Abstract
Consolidation of synaptic plasticity appears to require transcription, but how the nucleus is informed in this context remains unknown. Because NMDA receptor antagonists have been shown to interfere with action potential generation, the issue of whether or not a synaptically-generated signal is required for nuclear signaling is currently unresolved. Here we show that pharmacologic maintenance of action potentials during NMDA receptor blockade allows for NMDA receptor-independent transcription factor binding and arc gene expression, both of which were previously thought to be NMDA receptor dependent. These data suggest that types of signaling in the nucleus previously attributed to NMDA-receptor dependent synapse-to-nucleus signals can be initiated in the absence of NMDA-receptor dependent synaptic plasticity.
Keywords: action potential, calcium, arc/Arg3.1, CREB, LTP, hippocampus
Introduction
Transcription appears to be critical for the late phase of LTP [1,2], but how do we determine the mechanisms used by synapses to signal to the nucleus that potentiation has occurred? A widely held view is that a signal must be generated upon NMDA receptor activation and physically transported from the synapse to the nucleus after LTP is induced. In this scenario a signal is created at the synapse after LTP induction and is transported to the nucleus, where it enters and either directly or indirectly regulates transcription (i.e. NFkB, [3]). Evidence supporting this view is that blockade of NMDA-type ionotropic glutamate receptors, which are important for most types of LTP induction at synapses, inhibits transcription of many genes [4–6]. Recently, however, we have shown that NMDA receptor blockade has other effects in the cell, namely the inhibition of action potential generation from synaptic stimulation resulting in a blockade of signaling cascades such as the ERK MAPkinase pathway [7]. These data indicate that an alternative model should be considered seriously, one in which action potentials signal the nucleus directly through the resulting calcium influx from voltage-gated calcium channel signaling [8]. Thus one cannot infer that NMDA receptor blockade of transcription is due to signals transported from the synapse unless action potentials are carefully controlled [7].
The specific genes critical for LTP consolidation have yet to be identified, however, and so in the interim we consider the regulation of certain transcription factors and the activity-regulated gene arc/arg3.1. Arc is rapidly up-regulated after a learning episode [9] and has been closely associated with the later phases of learning and LTP [10,11], or LTD and homeostatic plasticity [12,13]. Induction of this gene previously has been shown to be NMDA-receptor dependent [5]. Here we show that when action potentials are pharmacologically maintained during an LTP-inducing stimulation, transcription factor binding and arc induction do not require NMDA receptors. These data demonstrate that synaptic stimulation induces many biochemical events related to transcription and that NMDA receptors need not be directly involved.
Methods
Slice preparation and electrical stimulation
Hippocampal slices (350 μm) were prepared from 5–7 week old Sprague-Dawley rats. Slices were cut on a vibratome at 4°C in artificial cerebrospinal fluid (ACSF) containing (in mM): KCl, 4; sucrose, 240; NaH2PO4, 1.25; NaHCO2, 26; CaCl2, 1; MgCl2, 3; glucose, 10; bubbled with 95/5% O2/CO2. Mini-slices of CA1 were microdissected in ice-cold cutting ACSF, after which they were placed in an interface recording chamber and perfused with standard ACSF (NaCl, 124; KCl, 4; NaH2PO4, 1.25; NaHCO2, 26; CaCl2, 2.5; MgCl2, 1.5; and glucose, 10) at 34°C for 2–3 hours prior to stimulation. After application of 10 μM bicuculline or bicuculline + 50 μM APV for 20–40 minutes, a concentric bipolar stimulating electrode (FHC) placed in the stratum radiatum was used to stimulate the mini-slices with a theta-burst pattern (130 μs duration, 100 μA). This pattern of stimulation is known to induce LTP in CA1 pyramidal neurons [14] and induces action potentials resistant to the inhibitory effects of APV [7]. LTP was effectively blocked by this concentration of APV, even in the presence of bicuculline (n=3, data not shown). This stimulus intensity and duration was found to evoke population spikes to the edge of the mini-slices, resulting in an estimated 60–80% of the cells being activated based on phospho-ERK staining [15]. Five (EMSA) or fifteen (qPCR) minutes after electrical stimulation, slices were removed from the chamber, snap-frozen on dry ice, and stored at −80°C. One control (non-stimulated) slice was removed and frozen for each stimulated slice to match for time after cutting and drug exposure. Electrical stimulation without bicuculline did not reliably induce arc, which was likely to be due to the fact that more action potentials are evoked across a greater area of the slice in the bicuculline case. Therefore, because the two conditions were not thought to represent similar degrees of stimulation, comparisons between cases stimulated in bicuculline and those stimulated without were not made.
