Abstract
Vascular adhesion protein-1 (VAP-1) is an endothelial adhesion molecule that possesses semicarbazide-sensitive amine oxidase (SSAO) activity and is involved in leukocyte recruitment. Leukocyte adhesion to retinal vessels is a predominant feature of experimentally induced diabetic retinopathy (DR). However, the role of VAP-1 in this process is unknown.
Diabetes was induced by i.p. injection of Streptozotocin in Long–Evans rats. The specific inhibitor of VAP-1, UV-002, was administered by daily i.p. injections. The expression of VAP-1 mRNA in the retinal extracts of normal and diabetic animals was measured by real time quantitative polymerase chain reaction (PCR). Firm leukocyte adhesion was quantified in retinal flatmounts after intravascular staining with concanavalin A (ConA). Leukocyte transmigration rate was quantified by in vivo acridine orange leukocyte staining (AOLS). In diabetic rats, the rate of leukocyte transmigration into the retinal tissues of live animals was significantly increased, as determined by AOLS. When diabetic animals were treated with daily injections of the VAP-1 inhibitor (0.3mg/kg), leukocyte transmigration rate was significantly reduced (P<0.05). However, firm adhesion of leukocytes in diabetic animals treated with the inhibitor did not differ significantly from vehicle-treated diabetic controls.
This work provides evidence for an important role of VAP-1 in the recruitment of leukocyte to the retina in experimental DR. Our results reveal the critical contribution of VAP-1 to leukocyte transmigration, with little impact on firm leukocyte adhesion in the retinas of diabetic animals. VAP-1 inhibition might be beneficial in the treatment of DR.
Keywords: Diabetic Retinopathy, Leukocyte Recruitment, Adhesion Molecules, Semicarbazide-Sensitive Amine Oxidase
Introduction
The prevalence of diabetes is worldwide and rapidly increasing [3]. Much of the morbidity and mortality associated with diabetes is due to complications of the disease, which virtually affect every organ in the body. For instance, diabetes is associated with significantly increased risk of visual loss through ocular complications, such as retinopathy, cataract and glaucoma [25]. The underlying causes of these complications are only beginning to be understood.
Diabetic retinopathy (DR) is a manifestation of diabetes in the microvasculature of the retina and a leading cause of adult vision loss [23,35]. DR is an inflammatory disease [10]. Some of the earliest pathological signs in experimental DR include leukocyte recruitment to the retinal vessels [4,12,22] and vascular leakage due to leukocyte accumulation [38]. Current treatments of DR are limited and there is an urgent need for the development of new therapeutic agents. Elucidating the molecular mediators of the inflammatory process in DR may provide novel therapeutic targets in the treatment or prevention of DR [1,36].
Inflammatory leukocyte recruitment occurs in a cascade-like fashion [5]. The initial rolling of leukocytes is followed by firm adhesion, and transmigration into interstitial tissue [5]. A number of specialized adhesion molecules, such as selectins, integrins, and immunoglobulins, fulfill distinct functions in the various stages of the recruitment [5,40]. Previously, ICAM-1, a member of the immunoglobulin superfamily, which binds to leukocyte β2 integrins, was shown to contribute to retinal leukocyte adhesion in experimental DR [4,22]. However, role of ICAM-1 in human DR is not well understood, as both elevated [18] and unaltered [9] levels of ICAM-1 have been reported in tissues from diabetic patients compared to normal controls.
Vascular adhesion protein-1 (VAP-1) is an endothelial adhesion molecule involved in leukocyte recruitment [13,31]. VAP-1 is a homodimeric sialylated glycoprotein expressed on the endothelium of human tissues such as skin, brain, lung, liver and heart under both normal and inflamed conditions [2,11,33,37]. Increased levels of both soluble and membrane-associated VAP-1 have been reported in a number of inflammatory diseases, including rheumatoid arthritis, inflammatory bowel disease, diabetes, and atherosclerosis [28].
VAP-1 has large homology with semicarbazide-sensitive amine oxidases (SSAO), enzymes that catalyze the deamination of primary amines such as methylamine and aminoaceton [39,41]. SSAO is a heart-shaped dimer having a unique topaquinone cofactor (a posttranslational modification of an intrinsic tyrosine) that is necessary for the catalytic reaction at the active site [16,32]. SSAO's active site generates toxic formaldehyde and methylglyoxal, hydrogen peroxide and ammonia [41], reactive chemicals and major reactive oxygen species [28]. Furthermore, VAP-1 cross-linking with mAbs, as well as addition of SSAO primary amine substrates (benzylamine; hydroxylamine), cause NF-κB activation in endothelial cells, leading to upregulation of proinflammatory adhesion molecules and chemokines [14].
Recently, we showed that VAP-1 is expressed on the endothelium of the retinal vessels and that it plays a critical role in the recruitment of leukocytes to the eye during acute inflammatory conditions, such as in endotoxin-induced uveitis [26]. In a laser-induced model of age-related macular degeneration (AMD), we found that VAP-1 blockade effectively reduces cytokine expression, macrophage recruitment, and the development of choroidal neovascularization (CNV) [27]. This suggests that VAP-1 may be an attractive molecular target in the prevention and treatment of ocular diseases with a vascular and inflammatory component, such as uveitis and AMD. However, VAP-1's role in DR remains unknown.
To study the role of VAP-1 in DR, we used the established streptozotocin (STZ)-induced model of diabetes in rodents [7]. STZ, an antibiotic produced from Streptomyces achromogenes, enters the cytoplasm via glucose transporter (GLUT) 2, which is the β-cell's glucose transporter in pancreas [34], and reduces insulin secretion through β-cell toxicity [15,24,29]. STZ-injected animals rapidly lose the ability to produce insulin and develop hyperglycemia, resembling the conditions found in type I diabetes. Although STZ-injected animals do not exhibit the entire pathology of the human diabetic patients, i.e. they do not develop proliferative DR, some of the earlier vascular changes, such as increased retinal leukostasis, vascular leakage, or cytokine expression occur in these animals.
