Abstract
The purpose of this study was to examine sex differences in sensitivity to nicotine’s reinforcing effects during adolescence, a hormone transition phase characterized by rapid and marked changes in levels of gonadal hormones. Male and female rats were trained to self-administer nicotine (5 or 10 µg/kg/infusion) under a fixed-ratio 1 schedule beginning on postnatal day 30. Following acquisition, responding was assessed under a progressive-ratio schedule until postnatal day 45 with blood sampling occurring prior to the first 5 sessions in order to determine the relationship between gonadal hormones (i.e., estradiol and progesterone in females and testosterone in males) and responding for nicotine. Under low dose conditions, a greater percentage of females than males acquired nicotine self-administration. Under progressive-ratio testing conditions, although adolescent females and males initially responded at similar levels, by the end of the adolescent testing period, females responded at higher levels than males to obtain nicotine infusions. Levels of responding under the progressive-ratio schedule were negatively associated with progesterone and positively associated with the ratio of estradiol to progesterone. These findings demonstrate an enhanced sensitivity in adolescent females as compared to adolescent males to nicotine’s reinforcing effects with evidence implicating circulating hormone levels as modulating this sensitivity.
Keywords: adolescence, estradiol, estrous cycle, motivation, nicotine, progesterone, progressive-ratio, self-administration, sex differences, testosterone
1. Introduction
Men are approximately one and a half times more likely than women to report past month use of a tobacco product and slightly more likely to smoke cigarettes (Substance Abuse and Mental Health Services Administration (SAMHSA 2007). However, among adolescents, the gender differential is eliminated with adolescent females aged 12 to 17 equal to males on reported rates of current smoking (9.7% vs. 10.0%, respectively). Moreover, accumulating evidence indicates that women are more vulnerable on certain aspects of nicotine dependence than are men. For example, compared to male smokers, female smokers take less time to become dependent after initial use, report shorter and less frequent abstinence periods, and have more difficulty quitting smoking (for review see Perkins 2001).
Results from studies with adult laboratory animals support a greater vulnerability to nicotine self-administration in females compared to males (Chaudhri et al. 2005; Donny et al. 2000; 2004; Rezvani et al. 2008), suggesting that differences observed between men and women smokers have a biological basis. For example, Donny et al. (2000) compared male and female rats on acquisition of nicotine self-administration and found that a greater percentage of females than males acquired self-administration under the low dose conditions. They also showed that females responded at higher levels than males on a progressive-ratio schedule suggesting that they were more motivated to obtain nicotine infusions than were males. Although this study did not find estrous cycle dependent changes in progressive-ratio responding for nicotine, studies conducted with humans suggest that ovarian hormones contribute to smoking behavior in women. For example, phase of the menstrual cycle has been shown to affect the subjective effects of smoking and smoking behavior (DeBon et al. 1995; Mello et al. 1987; Snively et al. 2000; but see Allen et al. 1996; Pomerleau et al. 1994; Marks et al. 1999) and to be predictive of nicotine withdrawal severity (Allen et al. 1999; O'Hara et al. 1989; Perkins et al. 2000) and vulnerability to craving and smoking relapse following abstinence (Pomerleau et al. 2000; Franklin et al. 2004; 2008; Carpenter et al. 2006; Allen et al. 2008a; b). Additionally, Sofuoglu et al. (2001) demonstrated that exogenously administered progesterone reduced the positive subjective effects of smoking and craving for cigarettes. Similar findings of an increased vulnerability in females as a function of hormonal phase have been reported in both laboratory animals and humans for a variety of other drugs of abuse (Lynch 2006; Lynch et al. 2002; Carroll et al. 2004; Becker and Hu 2008). These findings suggest that ovarian hormones modulate the reinforcing effects of drugs of abuse, including nicotine, raising the possibility that vulnerability to nicotine dependence may vary at hormone transition phases (e.g., adolescence, pregnancy, menopause).
Adolescence is a period of marked hormonal change that is associated with an apparent vulnerability to smoking initiation. Additionally, adolescents, particularly female adolescents, seem to progress more rapidly to nicotine dependence after initial use than adults (SAMHSA 2007) and show higher rates of dependence, even though adolescents report smoking fewer cigarettes than do adults (Tanski et al. 2004; Storr 2008). Despite these findings, and the fact that smoking generally begins during adolescence, most animal studies have focused on adult males (but see McQuown et al. 2007; Shram et al. 2008a; b; Levin et al. 2007; Adriani et al. 2003). As a result, very little information is available on sex differences in vulnerability to nicotine dependence during adolescence, and it is not yet known whether changes in vulnerability to nicotine dependence coincide with changes in hormones.
Adolescence in the rat is associated with the onset of puberty and has been broadly defined to include the few days preceding and following its onset (from postnatal day 28 to 42; Spear and Brake 2003). It is initiated by a surge in gonadal hormones on at around postnatal day 28 (e.g., GnRH, testosterone and estrogen; Corpechot et al. 1981). In adolescent females, vaginal opening occurs on postnatal day 35, and by postnatal day 45, females are randomly cycling (Ojeda et al. 1983). In males, following the surge in gonadal hormone, levels of testosterone continue to increase beginning at around PND 35 with the first free sperm appearing around postnatal day 45 (Ojeda et al. 1980).
An initial study conducted by Klein et al. (2004) in mice showed that oral nicotine consumption in g/kg was significantly greater in adolescent females as compared to adolescent males. Chen et al. (2007) recently showed in rats that adolescent males and females did not differ on acquisition of nicotine self-administration under low dose conditions when responding was assessed beginning during late adolescence (beginning between postnatal day 43 and 45). Levin and colleagues (2003) also examined nicotine self-administration in female rats during late adolescence (beginning between postnatal day 40 and 46) and showed that adolescent females rapidly acquired self-administration, although a comparison to males was not included. To date, no studies have examined estrous cycle phase in females or gonadal hormone levels in females or males with regard to nicotine self-administration during adolescence. Thus, the goal of this study was to explore sex differences and hormonal influences on acquisition of nicotine self-administration and subsequent motivation for nicotine as assessed under a progressive-ratio schedule in adolescent rats beginning at postnatal day 30 and ending at postnatal day 45. Estrous cycle phase was monitored throughout the study. Blood levels of estradiol and progesterone (females) and testosterone (males) were also examined prior to each of the first 5 progressive ratio sessions in order to determine the relationship between levels of circulating gonadal hormones and motivation for nicotine. Given previous findings of sex differences in adult rats and previous findings of hormonal influences in humans, we hypothesized that female rats would show an enhanced sensitivity to the reinforcing effects of nicotine as compared to male rats, and that ovarian hormones would underlie the enhanced sensitivity.
2. Methods
2.1 Subjects
Female and male Sprague Dawley rats arrived at the laboratory at postnatal day 22 with self-administration testing beginning at postnatal day 30 and continuing until postnatal day 45. Upon arrival at the laboratory, rats were individually housed in operant conditioning chambers (ENV-018M; Med Associates, St. Albans, VT), and maintained on a 12-hour light/dark cycle (lights on at 7-am) with free access to food and water. In order to ensure that acquisition occurred within the narrow window of time in which to study adolescence, rats were pre-trained to lever-press for sucrose pellets using methods previously described (Lynch 2008). Briefly, this training began following a 2-day habituation period (i.e., at postnatal day 25) wherein 30 male and 30 female rats were trained to lever-press for sucrose pellets (45-mg) during daily 23-hr sessions beginning at 12 pm. Training sessions were initiated daily by the introduction of the left lever into the operant chamber and responding was reinforced under a fixed-ratio 1 schedule with daily sessions continuing until rats obtained a minimum of 50 pellets in a session (typically occurred within the first 3 sessions). Rats were weighed on the day of surgery and for the 2 days that followed and there after, three times each week (i.e., Mon, Wed, and Friday). The experimental protocols were approved by the Animal Care and Use Committee of the University of Virginia and were conducted in accordance with guidelines set by the National Institutes of Health.
