Abstract
Background
The mechanism(s) for acute changes in electrophysiological properties of the atria during rapid pacing induced atrial fibrillation (AF) is not completely understood. We sought to evaluate the contribution of the intrinsic cardiac autonomic nervous system (ICANS) in acute atrial electrical remodeling and AF induced by six-hour rapid atrial pacing.
Methods and Results
Continuous rapid pacing (1200 bpm, 2× threshold, TH) was performed at the left atrial appendage. Group 1 (N=7): 6-hour pacing immediately followed by ganglionated plexi (GP) ablation; Group 2 (N=7): GP ablation immediately followed by 6-hour pacing; Group 3 (N=4): administration of autonomic blockers, atropine (1 mg/kg) and propranolol (0.6 mg/kg) immediately followed by 6-hour pacing. The effective refractory period (ERP) and window of vulnerability (WOV, in milliseconds), i.e., the difference between the longest and the shortest coupling interval of the premature stimulus which induced AF, were measured at 2×TH and 10×TH at the left atrium, right atrium and pulmonary veins every hour before and after GP ablation or autonomic blockade. In Group 1, ERP was markedly shortened in the first two hours and then stabilized both at 2×TH and 10×TH; however, WOV was progressively widened throughout the 6-hour period. After GP ablation, ERP was significantly longer than before ablation and AF could not be induced (WOV=0) at either 2×TH or 10×TH. In Group 2 and 3, rapid atrial pacing failed to shorten the ERP. AF could not be induced in 6/7 dogs in Group 2 and 4/4 dogs in Group 3 during the 6-hour pacing period.
Conclusion
The ICANS plays a crucial role in the acute stages of atrial electrical remodeling induced by rapid atrial pacing.
Keywords: atrial fibrillation, remodeling, autonomic nerve system
Introduction
In 1995, Wijffels and co-workers (1) found that continuous rapid atrial pacing in the goat heart leads to progressive shortening of the atrial effective refractory period (ERP) and increased duration of atrial fibrillation (AF) once it is induced. The longer the duration of atrial pacing, the longer the AF was maintained. This phenomenon, coined as “AF begets AF”, has attracted clinical attention because it accounts for the clinical observation that recurrent episodes of paroxysmal AF often progresses to more persistent forms of AF. In the past decade, tachycardia-induced atrial remodeling was found to be associated with alterations in expression of ion channels and changes in the structure of the atria (2, 3). Recently, several studies (4-11) from our institute demonstrated that the intrinsic cardiac autonomic nervous system (ICANS) plays an important role in the initiation and maintenance of AF. We hypothesized that the ICANS may be another remodeling mechanisms leading to the phenomenon of “AF begets AF”. Using a 6-hour rapid atrial pacing model modified from Wijffels et al (1), we examined the effect of the ICANS on the acute atrial remodeling process by ablation of the ganglionated plexi (GP) located in atrial epicardial fat pads and by administration of autonomic blockers.
Methods
Animal preparation
All animal studies were reviewed and approved by the institutional Animal Care and Use Committee of the University of Oklahoma Health Sciences Center. Eighteen adult mongrel dogs weighing 20∼25 kg were anesthetized with Na-pentobarbital, 50mg/kg, and ventilated with room air by a positive pressure respirator. Core body temperature was maintained at 36.5±1.5°C. Standard ECG leads were continuously recorded to determine the heart rate and rhythm. Blood pressure (BP) was continuously monitored via a pressure transducer positioned in the right femoral artery.
The chest was entered via a left thoracotomy at the 4th intercostal space. Multi-electrode catheters (Biosense-Webster, Diamond Bar, CA) were secured to allow recording at the left atrial appendage (LAA), left atrium (LA), left superior pulmonary vein (LSPV) and left inferior pulmonary vein (LIPV) (Fig. 1A). Similar electrode catheters were attached to right atrial appendage (RAA), right atrium (RA), right superior pulmonary vein (RSPV) and right inferior pulmonary vein (RIPV) through a right thoracotomy approach at the 4th intercostal space (Fig. 1B). All tracings from the electrode catheters were amplified and digitally recorded using a computer-based Bard Lab System (CR Bard, Inc, Billerica, MA, USA). Bipolar electrograms were filtered at 30 to 500 Hz. ECG filter settings were 0.1 to 250 HZ.
Figure 1.
