Abstract
Cell-based therapy for cancer is a promising new field. Among cell types that can be used for this purpose, mesenchymal stem cells (MSC) appear to hold great advantage for reasons including easier propagation in culture, possible genetic modification to express therapeutic proteins and preferential homing to sites of cancer growth upon in vivo transfer. The present study evaluated the potential of genetically modified MSC, constitutively expressing interferon (IFN)-β, in an immuno-competent mouse model of prostate cancer lung metastasis. A recombinant adeno-associated virus (rAAV) encoding mouse IFN-β was constructed and initially tested in vitro for high-level expression and bioactivity of the transgenic protein. MSC were transduced by the rAAV-IFN-β or GFP ex vivo and used as cellular vehicles to target lung metastasis of TRAMP-C2 prostate cancer cells in a therapy model. Cohorts of mice were sacrificed on days 30 and 75 to determine the effect of therapy by measurement of tumor volume, histology, immunohistochemistry, ELISA, and flow cytometry. Results indicated a significant reduction in the tumor volume in lungs following IFN-β expressing MSC therapy. Immunohistochemistry of the lung demonstrated increased tumor cell apoptosis and decreased tumor cell proliferation and blood vessel counts. A significant increase in the natural kill cell activity was observed following IFN-β therapy correlating the anti-tumor effect. Systemic level of IFN-β was not significantly elevated from this targeted cell therapy. These data demonstrate the potential of MSC-based IFN-β therapy for prostate cancer lung metastasis.
Keywords: Cancer gene therapy, Mesenchymal stem cells (MSC), recombinant adeno-associated virus, interferon beta, prostate cancer, lung metastasis
INTRODUCTION
Prostate cancer is the most commonly diagnosed cancer in adult males in the Western world and the second leading malignancy next to lung cancer.1 Improvements in early diagnosis have identified more patients with clinically localized disease.2 Local therapy such as radical prostatectomy improves the overall survival; however, most deaths from prostate cancer are due to metastases that are resistant to conventional therapies.3 Therefore, more effective therapies that can not only eradicate localized tumors but also prevent their metastasis are needed. One possible therapeutic molecule for prostate cancer is interferon-β, which is a member of the type I IFN family.4 IFN-β suppresses tumor cell growth by induction of differentiation, S-phase accumulation, and apoptosis.4-7 High concentrations of IFN-β are known to inhibit malignant cell growth in vitro.4,8 However, the therapeutic utility of IFN-β in vivo is limited by its excessive toxicity when administered systemically at high doses.9,10 Systemic IFN therapy for most solid tumors also has shown limited response since the short half-life of IFN-β protein could not reach the required concentrations to suppress tumor cell growth, down-regulate angiogenesis, and enhance host immune effector function.4,11 Thus, cell-based therapies are emerging as useful alternatives for interferon therapy for metastatic prostate disease.
Mesenchymal stem cells (MSC) are attractive cell therapy vehicles for the delivery of agents to tumor cells. Previous studies provide evidence supporting the rationale for genetically modified MSC to deliver therapeutic proteins directly into the tumor microenvironment to produce high concentrations of anti-tumor proteins directly within the tumor mass, which have been shown to blunt tumor growth kinetics in experimental animal models.12-16
The present study describes the potential of genetically modified MSC, constitutively expressing IFN-β in reducing tumor growth in a therapy model of prostate cancer lung metastasis. Targeted homing of MSC producing IFN-β, at tumor sites in the lungs was found to mediate anti-tumor effects by multiple mechanisms including induction of tumor apoptosis, anti-angiogenesis and by increasing natural killer (NK) cell activity. These results demonstrate the potential of cell-based IFN-β therapy for prostate cancer lung metastasis.
RESULTS
High-level expression and bioactivity of IFN-β produced by rAAV
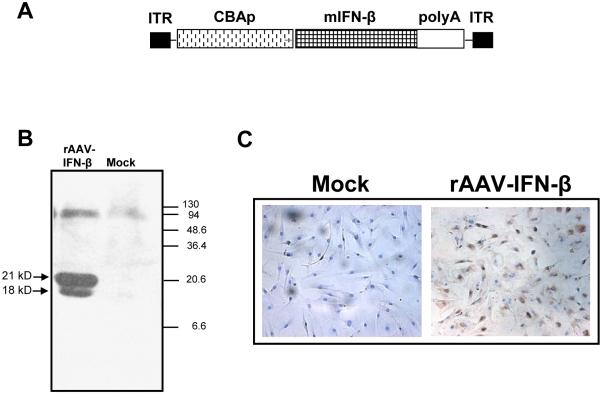
The open reading frame of murine IFN-β was subcloned in a rAAV vector under the CMV/chicken-β actin promoter (Fig 1A). High-level expression of the transgenic protein was confirmed by western blot analysis. 293 cells were mock-transduced or transduced with rAAV-encoding IFN-β. Forty-eighty hours later, supernatants from transduced cells were analyzed by western blot using an antibody for interferon-β. Results shown in Figure 1B indicting two bands of interferon-β with molecular weights 18 kD and 21 kD confirmed the presence of unglycosylated and glycosylated forms of the transgenic protein.