In this study, we investigate the expression of VAP-1 in the retinal tissues of normal and diabetic animals and its role in diabetic leukocyte recruitment using a novel and specific inhibitor.
Materials & Methods
Animals and Experimental Diabetes
Long–Evans rats (total n-numbers=109) weighing 200-250g (Charles River Laboratories, Inc., Wilmington, MA) received either a single intraperitoneal (i.p.) injection of STZ (60mg/kg; Sigma, St. Louis, MO) in 0.05mM citrate buffer (pH 4.5) or citrate buffer only after an overnight fast. All rats were kept with free access to water and standard laboratory chow in an air-conditioned room with a 12 hours light and dark cycle. Plasma glucose concentration was measured 24 hours after the STZ injection. Animals with non-fasting plasma glucose concentrations greater than 250mg/dl were considered diabetic. All animal experiments were conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Animal Care Committee of Massachusetts Eye & Ear Infirmary.
RNA Extraction and Real Time Quantitative PCR
The animals were euthanized at 2 and 4 weeks after either STZ (n=23) or vehicle (n=23) administration by overdose anesthesia and perfused with PBS (500ml/kg body weight (BW)). Eyes were immediately enucleated and the retinas were carefully dissected. Each retina was handled as a separate sample. Total RNA was extracted and reverse transcribed into cDNA for real-time PCR analysis (TaqMan gene expression system, with the Prism 7700 Sequence Detection System; Applied Biosystems), according to the manufacturer's protocol. Primers and TaqMan probes for rat VAP-1 (TaqMan Gene Expression Assays) and GAPDH (Pre-Developed TaqMan Assay Reagents) were purchased from Applied Biosystems, Inc. The cycling conditions were 50°C for 2 minutes, initial denaturation at 95°C for 10 minutes, and 40 cycles at 95°C for 15 seconds and 60°C for 1 minute. The quantity of VAP-1 mRNA expression was calculated by normalizing the CT (threshold cycle) of VAP-1 to the CT of the GAPDH in the same sample, according to the ΔΔCT method. Measurements were performed in triplicates.
VAP-1 Inhibition
To block VAP-1 we used the specific VAP-1 inhibitor, U-V002, developed and provided by R-tech Ueno, Ltd., Tokyo, Japan [26]. U-V002 has an IC50 of 0.007μM against human and 0.008μM against rat SSAO, while its IC50 against the functionally related monoamine oxidase (MAO)-A and MAO-B is >10μM [26]. SSAO Inhibitors are subdivided into the main groups of hydrazine derivatives, arylalkylamines, propenyl- and propargylamines, oxazolidinones, and haloalkylamines [17]. U-V002 is a new small molecule and a derivative of 1, 3-thiazole. After the STZ injection, the inhibitor (0.3mg/kg BW) was administered to the animals by daily i.p. injections. Control animals received the same regimen of the vehicle solution (R-tech Ueno, Ltd) [26].
Evaluation of Leukocyte Recruitment to the Retina
Leukocyte recruitment to the retina was investigated by the two established techniques, the in vivo acridine orange leukocyte staining (AOLS) [21] and Concanavalin A (ConA) staining [8].
AOLS
Retinal leukocyte transmigration was investigated, as described previously, with modification [19-21]. Briefly, 14 days after STZ or vehicle injection, the animals (n=5 to 6 in each group) were anesthetized with xylazine hydrochloride (10mg/kg) and ketamine hydrochloride (100mg/kg). AO (Sigma-Aldrich, St. Louis, MO), which emits a green fluorescence when it interacts with DNA, was injected into the rats via tail vein. AO fluorescently stains intravascular leukocytes and endothelial cells. By 30 minutes after the injection, the concentration of AO in the intravascular leukocytes and the endothelial cells significantly decreases due to the washout effect [21]. However, the leukocytes that leave the vasculature and transmigrate into the retinal parenchyma retain the staining and can thus be identified [21]. Thirty minutes after the injection, animals were euthanized with an overdose of anesthesia. Subsequently, eyes were enucleated and retinal flatmounts were prepared using a mounting medium (Vector Laboratories). The number of fluorescent dots in the retina within four separate circles of 800μm diameter adjacent to the optic disc was counted using fluorescence microscopy (DM RXA; Leica) as the number of leukocytes accumulated in the retina for each rat.
Quantification of Firm Leukocyte Adhesion by ConA Staining
Animals (n=6 in each group) were perfused with 100ml of PBS/kg BW to remove intravascular content, including non-adherent leukocytes. Subsequently, ConA (40μg/ml in PBS [pH 7.4], 5mg/kg BW) was perfused to label adherent leukocytes and vascular endothelial cells, followed by PBS perfusion (100ml of PBS/kg BW) to remove residual unbound lectin. The retinas were carefully removed and flatmounted in a mounting medium (Vector Laboratories). Each retina was imaged with a fluorescence microscope (DM RXA; Leica), and the total number of adherent leukocytes per retina was counted.
Confocal Microscopy
Confocal imaging was performed at 0.5μm slice thickness (z-stack), using a Leica microscope and a 20x oil immersion objective. Digital images were saved at 512×512 pixel resolution (x and y) and imported into ImageJ software (http://rsbweb.nih.gov/ij/) for three-dimensional (3D) reconstruction. Red and green channels were consolidated using the XOR image calculator and were further processed into an average image or a 3D stack.