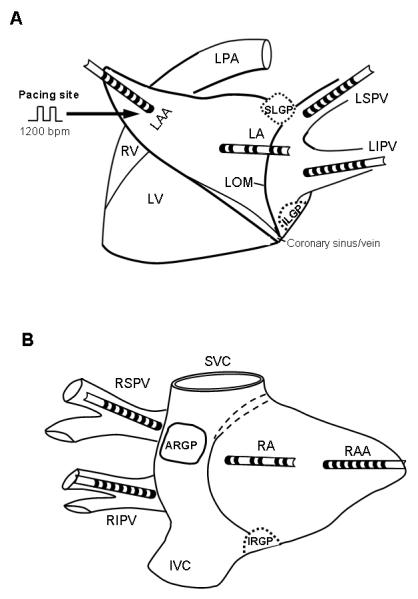
Schematic representation and catheter position in the atrium. (A) Left thoracotomy approach. SLGP: superior left ganglionated plexi (GP), located adjacent to the junction of left superior pulmonary vein (LSPV) and left atrium (LA); ILGP: inferior left GP located near the junction of left inferior pulmonary vein (LIPV) and LA. Multi-electrode catheters were sutured to the LSPV, LIPV, LA and left atrial appendage (LAA). Continuous rapid pacing (1200 bpm) was performed at the LAA. LV: left ventricle; RV: right ventricle; LOM: ligament of Marshall. (B) Right thoracotomy approach. ARGP: anterior right GP, located adjacent to the right superior pulmonary vein (RSPV) - atrial junction; IRGP: inferior right GP, located at the junction of the inferior vena cava (IVC) and both atria. SVC: superior vena cava. Similarly, multi-electrode catheters were sutured to RSPV, right inferior pulmonary vein (RIPV), right atrium (RA) and right atrial appendage (RAA).
Programmed stimulation
Rapid atrial pacing was delivered (1200 bpm, 2 × threshold, 1 ms in duration) at the LAA. After each pacing hour, rapid atrial pacing was temporarily stopped to measure the ERP and AF inducibility. Programmed stimulation at atrial myocardial sites or PV sleeves was performed using a Medtronic programmable stimulator (model 5328; Medtronic Inc. Minneapolis, MI). The ERP at an atrial pacing cycle length of 330 msec, was determined at both 2× diastolic threshold and 10× diastolic threshold (TH = 0.1 - 1.0 mA). The S1-S2 intervals were decreased from 150 msec to refractoriness initially by decrements of 10 msec (S1:S2=8:1). As the S1-S2 intervals approached the ERP, decrements were reduced to 1 msec. ERP Dispersion was defined as the coefficient of variation (standard deviation/mean) of the ERP at all 7 sites (LSPV, LIPV, LA, RSPV, RIPV, RA and RAA) (12).
We used the window of vulnerability (WOV) as a quantitative measure of AF inducibility (5). AF was defined as irregular atrial rates faster than 500 beats per minutes associated with irregular atrioventricular conduction lasting > 5 seconds (Supplement Fig. 1). During ERP measurements, if AF was induced by decremental S1S2 stimulation, the longest and the shortest S1-S2 interval (in msec) at which AF was induced were then determined (5). The difference between the two was designated as the WOV (5). The σWOV was counted as the sum of WOV acquired at 2×TH and 10×TH at all sites in each dog. The time for determining the ERP and WOV after each hour of pacing was less than 15 minutes in most cases (13.9±5.0 minutes).
GP ablation
The anterior right ganglionated plexi (ARGP) located in the fat pad at the RSPV-atrial junction was localized by applying high frequency stimulation (HFS; 20Hz, 0.1ms duration, 0.6-4.5 volts) with a bipolar stimulation/ablation probe electrode (AtriCure, West Chester, OH) (9-11). In this voltage range, progressive slowing of the sinus rate was observed directly related to the voltage applied. The same device was later used to deliver radiofrequency current in order to ablate the ARGP (9-11). The inferior right ganglionated plexi (IRGP) located at the junction of inferior vena cava and both atria, the superior left ganglionated plexi (SLGP) at the LSPV-atrial junction, the inferior left ganglionated plexi (ILGP) adjacent to the LIPV, and autonomic neural elements along the ligament of Marshall (LOM) were also localized by the effect of HFS which either slowed the sinus rate or suppressed the AV conduction. These areas were subsequently ablated using the same device. Complete ablation of each GP or LOM was verified by applying maximal strength of stimulation (12V) that failed to slow the sinus rate or inhibit the AV conduction. The ablation was limited to GP/fat pad, and the morphology and amplitude of the electrograms at the sites closest to the fat pad were not altered after ablation, indicating that the collateral tissue damage caused by GP ablation was minimal. The time for locating and ablating the GP/fat pad was less than 15 minutes in each dog.
Administration of autonomic blockers
Both parasympathetic and sympathetic systems were blocked by administration of autonomic blockers, atropine and propranolol, in 4 dogs prior to 6-hour rapid atrial pacing. Atropine and propranolol were administered according to the previous report by Wijffels et al (13). Atropine sulfate (Sigma-Aldrich Inc. St. Louis, MO) was infused intravenously in cumulative doses of 0.1, 0.3, 0.6, and 1.0 mg/kg in steps of 10 minutes. Propranolol HCl (Sigma-Aldrich Inc. St. Louis, MO) was given in a similar way in cumulative dosages of 0.1, 0.3, and 0.6 mg/kg.
Experiment protocol
Eighteen dogs were assigned into three groups: Group 1 (N=7): 6-hour atrial pacing immediately followed by GP (ARGP+IRGP+SLGP+ILGP+LOM) ablation;
Group 2 (N=7): GP (ARGP+IRGP+SLGP+ILGP+LOM) ablation immediately followed by atrial pacing for 6 hours;
Group 3 (N=4): Administration of autonomic blockers immediately followed by 6-hour atrial pacing.
The ERP and WOV were measured at LSPV, LIPV, LA, RSPV, RIPV, RA and RAA every hour during the 6-hours pacing period before and after GP ablation or autonomic blockade.