2.2. Surgery
Surgical implantation of a catheter (Silastic tubing; 0.51 mm o.d. and 0.94 mm o.d.; Dow Corning Corporation, Midland, MI) in the right jugular vein of each rat was conducted on postnatal day 28 under ketamine (90 mg/kg) and pentobarbital (5.0 mg/kg) anesthesia using methods previously described (Lynch 2008; Lynch and Taylor 2004). Catheter patency was tested for 2 consecutive days following surgery and thereafter every Mon, Wed, and Friday by flushing the catheter with a small amount of heparinized saline and then pulling back until blood appeared in the line. If a catheter was not patent (i.e., no blood appeared in the line), the rat was replaced until there were a minimum of 10 rats per group. Four of the 30 males and 5 of the 30 females lost catheter patency before acquisition testing was completed, and their data were excluded from all analyses.
2.3. Apparatus
After surgery animals were housed in 25 cm3 Med Associates testing chambers where they remained for the duration of the experiment. Each chamber contained an insertion for a drinking spout, a food jar, one standard response lever, and one retractable lever (Med Associates, St. Albans, VT). Stimulus lights (4.76 W) were located above each lever, and a house light (4.76 W) that was illuminated from 7-am to 7-pm was located at the top of the chamber. Each chamber was enclosed in a sound-attenuating wooden box that contained a fan for ventilation. A Med Associates infusion pump containing a 10-ml syringe for the nicotine solution was mounted inside the sound-attenuating chamber. Each syringe was connected with Tygon tubing to a swivel (Instech Laboratories Inc., Plymouth Meeting, PA) that was mounted at the top of the chamber. A tether (C313CS; Plastic Products, Roanoke, VA) was attached to the swivel and to the rat by a metal cannula (C3236; Plastic Products, Roanoke, VA) that was embedded in the center of a plastic covance infusion harness (Instech Laboratories Inc., Plymouth Meeting, PA). An IBM-compatible computer with Med-PC interface (Med Associates, St. Albans, VT) was used for programming and data collection and storage.
2.3 Drugs
Nicotine bitartrate was purchased from Sigma-Aldrich (St. Louis, MO). Nicotine was mixed in sterile saline solution and the pH was adjusted to 7.4 + 0.5 with NaOH. Doses (5 and 10 µg/kg) are expressed as free base weight which were adjusted for g/kg differences in weight by changing the delivery volume (i.e., infusion duration was set at 0.6 sec/100g of body weight). Nicotine solutions were made weekly and refrigerated, but they were added to the drug syringes at room temperature.
2.4 Acquisition of self-administration
Rats were assigned to self-administer one of two doses of nicotine (5 or 10 µg/kg/infusion) during daily self-administration training sessions that began at 12-pm on postnatal day 30. The 10 µg/kg dose of nicotine dose was selected based on previous research showing that responding in adult male rats is maximal at this dose (Corrigall and Coen 1989) and work showing that sex differences were revealed under similar low dose conditions (Donny et al. 2000). However, since initial findings from a pilot study revealed that adolescent rats rapidly acquired under this dose condition, a lower dose (5 µg/kg/infusion) was included to slow the acquisition process and maximize individual differences. Both doses were tested contemporaneously with random group assignment. Training sessions were initiated by the introduction of the left lever (nicotine-associated lever) into the operant conditioning chamber and each response on this lever produced an infusion of nicotine and the illumination of a stimulus light located above the lever. One non-contingently administered infusion was delivered at the beginning of each training session in order to signal the availability of nicotine and to induce responding (Campbell and Carroll 2000). Nicotine infusions were available under a fixed-ratio 1 schedule until a total of 20 infusions had been obtained. During sessions in which not all 20 infusions were obtained the lever was available for almost 24 hrs (sessions started at 12.00 h and ended at 11.50 h the next day) in order to increase the likelihood of acquisition. However, once an animal began responding for nicotine, all 20 infusions available were typically obtained within 3 hours (average session length was 2 h), and this typically occurred during the first hours of the session following the nicotine prime. At this point, the left lever was retracted until the next daily session. These training sessions continued until acquisition occurred (defined as 2 consecutive sessions in which all 20 infusions that were available were obtained) or until postnatal day 45.
2.5 Progressive-ratio self-administration
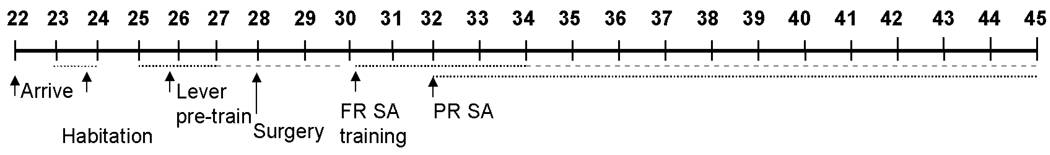
Progressive-ratio responding for nicotine was examined during daily self-administration sessions beginning following the second acquisition session and continuing until postnatal day 45. Each session began with the introduction of the left lever (nicotine-associated lever) into the operant conditioning chamber. Responses on this lever were reinforced under a progressive-ratio schedule where the response requirement to obtain a nicotine infusion (5 or 10 µg/kg) increased progressively throughout the session in the following steps: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, etc. (as described in Arnold and Roberts 1997). The last ratio completed, or breakpoint, was determined daily (defined by the number of infusions self-administered in a session). Although the lever was available for almost 24 h (sessions started at 12.00 h and ended at 11.50 h the next day), and the number of infusions available was not limited, responding typically ceased within 2–3 h (i.e., breakpoints were observed in an average of 2.5 h). The experimental time-line of these procedures is summarized in Figure 1.
Figure 1.
Experimental timeline used to examine acquisition of nicotine self-administration (SA) under a fixed-ratio (FR) 1 schedule and subsequent maintenance self-administration under a progressive-ratio (PR) schedule. Progressive-ratio testing was assessed once rats acquired nicotine self-administration under the fixed-ratio schedule, with sessions beginning as early as postnatal day 32.
2.6 Point of estrous determination and hormonal status verification
The point of estrous cycle phase was determined by microscopically examining vaginal swabs. Vaginal swabbing occurred approximately 30 minutes prior to the daily self-administration session beginning on postnatal day 35 (when vaginal opening occurs) using methods previously described (Lynch et al. 2000). Estrous cycle phase was determined on the basis of cell types present. Specifically, proestrus and estrus were characterized by the presence of predominantly nucleated epithelial cells and predominantly non-nucleated cornified epithelial cells, respectively. Metestrus and diestrus phase were categorized together and were characterized by the presence of predominately leukocytes and necrotic epithelia. Male rats were handled daily in a similar manner (i.e., their tails were raised and then they were gently poked in a similar region with a wet cotton swab).