Preparation of Nuclear Proteins
Nuclear proteins were isolated using a modification of the protocol as directed in the CelLytic NuCLEAR Extraction kit (Sigma). Twenty-one to twenty-four mini-slices from 3–4 rats were pooled for homogenization and nuclear preparation. Slices were homogenized in 600 μl lysis buffer (0.6 % Igepal, 10 mM HEPES, 10 mM KCl, 1.5 mM MgCl2, 1 mM DTT, protease inhibitor cocktail III (Calbiochem), and phosphatase inhibitor cocktail II (Calbiochem)) and spun at 9,000 × g for 20 min at 4° C to pellet nuclei. Nuclear proteins were extracted by resuspending the resulting pellet in 7 μl extraction buffer (extraction buffer from kit, 166 mM NaCl, 1 mM DTT, protease and phosphatase cocktails) and shaking the tubes on ice for 30 minutes. Nuclei were again spun at 18,000 × g for 15 minutes at 4° C, and the supernatant was divided into 1 μl aliquots and frozen for future EMSAs. A second extraction with the same buffer was performed on the pellet and the phosphorylation of ERK determined by Western blot to confirm electrical stimulation [7].
Electrophoretic Mobility Shift Assays (EMSAs)
Consensus oligonucleotides (AP-1, CBF, NFkB, SRE, CREB, and Ets (Santa Cruz Biotechnology, Inc.)) were end-labeled using a T4 polynucleotide kinase and γ-32P ATP according to the manufacturer’s instructions (New England Biolabs). Nuclear extracts from control and stimulated slices were incubated at room temperature with radiolabeled oligonucleotides in gel shift binding buffer (Promega) and subsequently resolved by non-denaturing electrophoresis on a 6% TBE gel at 4° C. Gels were dried overnight and visualized using a phosphorimager. Samples containing the bound DNA/protein complex were retarded within the gel. Images were analyzed using ImageQuant software (Amersham Biosciences), and bands representing specific binding were identified using mutant oligonucleotides that do not bind the specified transcription factors (Santa Cruz Biotechnology, Inc.). These bands are indicated in the figures with an arrow.
Quantitative real-time PCR
Total RNA was isolated from 21–26 frozen slices using RNAeasy Lipid Tissue Mini kit (Invitrogen) according to the manufacturer’s instructions, converted to cDNA, and stored for further analysis. Quantitative real time PCR experiments were performed using a Perkin Elmer ABI Prism 770 Sequence Detector using 2.5 μl cDNA with Power SYBR Green (Applied Biosystems) and forward and reverse primers (forward: 5′ TGA CTC ACA ACT GCC ACA CA-3′; reverse: 5′ TGA GGA AGC CAA AGG TGT TC-3′). Twenty-five μl reaction mixtures were held at 50° C for 2 minutes, 95° C for 10 minutes, and then 40 cycles at 95° C for 15 seconds and 1 minute at 60° C. GAPDH was used for normalization as an internal control. Data are presented as a mean fold change over the unstimulated control slices.
Results
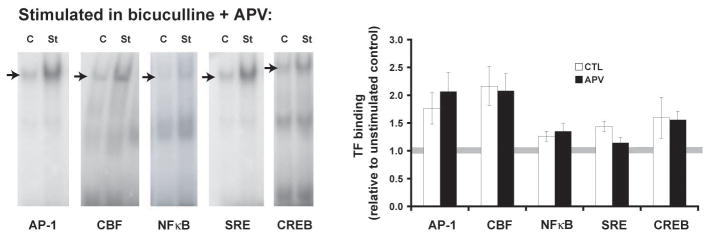
Activity-dependent genes such as arc are quickly transcribed after LTP-inducing stimulation, and so the transcription factors (TFs) regulating those genes can be studied using electrophoretic mobility shift assays (EMSAs). In previous work from our lab, we used transcription factor arrays to identify TFs of interest from rat hippocampal slices that had been electrically stimulated to induce LTP (Hudgins and Dudek, 2002 Society for Neuroscience abstracts). Using oligonucleotides with the consensus sequences to TF binding sites identified in the arrays and others known to be in the arc promoter region [16], we performed EMSAs on similar nuclear extracts to test for the role of the NMDA receptors. To test whether or not LTP-inducing stimulation could continue to activate TFs when action potentials were maintained, extracts were made from slices that had been electrically stimulated either in the presence or absence of APV, an NMDA receptor antagonist. This treatment consisted of eliminating fast synaptic inhibition with bicuculline, a GABA-A receptor antagonist that preserved action potentials during the continued NMDA receptor blockade.