Histologic Studies
The fat pads containing ARGP from animals after ablation (4 dogs) and without ablation (1 dog) were removed and fixed in neutral buffered formalin for 24 hours and processed into paraffin blocks. Cross sections of the ablated area were sectioned at 6 micrometer thickness and stained with hematoxylin and eosin. Digital photographs were taken. The panoramic view was produced by reconstruction of multiple images of the same piece of specimen by .Adobe Photoshop 7.0.
Statistical analysis
All values were expressed as mean ± standard deviation. Paired t-test was used for comparisons of ERP and WOV before and after GP ablation. Analysis of variance (ANOVA) for repeated measurements was used for comparisons of ERP or WOV among different pacing hours, and followed by post hoc testing (least significant differences) for comparisons of the ERP and WOV at the end of each subsequent hour of pacing versus ERP and WOV in the baseline state. Statistical significance was defined as p≤0.05.
Statement of Responsibility
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
The systolic and diastolic blood pressure were stable during the entire period of experiments, and no evident sign of heart failure was observed throughout the 6-hours pacing period.
ERP and WOV
In Group 1 (GP ablation after 6-hour pacing, Fig. 2), ERP was markedly shortened in the first two hours but longer periods of rapid pacing (3-6 hours) failed to further shorten the ERP at either 2×TH or 10×TH at each site (Fig. 2A-2G, ERP). In contrast, σWOV was progressively widened throughout the 6-hour pacing period (Fig. 2H). For instance, the ERP at LSPV was shortened from 125±19 ms in the baseline state to 96±14 ms at the end of 2 hours of pacing (2×TH; p<0.05). The ERP was stabilized at 90∼100 ms from the 3rd to the 6th pacing hours. The WOV at LSPV progressively increased from 5 ms in the baseline state to 319 ms at the end of the 6-hours pacing period (not shown in Fig. 2). After GP (ARGP+IRGP+SLGP+ILGP+LOM) ablation, ERP was lengthened to the baseline level and AF could no longer be induced (WOV=0) at all pacing sites at either 2×TH or 10×TH (Fig. 2, after GP ablation).
Figure 2.
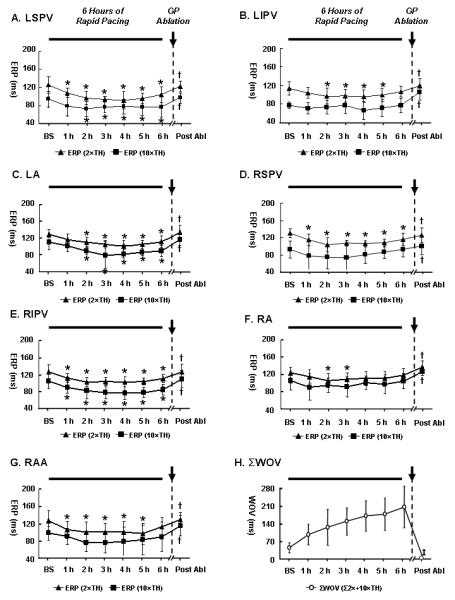
Changes of effective refractory period (ERP) and window of vulnerability (WOV) during 6-hour pacing and after GP (ARGP+IRGP+SLGP+ILGP+LOM) ablation in Group 1 (N=7). (A-G) The mean ERP of the 7 dogs (filled squares and triangles) was progressively shortened in the first two hours at 2×thresholds (TH) or 10×TH at all sites; however, longer period of pacing failed to further shorten the ERP. After GP (ARGP+IRGP+SLGP+ILGP+LOM) ablation, ERP were significantly longer than that prior to GP ablation and AF could no longer be induced (WOV=0) at any site at either 2×TH or 10×TH; (H) ΣWOV, serving as a quantitative measure of atrial fibrillation (AF) inducibility in the whole heart, was counted as the sum of WOV acquired at 2×TH and 10×TH at all sites. *: p<0.05 for comparisons of the ERP at the end of each hour of pacing vs. ERP in the baseline state (BS) at 2×TH or 10×TH; †: p<0.05 for comparisons of the ERP after GP ablation vs. ERP immediately prior to GP ablation at 2×TH or 10×TH. No significance was observed between the ERP in the baseline state and the ERP after GP ablation; ‡: p<0.01 for comparisons of the ΣWOV after GP ablation vs. ΣWOV immediately prior to GP ablation, respectively. The time for determining the ERP and WOV after hour of pacing was less than 15 minutes in most cases.
In Group 2 (GP ablation before 6-hour pacing, Fig. 3), rapid atrial pacing failed to shorten the ERP at any site (Fig. 3A-3G). AF could not be induced (WOV=0) in 6/7 dogs during the 6-hour pacing period, and was inducible in 1/7 with a cumulative WOV of only 10 ms at 10×TH (Fig. 3H).
Figure 3.