2.7 Hormone measurements
Hormone concentrations of testosterone were assessed in a subset of male rats (n=9) and hormone concentrations of estradiol and progesterone were assessed in a subset of female rats (n=12) using methods previously described (Lynch 2008; Feltenstein and See 2007). Blood samples (0.45 ml/sample) were obtained from the same catheter that was used for self-administration. Sampling occurred between 30 and 60 minutes prior to the progressive-ratio self-administration session beginning with the first session and continuing for an additional 4 sessions. Serum samples were stored at −20 degrees centigrade until the assays were performed. Radioimmunoassays were conducted by the Reproduction Ligand Assay and Analysis Core at the University of Virginia.
2.8 Data analysis
Acquisition of nicotine self-administration was compared between males and females using methods similar to those previously reported for cocaine, heroin, and methamphetamine (Perry et al. 2007; Roth and Carroll 2004; Lynch and Carroll 1999). Specifically, the rate of acquisition and percentage of female and male rats acquiring nicotine self-administration was compared at each dose between males and females using the Meier survival analysis followed by the Mantel-Cox rank statistic. Rats that did not meet the acquisition criterion within the testing period (i.e., by postnatal day 45) were excluded from all subsequent analyses. For rats that met the acquisition criterion, the number of inactive lever presses observed during the 2 sessions that met the acquisition criterion were compared between males and females using a 2-factor (dose and sex) ANOVA. Although the data from the progressive-ratio sessions are plotted as active lever responses, statistical comparisons were conducted on the number of infusions as per standard protocol (see Arnold and Roberts 1997). For the first 5 progressive-ratio sessions, the average number of infusions obtained was compared using repeated measures ANOVA with day as the within subjects factor and sex and dose as the between subjects factors. A similar analysis was used to compare the number of inactive lever presses during these same sessions. The relationship between the number of infusions obtained under the first 5 progressive-ratio sessions and estradiol and progesterone (females) and testosterone (males) concentrations was examined by calculating the Pearson Correlation Co-efficient for each subject. The one sample t-test was used to determine whether these correlations were significantly different from zero for each of hormone. In order to examine whether progressive-ratio responding for nicotine varied over the adolescent testing period, the average number of infusions and the number of inactive lever responses observed during the initial testing sessions (first three sessions which occurred between postnatal day 32 to 37) were compared to those obtained during the last three testing sessions (43–45) including only the animals that acquired self-administration prior to postnatal day 35 and animals that maintained patency through the adolescent testing period (Note: data from these animals were included in all other analyses). Specifically, 5 of the 6 males and 7 of the 9 females in the 5 µg/kg dose group that acquired nicotine self-administration acquired prior to postnatal day 35 and maintained catheter patency throughout the adolescent testing period. For the 10 µg/kg dose group, 8 of the 11 males and 6 of the 14 females that acquired self-administration acquired prior to postnatal day 35 and maintained catheter patency throughout the adolescent testing period (note that 3 of these females did not acquire until mid-to-late adolescence). The estrous cycle data were examined in female rats that maintained patency through late adolescence. Since rats were predominantly in the metestrus/diestrus phase of the estrous cycle, and most showed only 1 full cycle of proestrus to estrus to metestrus-diestrus, the number of infusions obtained under the progressive-ratio schedule was compared between the phases for the first full cycle. One rat did not exhibit a clear proestrus phase and was not included in the estrous cycle analysis. These data were analyzed by repeated measures ANOVA with subsequent pairwise comparisons made using the paired t-test controlling for family wise error. A similar analysis was used to examine phase differences in inactive lever responses. A p value < 0.05 indicated significant differences.
3. Results
3.1 Acquisition
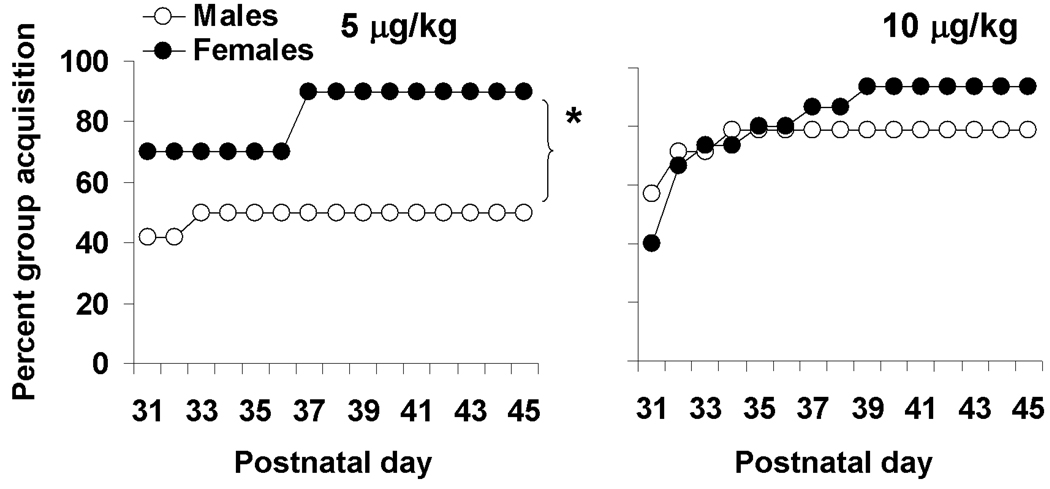
Acquisition of nicotine self-administration was achieved rapidly in both males and females under both dose conditions (Figure 2). Specifically, under the 5 µg/kg dose condition 5 of 12 males (42%) and 7 of 10 females (70%) met the acquisition criterion within the first 2 sessions. Under the 10 µg/kg dose condition 8 of 14 males (57%) and 6 of 15 females (40%) met the acquisition criterion within the first 2 sessions. Although a similar number of females and males acquired nicotine self-administration at the 10 µg/kg nicotine dose (79 versus 93%, respectively), a sex difference emerged at the 5 µg/kg nicotine dose with a greater percentage of females compared to males acquiring nicotine self-administration (90 versus 50%, respectively). A survival analysis revealed an effect of sex within the 5 µg/kg dose condition (χ2=6.6, p<0.05), but not under the 10 µg/kg dose condition (p>0.05) indicating that the difference between males and females on percent group acquisition under the low dose condition was significant. However, for both doses, males and females acquired self-administration at a similar rate. Specifically, of the rats that acquired self-administration under the 5 µg/kg dose condition, males acquired in a mean of 2.0 ± 0.0 days and females acquired in a mean of 3.3 ± 0.9 days. Of the rats that acquired self-administration under the 10 µg/kg condition, males acquired in a mean of 2.5 ± 0.3 days and females acquired in a mean of 3.7 ± 0.7 days. The number of inactive lever responses did not differ significantly between males and females for either the 5 µg/kg (8.3 ± 1.2, 3.2 ± 1.1, respectively) or 10 µg/kg (3.5 ± 1.2, 12.12 ± 5.2, respectively) dose condition (p>0.05). Both males and females quickly acquired sucrose self-administration (i.e., within 6 days and in a mean of 2.9 ± 0.3, 2.8 ± 0.3 days, respectively), and the mean number of days to acquire did not differ significantly between males and females (p>0.05). Thus, under the low dose conditions, a significantly greater percentage of females than males acquired nicotine self-administration.
Figure 2.
Data are presented as an inverse survival function to illustrate the percentage of female and male rats to meet the acquisition criterion by postnatal day 45 under the 5 and 10 µg/kg nicotine dose conditions. The asterisk indicates a significant sex difference (P<0.05). Insets show that number of inactive lever responses for male (open bars) and female (filled bars) rats for the 2 sessions that met the acquisition criterion. Each data point represents an N of 10 females and 12 males under the 5 µg/kg nicotine dose condition and an N of 15 females and 14 males under the 10 µg/kg nicotine dose condition.
