Abstract
Regions of genomic DNA containing repetitive nucleotide sequences can adopt a number of different structures in addition to the canonical B-DNA form: many of these non-B DNA structures are causative factors in genetic instability and human disease. Although chromosomal DNA replication through such repetitive sequences has been considered a major cause of non-B form DNA structure-induced genetic instability, it is also observed in non-proliferative tissues. In this review, we discuss putative mechanisms responsible for the mutagenesis induced by non-B DNA structures in the absence of chromosomal DNA replication.
Keywords: DNA structure, DNA replication, DNA repair, Transcription, Recombination, Genetic instability
INTRODUCTION
DNA has long been considered a passive “victim” of damage and mutagenesis. But studies over the past few decades have also suggested that the DNA itself can be a causative factor for genetic instability leading to expansion of repetitive units, or inducing gross genomic rearrangements (translocations, deletions, insertions, inversions, and duplications) that might cause human disease [1–3]. Many types of non-B DNA structures form at repetitive DNA sequences containing different repeat units [1]. For example, cruciform structures can form at inverted repeats when the duplex is unwound during replication, transcription, or repair, affording the single-stranded repeat sequence the opportunity to base pair with itself in an intramolecular fashion, thereby forming a hairpin or cruciform structure. Cruciform/hairpin structures have been found to induce DNA double strand breaks (DSBs), deletions, and translocations in prokaryotic cells [4,5] and in yeast, mice, and human cells [6–11]. Because purine bases can adopt a syn conformation, alternating pyrimidine-purine sequences such as GC or GT repeats can readily adopt left-handed Z-DNA structures with a zigzag arrangement of the DNA backbone [12,13]. Z-DNA-forming CG repeats cause small deletions within the repeats, likely due to slippage events during replication in bacteria [14], and can induce DSBs within or surrounding the repeats in mammalian cells, resulting in large-scale deletions (>100 bp) [15]. Polypurine-polypyrimidine elements with mirror repeat symmetry can adopt intramolecular triplex DNA structures (H-DNA), where unpairing of the duplex in half of the tract allows one of the single strands to pair in parallel with the purine strand in the remaining duplex segment, forming a three-stranded helix with the complement of the third strand remaining unpaired. H-DNA has been detected in vivo using both triplex antibodies and fluorescently labeled single-stranded DNA oligonucleotides [16,17]. We have shown that the endogenous H-DNA-forming sequence from the human c-MYC promoter caused a 20-fold increase in mutation frequency on a mutation reporter gene in mammalian cells [18]. Strand slippage events can occur at tandem repeats, particularly when the DNA within a loop can form intrastrand base pairs, such as in CAG/CTG triplet repeats. These structures can cause contraction or expansion of the tandem repeat units, and have been associated with at least 20 neurological diseases (reviewed in [19–23] and reviews within this issue). Expansion of simple repeats is also related with “fragile sites” in the genome, which are prone to DNA breaks, chromosomal translocations, deletions, and amplifications, and have been implicated in several hereditary disorders. For example, long CTG, CCG, or GAA tracts that are capable of adopting hairpin, Z-DNA [24], H-DNA [25] or sticky DNA [26] structures (i.e. two triplex-like-structures formed on GAA repeats), can cause chromosome breakage [1,27–29]. In addition, many non-B DNA structures formed at non-repetitive sequences are mapped in or near chromosome breakage “hotspots” [30–35], suggesting a relationship between non-B DNA structure and DNA breakage and chromosome translocation.
However, the mechanisms involved in expansion, deletion, and particularly DNA DSBs are still not clear, particularly in mammalian cells. Data obtained to date have revealed that the mechanisms involved in DNA structure-induced genetic instability are complex and more than one pathway is likely involved in processing non-B DNA structures. While some pathways are more dominant in certain cases, they may be less important under other conditions, depending on the type of DNA structure, the cis-elements nearby, and the levels of DNA replication, transcription, or the amounts of DNA damage and repair within close proximity to the non-B structures.
Generally, non-B DNA conformations are in higher energy states than B-DNA. To form most types of non-B DNA structures such as hairpins, cruciforms, H-DNA, G4 DNA (i.e. stable four-stranded structures formed at guanine-rich DNA sequences such as telomeres [36]), or slipped DNA, Watson-Crick base pairs in the B-form are separated prior to structure conversion, which is an energy-consuming process. Therefore, genomic DNA is likely in the B-form with Watson-Crick base pairing the majority of the time. However, when the DNA is unwrapped from nucleosome cores or the DNA duplex is separated during replication, the single strandedness required for non-B DNA structure conversion is generated, particularly in the lagging strand during DNA synthesis. In addition, many forms of non-B DNA structures appear to interfere with the processivity of DNA polymerases and cause replication fork stalling, which in turn further prolongs the existence of single strandedness and facilitates non-B DNA conformations. For example, H-DNA poses a strong barrier for DNA polymerases such as Taq [37] and polymerase alpha [38] and can pause or arrest DNA replication in mammalian cells [39,40]. 2D electrophoretic analysis of replication intermediates suggested that replication forks stall at hairpin structures formed at inverted repeats in bacteria, yeast, and mammalian cells [41,42]. These findings suggest that chromosomal DNA replication plays important roles in non-B DNA induced genetic instability.