Figure 1. Recombinant-AAV 6 expressing murine IFN-β.
An rAAV encoding human IFN-β under the control of CMV-chicken beta actin promoter was constructed using pSub201 vector (A). Expression of IFN-β was confirmed by transducing the vector in 293 cells and analyzing the culture supernatant by Western blot using an IFN-β antibody (B). Transduction efficiency of mouse MSC by rAAV- IFN-β was determined by in situ staining of the fixed cells using a rat, anti-mouse IFN-β antibody (C).
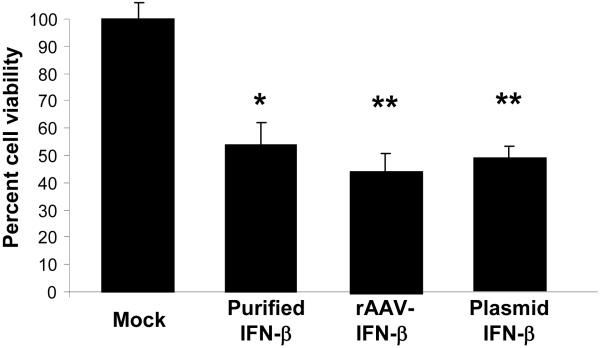
To determine the transduction efficiency of rAAV-IFN-β in mouse MSC, the cells were either mock-transduced or transduced with rAAV6-IFN-β at 1000 MOI. One week later, the cells were harvested, fixed on slides and immunohistochmistry were performed using a mouse interferon-β antibody. Result, indicating efficient transduction of mouse MSC by rAAV-IFN-β and expression of the transgenic protein is shown in Figure 1C. Interferon-β concentration was determined by ELISA and found to be 120 pg/ml, which amounts to approximately 8×10-4 pg/cell. To determine the bioactivity of rAAV produced IFN-β, an MTT assay was performed using TRAMP-C2 cells in vitro. Results of the proliferation assay indicated inhibition of TRAMP-C2 cell growth by 44% (P<0.004), following the addition of supernatant from rAAV6-IFN β transduced MSC containing approximately 4 ng of the vector produced cytokine. The inhibition observed was comparable to that using recombinant interferon-β protein (Figure 2).
Figure 2. Effects of rAAV produced IFN-β on cell proliferation.
The bioactivity of rAAV produced IFN-β was determined in vitro in TRAMP-C2 cells by incubating the cells in conditioned media obtained from MSC cultures that were either mock-transduced or transduced with rAAV encoding IFN-β or transfected with a plasmid vector encoding IFN-β. (*p<0.004 compared to mock).
Systemic application of MSC AAV-IFN-β reduced the growth of TRAMP-C2 prostate cancer cells in the lung
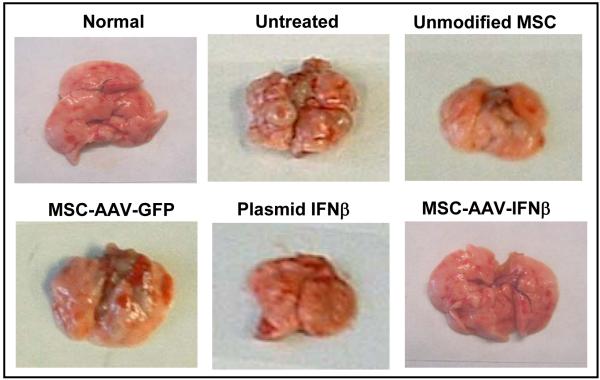
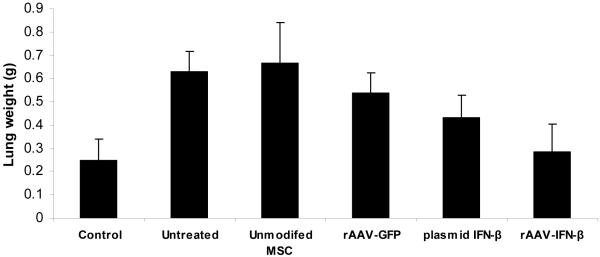
Cohorts of C57BL/6 mice were injected with 5×105 TRAMP-C2 cells via tail vain. Ten days later, mice were given no treatment or MSC (2×105) that were transduced with rAAV-IFN-β or rAAV-GFP. Cohorts of mice from each group were sacrificed at day-30 to determine the effect of therapy and remaining mice were sacrificed after 75 days. The number and size of tumor nodules in the lungs of untreated mice and that given MSC transduced with rAAV-GFP were significantly higher than in mice treated with MSC transduced with rAAV-IFN-β (p<0.0056 and p<0.0035 compared to untreated and rAAV-GFP treated groups respectively; Fig. 3A).
Figure 3. In vivo studies for anti-tumor activity.
Cohorts of C57BL/6 mice were injected with 5×105 TRAMP-C2 cells by tail vain. Ten days later, mice were given no treatment or MSC (2×105) that was transduced with rAAV-IFN-β or GFP. Cohorts of mice from each group (n=10) were sacrificed at day 30 to determine the effect of therapy remaining mice were sacrificed on day 75. Images represent lungs from indicated treatment group on day 30 (A) and mean lung volume on day 75 (B).