3.2 Progressive-ratio nicotine self-administration
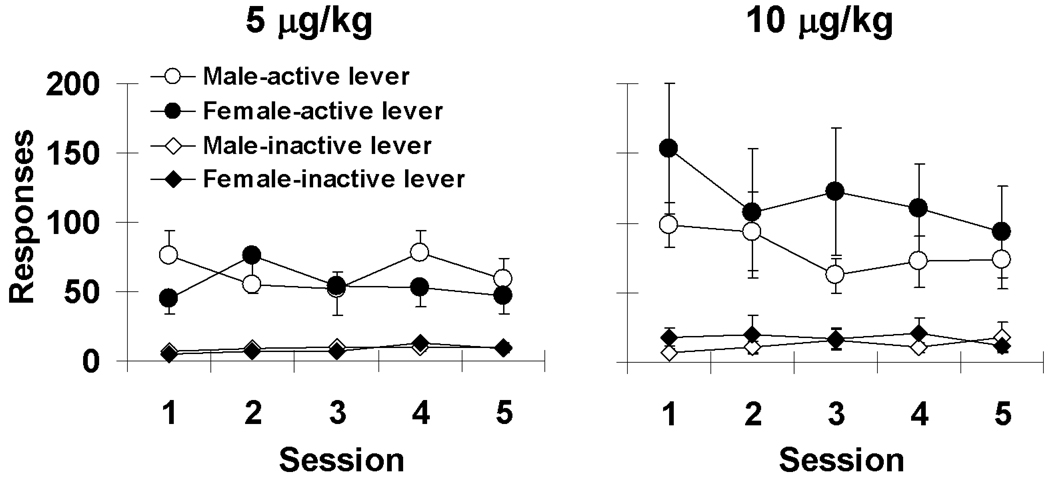
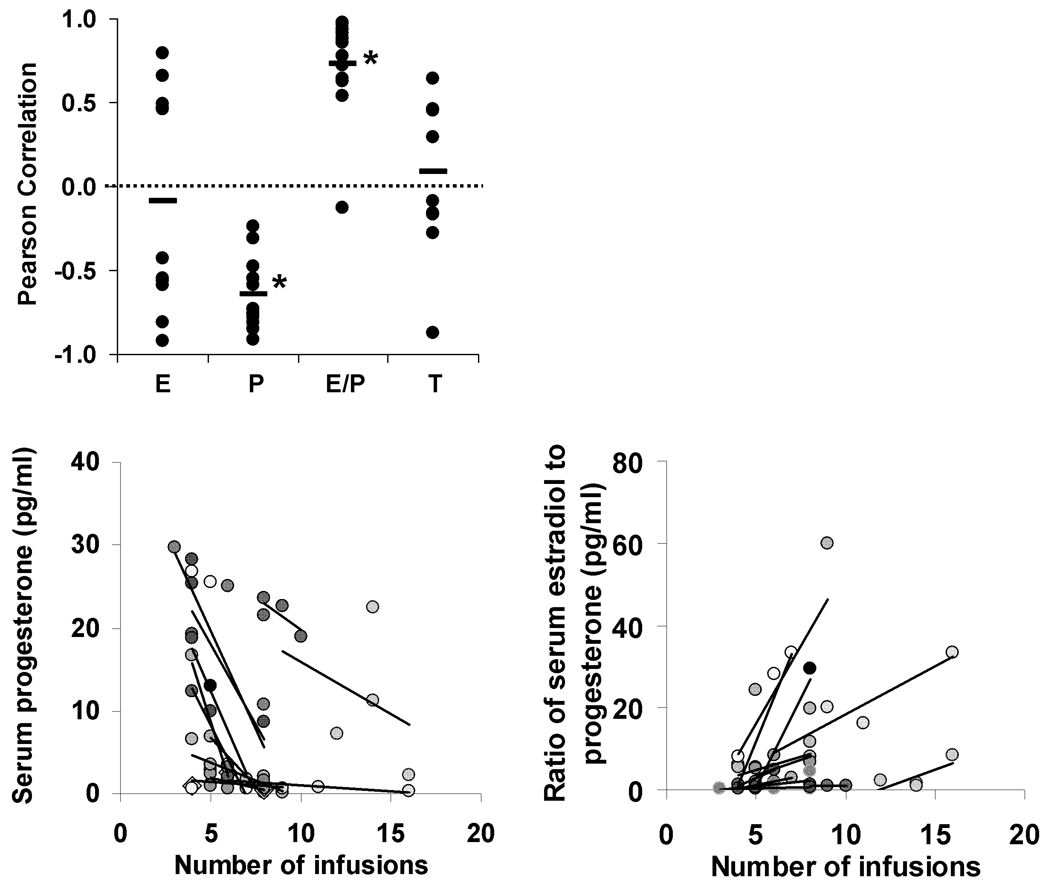
Levels of responding to obtain nicotine infusions during the first 5 progressive-ratio self-administration sessions did not differ significantly between males and females (Figure 3; p’s>0.05). However, within females, serum progesterone concentration and the ratio of estradiol to progesterone, but not estradiol, was associated with levels of responding during these first 5 sessions (Figure 4). These relationships did not vary systematically with dose. Although the overall relationships were variable, within each of the 12 rats tested, the number of infusions and progesterone concentration was negatively associated, and for 9 of these 12 rats, the association accounted for over 30% of the variability in the number of infusions (Figure 4, bottom left panel). The individual r values were −0.91, −0.85, −0.81, −0.78, −0.76, −0.75, −0.73, −0.59, −0.55, −0.48, −0.31, and −0.24. Similarly, for the ratio of estradiol to progesterone and number of infusions, a positive association was observed in 11 of out the 12 rats with the association accounting for over 30% of the variability in the number of infusions for 10 out of 12 rats (Figure 4, bottom right panel). The individual r values were 0.98, 0.97, 0.94, 0.91, 0.88, 0.86, 0.78, 0.72, 0.64, 0.63, 0.54, −0.13. A comparison of the Pearson Correlation Co-Efficients using separate one-sample t-tests revealed that these relationships were statistically significant for progesterone (t=10.5, df=11, p<0.001) and for the ratio of estradiol to progesterone (t=8.2, df=11, p<0.001). In males, there was no significant relationship between serum testosterone concentration and the number of infusions obtained (p>0.05). It should be noted that the data from the subset of rats for which hormonal data were assessed were statistically similar to the overall group in terms of days to acquire self-administration (3.1 ± 0.7 for females and 2.0 ± 0.0 for males) and numbers of infusions obtained under the progressive-ratio schedule (first 5 sessions; 7.3 ± .7 for females and 7.3 ± 0.5 for males).
Figure 3.
The average number of responses on the active and inactive levers for the first five progressive-ratio sessions under the 5 and 10 µg/kg nicotine dose conditions. Each data point represents an N of 9 females and 6 males under the 5 µg/kg nicotine dose condition and an N of 14 females and 11 males under the 10 µg/kg nicotine dose condition.
Figure 4.
(Top Panel) Data are plotted for each of the 12 females and 9 males for the relationship between serum hormones levels and the number of infusions obtained under the progressive-ratio schedule (E = estradiol; P = progesterone; T = testosterone). An asterisk indicates a significant difference from 0 (P<0.05). (Bottom Panels) Data are plotted for each of the 12 female rats for the relationship between serum concentration of progesterone (Left) and the ratio of estradiol to progesterone (Right) and the number of infusions obtained under the progressive-ratio schedule. Regression lines are also shown for each rat.
