humans. The use of a physiologically relevant model of acute inflammation further enhances the generalizability of these findings, which are relevant to other types of acute inflammatory pain.
List of abbreviations used
- COX
Cyclooxygenase
- G3PDH
Glyceraldehyde-3-phosphate dehydrogenase
- LPS
Lipopolysaccharides
- NSAID
Non-steroidal anti-inflammatory drug
- PG
Prostaglandin
- PMN
Polymorphonuclear
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson GD, Hauser SD, McGarity KL, Bremer ME, Isakson PC, Gregory SA. Selective inhibition of cyclooxygenase: (COX)-2 reverses inflammation and expression of interleukin-6 in rat adjuvant arthritis. J Clin Invest. 1996;97:2672–79. doi: 10.1172/JCI118717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buttar NS, Wang KK. The “aspirin” of the new millennium: cyclooxygenase inhibitors. Mayo Clin Proc. 2000;75:1027–38. doi: 10.4065/75.10.1027. [DOI] [PubMed] [Google Scholar]
- 3.Cooper SA, Beaver WT. A model to evaluate mild analgesic in oral surgery outpatients. Clin Pharmacol Ther. 1976;20:241–50. doi: 10.1002/cpt1976202241. [DOI] [PubMed] [Google Scholar]
- 4.Dionne RA, Berthold CW. Therapeutic uses of non-steroidal anti-inflammatory drugs in dentistry. Crit Rev Oral Biol Med. 2001;12:315–330. doi: 10.1177/10454411010120040301. [DOI] [PubMed] [Google Scholar]
- 5.Dionne RA. Evaluation of analgesic mechanisms and NSAIDs in the oral surgery model. In: Rainsford KD, Powanda MD, editors. Safety and Efficacy of Non-Prescription (OTC) Analgesics and NSAIDs. Kluwer Academic Publishers; London: 1994. pp. 105–117. [Google Scholar]
- 6.Fernandez-Morata JC, Mullol J, Fuentes M, Pujols L, Roca-Ferrer J, Xaubet A, Picado C. Regulation of cyclooxygenase-1 and cyclooxygenase-2 in human nasal mucosa. Effects of cytokines and dexamethasone. Clin Exp Allergy. 2004;30:1275–84. doi: 10.1046/j.1365-2222.2000.00891.x. [DOI] [PubMed] [Google Scholar]
- 7.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5(6):698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 8.Gilroy DW, Tomlinson A, Willoughby DA. Differential effects of inhibition of isoforms of cyclooxygenase (COX-1, COX-2) in chronic inflammation. Inflamm Res. 1998;47:79–85. doi: 10.1007/s000110050285. [DOI] [PubMed] [Google Scholar]
- 9.Gilroy DW, Tomlinson A, Willoughby DA. Differential effects of inhibitors of cyclooxygenase (cyclooxygenase 1 and cyclooxygenase 2) in acute inflammation. Eur J Pharmacol. 1998;355:211–7. doi: 10.1016/s0014-2999(98)00508-1. [DOI] [PubMed] [Google Scholar]
- 10.Gordon SM, Brahim JS, Rowan J, Kent A, Dionne RA. Peripheral prostanoid levels and nonsteroidal anti-inflammatory drug analgesia: replicate clinical trials in a tissue injury model. Clin Pharmacol Ther. 2002;72:175–83. doi: 10.1067/mcp.2002.126501. [DOI] [PubMed] [Google Scholar]
- 11.Habib A, Creminon C, Frobert Y, Grassi J, Pradelles P, Maclouf J. Demonstration of an inducible cyclooxygenase in human endothelial cells using antibodies raised against carboxyl-terminal region of cyclooxygenase-2. J Biol Chem. 1993;268:23448–54. [PubMed] [Google Scholar]
- 12.Hargreaves KM, Costello A. Glucocorticoids suppress levels of immunoreactive bradykinin in inflamed tissue as evaluated by microdialysis probes. Clin Pharmacol Ther. 1990;48:168–78. doi: 10.1038/clpt.1990.132. [DOI] [PubMed] [Google Scholar]