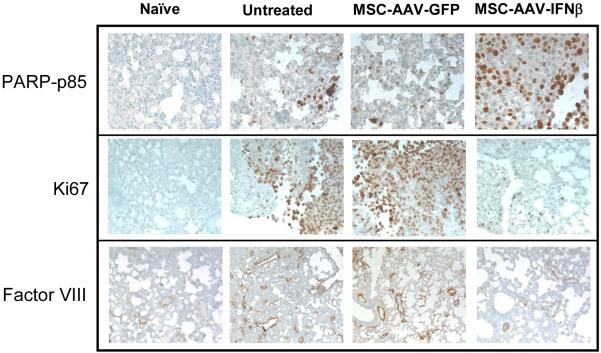
Results of immunohistochemistry indicated increased apoptosis of tumor cells in the lungs of mice treated with MSC, transduced with AAV-IFN-β compared to untreated and AAV-GFP treated groups. Staining with Factor-VIII related antigen for blood vessels indicated significantly higher blood vessel counts in untreated and MSC-AAV-GFP group compared to the MSC-AAV-IFN-β group. Similarly, the number of proliferating tumor cells was found to be significantly higher in naïve and AAV-GFP groups compared to the AAV-IFN-β group (Figure 4A).
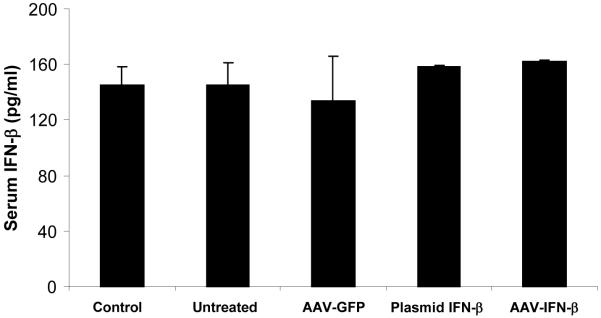
Figure 4. Immunochemistry of lungs with TRAMP-C2 cells for apoptosis, cell proliferation and neovasculature and systemic IFN-β level following therapy.
For immunohistochemical analysis of tumor cell proliferation, apoptosis and microvessel density, lungs were paraffin-embedded and sectioned. Staining was performed with anti-PARP-p85 fragment polyclonal antibody for apoptosis, anti-Ki67 antibody for proliferation, and Factor-VIII antibody for endothelial cells. Slides were slightly counterstained with haematoxylin (A). An ELISA was performed with serum samples from mice in different groups, obtained on day-20 following MSC administration (B).
rAAV-IFN-β transduced MSC show superior anti-tumor effect compared to IFN-β plasmid transfected MSC
We also tested in parallel, in vitro and in vivo efficiency of MSC transfected with an expression plasmid. Results of the cell proliferation assay by MTT method showed comparable growth inhibition to that of rAAV-IFN-β and purified protein (Figure 2). However, in vivo studies with IFN-β plasmid transfected MSC demonstrated more lung metastasis compared to the rAAV-IFN-β group, and more blood vessel growth by immunohistochemistry. The treatment efficacy of IFN-β plasmid transfected MSC was not significant compared to the naïve group (P<0.3) (Figure 3B).
Serum IFN-β levels
Serum samples were collected at different time points following MSC therapy (on days 10, 20, 30, 40 and 50) and used for measuring IFN-β levels by ELISA. Results showed no significant difference in systemic IFN-β level after MSC injection in different groups at different time points. Representative data on serum IFN-β levels on day-20 is shown in Figure 4B (p>0.05).
MSC homing to tumors in lungs and expression of IFN-β
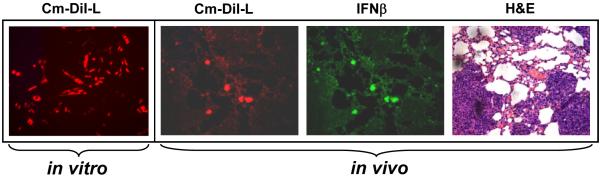
In order to track the homing of transplanted MSC to tumor cells in the lung metastasis model, MSC were labeled with the cell tracker dye CM-DiI prior to in vivo administration (Figure 5). Twenty days after the administration of CM-DiI labeled MSC-IFN-β in mice, the tissues were harvested and paraffin sections were made. Microscopic analysis of lung section provided evidence for homing of MSC to the tumor site in the lungs as shown in Figure 5. Expression of IFN-β in MSC that homed to lungs was also confirmed by staining with an IFN-β primary antibody, followed by incubation with Alexa-Fluor-488 conjugated secondary antibody (green).
Figure 5. Immunofluorescence analysis of MSC homing to tumors in the lung, and expression of IFN-β.
MSC were labeled with the cell tracker dye CM-DiI (Red) prior to in vivo administration. C57BL/6 mice bearing TRAMP-C2 tumors in the lungs were injected with 2×105MSC transduced with rAAV-IFN-β and labeled with CM-DiI. Twenty days later, mice were sacrificed and lungs harvested, and paraffin sections made. The sections were stained with IFN-β antibody (green). Tumor-homed MSC were identified from CM-DiI staining (Red). H&E staining indicates areas of the tumor in the lungs.