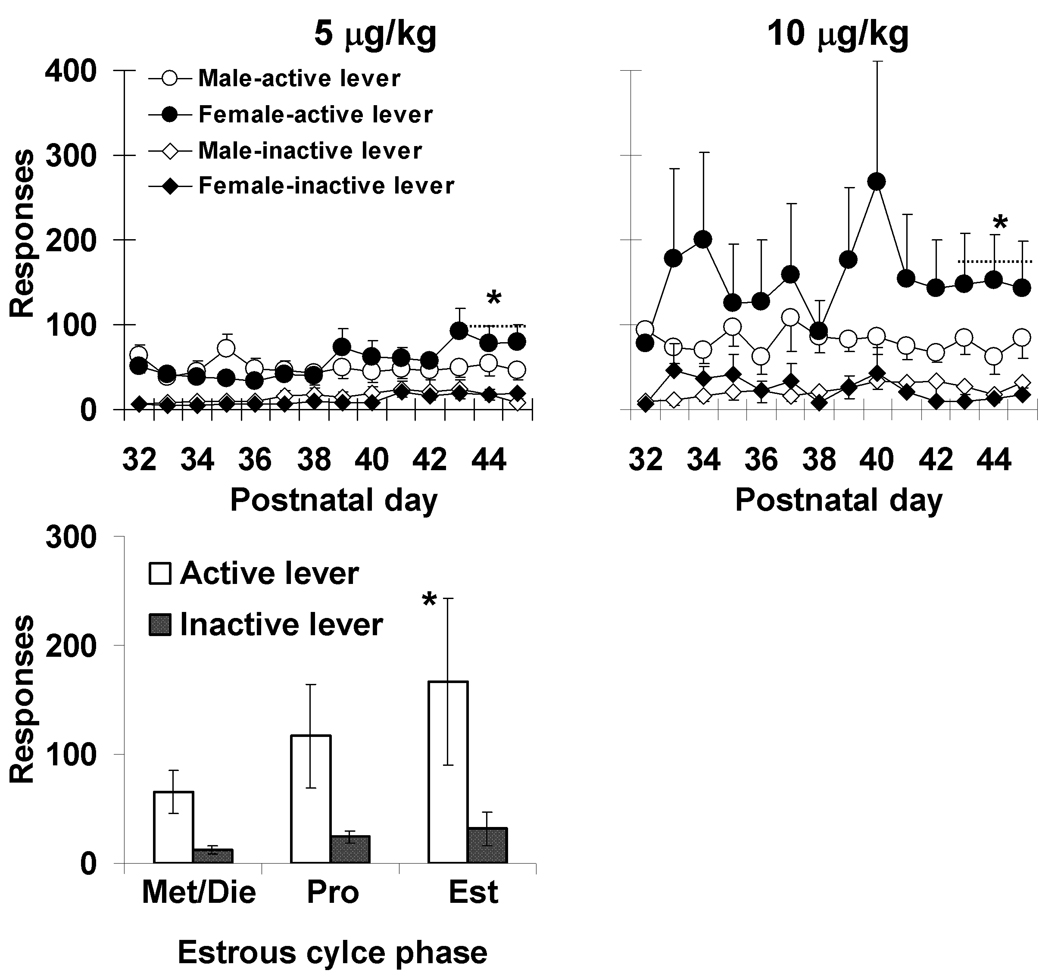
Although males and females did not initially differ on progressive-ratio responding for nicotine, a sex difference became apparent toward the end of the adolescent testing period (Figure 5). A comparison of the average number of infusions obtained by male and female rats during the first 3 progressive ratio sessions (averaged from the first 3 progressive-ratio sessions that occurred during postnatal day 32–37) with those obtained during the last 3 sessions (averaged from postnatal day 43 to 45) revealed a significant interaction of sex by time (F (1,22) = 7.3, p<0.05), but non-significant effects of dose and sex and their interaction (p’s >0.05). Subsequent comparison within each time-point revealed a significant effect of sex the last three sessions (F (1,22) = 8.2, p,0.05), but not during the initial three sessions (p>0.05). Levels of responding under the progressive-ratio schedule also varied with estrous cycle phase (Figure 5, bottom panel; F (1,9) = 22.2, p<0.001) with rats responding at the highest levels during estrus as compared to during other phases of their cycle (estrus versus metestrus/diestrus: t=4.7, df=10, p<0.001; estrus versus proestrus: t=2.2, df=10, p=.05). This effect did not vary systemically with dose. Inactive lever responding did not differ significantly between males and females at any point during the study (p’s>0.05).
Figure 5.
(Top Panels) The average number of active and inactive lever responses observed during the progressive-ratio nicotine self-administration sessions as function of postnatal day across the adolescent testing period under the 5 and 10 µg/kg nicotine dose conditions. The asterisk indicates a significant interaction of sex by time (P<0.05). Each data point represents an N of 7 females and 5 males under the 5 µg/kg nicotine dose condition and an N of 6 females and 8 males under the 10 µg/kg nicotine dose condition. Vertical bars with asterisks represents a significant effect of sex for last three sessions (P<0.05). (Bottom Panel) The average number of active and inactive lever responses observed under the progressive-ratio schedule as function of phase of the estrous cycle (Met/Die = metestrus/diestrus; Pro = proestrus; Est = estrus). The asterisk indicates a significant difference in the number of infusions obtained between estrus and the other 2 phases (P’s≤0.05). Each bar represents an N of 12.
4. Discussion
The goal of this study was to explore sex differences and hormonal influences on acquisition of nicotine self-administration and subsequent motivation for nicotine during adolescence. The results were consistent with our hypotheses that female rats would show an enhanced sensitivity to the reinforcing effects of nicotine and that ovarian hormones would underlie the enhanced sensitivity. Under the low dose condition, a greater percentage of females than males acquired nicotine self-administration. Sex differences in progressive-ratio responding for nicotine, though not initially apparent, emerged at the end of adolescent testing period suggesting that sex differences in motivation for nicotine may result from changes in ovarian hormones. Consistent with this idea, we also observed the highest levels of responding under the progressive-ratio schedule during estrus, a negative association of progesterone and progressive-ratio responding for nicotine, and a positive association of the ratio of estradiol to progesterone and progressive-ratio responding for nicotine. Together these findings suggest that adolescent females are more sensitive than adolescent males to the reinforcing effects of nicotine and that ovarian hormones contribute to this enhanced sensitivity. These differences are unlikely to be due to a general learning difference or to a difference in general activity in that males and females did not differ on rates of acquisition of responding for sucrose reinforcement or on levels of responding on the inactive lever at any point during the study. These differences are also unlikely attributable to a general motivational difference given our previous work under similar experimental conditions showing that adolescent males and females did not differ on progressive-ratio responding for sucrose reward (Lynch 2008).