Five minutes post-stimulation we found that NMDA receptors were not required for the increase in TF binding to AP-1, CBF, CREB, or NFκB consensus sequence oligonucleotides when action potentials were maintained (Figure 1). The effect of NMDA receptor blockade on SRE binding trended toward, but did not reach, significance (p=0.04, α=0.01). Thus, synaptic stimulation induces rapid transcription factor binding to at least several of the consensus sequences related to plasticity-regulated genes, and the binding is independent of NMDA receptor activation.
Fig. 1.
Stimulation-induced transcription factor binding does not require NMDA receptors (or LTP) if action potentials are maintained. To insure that action potentials were maintained during NMDA receptor antagonist exposure (50 μM D-APV), synaptic stimulation was delivered in a theta burst pattern of stimulation (TBS) with bicuculline [7]. Hippocampal CA1 mini-slices were sampled 5 minutes after stimulation. Nuclear protein extracts were assessed for transcription factor binding by electrophoretic mobility shift assays (EMSAs). Example gels are shown on the left, with arrows marking the bands representing specific binding. Plotted on the right are data from 8–11 (control) and 7–8 (APV) separate EMSAs. Transcription factor binding in APV-treated slices are not significantly different from the slices without the drug. Binding to SRE is not affected by APV at this time point (p=0.04, a=0.01).
Based on this data, we sought to determine whether a similar response could be seen with a gene whose induction had been previously shown to be NMDA receptor-dependent [5,17]. Using real-time quantitative PCR analysis, we tested whether arc, an immediate early gene, could be induced in the absence of NMDA receptor activation while maintaining action potentials with bicuculline. Like transcription factor binding, we found that LTP-inducing stimulation in the presence of bicuculline led to an increase in arc transcription, and it was unaffected by the NMDA receptor blockade (Figure 2). L-type voltage sensitive calcium channels were likely contributing, at least in part, to the calcium levels in the postsynaptic neurons: nifedipine, a blocker of voltage-gated calcium channels, significantly reduced the stimulation-induced increase in arc in APV and bicuculline (APV/bicuculline: 1.53±0.12; APV/bicuculline/10 μM nifedipine: 1.25±0.11; p<0.018). We conclude that NMDA receptors are not necessary for stimulation-induced changes in arc transcription when action potentials are preserved.
Fig. 2.
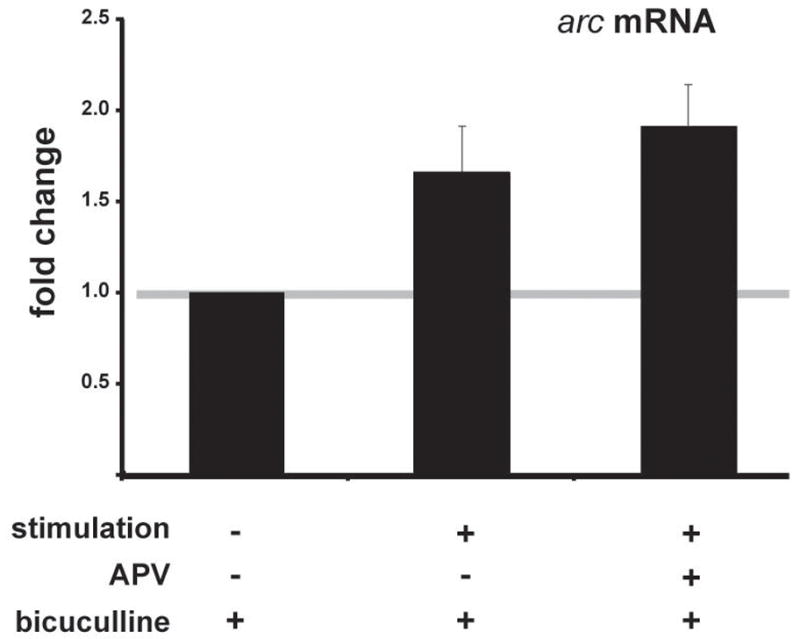
Stimulation-induced arc expression does not require NMDA receptors if action potentials are maintained. To insure that action potentials were maintained during NMDA receptor antagonist exposure (50 μM D-APV), synaptic stimulation was delivered in a theta burst pattern of stimulation (TBS) with bicuculline [7]. Hippocampal CA1 mini-slices were sampled 15 minutes after stimulation. mRNA was purified from pooled slices, cDNA prepared and real-time quantitative PCR performed. Plotted are data from 4 separate determinations. Stimulated arc levels in the APV-treated slices are not significantly different from the stimulated levels in slices without the drug.