IFN-β expressing MSC therapy was found to increase NK cell activity
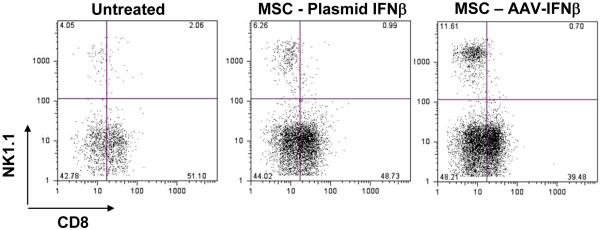
Since the present study was performed in immunocompetent mice, and based on the role of IFN-β in activating immune effectors, we determined the number of CD8+ T cells and NK cells, 20 days after initiation of the therapy. Mononuclear cells (MNC) from the lung and spleen were isolated and stained with anti-CD8 and anti-NK1.1 antibodies and analyzed by flow cytometry. About 12% of lung MNC were NK cells in MSC-IFN-β treated mice, whereas in untreated and AAV-GFP treated mice there was 4% of NK cells in the lung. There was no significant difference in CD8+ T cell population between the groups. Mice that were treated with MSC-IFN-β plasmid exhibited only a slight increase in NK cells in the lung, which was not statistically significant (Figure 6).
Figure 6. Determination of immune effectors in the lung following IFN-β therapy.
MSC, transduced with rAAV-IFN-β or transfected with a plasmid encoding IFN-β were injected intravenously into C57BL/6 mice with lung metastases of TRAM-C2 cells. Twenty days later, mononuclear cells from the lung were isolated and stained with anti-CD8 and anti-NK1.1 antibodies and analyzed by flow cytometry.
DISCUSSION
The utility of many biological agents as recombinant proteins for the treatment of metastatic prostate disease is often limited by their short half-life or excessive toxicity. Therefore, more effective therapies that can provide sustained anti-tumor effects on metastatic tumors can increase patient survival. Studies have shown that MSC, engrafted in tumors, could potentially serve as delivery vehicles of anticancer agents to malignant cells.13,16 The rationale for using bone marrow-derived stem cells for delivering therapies to tumors is based on the concept that bone marrow is a source of circulating stem cells that are recruited from the blood into peripheral solid organs in times of tissue stress or injury.17-20 Because the microenvironment of solid tumor is similar to the environment of injured tissue,21,22 it is believed that that solid tumors may provide a permissive environment for the engraftment of exogenously given MSC.13 Thus, tumor homing property of MSC provides an attractive opportunity for targeted delivery of transgenes into the sites of tumor growth.
Mesenchymal stem cells are attractive as a cellular vehicle for the expression of therapeutic proteins by gene transfer because of several advantages such as their ease in harvesting, isolation and expansion, transduciblity with viral vectors, systemic or local delivery and refractory to host immune responses.23,24 Recent studies have reported successful engraftment of ex vivo transduced MSC at the site of lung metastasis of breast cancer, gliomas, colorectal tumors, and ovarian tumors in animal models.25-28 MSC are capable of long-term transgene expression.29 Genetic modification of MSC has been used to overcome the problem of limited viability and engraftment of the transplanted cells. Indeed, genetic modification can improve survival, metabolic characteristics, proliferative capacity or differentiation of MSC. In addition, MSC can be engineered to serve as vehicles for gene therapy in which secreted proteins can exert paracrine or endocrine actions that may augment therapeutic benefits. A recent study has shown that MSC of various origins can also stimulate the growth of tumor cells Karnoub et al.30 Hence, it is important to obtain high-efficiency transduction MSC and expression of transgenic protein with anti-tumor property for therapeutic application. We have recently demonstrated high-efficiency transduction of MSC by rAAV.31
IFN-β is a potent anti-angiogenic and anti-metastatic molecule. It not only inhibits growth and migration of endothelial cells, but also down-regulates expression of molecules for angiogenesis and invasion.32, 33 Prostate cancer cells and several other types of tumor cells are more sensitive to IFN-β than IFN-α.34-36 Studies have shown that in vitro modified MSC can deliver IFN-β into the tumor microenvironment and IFN-β exerts local paracrine effect after it is delivered into tumors.
The present study demonstrates that intravenous injection of MSC, genetically modified to produce IFN-β using a rAAV, into mice with established TRAMP-C2 pulmonary metastases suppressed the tumor growth. Despite the anti-tumor effects observed, there was an absence of higher systemic levels of the transgenic protein suggesting the local effects of MSC-produced IFN-β in the tumor microenvironment. This, in fact, is advantageous because it may reduce undesired side effects such as cell toxicity encountered in IFN-β protein therapy. A combination of mechanisms including tumor apoptosis, anti-angiogenesis and induction of NK cell activity was found to effect the anti-tumor activity of this pleiotrophic cytokine. IFN-β is known to cause anti-angiogenic effect by blocking endothelial cell migration and downregulation of FGF gene expression.33,37 The anti-tumor effects of IFNs through immune stimulation is known to be mediated by induction of MHC class 1 expression, increasing the activity of cytolytic T cells, macrophages and NK cells.36,37 Although there was a significant increase in the NK cell population in the lungs of mice treated with MSC producing IFN-β, there was no significant difference in the CD8+ T cell population. This data, while implying the role of innate effectors, cannot completely rule out the possible role of adaptive immune response through CTL. It remains possible that the ratio of tumor-specific CTL to total CD8+ cells measured was not relatively substantial. The indirect effect of IFN-β by upregulating immune effectors will also be advantageous in situations where metastatic tumor cells develop resistance to anti-proliferative effects of the cytokine.