Western Blot
To obtain tissues, animals (n=4 in each group) were perfused with PBS (500ml/kg body weight (BW)), and eyes were enucleated immediately after perfusion. Retina and choroid were microsurgically isolated and placed into 100μl of lysis buffer (mammalian cell lysis kit MCL 1, Sigma Chemical Co,St.Louis, MO), supplemented with protease and phosphatase inhibitors (Sigma), and sonicated. The lysate was centrifuged (12000rpm, 15 minutes, 4°C) and the supernatant was collected. Each sample containing equal amount of total protein, quantified by protein assay (Bio-Rad Laboratories, Inc, CA), was separated by SDS-PAGE (sodium dodecylsulfate-polyacrylamide gel electrophoresis), and electroblotted to PVDF (polyvinylidene fluoride) membranes (Invitrogen, Carlsbad, CA). To block the nonspecific binding, the membranes were washed with 5% skim milk and subsequently incubated with a rabbit polyclonal Ab against VAP-1 (H-43, 1μg/ml, Santa Cruz Biotechnology, Santa Cruz, CA) or a mAb against β-tubulin (1.5μg/ml; Abcam, Cambridge, MA) at 4°C overnight, followed by incubation with a horseradish peroxidase-conjugated donkey or sheep Ab against rabbit or mouse IgG (1:2000; GE Healthcare UK limited, Buckinghamshire, UK). The signals were visualized with chemiluminescence (ECL kit; GE Healthcare UK limited, Buckinghamshire, UK) according to the manufacturer's protocol.
Enzyme-Linked Immunosorbent Assay (ELISA)
Two weeks after diabetes induction, animals (n=4 in each group) were sacrificed with an overdose of anesthesia, and eyes were immediately enucleated. The retina was microsurgically isolated, placed in 150μl of lysis buffer (mammalian cell lysis kits, SIGMA) and sonicated. The lysate was centrifuged at 12000rpm for 15 minutes at 4°C, and the ICAM-1 levels in the supernatant were determined using an ICAM-1 kit (Quantikine; R&D Systems, Minneapolis, MN), according to the manufacturer's protocol. Total protein concentration in the supernatant was determined using a protein assay kit (Bio-Rad Laboratories) and was used to normalize the ICAM-1 values.
Statistical Analysis
All results are expressed as mean±SEM with n-numbers as indicated. Student's t-test was used for statistical comparison between the groups. Differences between the means were considered statistically significant when the probability values were <0.05.
Results
VAP-1 Expression in Diabetic Retina
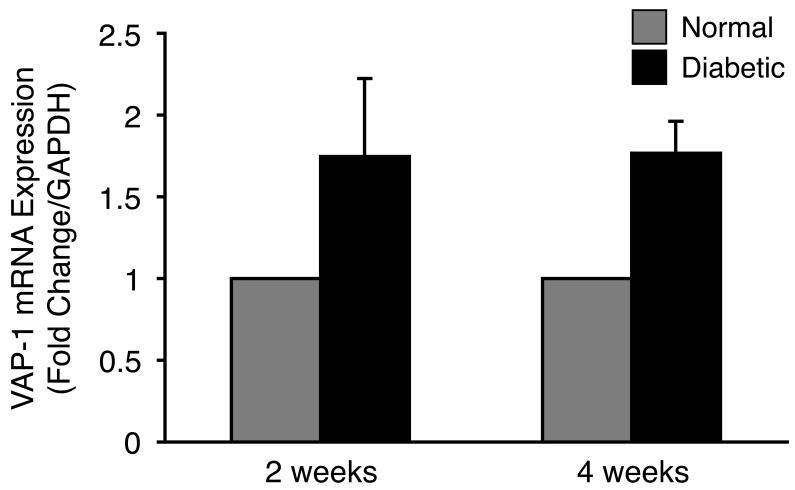
To investigate the expression of VAP-1 in the retina during diabetes, we harvested retinas of normal and diabetic animals 2 and 4 weeks after STZ injection and analyzed the level of mRNA expression by real-time PCR. Two weeks after STZ injection we found a 1.75-fold higher VAP-1 expression in the retinas of diabetic animals compared to that of normal controls (n=11 in each group; P=0.25; Fig. 1). Similarly, 4 weeks after the STZ injection, retinal VAP-1 mRNA expression was 1.8-fold higher in the diabetic animals compared to the normal controls, however, the difference did not reach statistical significance (n=12 in each group; P=0.08; Fig. 1).
Figure 1. Retinal VAP-1 mRNA Expression in Normal and Diabetic Animals.
Quantitative real-time PCR analysis showing increased retinal VAP-1 expression in diabetic animals compared to normal controls. Values are mean±SEM (n=11 and 12 in each group, 2 and 4 weeks after diabetes induction, respectively). * P<0.05.
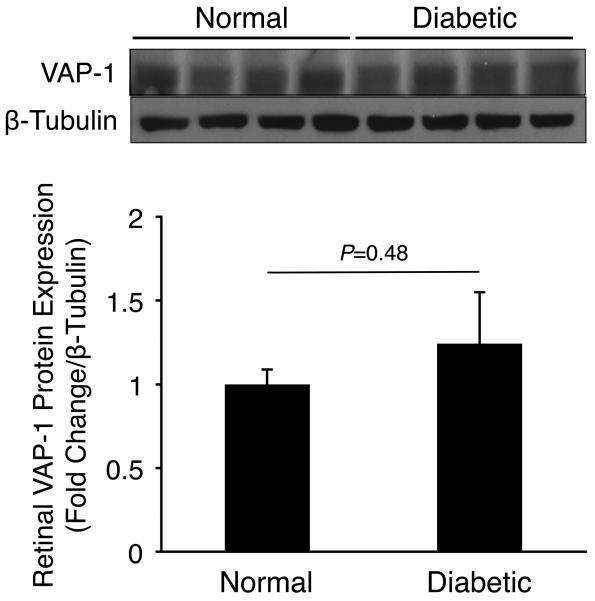
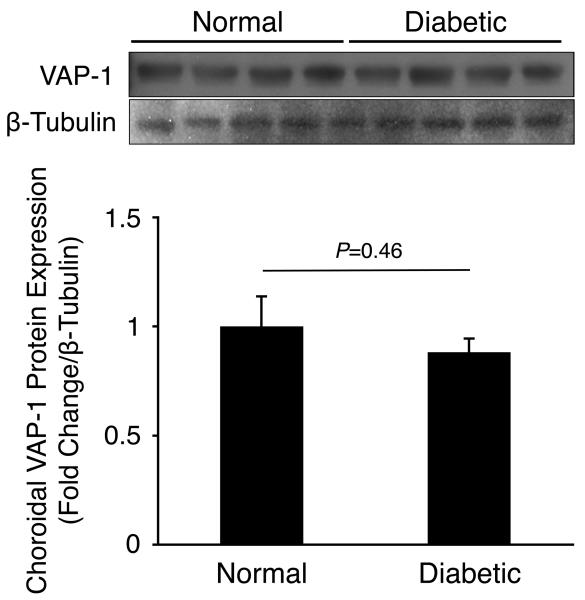
To investigate VAP-1 protein expression in experimentally induced DR, we harvested retinal and choroidal tissues from normal and 2 weeks diabetic animals and performed western blotting for VAP-1 and β-tubulin. Normalized VAP-1 protein levels in retinal (1.24±0.3 fold change, n=4) or choroidal tissues (0.88±0.06 fold change, n=4) of diabetic animals did not differ significantly from those of normal controls (P=0.48 and P=0.46, respectively; Fig. 2).