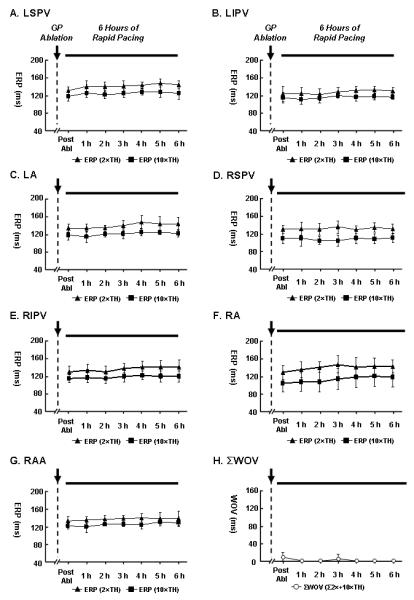
Changes of ERP and WOV after GP (ARGP+IRGP+SLGP+ILGP+LOM) ablation followed by 6-hour atrial pacing in Group 2 (N=7). No shortening of ERP was observed during the pacing period. AF could not be induced (WOV=0) in 6/7 dogs during the 6-hour pacing, and was inducible in 1/7 with WOV of only 10 ms at 10×TH. Abbreviations as Figure 2.
In Group 3 (administration of autonomic blockers before 6-hour pacing, Fig. 4), rapid atrial pacing could not shorten the ERP at any site (Fig. 4A-4G). AF could not be induced during the 6-hour pacing period after infusion of atropine and propranolol in all 4 dogs (Fig. 4H).
Figure 4.

Changes of ERP and WOV after administration of autonomic blockers followed by 6-hour atrial pacing in Group 3 (N=4). Atropine sulfate was infused intravenously in cumulative doses of 0.1, 0.3, 0.6, and 1.0 mg/kg in steps of 10 minutes. Propranolol HCl was given in a similar way in cumulative dosages of 0.1, 0.3, and 0.6 mg/kg. Six-hour rapid atrial pacing failed to shorten the ERP or induce AF at any site.
ERP dispersion
In Group 1, the ERP dispersion was increased at the first hour and then stabilized during the 2nd-6th hours after pacing was started. The increase in ERP dispersion induced by 6-hour pacing was eliminated by GP ablation (Table 1). However, these changes did not achieve statistical significance (ANOVA; end of the 6th pacing hour vs. immediately after GP ablation; 2×TH: P=0.08; 10TH: P=0.10). In Group 2, after GP were ablated, 6-hour pacing failed to increase the ERP dispersion at all (Table 2).
Table 1.
Effective Refractory Period (ERP) Dispersion during 6-hour Pacing and after Ganglionated Plexi (GP) Ablation in Group 1 (N=7)
| Baseline | 1sthour pacing |
2ndhour pacing |
3rdhour pacing |
4thhour pacing |
5thhour pacing |
6thhour pacing |
GP ablation | |
|---|---|---|---|---|---|---|---|---|
| 2×TH | 0.08±0.05 | 0.11±0.04 | 0.11±0.05 | 0.1±0.03 | 0.12±0.05 | 0.11±0.06 | 0.13±0.05 | 0.06±0.02 |
| 10×TH | 0.17±0.06 | 0.26±0.09 | 0.21±0.06 | 0.23±0.15 | 0.22±0.1 | 0.19±0.07 | 0.23±0.05 | 0.13±0.04 |
ERP dispersion was taken as the coefficient of variation (standard deviation/mean) of the ERP at all 7 sites (LSPV, LIPV, LA, RSPV, RIPV, RA and RAA). TH: threshold.
Table 2.
ERP Dispersion after GP ablation followed by 6-hour atrial pacingin Group 2(N=7)
| GP ablation | 1sthour pacing |
2ndhour pacing |
3rdhour pacing |
4thhour pacing |
5thhour pacing |
6thhour pacing |
|
|---|---|---|---|---|---|---|---|
| 2×TH | 0.11±0.05 | 0.12±0.05 | 0.12±0.04 | 0.12±0.04 | 0.11±0.03 | 0.11±0.04 | 0.11±0.02 |
| 10×TH | 0.13±0.02 | 0.15±0.03 | 0.13±0.01 | 0.14±0.03 | 0.12±0.02 | 0.12±0.04 | 0.11±0.03 |
ERP dispersion was taken as the coefficient of variation (standard deviation/mean) of the ERP at all 7 sites (LSPV, LIPV, LA, RSPV, RIPV, RA and RAA). Abbreviations are as Table 1.
Histologic Studies
Fig. 5 illustrates an example of the histologic studies of the fat pad containing ARGP after ablation or without ablation. After GP ablation, the autonomic ganglia were destroyed and the damage to the surrounding myocardial tissue was minimal.
Figure 5.