Engraftment of intravenously administered MSC in any of the healthy organs, including heart, liver, spleen, kidney, brain and muscle, was not observed in the present study. This finding is consistent with earlier reports that systemically administered MSC poorly engraft in healthy tissues.38 This property adds advantage towards using MSC to express IFN-β and avoid organ toxicity. Thus, preferential homing of MSC to tumor sites and the potential of genetically modified MSC therapy for anti-tumor effects could also be translated for treating other primary and metastatic tumors. Prior to clinical translation of this strategy, more studies need to be done to determine the longevity of genetically modified MSC and its long-term effects in vivo.
MATERIAL AND METHODS
Cell lines and Reagents
The human embryonal kidney cell line HEK293 and the mouse metastatic prostate cancer cell line TRAMP-C2, were purchased from the American Type Culture Collection (Manassas, VA). The 293 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), penicillin (100 U/ml), and streptomycin (100 μg/ml) (Cellgro, Herndon, VA) and the TRAMP-C2 cells were maintained in DMEM with 4.5 g/L glucose (Media, Inc., Herndon), 5% fetal bovine serum, 5% Nu-serum IV (Becton, Bedford, MA),10-8 mol/L Dihydrotestosterone (Sigma-Aldrich, St. Louis, MO), insulin (Sigma-Aldrich) and 25 μg/ml penicillin/streptomycin (Cellgro, Herndon, VA) at 37°C in a CO2 atmosphere. Restriction endonucleases and other modifying enzymes were purchased from either New England Biolabs (Beverly, MA) or Promega Corporation (Madison, WI). Anti PARP-p85 antibody was purchased from Promega, the Ki67 antibody from Abcam (Cambridge, MA), Factor VIII antibody from Ventana (Tucson, AZ) and the IFN-β antibody from United States Biological (Swampscott, MA)
Vector construction, packaging and purification
The open reading frame of mouse IFN-β was amplified by PCR from the plasmid pmGβ-3.1 (kind gift of Dr. Taniguchi, Cancer Institute, Japanese Foundation for Cancer Research, Japan) and cloned in a recombinant AAV plasmid pAAV-CA containing the CMV/chicken beta-actin promoter. Nucleotide sequence of the cloned fragment was verified by automated sequencing. Packaging of rAAV-IFN-β was performed by transient transfection in 293 cells with the helper plasmid pDP6 as described.39 Purification of rAAV was done by iodixanol density-gradient and heparin affinity chromatography as described.40 Particle titers of the rAAV were determined by quantitative slot blot and real-time polymerase chain reaction (PCR).41
Western blot
rAAV-IFN-β was transduced to 293 cells at a multiplicity of infection (MOI) of 1000. After 48 hrs, mock-transduced and vector-transduced cells were lysed using lysis buffer (10 mM Tris-Cl, pH 7.4, 0.15 M NaCl, 5 mM EDTA, and 1% Triton X-100) containing 100 μg/ml of protease cocktail inhibitor (Sigma-Aldrich; St. Louis, MO). Protein content of the lysates was determined using Bio-Rad Dc protein assay kit. Ten microgram of protein from each sample were separated in 10% SDS-PAGE; subsequently transblotted onto polyvinylidene fluoride (PVDF) membrane. The membranes were blocked in blocking buffer (5% powdered nonfat milk in phosphate-buffered saline (PBS) containing 0.05% Tween-20. The blot was probed with a monoclonal, rat anti-mouse IFN-β antibody followed by binding with an anti-rat secondary antibody (Molecular Probes, Eugene, OR). Interferon-β protein band was detected with an enhanced chemiluminescence kit (ECL; Amersham Biosciences/GE Healthcare, Little Chalfont, UK).
Isolation and culturing of mouse MSC
C57BL/6 mice were purchased from the National Cancer Institute-Frederick Cancer Research Facility (Frederick, MD, USA). All animal protocols were performed following the guidelines of the Institutional Animal Care and Use Committee. To obtain bone marrow stromal cells, 6- to 8-wk-old male mice were sacrificed, bone marrow was flushed from the femur and tibia, and the marrow mononuclear cells were purified by ficoll gradient. Bone marrow stromal cells were grown in α-MEM containing 10% FBS and supplemented with 10-9 M fibroblast growth factor-2 to maintain cells in pluripotent and undifferentiated state.42,43 Periodically, floating cells were removed and fresh medium replenished. Residual macrophages from the MSC culture were removed by IMAC using anti-mouse CD11b beads (BD Biosciences, San Diego, CA). After 14 days, the adherent stromal cells were split before attaining confluence to avoid possible onset of differentiation. The cells were routinely prepared and used for in vitro and in vivo studies as low passage cultures (passages 4-8). Undifferentiated MSC were transduced with 1000 MOI of rAAV6-IFN-β or rAAV6-GFP. Before transduction, cells were removed off the growth medium and washed once with serum-free Opti-MEM (Gibco-BRL, Gaithersburg, MD). Virus infection was performed in 125 μl of Opti-MEM for 2 h at 37°C after which complete medium with FGF2 was added. One week later, cells were harvested by tryspinization and resuspended in PBS for injection. In situ staining of IFN-β in cultured MSC following transduction was performed using a rat anti-mouse IFN-β antibody.