Figure 2. Retinal and Choroidal VAP-1 Expression in Normal and Diabetic Animals.
Western blot analysis of VAP-1 and β-tubulin expression in (A) retinal and (B) choroidal samples of normal and 2 weeks diabetic animals (n=4 in each group).
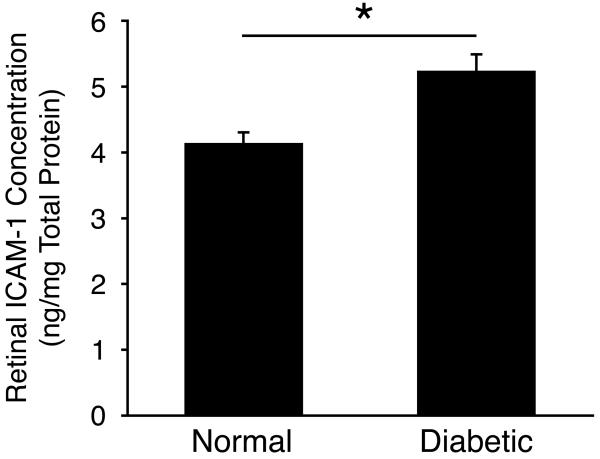
In comparison, ICAM-1 expression in retinal tissues of diabetic animals (5.22±0.29ng/mg total protein, n=4) was significantly higher than in normal controls (4.12±0.13ng/mg total protein, n=4; P=0.02; Fig. 3).
Figure 3. Retinal ICAM-1 Expression in Diabetes.
ICAM-1 protein expression in retinal tissues of normal and diabetic animals (n=4 in each group).
Effect of VAP-1 inhibitor on diabetic status
To examine, whether the daily treatments with the specific VAP-1 inhibitor, UV002, affects growth or metabolic state we measured BW and BG levels in normal and diabetic animals. STZ-injected diabetic animals showed significantly higher BG levels (417±33mg/dl, n=6, P<0.01) compared with the normal controls (114.0±2.9mg/dl, n=6) 2 weeks after the injection (Table 1). STZ-injected animals showed less BW gain (235±16g, n=6, P<0.01, Table1) than the normal controls (379±11g, n=6) 2 weeks after STZ injection. There was no significant difference in BW (287±10 vs. 278±11g, P=0.6, n=6 in each group) and BG level (420±19 vs. 419±22mg/dl, P=0.4, n=6 in each group) between the diabetic animals with or without inhibitor treatment (Table 1).
Table 1. Body weight and blood glucose levels in normal and diabetic animals with various treatments.
Day 0, 7, 14 refers to the time point after STZ injection in the diabetic groups. The BG values at day 0 are after overnight fasting, while at day 7 and 14 the values are regular non-fasting levels. Values are mean±SEM, n=6 in each group.
| Day 0 | Day 7 | Day 14 | ||
|---|---|---|---|---|
| Normal | Body Weight (g) |
201±4 | 310±8 | 379±11 |
| Blood Glucose (mg/dl) |
65±3 | 132±16 | 114±3 | |
| Diabetic | Body Weight (g) |
196±3 | 228±12 | 235±16 |
| Blood Glucose (mg/dl) |
70±6 | 407±19 | 417±33 | |
|
Diabetic + Vehicle |
Body Weight (g) |
203±3 | 253±9 | 278±11 |
| Blood Glucose (mg/dl) |
70±7 | 430±42 | 419±22 | |
|
Diabetic + VAP-1 Inhibitor |
Body Weight (g) |
210±3 | 265±6 | 287±10 |
| Blood Glucose (mg/dl) |
79±4 | 400±15 | 421±19 |
Role of VAP-1 for Leukostasis in the Retinal Vessels of Diabetic Animals
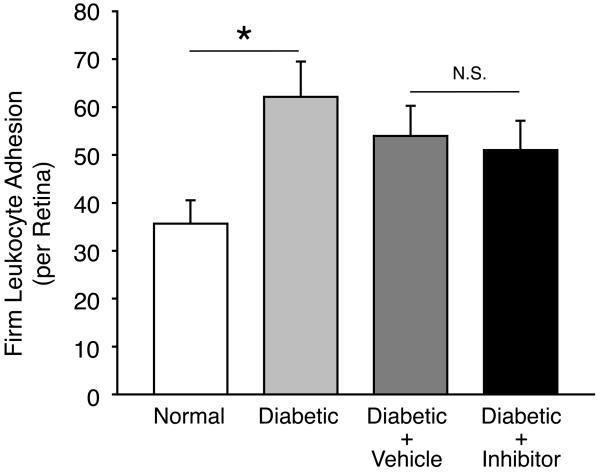
To investigate the role of VAP-1 in the leukocyte recruitment to the retina, we quantified firm leukocyte adhesion in the retinal vessels of untreated and inhibitor-treated diabetic animals using the ConA staining technique (Fig. 4A). Two weeks after STZ administration, a significantly higher number of leukocytes firmly adhered to the retinal vessels of diabetic animals (62±7cells/retina; n=6; P<0.05) compared to normal controls (36±5cells/retina; n=6). However, diabetic animals treated with daily injections of the VAP-1 inhibitor did not show a difference in the number of adhering leukocytes compared with the vehicle-treated diabetic animals (51±6cells/retina vs. 54±6cells/retina; n=6 in each group; P=0.7) (Fig. 4B). The difference in the number of firmly adhering leukocytes in the retinal vessels of diabetic animals with or without vehicle-treatment was not statistically significant (P=0.2).