Discussion
Long-term potentiation, particularly its later transcription-dependent phases, has been a major focus of research in the search for the cellular mechanisms of long-term memory. Due to the evidence showing that new RNA is transcribed in the nucleus in response to LTP and learning [9,18], we have examined how synapses signal a need for new RNA synthesis in response to synaptic plasticity. Based on experiments demonstrating that NMDA receptor antagonists inhibit transcription of many genes [4,5,19], a reasonable conclusion has been that a signal originating at synapses translocates to the nucleus to induce transcription. Also, certain proteins have recently been demonstrated to be capable of such translocation from synapses [3,20]. The inhibitory effects of NMDA receptor antagonists on action potential generation, however, had not been considered until recently [7]. This inhibitory effect on action potentials only becomes an issue if one proposes that action potentials, through calcium, are instead the critical signals regulating rapid transcription of plasticity-related genes. Because action potentials alone are sufficient to induce a myriad of signaling pathways and rescue of late-phase LTP [21], though, the idea of action potential-dependent transcription must now be considered. Here we have provided evidence consistent with the idea that an NMDA receptor-independent signal is sufficient to signal the nucleus and commence transcription of an important plasticity-related gene. These data support our hypothesis that NMDA receptor-independent mechanisms, such as calcium increases generated by action potentials, can provide a large, fast signal in the nucleus that would favorably permit rapid induction of transcription. Signals transported from synapses would have little or no targeting advantage over a cell-wide signal, as synaptic “tagging” after LTP would be critical no matter how a signal reached the nucleus [22,23].
These data support our hypothesis that gene expression can occur irrespective of NMDA receptor-dependent plasticity for at least one immediate early gene. Because bicuculline may have some effects on the integrative properties of dendrites that we are not measuring, our data do not rule out a role for aberrant activation of another signaling pathway, such as activation of ERK through the Brain Derived Neurotrophic Factor (BDNF). The pathway would, however, be synaptic plasticity independent- recall that LTP (and LTD) are blocked in our experiments with APV. We do know, though, that bicuculline by itself, in the absence of electrical stimulation, does not lead to the activation of ERK [7]. One way relevant transcription could be initiated is with the activation of kinases by calcium: CAMKIV is concentrated in the nucleus and has been implicated in late-LTP and learning [24], while CAMKI reportedly is able to translocate to the nucleus from the cytosol very rapidly [25]. Similarly, calcium-dependent cyclic AMP synthesis and subsequent activation of PKA could also mediate transcription factor binding, as PKA activity is necessary for late-LTP and memory [26]. PKA’s speed of activation in the nucleus in response to neuronal activity is unknown, however. Further study will be required to work out the details of nuclear engagement once specific necessary genes and TFs are identified.
Conclusion
These data indicate that when action potentials are carefully controlled, NMDA receptor-dependent signals from synapses are unnecessary for fast stimulation of transcription factor binding and transcription of arc. We propose that an action potential-mediated calcium increase, or other similar non-NMDA receptor dependent processes, initiate the rapid regulation of genes necessary for consolidation of synaptic plasticity.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences
We thank Dr. Mineyoshi Aoyama for help with the qPCR and Dr. G. Jean Harry for advice on the data analysis.