The differences observed here between adolescent male and female rats are consistent with findings in adult rats (Donny et al. 2000; Rezvani et al. 2008) with evidence suggesting that females are more sensitive to the reinforcing effects of nicotine. These findings of sex differences for nicotine self-administration in adolescent rats are also consistent with previous work with other drugs of abuse, such as cocaine. For example, we recently showed that a greater percentage of adolescent females acquired cocaine self-administration as compared to adolescent males and that they responded at higher levels under a progressive-ratio schedule to obtain cocaine infusions as compared to males (Lynch 2008). However, one interesting difference between the present findings and the previous findings with cocaine is that the sex difference in cocaine self-administration was relatively constant across the adolescent testing period, whereas with nicotine, differences in progressive-ratio responding for nicotine become more pronounced at the end of the adolescent testing period. One possible explanation for this difference is that the roles of progesterone and estradiol in modulating the reinforcing effects of cocaine versus nicotine may be different. This interpretation is in line with our present data with nicotine showing that progesterone and the ratio of estradiol to progesterone, but not estradiol, was associated with progressive-ratio responding for nicotine and our previous work with cocaine showing that estradiol, but not progesterone or the ratio of estradiol to progesterone, was associated with progressive-ratio responding for cocaine. Given that the time-course for changes in estradiol and progesterone are different (e.g., progesterone rapidly increases on the day of vaginal opening and further increases to adult levels over the next several days; whereas, estradiol levels are relatively high at the beginning of adolescence but begin to show more regular fluctuations and stabilize out at adult levels at the end of adolescence; Dohler and Wuttke 1974), it is not surprising that the behavioral changes are also different. Taken together, these results suggests that in females nicotine’s reinforcing effects may be altered by changes in circulating levels of hormones that occur during adolescence, particularly with regard to progesterone.
Interestingly, a sex difference was observed under the acquisition paradigm but not during initial sessions under the progressive-ratio schedule. One explanation is that the acquisition paradigm may be more sensitive than the progressive-ratio schedule for detecting individual differences in sensitivity to the reinforcing effects of nicotine. Also, since the question addressed with each paradigm is slightly different, they may be measuring different aspects of nicotine’s reinforcing effects. Specifically, with the acquisition paradigm the question addressed is “which animals can detect the reinforcing effects of this low drug dose?” and with the progressive ratio schedule the question addressed is “which animals are more motivated to obtain drug infusions”. Another possible explanation is that sex differences in nicotine metabolism may have been relevant during initial exposure, but not following repeated exposure. Evidence to support this idea is provided by results showing that although females initially have higher brain levels of nicotine following similar g/kg doses of nicotine, over time or following chronic exposure, this sex difference disappears (Rosecrans 1972; Rosecrans and Schechter 1972; also see Donny et al. 2000). The sex difference that emerged during the later progressive ratio sessions, however, cannot be readily explained by differences in nicotine metabolism. More research is needed to examine sex differences in nicotine metabolism during adolescence.
The findings showing that progressive-ratio responding for nicotine varied with estrous cycle phase and as a function of estradiol and progesterone levels suggest that ovarian hormones are critical for modulating nicotine’s reinforcing effects in females. These findings are consistent with previous work in humans (Sofuoglu et al. 2001; DeBon et al. 1995; Mello et al. 1987; Snively et al. 2000), and with other psychostimulant drugs (Roberts et al. 1989). They are also consistent with neurochemical studies showing that dopaminergic signaling in the striatum which is believed to underlie nicotine’s reinforcing effects, is enhanced by estradiol but inhibited by progesterone (Feng et al. 2004; Nuwayhid and Werling 2003; Becker 1999). However, these findings are contrary to a previous study with nicotine self-administration in adult female rats showing that progressive-ratio responding did not vary with estrous cycle phase (Donny et al. 2000). In the previous study, the authors cautioned that the negative result may have been attributable to heterogeniety within each cycle in that the data were averaged over time for each of the cycle phases. In the present study, because so few regular cycles were observed, we examined the first full estrous cycle, and in doing so, we may have minimized the variability with each rat. Another possible explanation for the divergent results between the present study and the previous one is that the role ovarian hormones may be particularly apparent during adolescence as compared to during adulthood although the present data showing that sex differences in progressive-ratio responding for nicotine emerge at the end of adolescent testing period would argue against this possibility. It is also important to note that the doses tested, stimulus cues that were associated with nicotine, as well as the session length used in this study, were different from those used in the previous study, and such differences may account for the divergent results.
The present findings indicate that testosterone does not predict nicotine self-administration in males. This finding is somewhat surprising given that several previous studies in humans have found a positive association of testosterone and cigarette smoking in both adolescents and adults (Martin et al. 2001; Bauman et al. 1992; Dai et al. 1988). Previous work with rats has also shown that chronic nicotine administration is associated with lower testosterone levels (Kavitharaj et al. 1999). These results are, however, consistent with previous work with other drugs of abuse, such as cocaine where studies consistently demonstrate no relationship between testosterone levels and self-administration (Lynch 2008; Caine et al. 2004, Hu et al. 2004).
Based on our previous experience with nicotine self-administration in adults (Lynch and Carroll and unpublished results) and based in previous findings from others (e.g., Lanza et al. 2004) showing that acquisition of nicotine self-administration can be a lengthy process, a lever press training protocol was used in order to ensure that the behavioral data on acquisition and subsequent maintenance responding would be obtained within the narrow window of time that we had to study adolescence. A limitation of this lever-press training protocol, however, is that it may have differentially affected subsequent responding in males versus females. We attempted to minimize the likelihood that this would be a problem by using a short pre-training period and food satiated rats, and under these conditions we did show that males and females did not differ on the acquisition of lever-press responding. That said, however, we still cannot rule out the possibility that a sex difference in extinction of sucrose-reinforcement may have contributed to our findings, particularly for acquisition of nicotine self-administration. Individual housing may also have impacted our findings given that social isolation is a stressor that is known to impact sensitivity to the reinforcing effects of drugs of abuse (e.g., Kosten et al. 2000) with evidence indicating sex-dependent differences (e.g., Kosten et al. 2004). However, because the previous work with regard to sex differences is on the effects of neonatal isolation, it is not clear whether social isolation during adolescence underlies the current findings. It is also important to note although some studies have found that early life stress has a greater impact on drug-related behavior in females than males (e.g., Kosten et al. 2004), others have shown that the effects are equal (Weiss et al. 2001), or even greater in males compared to females (Lynch et al. 2005; McCormick and colleagues 2004; 2005). Another limitation with the present study is that because the animals gained weight so rapidly within the adolescent testing period, catheter patency was an issue which impacted the number of animals that maintained patency throughout the study and prevented an examination of changes in nicotine self-administration during the transition from late adolescence to adulthood. Further research is necessary to resolve these issues.
In summary, these findings indicate that adolescent female rats are more sensitive to the reinforcing effects of nicotine with evidence to suggest that circulating progesterone and estradiol levels, at least in part, mediate the enhanced sensitivity in females. These data also suggest that motivation for nicotine in females changes over the adolescent period possibility as a result of the rapid change in circulating hormones that occurs during this important hormone transition phase. More research is needed to determine whether hormonal changes during adolescence, particularly during the transition from late adolescence to adulthood, coincide with changes in sensitivity to nicotine’s reinforcing effects.