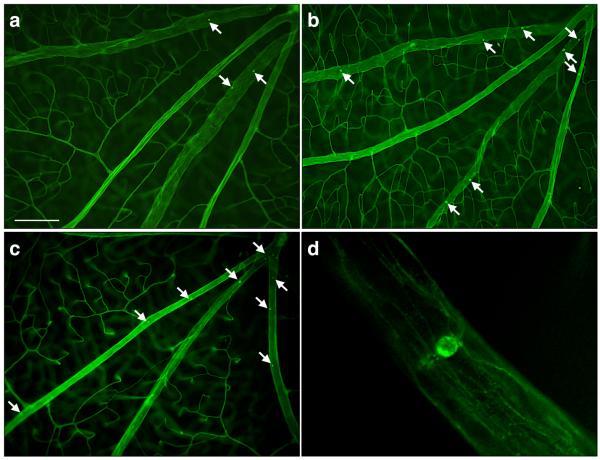
Figure 4. Contribution of VAP-1 to Firm Leukocyte Adhesion in the Retinal Vessels During Diabetes.
(A) Representative micrographs of the ConA-stained flat-mounted retinas obtained from control and diabetic animals with or without VAP-1 inhibitor treatment. a) Normal control, b) Diabetic + vehicle, c) Diabetic + VAP-1 inhibitor, and d) Magnified view of a firmly adhering leukocyte in the retinal vein of a diabetic animal. Arrows depict firmly adhering leukocytes in the retinal vessels. Bar, 100μm. (B) Bars represent the average numbers of firmly adhering leukocytes in retinal vessels of normal and diabetic animals, treated with VAP-1 inhibitor or control from ConA-stained whole retinal flat-mounts. Error bars represent SEM (n=6 in each group), * P<0.05; N.S., not significant.
Role of VAP-1 in Transmigration of Leukocyte in Diabetic Retina
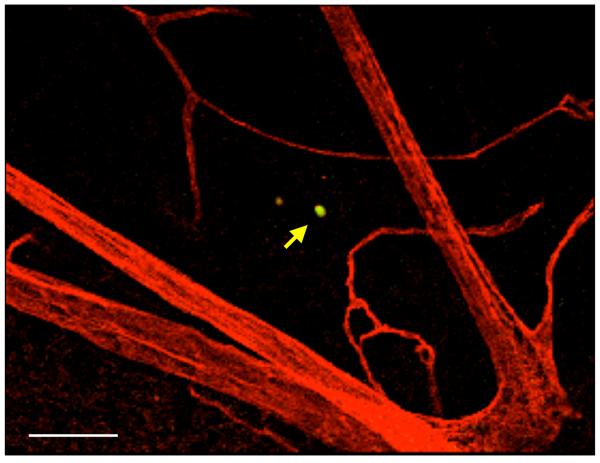
To investigate the role of VAP-1 in the later stages of leukocyte recruitment cascade, the transendothelial migration and diapedesis in the extracellular matrix, we quantified the leukocyte transmigration rate in the retinal tissues of normal and diabetic animals with and without VAP-1 inhibition using the established in vivo AO staining. AO-stained transmigrated leukocytes emitted a bright signal in epifluorescence microscopy of the flat-mounted retinas that allowed the quantification of their numbers. To examine the spatial relation of transmigrated leukocytes and retinal vasculature, we performed confocal microscopy of flatmounted retinas from AO-injected diabetic animals, in which the endothelium was stained with rhodamine conjugated ConA. Confocal microspcopy revealed that the AO-stained leukocytes were indeed outside of the vessels (Fig. 5A).
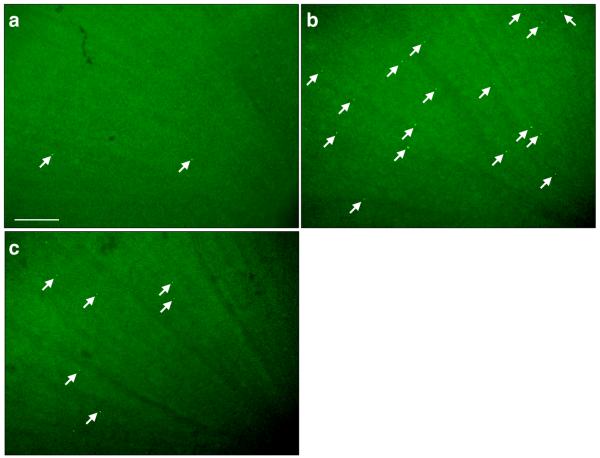
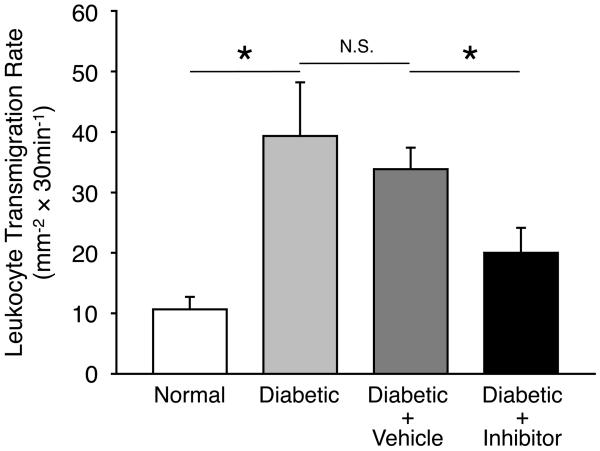
Figure 5. Impact of VAP-1 Inhibition on the Retinal Leukocyte Extravasation Rate During Diabetes.