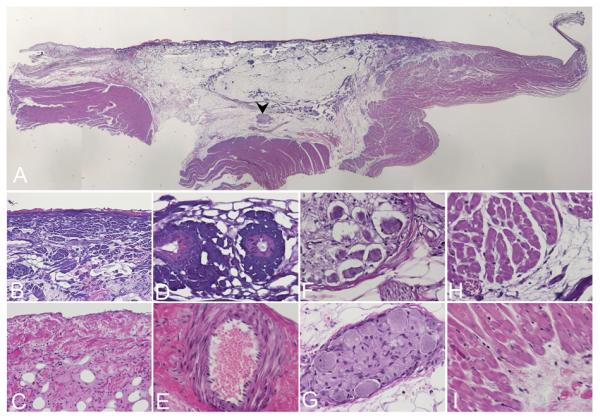
Example of histologic examination of the fat pad containing ARGP with or without ablation. (A) Panoramic view demonstrates the range of the ablation. Note the purple color of the collagen in the ablated area at the center in comparison to the non-ablated area at the periphery. Arrow: ablated area; Arrow head: ablated ganglion. (B) Collagen fibers under the epicardium in the ablated area turned deep purple and lost histologic details. (C) Non-ablated epicardium for comparison. (D)The blood vessels in the ablated area also turned dark purple and lost histologic details. (E) Non-ablated blood vessels for comparison. (F) In the ablated ganglion indicated by the arrow head in (A), the ganglioinic cells have contracted morphology with large vacuoles around the cells. (G) Non-ablated ganglion for comparison. (H) The myocardial cells are structurally intact although the collagen adjacent to them has purple changes. (I) Non-ablated myocardial muscle for comparison. Original magnification is X1 in (A), X20 in (B) and (C), x40 in (D) to (I). Panel A is generated by reconstruction from multiple images with Adobe Photoshop. Panel (C), (E), (G), (I) are taken from a control animal without ablation.
Discussion
Main findings
In this acute study of AF facilitated by rapid atrial pacing, the ERP was markedly shortened in the first two hours and the WOV was progressively widened throughout the 6-hour pacing period (Fig. 2). Ablation of the four main GP and LOM in the atria reversed the effects of ERP shortening and also eliminated AF inducibility at all sites. Moreover, in the animals receiving GP ablation or autonomic blockade first, rapid atrial pacing failed to shorten the ERP and AF could not be induced with programmed stimulation in 6/7 dogs (Group 2) and 4/4 dogs (Group 3) during the 6-hour pacing period. These results strongly suggest that the ICANS, specifically the GP, plays a critical role in acute atrial electrical remodeling induced by rapid atrial pacing.
Mechanisms underlying “AF begets AF”
The mechanism underlying AF was considered to be multiple wavelet reentry since Moe et al 14) proposed that hypothesis. However, recent studies (15, 16) showed that rapid focal discharges particularly from the PVs are the initiators of AF in many patients with paroxysmal or persistent AF. In an animal model of rapid atrial pacing, Morillo et al. (17) reported that an area in the posterior LA near the PV-LA junction uniformly had the shortest AF cycle length, which was presumably a focal driver for maintenance of AF. In another group of studies using a computerized high-density electrode mapping array, Zhou et al. (18) and Chou et al. (19) demonstrated intermittent focal discharges at the PVs which cannot be suppressed by ibutilide, a class III antiarrhythmic drug, in a canine model of AF induced by chronic rapid atrial pacing. These observations raised the possibility that both multi-wave reentry and rapid focal discharges may be the mechanisms operative in the phenomenon of “AF begets AF”.
Recent studies from our laboratory (4, 10, 11) have shown that focal AF can be induced or eliminated by stimulating or interrupting the ICANS. Within the ICANS, the GP contains the greatest number of neural elements, both parasympathetic and sympathetic. Along with the results presented in this study, we hypothesize that GP ablation diminishes the trigger activities and prevents the shortening of ERP across the atrium, leading to more homogenous distribution of ERP which prevents multi-wave reentry. In other words, GP ablation may diminish or eliminate AF resulting from both rapid focal discharges and multi-wave reentry.
Mechanisms underlying atrial electrical remodeling induced by acute rapid atrial pacing (≤ 6 hours)
Both structural remodeling and electrical remodeling have been demonstrated to facilitate the initiation and maintenance of AF in the atrial pacing model (3, 16, 20). Tachycardia-induced structural remodeling cannot explain the results presented in this study since the structural changes of atria would require at least several days of pacing. Atrial electrical remodeling is thought to result mainly from Ca2+ overload induced by frequent depolarizations (rapid pacing), leading to alterations of ion channels, especially decreased ICa-L which subsequently shorten the atrial action potential duration and refractoriness (2, 3, 21, 22). However, electrical remodeling of the atria cannot be explained solely by Ca2+ overload and a decrease of ICa-L induced by frequent depolarizations as it was prevented by interruption of the ICANS (Group 2 and Group 3) prior to the initiation of atrial pacing in the present study. It has been proposed that increased ERP dispersion plays a vital role in the chronic atrial pacing model (12). In the present study, we observed a trend of increase in the ERP dispersion which was eliminated by GP ablation in Group 1 (Table 1). The differences did not reach statistical significance, possibly due to a relatively short duration of pacing (6 hours) and a small sample size (N=7). In Group 2 animals, the GP were ablated first, this trend in ERP dispersion increase, as observed in Group 1 animals, was not observed (Table 2). These findings imply that the antifibrillatory effect of GP ablation may result, in part, from reducing the ERP dispersion.