Acknowledgments
We would like to thank Florence Breslin for her excellent technical assistance. This work was supported by the Virginia Youth Tobacco Project Small Grants Program and by the University of Virginia. The author has no financial relationships to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, Piazza PV. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J Neurosci. 2003;23(11):4712–4716. doi: 10.1523/JNEUROSCI.23-11-04712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SS, Allen AM, Pomerleau CS. Influence of phase-related variability in premenstrual symptomatology, mood, smoking withdrawal and smoking behavior during ad libitum smoking, on smoking cessation outcome. Addict Behav. 2008a doi: 10.1016/j.addbeh.2008.08.009. print copy in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SS, Bade T, Center B, Finstad D, Hatsukami D. Menstrual phase effects on smoking relapse. Addiction. 2008b;103(5):809–821. doi: 10.1111/j.1360-0443.2008.02146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SS, Hatsukami DK, Christianson D, Nelson D. Withdrawal and pre-menstrual symptomatology during the menstrual cycle in short-term smoking abstinence: effects of menstrual cycle on smoking abstinence. Nicotine Tob Res. 1999;1(2):129–142. doi: 10.1080/14622299050011241. [DOI] [PubMed] [Google Scholar]
- Allen SS, Hatsukami D, Christianson D, Nelson D. Symptomatology and energy intake during the menstrual cycle in smoking women. J Subst Abuse. 1996;8(3):303–319. doi: 10.1016/s0899-3289(96)90170-4. [DOI] [PubMed] [Google Scholar]
- Arnold JM, Roberts DC. A critique of fixed and progressive-ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57(3):441–447. doi: 10.1016/s0091-3057(96)00445-5. (1997) [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29(1):36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman KE, Foshee VA, Haley NJ. The interaction of sociological and biological factors in adolescent cigarette smoking. Addictive Behaviors. 1992;17:459–467. doi: 10.1016/0306-4603(92)90006-h. [DOI] [PubMed] [Google Scholar]
- Caine SB, Bowen CA, Yu G, Zuzga D, Negus SS, Mello NK. Effect of gonadectomy and gonadal hormone replacement on cocaine self-administration in female and male rats. Neuropsychopharmacology. 2004;29(5):929–942. doi: 10.1038/sj.npp.1300387. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Carroll ME. Acquisition of drug self-administration: environmental and pharmacological interventions. Exp Clin Psychopharmacol. 2000;8(3):312–325. doi: 10.1037//1064-1297.8.3.312. [DOI] [PubMed] [Google Scholar]
- Carpenter MJ, Upadhyaya HP, LaRowe SD, Saladin ME, Brady KT. Menstrual cycle phase effects on nicotine withdrawal and cigarette craving: a review. Nicotine Tob Res. 2006;8(5):627–638. doi: 10.1080/14622200600910793. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, Cosgrove KP. Sex and estrogen influence drug abuse. Trends Pharmacol Sci. 2004;25(5):273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology. 2002;161(3):304–313. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Lac ST. Acquisition of i.v. amphetamine and cocaine self-administration in rats as a function of dose. Psychopharmacology. 1997;129(3):206–214. doi: 10.1007/s002130050182. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Allen SS, Sved AF, Perkins KA. Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats. Psychopharmacology. 2005;180(2):258–266. doi: 10.1007/s00213-005-2152-3. [DOI] [PubMed] [Google Scholar]
- Chen H, Matta SG, Sharp BM. Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacology. 2007;32(3):700–709. doi: 10.1038/sj.npp.1301135. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology. 1989;99(4):473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Dai WS, Gutai JP, Kuller LH, Cauley JA. Cigarette smoking and serum sex hormones in men. Am J Epidemiol. 1988;128(4):796–805. doi: 10.1093/oxfordjournals.aje.a115033. [DOI] [PubMed] [Google Scholar]
- DeBon M, Klesges RC, Klesges LM. Symptomatology across the menstrual cycle in smoking and nonsmoking women. Addict Behav. 1995;20(3):335–343. doi: 10.1016/0306-4603(94)00070-f. [DOI] [PubMed] [Google Scholar]
- Donny EC, Lanza ST, Balster RL, Collins LM, Caggiula A, Rowell PP. Using growth models to relate acquisition of nicotine self-administration to break point and nicotinic receptor binding. Drug Alcohol Depend. 2004;75(1):23–35. doi: 10.1016/j.drugalcdep.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, Mielke MM, Hoffman A, McCallum S. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology. 2000;151(4):392–440. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- Döhler KD, Wuttke W, Serum LH. FSH, prolactin and progesterone from birth to puberty in female and male rats. Endocrinology. 1974;94(4):1003–1008. doi: 10.1210/endo-94-4-1003. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend. 2007;89(2–3):183–189. doi: 10.1016/j.drugalcdep.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng XQ, Dong Y, Fu YM, Zhu YH, Sun JL, Wang Z, Sun FY, Zheng P. Progesterone inhibition of dopamine-induced increase in frequency of spontaneous excitatory postsynaptic currents in rat prelimbic cortical neurons. Neuropharmacology. 2004;46(2):211–222. doi: 10.1016/j.neuropharm.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Napier K, Ehrman R, Gariti P, O'Brien CP, Childress AR. Retrospective study: influence of menstrual cycle on cue-induced cigarette craving. Nicotine Tob Res. 2004;6(1):171–175. doi: 10.1080/14622200310001656984. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Ehrman R, Lynch KG, Harper D, Sciortino N, O'Brien CP, Childress AR. Menstrual cycle phase at quit date predicts smoking status in an NRT treatment trial: a retrospective analysis. J Womens Health. 2008;17(2):287–292. doi: 10.1089/jwh.2007.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Maccari S, Le Moal M, Simon H, Piazza PV. Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res. 1995;698(1–2):46–52. doi: 10.1016/0006-8993(95)00788-r. [DOI] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29(1):81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31(1):129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Kavitharaj NK, Vijayammal PL. Nicotine administration induced changes in the gonadal functions in male rats. Pharmacology. 1999;58:2–7. doi: 10.1159/000028262. [DOI] [PubMed] [Google Scholar]
- Klein LC, Stine MM, Vandenbergh DJ, Whetzel CA, Kamens HM. Sex differences in voluntary oral nicotine consumption by adolescent mice: a dose-response experiment. Pharmacol Biochem Behav. 2004;78(1):13–25. doi: 10.1016/j.pbb.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ, Kehoe P. Enhanced acquisition of cocaine self-administration in adult rats with neonatal isolation stress experience. Brain Res. 2000;875(1–2):44–50. doi: 10.1016/s0006-8993(00)02595-6. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Sanchez H, Zhang XY, Kehoe P. Neonatal isolation enhances acquisition of cocaine self-administration and food responding in female rats. Behav Brain Res. 2004;151(1–2):137–149. doi: 10.1016/j.bbr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Lanza ST, Donny EC, Collins LM, Balster RL. Analyzing the acquisition of drug self-administration using growth curve models. Drug Alcohol Depend. 2004;75(1):11–21. doi: 10.1016/j.drugalcdep.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH, Montoya D, Rose JE, Swartzwelder HS. Adolescent-onset nicotine self-administration modeled in female rats. Psychopharmacology. 2003;169(2):141–149. doi: 10.1007/s00213-003-1486-y. [DOI] [PubMed] [Google Scholar]