(A) Three dimensional reconstruction of confocal sections from the retina of a diabetic animal, 30 minutes after systemic AO injection and cardiac perfusion with rhodamine conjugated ConA. Arrow indicates an AO-stained leukocyte transmigrated out of retinal vasculature. Bar, 100μm. (B) Representative micrographs of the flatmounted retinas obtained from control and diabetic animals with or without VAP-1 inhibitor treatment. a) Normal control, b) Diabetic + vehicle, and c) Diabetic + VAP-1 inhibitor. Arrows depict transmigrated leukocytes into the retinal tissues during the 30 minutes window after AO injection. Bar, 100μm. (C) Averages of leukocyte transmigration rates in normal (n=6) and diabetic animals (n=6), treated with vehicle (n=6) or VAP-1 inhibitor (n=5). Values are mean±SEM. * P<0.05.
In diabetic animals, the rate of leukocyte transmigration (39±9cells/0.5h; n=6) was significantly higher compared to that in normal controls (11±2cells/0.5h; n=6; P=0.02). When diabetic animals received daily injections of the VAP-1 inhibitor for 1 week, the leukocyte transmigration rate was significantly reduced by 41% (20±4cells; n=5; P<0.03) compared with the vehicle-treated diabetic animals (34±4cells; n=6; Fig. 5B&C). The difference in the rate of leukocyte transmigration in the retinas of diabetic animals with or without vehicle-treatment was not statistically significant (P=0.6).
Discussion
DR, a devastating complication of diabetes, is an inflammatory disease with deleterious growth of new vessels. Recently, we showed a critical role for VAP-1 in acute ocular inflammation [26] and angiogenesis [27]. However, whether VAP-1 contributes to the pathogenesis of DR is unknown. In this work we investigated the contribution of VAP-1 to the inflammatory leukocyte recruitment in experimental DR.
Our results indicate a critical role for VAP-1 in the regulation of leukocyte transmigration rate in the retinas of diabetic animals. However, firm leukocyte adhesion to the retinal vasculature in diabetic animals was surprisingly unaffected by VAP-1 inhibition. This suggests that during chronic low-grade inflammation, such as found in experimental diabetes, VAP-1 principally regulates the step of leukocyte transmigration, with little influence on the preceding step of firm adhesion. However, contribution of VAP-1 to leukocyte rolling in chronic inflammation remains to be investigated. This provides a clear distinction between the role of VAP-1 in acute vs. chronic inflammation. During acute inflammation VAP-1 regulates both firm adhesion and transmigration [26], while in chronic low-grade inflammation, such as found during diabetes, VAP-1 may only regulate transmigration.
Previously we showed significantly increased retinal VAP-1 expression in acute inflammation [26]. In contrast, in diabetic animals retinal VAP-1 mRNA expression showed a trend to higher levels that did not reach statistical significance. Similarly, VAP-1 protein levels in retinal and choroidal tissues of normal vs. diabetic animals did not differ significantly. Changes in VAP-1 expression may not be detectable during diabetes due to the milder nature of the inflammatory processes in DR compared to the conditions found in acute inflammation, for instance in uveitis [26].
Transmigration of leukocytes from the peripheral blood into the tissues of various organs is central to immune function. The details of the transmigration step are only beginning to be understood. As one of the concluding steps of the recruitment cascade, leukocyte transmigration is impacted by an array of factors influencing the preceding steps, such as tethering, rolling, or firm adhesion [30]. For instance, the endothelial adhesion molecules, ICAM-1 and VCAM-1, are known to be upregulated during inflammation and facilitate the recruitment of immune cells to the retina. Recently, activated leukocyte cell adhesion molecule (ALCAM or CD166) was identified as a critical mediator of leukocyte migration across the barrier-privileged vessels of the brain [6]. ALCAM blockade reduces the severity and delays the time of onset of experimental autoimmune encephalomyelitis [6], underlining the significance of targeting adhesion molecules in prevention and treatment of inflammatory diseases of CNS.
However, the study of transmigration in vivo is complicated by the challenge to clearly distinguish leukocytes at the various stages of recruitment and in a statistically meaningful manner. By combining the strengths of two in vivo detection techniques, the in situ ConA staining and the in vivo AO staining, we have been able to reveal the isolated role of VAP-1 in regulating the leukocyte transmigration rate in the diabetic retina. While ConA staining provides a quantitative measure for firmly adhering leukocytes to the retinal vessels, AO staining is a sensitive technique, which allows quantification of the number of transmigrated leukocytes from the entire vascular bed of a retina within a time window.
Due to the immune privileged status of the eye, few immune cells transmigrate into the retina under physiological conditions. However, during various diseases, such as uveitis, DR, or age-related macular degeneration (AMD), large numbers of immune cells cross the blood-retinal barrier and migrate into the neuronal retina. In such scenarios, perivascular leukocyte infiltration is believed to be the cause of considerable harm to the neurons. Understanding the molecular mechanisms that facilitate the transmigration step may help us identify ways to protect the fragile retinal neurons from the deleterious impact of immune cell invasion.
In summary, this work provides evidence for a role for VAP-1 in the inflammatory outcome of DR. Specifically, VAP-1 plays a central role in regulating leukocyte transmigration rate during chronic low-grade inflammation. VAP-1 inhibition may be beneficial in the treatment and prevention of DR.