In the study of Wijffels et al (1), they postulated that the changes in activity or sensitivity of the ICANS may be one of the mechanisms underlying the electrical remodeling induced by rapid atrial pacing. They later (13) found that administration of autonomic blockers, atropine and propranolol, partially reverse the pacing-induced shortening of ERP. Of note, atropine or proprannolol was administrated after AF had sustained for 1-3 days after chronic atrial pacing. The discrepancy between the results of that study (chronic pacing) and the present study (acute pacing) suggests that different mechanisms may underlie the atrial remodeling induced by short-term and long-term pacing. Alterations of ion channel expression and changes in atrial structure play a predominant role in atrial remodeling induced by longer periods of pacing, whereas changes in the ICANS may be an important factor in atrial remodeling induced by short-term rapid pacing.
The present study indicated that ICANS is strongly associated with acute atrial remodeling induced by rapid atrial pacing. As shown in Group 2 and Group 3, with the blockade of ICANS by GP ablation or administration of autonomic blockers, atrial electrical remodeling (shortening of ERP and increasing vulnerability of AF) was prevented. We hypothesize that ERP shortening is due in part to the local release of autonomic neurotransmitters, particularly acetylcholine, at the nerve endings which are distributed heterogeneously within the atria and PVs. Therefore, GP ablation and autonomic blockade would not only prolong ERP but also resist the effect of autonomic neurotransmitters in response to rapid atrial pacing. Several possible mechanisms may be responsible for the observation that AF was prevented by blockade of ICANS: (1) A large portion of the atria was protected by the significantly prolonged ERP induced by blockade of ICANS; in other words, multiple wavelets may not be able to propagate to a large portion of the atria, leading to a significant reduction of the functional critical mass for AF to sustain; (2) The innervation and activity of the ICANS may be increased by rapid atrial pacing as shown by Jayachandran et al. (23) that rapid atrial pacing induced a heterogeneous increase in innervation and activity of atrial sympathetic nervous system, termed as “autonomic remodeling”, which potentially can facilitate the development of AF. In the present study, GP ablation caused uniformity of ERP at several sites. This effect, in turn, would decrease dispersion of refractoriness creating less favorite conduction for AF inducibility. (3) Ion channels modulated by autonomic tone, such as IKH (a constitutively active component of IK,ACh), which plays a role in atrial electrical remodeling, may be inhibited by the interruption of the ICANS (24, 25).
Clinical Implications
GP have been shown to be the “integration centers” to modulate electrophysiological functions of the ICANS (9, 10). Inhibition of the ICANS prevents atrial electrical remodeling induced by short-term rapid atrial pacing. The ICANS may play an important role in the progression of paroxysmal AF lasting from several minutes to several hours, to persistent AF lasting several days. This progression may be prevented by interruption of the ICANS, specifically ablation of the GP. Moreover, in persistent AF patients with mildly to moderately remodeled atria, GP ablation might reverse the remodeling process and impede or reverse the progression of AF.
Study Limitations
Firstly, the present study strongly suggests that ICANS is associated in the acute electrical remodeling induced by rapid atrial pacing; however, we did not have recordings of neural activity to provide direct evidence for the involvement of autonomic activation in these pathophysiological processes. Further study will be needed to provide neurophysiologic evidence of the involvement of the ICANS. Secondly, in Group 1, reverse remodeling might have occurred during the period when localization and ablation of the GP, leading to lengthening of the ERP and shortening of the WOV. A set of control experiments were then performed. After 6-hour pacing, GP were not ablated. Instead, the mean ERP and cumulative WOV were measured 30 and 60 minutes after termination of pacing. The mean ERP and cumulative WOV did not significantly change within one hour after the pacing was discontinued (Fig. 6). This observation indicates that, compared with GP ablation, the reverse remodeling occurred during locating and ablating the GP (<15 minutes) was a minor factor contributing to the change of ERP and WOV after GP ablation. Thirdly, the large numbers of tests were performed in small sample sizes (N=7), which might have affected the statistical power to detect differences of ERP and ERP dispersion among 6 pacing hours. Finally, the long-term effects of autonomic denervation will acquire future studies to elucidate the role of the ICANS in chronic atrial pacing models.
Figure 6.
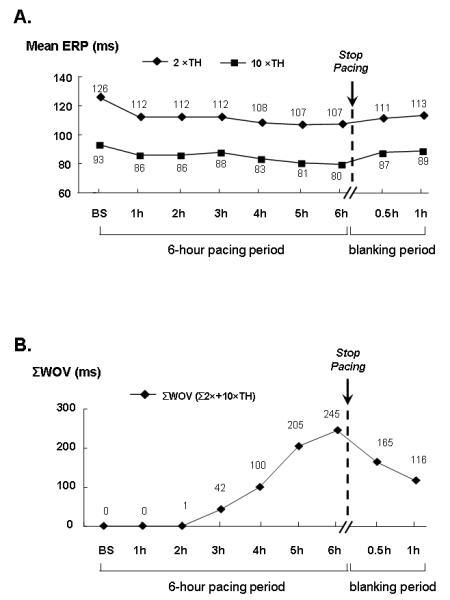
Changes of mean ERP and Cumulative WOV (ΣWOV) before and after stopping pacing without GP ablation in one dog. (A) After a 30-min blanking period, the mean ERP of the 7 sites (LSPV, LIPV, LA, RAA, RA, RSPV, RIPV) showed a slight but not significant increase in comparison with the value at the end of 6-hour pacing. The mean ERP did not return to the baseline value even after a one-hour blanking period. (B) The ΣWOV (i.e. the overall WOV from the above 7 sites) decreased to 165 ms after a 30-min blanking period; importantly, it remained 116 ms longer than the baseline state after one hour blanking period. This observation indicates that, compared with GP ablation (Fig. 2, after GP ablation), the reverse remodeling occurred during locating and ablating the GP (<15 minutes) was a minor factor contributing to the change of ERP and WOV after GP ablation.