MTT assay
TRAMP-C2 cells were seeded in 96-well plates (Falcon 3072, Becton-Dickinson, Lincoln Park, NJ) at 7.5×103 cells per well and incubated at 37°C for 24 hours. Supernatants from mock-transduced and rAAV6-IFN-β transduced MSC were added to the plate, and returned to the incubator for 3 days. MTT dye was added to each well for the last 4 hrs of treatment. The reaction was stopped with the addition of DMSO, and the optical density was determined at 570 nm on a multiwell plate reader (Bio-Rad, Model 3550, Mississauga, Canada). Background absorbance of the medium in the absence of cells was subtracted. All samples were assayed in triplicate, and the mean for each experiment was calculated. Results were expressed as a percentage of control, which was considered as 100%.
In vivo studies
Eight-week-old male C57BL/6 mice were purchased from the National Cancer Institute-Frederick Cancer Research Facility (Frederick, MD). Handling of the animals was done in accordance with the guidelines of the Institutional Animal Care and Use Committee. For establishing lung metastasis, 5×105 TRAMP-C2 cells were injected by tail vein into each mouse. Ten days later, when tumors were established in the lungs, 5×105 MSC that were unmodified or transduced with rAAV6-IFN-β or rAAV6-GFP were given by i.v. injection through the tail vein in a volume of 200 μl. The MSC injection was given two times.
Immunohistochemistry
Cohorts of mice were sacrificed at two different time points (30 days and 75 days after NSC injection) and tissues were harvested and 5-μm thick paraffin sections were made. Tissue sections were dewaxed in xylene, and then rehydrated in graded alcohol. Endogenous peroxidase activity was blocked with 0·3% (v/v) hydrogen peroxide in methanol before washing the slides in water. Slides were incubated in citrate buffer (pH-6.0) for 20 minutes in a steamer for antigen retrieval for Ki67, anti-PARP-p85 and IFN-β. For Factor VIII-related antigen, pepsin antigen retrieval was used by incubating slides in 0.5 mg/ml pepsin A in pH 2.0 HCl (0.01N) for 15 minutes at 37 °C. Non-specific binding was blocked by incubation in 5% (v/v) normal serum (depending on the animal in which the secondary antibody was raised). The primary antibodies were used in dilutions: Ki67, 1:50; anti-PARP p85 fragment, 1:100; Factor VIII-related antigen, 1:40 and IFN-β, 1:500. Following binding with primary antibodies, slides were incubated with respective secondary antibodies conjugated to horseradish peroxidase. The bound antibody was visualized using the peroxidase-based Vectastain Elite ABC Kit (Vector Laboratories Ltd, Peterborough, UK). The substrate reaction was stopped by washing the slides in running water. Finally, the slides were lightly counterstained in haematoxylin.
Serum ELISA
Blood was collected from mice at different time points and sera were stored frozen in -80°C until use. To quantitate the amount of IFN-β protein, sera were used in triplicates in an ELISA, specific for mouse IFN-β (PBL Biomedical Laboratories; Piscataway, N.J.) in accordance with the manufacturer’s protocol and analyzed at an absorbance of 450 nm. The minimum sensitivity of detection was 15.6 pg/ml.
Determination of MSC homing to lung
MSC homing to tumor tissue in the lung in vivo was determined by labeling with the fluorescent dye CM-DiI (Molecular Probes, Eugene, OR) prior to in vivo administration. The dye was reconstituted at a concentration of 1 μg/μl in dimethyl sulfoxide (DMSO). Cultured MSC were trypsinized, washed with PBS, and resuspended at a concentration of 106 cells 1 ml Dulbecco’s PBS containing 2 μg CM-DiI dye. Cells were labeled by incubation at 37°C for 5 minutes followed by 15 minutes at 4°C in the dark. Unincorporated dye was then removed by centrifugation at 300 g for 5 minutes followed by 2 washes in PBS. Cells were resuspended in PBS and injected by tail vein to mice bearing TRAMP-C2 tumors in the lung. Two weeks later, mice were sacrificed and 5-μm thick paraffin sections were made of lungs. Visualization of labeled MSC was based on fluorescence of CM-Dil dye (Red), and the expression of IFN-β was determined by using a fluorescent secondary antibody (Green) following incubation with the IFN-β primary antibody. The stained slides were analyzed in a Leica DM 2RB fluorescence microscope.
Analysis of immune effectors
Spleens and lungs from mice from different groups were disrupted using wire mesh screens, and erythrocytes were lysed by treatment with lysis buffered (8.29 g NH4Cl, 1.0 g KHCO3, and 0.037 g EDTA/liter). Mononuclear cells were centrifuged at 1500 rpm for 5 min. The cells were washed in PBS containing 2% (w/v) BSA and 0.2% (w/v) NaN3 and stained with FITC-conjugated anti-NK1.1 and anti-CD8 APC antibodies (BD PharMingen, San Diego, CA). The stained cells were subjected to FACS analysis to enumerate the percentage of positive cells.