Supplementary Material
Acknowledgements
We thank Alexander Schering for compiling the 3D confocal images. This work was supported by NIH grants AI050775 (AHM) and HL086933 (Alan Cross, University of Maryland), research funds from RTECH-UENO, Bausch & Lomb Fellowships (K.N. and S.N.), a Fellowship Award from the Japan Eye Bank Association (K.N. and S.N.), and NEI core grant EY14104. We are indebted to the Massachusetts Lions Eye Research Fund Inc. for generous funds provided for laboratory equipment used in this project. We would like to thank Marion W. and Edward F. Knight AMD Fund and Research to Prevent Blindness for their support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Aiello LM. Perspectives on diabetic retinopathy. Am J Ophthalmol. 2003;136:122–35. doi: 10.1016/s0002-9394(03)00219-8. [DOI] [PubMed] [Google Scholar]
- 2.Akin E, Aversa J, Steere AC. Expression of adhesion molecules in synovia of patients with treatment-resistant lyme arthritis. Infect Immun. 2001;69:1774–80. doi: 10.1128/IAI.69.3.1774-1780.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med. 1997;14(Suppl 5):S1–85. [PubMed] [Google Scholar]
- 4.Barouch FC, Miyamoto K, Allport JR, Fujita K, Bursell SE, Aiello LP, Luscinskas FW, Adamis AP. Integrin-mediated neutrophil adhesion and retinal leukostasis in diabetes. Invest Ophthalmol Vis Sci. 2000;41:1153–8. [PubMed] [Google Scholar]
- 5.Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–6. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 6.Cayrol R, Wosik K, Berard JL, Dodelet-Devillers A, Ifergan I, Kebir H, Haqqani AS, Kreymborg K, Krug S, Moumdjian R, Bouthillier A, Becher B, Arbour N, David S, Stanimirovic D, Prat A. Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nat Immunol. 2008;9:137–45. doi: 10.1038/ni1551. [DOI] [PubMed] [Google Scholar]
- 7.Fukushi S, Merola LO, Kinoshita JH. Altering the course of cataracts in diabetic rats. Invest Ophthalmol Vis Sci. 1980;19:313–5. [PubMed] [Google Scholar]
- 8.Hafezi-Moghadam A, Noda K, Almulki L, Iliaki EF, Poulaki V, Thomas KL, Nakazawa T, Hisatomi T, Miller JW, Gragoudas ES. VLA-4 blockade suppresses endotoxin-induced uveitis: in vivo evidence for functional integrin up-regulation. Faseb J. 2007;21:464–74. doi: 10.1096/fj.06-6390com. [DOI] [PubMed] [Google Scholar]
- 9.Hughes JM, Brink A, Witmer AN, Hanraads-de Riemer M, Klaassen I, Schlingemann RO. Vascular leucocyte adhesion molecules unaltered in the human retina in diabetes. Br J Ophthalmol. 2004;88:566–72. doi: 10.1136/bjo.2003.021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishida S, Usui T, Yamashiro K, Kaji Y, Ahmed E, Carrasquillo KG, Amano S, Hida T, Oguchi Y, Adamis AP. VEGF164 is proinflammatory in the diabetic retina. Invest Ophthalmol Vis Sci. 2003;44:2155–62. doi: 10.1167/iovs.02-0807. [DOI] [PubMed] [Google Scholar]
- 11.Jaakkola K, Jalkanen S, Kaunismaki K, Vanttinen E, Saukko P, Alanen K, Kallajoki M, Voipio-Pulkki LM, Salmi M. Vascular adhesion protein-1, intercellular adhesion molecule-1 and P-selectin mediate leukocyte binding to ischemic heart in humans. J Am Coll Cardiol. 2000;36:122–9. doi: 10.1016/s0735-1097(00)00706-3. [DOI] [PubMed] [Google Scholar]
- 12.Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am J Pathol. 2001;158:147–52. doi: 10.1016/S0002-9440(10)63952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koskinen K, Vainio PJ, Smith DJ, Pihlavisto M, Yla-Herttuala S, Jalkanen S, Salmi M. Granulocyte transmigration through the endothelium is regulated by the oxidase activity of vascular adhesion protein-1 (VAP-1) Blood. 2004;103:3388–95. doi: 10.1182/blood-2003-09-3275. [DOI] [PubMed] [Google Scholar]
- 14.Lalor PF, Sun PJ, Weston CJ, Martin-Santos A, Wakelam MJ, Adams DH. Activation of vascular adhesion protein-1 on liver endothelium results in an NF-kappaB-dependent increase in lymphocyte adhesion. Hepatology. 2007;45:465–74. doi: 10.1002/hep.21497. [DOI] [PubMed] [Google Scholar]
- 15.Liu K, Paterson AJ, Chin E, Kudlow JE. Glucose stimulates protein modification by O-linked GlcNAc in pancreatic beta cells: linkage of O-linked GlcNAc to beta cell death. Proc Natl Acad Sci U S A. 2000;97:2820–5. doi: 10.1073/pnas.97.6.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marttila-Ichihara F, Smith DJ, Stolen C, Yegutkin GG, Elima K, Mercier N, Kiviranta R, Pihlavisto M, Alaranta S, Pentikainen U, Pentikainen O, Fulop F, Jalkanen S, Salmi M. Vascular amine oxidases are needed for leukocyte extravasation into inflamed joints in vivo. Arthritis Rheum. 2006;54:2852–62. doi: 10.1002/art.22061. [DOI] [PubMed] [Google Scholar]
- 17.Matyus P, Dajka-Halasz B, Foldi A, Haider N. Semicarbazide-sensitive amine oxidase: current status and perspectives. Curr. Med. Chem. 2004 doi: 10.2174/0929867043365305. [DOI] [PubMed] [Google Scholar]
- 18.McLeod DS, Lefer DJ, Merges C, Lutty GA. Enhanced expression of intracellular adhesion molecule-1 and P-selectin in the diabetic human retina and choroid. Am J Pathol. 1995;147:642–53. [PMC free article] [PubMed] [Google Scholar]
- 19.Miyahara S, Kiryu J, Miyamoto K, Katsuta H, Hirose F, Tamura H, Musashi K, Honda Y, Yoshimura N. In vivo three-dimensional evaluation of leukocyte behavior in retinal microcirculation of mice. Invest Ophthalmol Vis Sci. 2004;45:4197–201. doi: 10.1167/iovs.04-0192. [DOI] [PubMed] [Google Scholar]