Conclusions
We conclude that blockade of the ICANS before or after 6-hour pacing prevents or reverses the process of atrial electrical remodeling induced by short-term atrial pacing. Some of the mechanisms operative in the atrial electrical remodeling induced by rapid pacing in the normal dogs may be altered by the effects of interruption of the ICANS. The actions or hyperactivity of the ICANS itself may be a crucial element in acute atrial remodeling. These findings suggest that the ICANS is crucial for the process of “AF begets AF”, at least, in the acute phase.
Supplementary Material
Acknowledgments
We are grateful to Andrea Mosley, Joseph Klimkoski and Dr. Tushar Sharma for technical assistance.
Funding Sources This work was supported in part by grant 0650077Z from the American Heart Association (SSP), grant K23HL069972 from the National Heart, Lung and Blood Institute (SSP), grant from the Helen and Wil Webster Arrhythmia Research Fund of the University of Oklahoma Foundation (BJS) and grant 200750730309 from Disciplinary Leadership in Scientific Research Project of Wuhan City, China (ZL, HJ).
Footnotes
Conflict of Interest Disclosures: None.
Subject Code: Basic Science Research/ [132] Arrhythmias-basic studies
Reference
- 1.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–68. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 2.Yue L, Melnyk P, Gaspo R, Wang Z, Nattel S. Molecular mechanisms underlying ionic remodeling in a dog model of atrial fibrillation. Circ Res. 1999;84:776–84. doi: 10.1161/01.res.84.7.776. [DOI] [PubMed] [Google Scholar]
- 3.Bosch RF, Scherer CR, Rüb N, Wöhrl S, Steinmeyer K, Haase H, Busch AE, Seipel L, Kühlkamp V. Molecular mechanisms of early electrical remodeling: transcriptional downregulation of ion channel subunits reduces I(Ca,L) and I(to) in rapid atrial pacing in rabbits. J Am Coll Cardiol. 2003;41:858–69. doi: 10.1016/s0735-1097(02)02922-4. [DOI] [PubMed] [Google Scholar]
- 4.Po SS, Scherlag BJ, Yamanashi WS, Edwards J, Zhou J, Wu R, Geng N, Lazzara R, Jackman WM. Experimental model for paroxysmal atrial fibrillation arising at the pulmonary vein-atrial junctions. Heart Rhythm. 2006;3:201–8. doi: 10.1016/j.hrthm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Zhou J, Scherlag BJ, Edwards J, Jackman WM, Lazzara R, Po SS. Gradients of Atrial Refractoriness and Inducibility of Atrial Fibrillation due to Stimulation of Ganglionated Plexi. J Cardiovasc Electrophysiol. 2007;18:83–90. doi: 10.1111/j.1540-8167.2006.00679.x. [DOI] [PubMed] [Google Scholar]
- 6.Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005;2:624–31. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Po SS, Li Y, Tang D, Liu H, Geng N, Jackman WM, Scherlag B, Lazzara R, Patterson E. Rapid and stable re-entry within the pulmonary vein as a mechanism initiating paroxysmal atrial fibrillation. J Am Coll Cardiol. 2005;45:1871–7. doi: 10.1016/j.jacc.2005.02.070. [DOI] [PubMed] [Google Scholar]
- 8.Scherlag BJ, Nakagawa H, Jackman WM, Yamanashi WS, Patterson E, Po S, Lazzara R. Electrical stimulation to identify neural elements on the heart: their role in atrial fibrillation. J Interv Card Electrophysiol. 2005;(Suppl 1):37–42. doi: 10.1007/s10840-005-2492-2. [DOI] [PubMed] [Google Scholar]
- 9.Hou Y, Scherlag BJ, Lin J, Zhou J, Song J, Zhang Y, Patterson E, Lazzara R, Jackman WM, Po SS. Interactive atrial neural network: Determining the connections between ganglionated plexi. Heart Rhythm. 2007;4:56–63. doi: 10.1016/j.hrthm.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Hou Y, Scherlag BJ, Lin J, Zhang Y, Lu Z, Truong K, Patterson E, Lazzara R, Jackman WM, Po SS. Ganglionated plexi modulate extrinsic cardiac autonomic nerve input: effects on sinus rate, atrioventricular conduction, refractoriness, and inducibility of atrial fibrillation. J Am Coll Cardiol. 2007;50:61–8. doi: 10.1016/j.jacc.2007.02.066. [DOI] [PubMed] [Google Scholar]