- Levin ED, Lawrence SS, Petro A, Horton K, Rezvani AH, Seidler FJ, Slotkin TA. Adolescent vs. adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicol Teratol. 2007;29(4):458–465. doi: 10.1016/j.ntt.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology. 2008;197(2):237–246. doi: 10.1007/s00213-007-1028-0. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Sex differences in vulnerability to drug self-administration. Exp Clin Psychopharmacol. 2006;14(1):34–41. doi: 10.1037/1064-1297.14.1.34. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Taylor JR. Sex differences in the behavioral effects of 24-h/day access to cocaine under a discrete trial procedure. Neuropsychopharmacology. 2004;29(5):943–951. doi: 10.1038/sj.npp.1300389. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164(2):121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology. 1999;144(1):77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Arizzi MN, Carroll ME. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology. 2000;152(2):132–139. doi: 10.1007/s002130000488. [DOI] [PubMed] [Google Scholar]
- Marks JL, Pomerleau CS, Pomerleau OF. Effects of menstrual phase on reactivity to nicotine. Addict Behav. 1999;24(1):127–134. doi: 10.1016/s0306-4603(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Martin CA, Logan TK, Portis C, Leukefeld CG, Lynam D, Staton M, Brogli B, Flory K, Clayton RR. The association of testosterone with nicotine use in young adult females. Addict Behav. 2001;26(2):279–283. doi: 10.1016/s0306-4603(00)00094-0. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Robarts D, Kopeikina K, Kelsey JE. Long-lasting, sex- and age-specific effects of social stressors on corticosterone responses to restraint and on locomotor responses to psychostimulants in rats. Horm Behav. 2005;48(1):64–74. doi: 10.1016/j.yhbeh.2005.01.008. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Robarts D, Gleason E, Kelsey JE. Stress during adolescence enhances locomotor sensitization to nicotine in adulthood in female, but not male, rats. Horm Behav. 2004;46(4):458–466. doi: 10.1016/j.yhbeh.2004.05.004. [DOI] [PubMed] [Google Scholar]
- McQuown SC, Belluzzi JD, Leslie FM. Low dose nicotine treatment during early adolescence increases subsequent cocaine reward. Neurotoxicol Teratol. 2007;29(1):66–73. doi: 10.1016/j.ntt.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Palmieri SL. Cigarette smoking by women: interactions with alcohol use. Psychopharmacology. 1987;93(1):8–15. doi: 10.1007/BF02439579. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Sholar MB, Mutschler NH, Jaszyna-Gasior M, Goletiani NV, Siegel AJ, Mello NK. Effects of intravenous cocaine and cigarette smoking on luteinizing hormone, testosterone, and prolactin in men. J Pharmacol Exp Ther. 2003;307(1):339–348. doi: 10.1124/jpet.103.052928. [DOI] [PubMed] [Google Scholar]
- Nuwayhid SJ, Werling LL. Steroids modulate N-methyl-D-aspartate-stimulated [3H] dopamine release from rat striatum via sigma receptors. J Pharmacol Exp Ther. 2003;306(3):934–940. doi: 10.1124/jpet.103.052324. [DOI] [PubMed] [Google Scholar]
- O'Hara P, Portser SA, Anderson BP. The influence of menstrual cycle changes on the tobacco withdrawal syndrome in women. Addict Behav. 1989;14(6):595–600. doi: 10.1016/0306-4603(89)90001-4. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Andrews WW, Advis JP, White SS. Recent advances in the endocrinology of puberty. Endocr Rev. 1980;1(3):228–257. doi: 10.1210/edrv-1-3-228. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Smoking cessation in women. Special considerations. CNS Drugs. 2001;15(5):391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Levine M, Marcus M, Shiffman S, D'Amico D, Miller A, Keins A, Ashcom J, Broge M. Tobacco withdrawal in women and menstrual cycle phase. J Consult Clin Psychol. 2000;68(1):176–180. doi: 10.1037/0022-006X.68.1.176. [DOI] [PubMed] [Google Scholar]
- Perry JL, Anderson MM, Nelson SE, Carroll ME. Acquisition of i.v. cocaine self-administration in adolescent and adult male rats selectively bred for high and low saccharin intake. Physiol Behav. 2007;91(1):126–133. doi: 10.1016/j.physbeh.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau CS, Cole PA, Lumley MA, Marks JL, Pomerleau OF. Effects of menstrual phase on nicotine, alcohol, and caffeine intake in smokers. J Subst Abuse. 1994;6(2):227–234. doi: 10.1016/s0899-3289(94)90253-4. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Mehringer AM, Marks JL, Downey KK, Pomerleau OF. Effects of menstrual phase and smoking abstinence in smokers with and without a history of major depressive disorder. Addict Behav. 2000;25(4):483–497. doi: 10.1016/s0306-4603(99)00075-1. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Eddins D, Slade S, Hampton DS, Christopher NC, Petro A, Horton K, Johnson M, Levin ED. Neonatal 6-hydroxydopamine lesions of the frontal cortex in rats: persisting effects on locomotor activity, learning and nicotine self-administration. Neuroscience. 2008;154(3):885–897. doi: 10.1016/j.neuroscience.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive-ratio schedule in rats. Psychopharmacology. 1989;98(3):408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Rosecrans JA. Brain area nicotine levels in male and female rats with different levels of spontaneous activity. Neuropharmacology. 1972;11(6):863–870. doi: 10.1016/0028-3908(72)90045-7. [DOI] [PubMed] [Google Scholar]
- Rosecrans JA, Schechter MD. Brain area nicotine levels in male and female rats of two strains. Arch Int Pharmacodyn Ther. 1972;196(1):46–54. [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive-ratio schedule in rats. Psychopharmacology. 2004;172(4):443–449. doi: 10.1007/s00213-003-1670-0. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Li Z, Lê AD. Age differences in the spontaneous acquisition of nicotine self-administration in male Wistar and Long-Evans rats. Psychopharmacology. 2008a;197(1):45–58. doi: 10.1007/s00213-007-1003-9. [DOI] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Lê AD. Nicotine self-administration, extinction responding and reinstatement in adolescent and adult male rats: evidence against a biological vulnerability to nicotine addiction during adolescence. Neuropsychopharmacology. 2008b;33(4):739–748. doi: 10.1038/sj.npp.1301454. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26(3–4):163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Södersten P. Receptive behavior in developing female rats. Horm Behav. 1975;6(4):307–317. doi: 10.1016/0018-506x(75)90001-x. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: Age-dependent behavior and psychopharmacological responsivity in rats. Devel. Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Snively TA, Ahijevych KL, Bernhard LA, Wewers ME. Smoking behavior, dysphoric states and the menstrual cycle: results from single smoking sessions and the natural environment. Psychoneuroendocrinology. 2000;25(7):677–691. doi: 10.1016/s0306-4530(00)00018-4. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Babb DA, Hatsukami DK. Progesterone treatment during the early follicular phase of the menstrual cycle: effects on smoking behavior in women. Pharmacol Biochem Behav. 2001;69(1–2):299–304. doi: 10.1016/s0091-3057(01)00527-5. [DOI] [PubMed] [Google Scholar]
- Storr CL. Characteristics associated with rapid transition to tobacco dependence in youth. Nicotine Tob Res. 2008;10(6):1099–1104. doi: 10.1080/14622200802087556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of Applied Studies. Rockville, MD: NHSDA Series H-21, DHHS; 2007. Substance Abuse and Mental Health Services Administration Overview of Findings from the 2006 National Survey on Drug Use and Health. Publication No SMA 03-3774. [Google Scholar]
- Tanski SE, Prokhorov AV, Klein JD. Youth and tobacco. Minerva Pediatr. 2004;56(6):553–565. [PubMed] [Google Scholar]
- Weiss IC, Domeney AM, Heidbreder CA, Moreau JL, Feldon J. Early social isolation, but not maternal separation, affects behavioral sensitization to amphetamine in male and female adult rats. Pharmacol Biochem Behav. 2001;70(2–3):397–409. doi: 10.1016/s0091-3057(01)00626-8. [DOI] [PubMed] [Google Scholar]