ACKNOWLEDGEMENTS
Financial support from National Institutes of Health grants R01CA98817, R01AR50251 and the U.S. Army Department of Defense grants BC044440 and PC050949 is gratefully acknowledged.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer Statistics. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Miller DC, Spencer BA, Ritchey J, et al. Treatment choice and quality of care for men with localized prostate cancer. Med Care. 2007;45:401–409. doi: 10.1097/01.mlr.0000255261.81220.29. [DOI] [PubMed] [Google Scholar]
- 3.Webster WS, Small EJ, Rini BI, Kwon ED. Prostate cancer immunology: biology, therapeutics, and challenges. J Clin Oncol. 2005;23:8262–8269. doi: 10.1200/JCO.2005.03.4595. [DOI] [PubMed] [Google Scholar]
- 4.Pestka S, Langer JA, Zoon KC, Samuel CE. Interferons and their actions. Annu Rev Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- 5.Dong Z, Greene G, Pettaway C, et al. Suppression of angiogenesis, tumorigenicity, and metastasis by human prostate cancer cells engineered to produce interferon-β. Cancer Research. 1999;59:872–879. [PubMed] [Google Scholar]
- 6.Qin XQ, Runkel L, Deck C, DeDios C, Barsoum J. Interferon-β induces S phase accumulation selectively in human transformed cells. J Interferon Cytokine Res. 1997;17:355–367. doi: 10.1089/jir.1997.17.355. [DOI] [PubMed] [Google Scholar]
- 7.Lokshin A, Mayotte JE, Levitt ML. Mechanism of interferon β-induced squamous differentiation and programmed cell death in human non-small-cell lung cancer cell lines. J Natl Cancer Inst. 1995;87:206–212. doi: 10.1093/jnci/87.3.206. [DOI] [PubMed] [Google Scholar]
- 8.Wong VL, Rieman DJ, Aronson L, Dalton BJ, Greig R, Anzano MA. Growth-inhibitory activity of interferon-β against human colorectal carcinoma cell lines. Int J Cancer. 1989;43:526–530. doi: 10.1002/ijc.2910430331. [DOI] [PubMed] [Google Scholar]
- 9.Einhorn S, Grander D. Why do so many cancer patients fail to respond to interferon therapy? J Interferon Cytokine Res. 1996;16:275–281. doi: 10.1089/jir.1996.16.275. [DOI] [PubMed] [Google Scholar]
- 10.Salmon P, Le Cotonnec JY, Galazka A, Abdul-Ahad A, Darragh A. Pharmacokinetics and pharmacodynamics of recombinant human interferon-beta in healthy male volunteers. J Interferon Cytokine Res. 1996;16:759–764. doi: 10.1089/jir.1996.16.759. [DOI] [PubMed] [Google Scholar]
- 11.Le PC, Genin P, Baines MG, Hiscott J. Interferon activation and innate immunity. Rev Immunogenet. 2000;2:374–386. [PubMed] [Google Scholar]
- 12.Studeny M, Marini FC, Champlin RE, et al. Bone marrow derived mesenchymal stem cells as vehicles for interferon-β; Delivery into Tumors. Cancer Research. 2002;62:3603–3608. [PubMed] [Google Scholar]
- 13.Studeny M, Marini FC, Dembinski JL, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. Journal of the National Cancer Institute. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 14.Kassem M, Kristiansen M, Abdallah BM. Mesenchymal stem cells: cell biology and potential use in therapy. Basic and Clinical Pharmacol Toxicology. 2004;95:209–214. doi: 10.1111/j.1742-7843.2004.pto950502.x. [DOI] [PubMed] [Google Scholar]
- 15.Pereboeva L, Curiel DT. Cellular vehicles for cancer gene therapy: current status and future potential. BioDrugs. 2004;18:361–385. doi: 10.2165/00063030-200418060-00003. [DOI] [PubMed] [Google Scholar]
- 16.Nakamizo A, Marini F, Amano T, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Research. 2005;65:3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 17.Mahmood A, Lu D, Wang L, Li Y, Lu M, Chopp M. Treatment of traumatic brain injury in female rats with intravenous administration of bone marrow stromal cells. Neurosurgery. 2001;49:1196–1203. [PubMed] [Google Scholar]
- 18.Li Y, Chopp M, Chen J, et al. Intrastriatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab. 2000;20:1311–1319. doi: 10.1097/00004647-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Korbling M, Estrov Z. Adult stem cells for tissue repair-a new therapeutic concept? N Engl J Med. 2003;349:570–582. doi: 10.1056/NEJMra022361. [DOI] [PubMed] [Google Scholar]