- 13.Ichitani Y, Holmberg K, Maunsbach AB, Haeggstrom JZ, Samuelsson B, De Witt D, Hokfelt T. Cyclooxygenase-1 and cyclooxygenase-2 expression in rat kidney and adrenal gland after stimulation with systemic lipopolysaccharide: in situ hybridization and immunocytochemical studies. Cell Tissue Res. 2001;303:235–52. doi: 10.1007/s004410000296. [DOI] [PubMed] [Google Scholar]
- 14.Khan AA, Brahim JS, Rowan JS, Dionne RA. In vivo selectivity of a selective cyclooxygenase 2 inhibitor in the oral surgery model. Clin Pharmacol Ther. 2002;72:44–9. doi: 10.1067/mcp.2002.125560. [DOI] [PubMed] [Google Scholar]
- 15.Chuang BP, Gordon SM, Wang Xiao-Min, Rowan J, Brahim J, Dionne RA. Paradoxical effects of bupivacaine on PGE2 release and COX gene expression produces hyperalgesia following local anesthetic offset. J Oral Maxillofac Surg. 2006 submitted. [Google Scholar]
- 16.Langenbach R, Morham SG, Tiano HF, Loftin CD, Ghanayem BI, Chulada PC, Mahler JF, Lee CA, Goulding EH, Kluckman KD, Kim HS, Smithies O. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell. 1995;83:483–92. doi: 10.1016/0092-8674(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 17.Lee Y, Kim H, Wu T, Wang X, Dionne RA. Genetically mediated inter-individual variation in analgesic responses to COX inhibitory drug. Clinical Pharmacol Therap. 2006;79:407–18. doi: 10.1016/j.clpt.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Liu SF, Newton R, Evans TW, Barnes PJ. Differential regulation of cyclo-oxygenase-1 and cyclo-oxygenase-2 gene expression by lipopolysaccharide treatment in vivo in the rat. Clin Sci London. 1996;90:301–6. doi: 10.1042/cs0900301. [DOI] [PubMed] [Google Scholar]
- 19.Lukiw WJ, Bazan NG. Cyclooxygenase 2 RNA message abundance, stability, and hypervariability in sporadic Alzheimer neocortex. J Neurosci Res. 1997;50:937–45. doi: 10.1002/(SICI)1097-4547(19971215)50:6<937::AID-JNR4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 20.Maloney CG, Kutchera WA, Albertine KH, McIntyre TM, Prescott SM, Zimmerman GA. Inflammatory agonists induce cyclooxygenase type 2 expression by human neutrophils. J Immunol. 1998;160:1402–10. [PubMed] [Google Scholar]
- 21.McAdam BF, Mardinin IA, Habib A, Burke A, Lawson A, Kapoor A, Fitzgerald GA. Effect of regulated expression of human cyclooxygenase isoforms on eicosanoid and isoeicosanoid production in inflammation. J Clin Invest. 2000;105:1473–82. doi: 10.1172/JCI9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakao S, Ogata Y, Modeer T, Segawa M, Furuyama S, Sugiya H. Bradykinin induces a rapid cyclooxygenase-2 mRNA expression via Ca2+ mobilization in human gingival fibroblasts primed with interleukin-1 beta. Cell Calcium. 2001;29:446–52. doi: 10.1054/ceca.2001.0206. [DOI] [PubMed] [Google Scholar]
- 23.Noguchi K, Shitashige M, Endo H, Kondo H, Yotsumoto Y, Izumi Y, Nitta H, Ishikawa I. Involvement of cyclooxygenase-2 in serum-induced prostaglandin production by human oral gingival epithelial cells. J Periodontal Res. 2001;36:124–30. doi: 10.1034/j.1600-0765.2001.360209.x. [DOI] [PubMed] [Google Scholar]
- 24.Noguchi K, Shitashige M, Yanai M, Morita I, Nishihara T, Murota S, Ishikawa I. Prostaglandin production via induction of cyclooxygenase-2 by human gingival fibroblasts stimulated with lipopolysaccharides. Inflammation. 1996;20:555–68. doi: 10.1007/BF01487046. [DOI] [PubMed] [Google Scholar]