- 20.Miyahara S, Kiryu J, Tsujikawa A, Katsuta H, Nishijima K, Miyamoto K, Yamashiro K, Nonaka A, Honda Y. Argatroban attenuates leukocyte- and platelet-endothelial cell interactions after transient retinal ischemia. Stroke. 2003;34:2043–9. doi: 10.1161/01.STR.0000083052.01361.3D. [DOI] [PubMed] [Google Scholar]
- 21.Miyahara S, Kiryu J, Yamashiro K, Miyamoto K, Hirose F, Tamura H, Katsuta H, Nishijima K, Tsujikawa A, Honda Y. Simvastatin inhibits leukocyte accumulation and vascular permeability in the retinas of rats with streptozotocin-induced diabetes. Am J Pathol. 2004;164:1697–706. doi: 10.1016/S0002-9440(10)63728-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyamoto K, Khosrof S, Bursell SE, Rohan R, Murata T, Clermont AC, Aiello LP, Ogura Y, Adamis AP. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci U S A. 1999;96:10836–41. doi: 10.1073/pnas.96.19.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore TC, Moore JE, Kaji Y, Frizzell N, Usui T, Poulaki V, Campbell IL, Stitt AW, Gardiner TA, Archer DB, Adamis AP. The role of advanced glycation end products in retinal microvascular leukostasis. Investigative Ophthalmology & Visual Science. 2003;44:4457–64. doi: 10.1167/iovs.02-1063. [DOI] [PubMed] [Google Scholar]
- 24.Murata M, Takahashi A, Saito I, Kawanishi S. Site-specific DNA methylation and apoptosis: induction by diabetogenic streptozotocin. Biochem Pharmacol. 1999;57:881–7. doi: 10.1016/s0006-2952(98)00370-0. [DOI] [PubMed] [Google Scholar]
- 25.Negi A, Vernon SA. An overview of the eye in diabetes. J R Soc Med. 2003;96:266–72. doi: 10.1258/jrsm.96.6.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noda K, Miyahara S, Nakazawa T, Almulki L, Nakao S, Hisatomi T, She H, Thomas KL, Garland RC, Miller JW, Gragoudas ES, Kawai Y, Mashima Y, Hafezi-Moghadam A. Inhibition of vascular adhesion protein-1 suppresses endotoxin-induced uveitis. Faseb J. 2008;22:1094–103. doi: 10.1096/fj.07-9377com. [DOI] [PubMed] [Google Scholar]
- 27.Noda K, She H, Nakazawa T, Hisatomi T, Nakao S, Almulki L, Zandi S, Miyahara S, Ito Y, Thomas KL, Garland RC, Miller JW, Gragoudas ES, Mashima Y, Hafezi-Moghadam A. Vascular adhesion protein-1 blockade suppresses choroidal neovascularization. Faseb J. 2008;22:2928–35. doi: 10.1096/fj.07-105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Sullivan J, Unzeta M, Healy J, O'Sullivan MI, Davey G, Tipton KF. Semicarbazide-sensitive amine oxidases: enzymes with quite a lot to do. Neurotoxicology. 2004;25:303–15. doi: 10.1016/S0161-813X(03)00117-7. [DOI] [PubMed] [Google Scholar]
- 29.Ohkuwa T, Sato Y, Naoi M. Hydroxyl radical formation in diabetic rats induced by streptozotocin. Life Sci. 1995;56:1789–98. doi: 10.1016/0024-3205(95)00150-5. [DOI] [PubMed] [Google Scholar]
- 30.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–15. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 31.Salmi M, Jalkanen S. A 90-kilodalton endothelial cell molecule mediating lymphocyte binding in humans. Science. 1992;257:1407–9. doi: 10.1126/science.1529341. [DOI] [PubMed] [Google Scholar]
- 32.Salmi M, Jalkanen S. Cell-surface enzymes in control of leukocyte trafficking. Nat Rev Immunol. 2005;5:760–71. doi: 10.1038/nri1705. [DOI] [PubMed] [Google Scholar]
- 33.Salmi M, Kalimo K, Jalkanen S. Induction and function of vascular adhesion protein-1 at sites of inflammation. J Exp Med. 1993;178:2255–60. doi: 10.1084/jem.178.6.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schnedl WJ, Ferber S, Johnson JH, Newgard CB. STZ transport and cytotoxicity. Specific enhancement in GLUT2-expressing cells. Diabetes. 1994;43:1326–33. doi: 10.2337/diab.43.11.1326. [DOI] [PubMed] [Google Scholar]
- 35.Sheetz MJ, King GL. Molecular understanding of hyperglycemia's adverse effects for diabetic complications. Jama. 2002;288:2579–88. doi: 10.1001/jama.288.20.2579. [DOI] [PubMed] [Google Scholar]
- 36.Sinclair SH, Delvecchio C. The internist's role in managing diabetic retinopathy: screening for early detection. Cleve Clin J Med. 2004;71:151–9. doi: 10.3949/ccjm.71.2.151. [DOI] [PubMed] [Google Scholar]
- 37.Singh B, Tschernig T, van Griensven M, Fieguth A, Pabst R. Expression of vascular adhesion protein-1 in normal and inflamed mice lungs and normal human lungs. Virchows Arch. 2003;442:491–5. doi: 10.1007/s00428-003-0802-6. [DOI] [PubMed] [Google Scholar]
- 38.Skondra D, Noda K, Almulki L, Tayyari F, Frimmel S, Nakazawa T, Kim IK, Zandi S, Thomas KL, Miller JW, Gragoudas ES, Hafezi-Moghadam A. Characterization of Azurocidin as a Permeability Factor in the Retina: Involvement in VEGF-Induced and Early Diabetic Blood-Retinal Barrier Breakdown. Invest Ophthalmol Vis Sci. 2008;49:726–31. doi: 10.1167/iovs.07-0405. [DOI] [PubMed] [Google Scholar]
- 39.Smith DJ, Salmi M, Bono P, Hellman J, Leu T, Jalkanen S. Cloning of vascular adhesion protein 1 reveals a novel multifunctional adhesion molecule. J Exp Med. 1998;188:17–27. doi: 10.1084/jem.188.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 41.Yu PH, Wright S, Fan EH, Lun ZR, Gubisne-Harberle D. Physiological and pathological implications of semicarbazide-sensitive amine oxidase. Biochim Biophys Acta. 2003;1647:193–9. doi: 10.1016/s1570-9639(03)00101-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.