- 20.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bissell MJ, Radisky D. Putting tumors in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ben-Baruch A. Host microenvironment in breast cancer development: inflammatory cells, cytokines and chemokines in breast cancer progression: reciprocal tumor-microenvironment interactions. Breast Cancer Res. 2003;5:31–36. doi: 10.1186/bcr554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080–2087. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- 24.Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogeneic rejection. J Inflamm. 2005;2:8–19. doi: 10.1186/1476-9255-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoff-Khalili MA, Rivera AA, Mathis JM, et al. Mesenchymal stem cells as a vehicle for targeted delivery of CRAds to lung metastases of breast carcinoma. Breast Cancer Res Treat. 2007;105:157–167. doi: 10.1007/s10549-006-9449-8. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura K, Ito Y, Kawano Y, et al. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004;11:1155–1164. doi: 10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- 27.Hung SC, Deng WP, Yang WK, et al. Mesenchymal stem cell targeting of microscopic tumors and tumor stroma development monitored by noninvasive in vivo positron emission tomography imaging. Clin Cancer Res. 2005;11:7749–7756. doi: 10.1158/1078-0432.CCR-05-0876. [DOI] [PubMed] [Google Scholar]
- 28.Komarova S, Kawakami Y, Stoff-Khalili MA, Curiel DT, Pereboeva L. Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol Cancer Ther. 2006;5:755–766. doi: 10.1158/1535-7163.MCT-05-0334. [DOI] [PubMed] [Google Scholar]
- 29.Zhang XY, La Russa VF, Bao L, Kolls J, Schwarzenberger P, Reiser J. Lentival vectors for sustained transgene expression in human bone marrow-derived stromal cells. Mol Ther. 2002;5:555–565. doi: 10.1006/mthe.2002.0585. [DOI] [PubMed] [Google Scholar]
- 30.Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 31.Kumar S, Mahendra G, Nagy TR, Ponnazhagan S. Osteogenic differentiation of recombinant adeno-associated virus 2-transduced murine mesenchymal stem cells and development of an immunocompetent mouse model for ex vivo osteoporosis gene therapy. Hum Gene Ther. 2004;15:1197–1206. doi: 10.1089/hum.2004.15.1197. [DOI] [PubMed] [Google Scholar]
- 32.Singh RK, Gutman M, Bucana CD, Sanchez R, Llansa N, Fidler IJ. Interferons α and β down-regulate the expression of basic fibroblast growth factor in human carcinomas. Proc Natl Acad Sci USA. 1995;92:4562–4566. doi: 10.1073/pnas.92.10.4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gohji K, Fidler IJ, Tsan R, et al. Human recombinant interferons-β and -γ decrease gelatinase production and invasion by human KG-2 renal-carcinoma cells. Int J Cancer. 1994;58:380–384. doi: 10.1002/ijc.2910580313. [DOI] [PubMed] [Google Scholar]
- 34.Sica G, Fabbroni L, Castagnetta L, Cacciatore M, Pavone-Macaluso M. Antiproliferative effect of interferons on human prostate carcinoma cell lines. Urol. Res. 1989;17:111–115. doi: 10.1007/BF00262031. [DOI] [PubMed] [Google Scholar]
- 35.Belhumeur P, Lanoix J, Blais Y, Forget D, Steyaert A, Skup D. Action of spontaneously produced β interferon in differentiation of embryonal carcinoma cells through an autoinduction mechanism. Mol Cell Biol. 1993;13:2846–2857. doi: 10.1128/mcb.13.5.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johns TG, Mackay IR, Callister KA, Hertzog PJ, Devenish RJ, Linnane AW. Antiproliferative potencies of interferons on melanoma cell lines and xenografts: higher efficacy of interferon β. J Natl Cancer Inst. 1992;84:1185–1190. doi: 10.1093/jnci/84.15.1185. [DOI] [PubMed] [Google Scholar]
- 37.Albini A, Marchisone C, Del Grosso F, et al. Inhibition of angiogenesis and vascular tumor growth by interferon-producing cells: a gene therapy approach. Am J Pathol. 2000;156:1381–1393. doi: 10.1016/S0002-9440(10)65007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- 39.Grimm D, Kern A, Rittner K, Kleinschmidt JA. Novel tools for production and purification of recombinant adeno-associated virus vectors. Hum Gene Ther. 1998;9:2745–2760. doi: 10.1089/hum.1998.9.18-2745. [DOI] [PubMed] [Google Scholar]
- 40.Zolotukhin S, Byrne BJ, Mason E, et al. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]
- 41.Ponnazhagan S, Mahendra G, Kumar S, et al. Adeno-associated virus 2-mediated antiangiogenic cancer gene therapy: long-term efficacy of a vector encoding angiostatin and endostatin over vectors encoding a single factor. Cancer Res. 2004;64:1781–1787. doi: 10.1158/0008-5472.can-03-1786. [DOI] [PubMed] [Google Scholar]
- 42.Kalajzcic I, Kalajzcic J, Hurley M, Licthler A, Rowe D. Stage specific inhibition of osteoblast lineage differentiation by FGF2 and Noggin. J Cell Biochem. 2003;88:1168–1176. doi: 10.1002/jcb.10459. [DOI] [PubMed] [Google Scholar]
- 43.Kumar S, Ponnazhagan S. Bone homing of mesenchymal stem cells by ectopic alpha4 integrin expression. FASEB J. 2007;21:3917–3927. doi: 10.1096/fj.07-8275com. [DOI] [PubMed] [Google Scholar]