References
- 1.Frey U, Frey S, Schollmeier F, Krug M. Influence of actinomycin D, a RNA synthesis inhibitor, on long-term potentiation in rat hippocampal neurons in vivo and in vitro. J Physiol. 1996;490:703–711. doi: 10.1113/jphysiol.1996.sp021179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen PV, Abel T, Kandel ER. Requirement of a critical period of transcription for induction of a late phase of LTP. Science. 1994;265:1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- 3.Meffert M, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D. NF-KB functions in synaptic signaling and behavior. Nat Neurosci. 2003;6:1072–1078. doi: 10.1038/nn1110. [DOI] [PubMed] [Google Scholar]
- 4.Matsuo R, Murayama A, Saitoh Y, Sakaki Y, Inokuchi K. Identification and cataloging of genes induced by long-lasting long-term potentiation in awake rats. J Neurochem. 2000;74:2239. doi: 10.1046/j.1471-4159.2000.0742239.x. [DOI] [PubMed] [Google Scholar]
- 5.Steward O, Worley PF. Selective Targeting of Newly Synthesized Arc mRNA to Active Synapses Requires NMDA Receptor Activation. Neuron. 2001;30:227–240. doi: 10.1016/s0896-6273(01)00275-6. [DOI] [PubMed] [Google Scholar]
- 6.Worley PF, Bhat RV, Baraban JM, Erickson CA, McNaughton BL, Barnes CA. Thresholds for synaptic activation of transcription factors in hippocampus: correlation with long-term enhancement. J Neurosci. 1993;13:4776–4786. doi: 10.1523/JNEUROSCI.13-11-04776.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao M, Adams JP, Dudek SM. Pattern-dependent role of NMDA receptors in action potential generation: consequences on extracellular signal-regulated kinase activation. J Neurosci. 2005;25:7032–7039. doi: 10.1523/JNEUROSCI.1579-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams JP, Dudek SM. Late-phase long-term potentiation: getting to the nucleus. Nat Rev Neurosci. 2005;6:737–743. doi: 10.1038/nrn1749. [DOI] [PubMed] [Google Scholar]
- 9.Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- 10.Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 11.Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, et al. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rial Verde EM, Lee-Osbourne J, Worley PF, Malinow R, Cline HT. Increased expression of the immediate-early gene arc/arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron. 2006;52:461–474. doi: 10.1016/j.neuron.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, et al. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52:475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986;368:347–350. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
- 15.Dudek SM, Fields RD. Mitogen-activated protein kinase/extracellular signal-regulated kinase activation in somatodendritic compartments: roles of action potentials, frequency, and mode of calcium entry. J Neurosci. 2001;21:RC122. doi: 10.1523/JNEUROSCI.21-02-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waltereit R, Dammermann B, Wulff P, Scafidi J, Staubli U, Kauselmann G, et al. Arg3.1/Arc mRNA induction by Ca2+ and cAMP requires protein kinase A and mitogen-activated protein kinase/extracellular regulated kinase activation. J Neurosci. 2001;21:5484–5493. doi: 10.1523/JNEUROSCI.21-15-05484.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Worley PF, Cole AJ, Saffen DW, Baraban JM. Regulation of immediate early genes in brain: role of NMDA receptor activation. Prog Brain Res. 1990;86:277–285. doi: 10.1016/s0079-6123(08)63184-2. [DOI] [PubMed] [Google Scholar]
- 18.Frey U, Krug M, Brodemann R, Reymann K, Matthies H. Long-term potentiation induced in dendrites separated from rat’s CA1 pyramidal somata does not establish a late phase. Neuroscience Letters. 1989;97:135–139. doi: 10.1016/0304-3940(89)90152-3. [DOI] [PubMed] [Google Scholar]
- 19.Cole AJ, Saffen DW, Baraban JM, Worley PF. Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature. 1989;340:474–476. doi: 10.1038/340474a0. [DOI] [PubMed] [Google Scholar]
- 20.Jordan BA, Fernholz BD, Khatri L, Ziff EB. Activity-dependent AIDA-1 nuclear signaling regulates nucleolar numbers and protein synthesis in neurons. Nat Neurosci. 2007;10:427–435. doi: 10.1038/nn1867. [DOI] [PubMed] [Google Scholar]
- 21.Dudek SM, Fields RD. Somatic action potentials are sufficient for late-phase LTP-related cell signaling. Proc Natl Acad Sci U S A. 2002;99:3962–3967. doi: 10.1073/pnas.062510599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alarcon JM, Barco A, Kandel ER. Capture of the late phase of long-term potentiation within and across the apical and basilar dendritic compartments of CA1 pyramidal neurons: synaptic tagging is compartment restricted. J Neurosci. 2006;26:256–264. doi: 10.1523/JNEUROSCI.3196-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sajikumar S, Navakkode S, Frey JU. Identification of compartment- and process-specific molecules required for “synaptic tagging” during long-term potentiation and long-term depression in hippocampal CA1. J Neurosci. 2007;27:5068–5080. doi: 10.1523/JNEUROSCI.4940-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang H, Sun LD, Atkins CM, Soderling TR, Wilson MA, Tonegawa S. An important role of neural activity-dependent CaMKIV signaling in the consolidation of long-term memory. Cell. 2001;106:771–783. doi: 10.1016/s0092-8674(01)00497-4. [DOI] [PubMed] [Google Scholar]
- 25.Sakagami H, Kamata A, Nishimura H, Kasahara J, Owada Y, Takeuchi Y, et al. Prominent expression and activity-dependent nuclear translocation of Ca2+/calmodulin-dependent protein kinase Idelta in hippocampal neurons. Eur J Neurosci. 2005;22:2697–2707. doi: 10.1111/j.1460-9568.2005.04463.x. [DOI] [PubMed] [Google Scholar]
- 26.Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchuladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]