- 11.Lin J, Scherlag BJ, Zhou J, Lu Z, Patterson E, Jackman WM, Lazzara R, Po SS. Autonomic mechanism to explain complex fractionated atrial electrograms (CFAE) J Cardiovasc Electrophysiol. 2007;18:1197–205. doi: 10.1111/j.1540-8167.2007.00976.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee KW, Everett TH, 4th, Rahmutula D, Guerra JM, Wilson E, Ding C, Olgin JE. Pirfenidone prevents the development of a vulnerable substrate for atrial fibrillation in a canine model of heart failure. Circulation. 2006;114:1703–12. doi: 10.1161/CIRCULATIONAHA.106.624320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wijffels MC, Kirchhof CJ, Dorland R, Power J, Allessie MA. Electrical remodeling due to atrial fibrillation in chronically instrumented conscious goats: roles of neurohumoral changes, ischemia, atrial stretch, and high rate of electrical activation. Circulation. 1997;96:3710–20. doi: 10.1161/01.cir.96.10.3710. [DOI] [PubMed] [Google Scholar]
- 14.Moe GK, Abildskov JA. Atrial fibrillation as a self-sustaining arrhythmia independent of focal discharge. Am Heart J. 1959;58:59–70. doi: 10.1016/0002-8703(59)90274-1. [DOI] [PubMed] [Google Scholar]
- 15.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 16.Patterson E, Jackman WM, Beckman KJ, Lazzara R, Lockwood D, Scherlag BJ, Wu R, Po S. Spontaneous Pulmonary Vein Firing in Man: Relationship to Tachycardia-Pause Early Afterdepolarizations and Triggered Arrhythmia in Canine Pulmonary Veins In Vitro. J Cardiovasc Electrophysiol. 2007;18:1067–75. doi: 10.1111/j.1540-8167.2007.00909.x. [DOI] [PubMed] [Google Scholar]
- 17.Morillo CA, Klein GJ, Jones DL, Guiraudon CM. Chronic rapid atrial pacing. Structural, functional, and electrophysiological characteristics of a new model of sustained atrial fibrillation. Circulation. 1995;91:1588–95. doi: 10.1161/01.cir.91.5.1588. [DOI] [PubMed] [Google Scholar]
- 18.Zhou S, Chang CM, Wu TJ, Miyauchi Y, Okuyama Y, Park AM, Hamabe A, Omichi C, Hayashi H, Brodsky LA, Mandel WJ, Ting CT, Fishbein MC, Karagueuzian HS, Chen PS. Nonreentrant focal activations in pulmonary veins in canine model of sustained atrial fibrillation. Am J Physiol Heart Circ Physiol. 2002;283:H1244–52. doi: 10.1152/ajpheart.01109.2001. [DOI] [PubMed] [Google Scholar]
- 19.Chou CC, Zhou S, Tan AY, Hayashi H, Nihei M, Chen PS. High-density mapping of pulmonary veins and left atrium during ibutilide administration in a canine model of sustained atrial fibrillation. Am J Physiol Heart Circ Physiol. 2005;289:H2704–13. doi: 10.1152/ajpheart.00537.2005. [DOI] [PubMed] [Google Scholar]
- 20.Neuberger HR, Schotten U, Blaauw Y, Vollmann D, Eijsbouts S, van Hunnik A, Allessie M. Chronic atrial dilation, electrical remodeling, and atrial fibrillation in the goat. J Am Coll Cardiol. 2006;47:644–53. doi: 10.1016/j.jacc.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita T, Murakawa Y, Hayami N, Fukui E, Kasaoka Y, Inoue M, Omata M. Short-term effects of rapid pacing on mRNA level of voltage-dependent K(+) channels in rat atrium: electrical remodeling in paroxysmal atrial tachycardia. Circulation. 2000;101:2007–14. doi: 10.1161/01.cir.101.16.2007. [DOI] [PubMed] [Google Scholar]
- 22.Cha TJ, Ehrlich JR, Chartier D, Qi XY, Xiao L, Nattel S. Kir3-based inward rectifier potassium current: potential role in atrial tachycardia remodeling effects on atrial repolarization and arrhythmias. Circulation. 2006;113:1730–7. doi: 10.1161/CIRCULATIONAHA.105.561738. [DOI] [PubMed] [Google Scholar]
- 23.Jayachandran JV, Sih HJ, Winkle W, Zipes DP, Hutchins GD, Olgin JE. Atrial fibrillation produced by prolonged rapid atrial pacing is associated with heterogeneous changes in atrial sympathetic innervation. Circulation. 2000;101:1185–91. doi: 10.1161/01.cir.101.10.1185. [DOI] [PubMed] [Google Scholar]
- 24.Cha TJ, Ehrlich JR, Chartier D, Qi XY, Xiao L, Nattel S. Kir3-based inward rectifier potassium current: potential role in atrial tachycardia remodeling effects on atrial repolarization and arrhythmias. Circulation. 2006;113:1730–7. doi: 10.1161/CIRCULATIONAHA.105.561738. [DOI] [PubMed] [Google Scholar]
- 25.Yeh YH, Qi X, Shiroshita-Takeshita A, Liu J, Maguy A, Chartier D, Hebert T, Wang Z, Nattel S. Atrial tachycardia induces remodelling of muscarinic receptors and their coupled potassium currents in canine left atrial and pulmonary vein cardiomyocytes. Br J Pharmacol. 2007;152:1021–32. doi: 10.1038/sj.bjp.0707376. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