- 25.Ristimaki A, Narko K, Hla T. Down-regulation of cytokine-induced cyclo-oxygenase-2 transcript isoforms by dexamethasone: evidence for post-transcriptional regulation. Biochem J. 1996;318(Pt 1):325–31. doi: 10.1042/bj3180325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roszkowski MT, Swift JQ, Hargreaves KM. Effect of NSAID administration on tissue levels of immunoreactive prostaglandin E2, leukotriene B4, and (S)-flurbiprofen following extraction of impacted third molars. Pain. 1997;73:339–45. doi: 10.1016/S0304-3959(97)00120-6. [DOI] [PubMed] [Google Scholar]
- 27.Seibert K, Zhang Y, Leahy K, Hauser S, Masferrer J, Isakson P. Distribution of COX-1 and COX-2 in normal and inflamed tissues. Adv Exp Med Biol. 1997;400A:167–70. doi: 10.1007/978-1-4615-5325-0_24. [DOI] [PubMed] [Google Scholar]
- 28.Simon AM, Manigrasso MB, O'Connor JP. Cyclo-oxygenase 2 function is essential for bone fracture healing. J Bone Miner Res. 2002;17:963–76. doi: 10.1359/jbmr.2002.17.6.963. [DOI] [PubMed] [Google Scholar]
- 29.Svensson CI, Yaksh TL. The spinal phospholipase-cyclooxygenase-prostanoid cascade in nociceptive processing. Annu Rev Pharmacol Toxicol. 2002;42:553–83. doi: 10.1146/annurev.pharmtox.42.092401.143905. [DOI] [PubMed] [Google Scholar]
- 30.Swift JQ, Garry MG, Roszkowski MT, Hargreaves KM. Effect of flurbiprofen on tissue levels of immunoreactive bradykinin and acute postoperative pain. J Oral Maxillofac Surg. 1993;51:112–6. doi: 10.1016/s0278-2391(10)80002-3. discussion 116-7. [DOI] [PubMed] [Google Scholar]
- 31.Tilley SL, Coffman TM, Koller BH. Mixed messages: modulation of inflammation and immune responses by prostaglandins and thromboxanes. J Clin Invest. 2001;108:15–23. doi: 10.1172/JCI13416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomlinson A, Appleton I, Moore AR, Gilroy DW, Willis D, Mitchell JA, Willoboughy DA. Cyclo-oxygenase and nitric oxide synthase isoforms in rat carrageenin-induced pleurisy. Br J Pharmacol. 1994;113:693–98. doi: 10.1111/j.1476-5381.1994.tb17048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vane JR, Botting RM. Mechanism of action of non-steroidal anti-inflammatory drugs. Am J Med. 1998;104:2S–8S. doi: 10.1016/s0002-9343(97)00203-9. [DOI] [PubMed] [Google Scholar]
- 34.Vane JR, Mitchell JA, Appleton I, Tomlinson A, Bishop-Bailey D, Croxtall J, Willoboughy DA. Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proc Natl Acad Sci U S A. 1994;91:2046–50. doi: 10.1073/pnas.91.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallace JL, Bak A, McKnight W, Asfaha S, Sharkey KA, MacNaughton WK. Cyclooxygenase-1 contributes to inflammatory responses in rat and mice: implications for GI toxicity. Gastroenterol. 1998;115:101–9. doi: 10.1016/s0016-5085(98)70370-1. [DOI] [PubMed] [Google Scholar]
- 36.Yucel-Lindberg T, Brunius G, Wondimu B, Anduren I, Modeer T. Enhanced cyclooxygenase-2 mRNA expression in human gingival fibroblasts induced by cell contact with human lymphocytes. Eur J Oral Sci. 2001;109:187–92. doi: 10.1034/j.1600-0722.2001.00999.x. [DOI] [PubMed] [Google Scholar]
- 37.Yucel-Lindberg T, Ahola H, Carlstedt-Duke J, Modeer T. Involvement of tyrosine kinases on cyclooxygenase expression and prostaglandin E2 production in human gingival fibroblasts stimulated with interleukin-1beta and epidermal growth factor. Biochem Biophys Res Commun. 1999;257:528–32. doi: 10.1006/bbrc.1999.0523. [DOI] [PubMed] [Google Scholar]
- 38.Yucel-Lindberg T, Ahola H, Nilsson S, Carlstedt-Duke J, Modeer T. Interleukin-1 beta induces expression of cyclooxygenase-2 mRNA in human gingival fibroblasts. Inflammation. 1995;19:549–60. doi: 10.1007/BF01539135. [DOI] [PubMed] [Google Scholar]