Instability at triplet repeats often occurs in highly proliferative tissues [43,44] or in rapidly dividing cells [45,46], and are relatively stable in differentiated skeletal muscle cells [47–49], supporting a role for replication in triplet repeat stability. CAG or CTG triplets can form hairpins with loops containing a mismatch once in every two C–G pairings. Such a structure may be the result of primer-template slippage in DNA replication and can promote further slippage events [50] giving rise to repeat expansion seen in human disease. In addition, the location and orientation of the triplet repeat relative to the replication origin have dramatic effects on the frequency and types of instability [42,51–56]. Mutations in genes involved in DNA replication, e.g., FEN-1 [57] or DNA polymerases [58] can have dramatic effects on triplet repeat stability. These data clearly implicate semi-conservative chromosomal DNA replication in non-B DNA-induced genetic instability.
Although the replication slippage model of triplet repeat (or other direct repeats) instability has been considered a major mechanism of repeat instability [59], sequences capable of forming non-B DNA structures can induce instability in non-proliferative tissues as well, suggesting a chromosomal replication-independent process(es) leading to DNA structure-related instability. For example, CAG repeat expansion occurs in the brain [60–62], even though all neuronal cells do not divide, and different regions of brain exhibited different levels of CAG repeat expansion [61,62]. The CAG expansion was even larger in muscle tissue than in lymphocytes from patients with Kennedy’s disease and Myotonic dystrophy [48,63]. In cultured cells derived from different tissues of transgenic mice carrying long CAG(162) repeats in the chromosome, CAG repeats exhibited different rates of expansion and no correlation between repeat instability and the cell proliferation rate was found [64]. In addition, we found that H-DNA and Z-DNA structures induced DSBs and large-scale deletions and rearrangements of plasmid DNA in replication-deficient HeLa cell extracts ([15] and our unpublished results). Moreover, Z-DNA and H-DNA induced a higher proportion of large-scale deletions in replication-deficient plasmids than on those recovered from replicating plasmids ([15] and our unpublished results). Obviously, chromosomal DNA replication-independent mechanisms also play important roles in non-B DNA structure-induced mutagenesis, particularly in related neurological disorders. In this review, we summarize different pathways of non-B DNA structure-induced mutagenesis associated with DNA damage and repair.
DNA REPAIR-STIMULATED NON-B DNA STRUCTURE FORMATION
As discussed above, genomic DNA remains in the B-form the majority of the time and needs to be unwrapped from nucleosomes and separated into single strands prior to conversion into non-B DNA structures, such as slippage loops, hairpins/cruciforms, H-DNA, and G4 structures. DNA damage occurs frequently on genomic DNA either spontaneously or induced by endogenous or exogenous damaging agents. DNA repair processes at or near the repetitive sequences could give rise to non-B DNA conformations and induce structure-related genetic instability. This “repair stimulated DNA structure formation” model may not apply to those structures that require torsional stress from negative supercoiling within an intact duplex, such as Z-DNA.
DSBs resulting from many endogenous or exogenous factors occur frequently in vivo, and are strong signals for the recruitment of the DNA repair machinery to the breakpoints. Repair of DSBs thousands of base-pairs away from an inverted repeat in the yeast chromosome resulted in 5′-to-3′ degradation at the breakpoint and exposure of single-stranded inverted repeat DNA. Folding the ssDNA to form a hairpin structure could bring the two ends of complimentary strands at the breakpoint together, resulting in a large hairpin structure. Resolution of the structure and bi-directional replication could result in amplification of large chromosomal regions [65–67]. Alternatively, the single-stranded repair intermediates could initiate a pairing interaction between two inverted repeats from different chromosomes [68]. Both processes could result in the formation of dicentric chromosomes and would lead to gross chromosomal rearrangements, including translocations, truncations, and amplifications.
When DSBs were introduced within or near a CTG repeat tract on plasmids prior to being transfected into mammalian cells, they were predominantly processed into deletions of the repeat and flanking regions. Longer repeats induced a greater proportion of deletions and expansions in the repeats than did shorter ones [CTG(79) > CTG(47) > CTG(17)], and the deletion areas were larger in those induced by the longer repeats, consistent with the higher inherent capability of longer CTG repeats to form more stable non-B DNA structures [69]. DSBs centrally located within CTG or CGG repeats also increased deletions of the repeating sequence in RecA or RecBC-deficient E. coli [70]. A nick or a short 15 nt gap within the CTG repeats did not induce expansions or deletions; however, a longer gap of 30 nt, leaving a 30 nt single-stranded CAG repeat which can form a stable hairpin structure, caused a distinct 30 nt deletion of the entire looped region [70], suggesting that hairpin formation at the repair intermediate is an essential step for repair-stimulated CTG instability [71]. Repair of a DSB within a GAA repeat, which is not able to form an intrastrand hairpin structure, also increased repeat deletions. But unlike DSB repair near CTG or CCG repeats, DSB repair outside the GAA repeat tract did not affect instability [72]. Moreover, repair of DSBs within the repeat resulted in deletion lengths equivalent to the distance of the DSB to the closer end of the repeat tract, suggesting a single-strand annealing mechanism of DSB repair [72].
Alternatively, in bacteria, yeast, or mammalian cells in late S phase where homologous templates are available, the single-stranded repeat sequence derived from the DSB can invade a homologous template, and slippage loops (or hairpin structures if the sequence supports intrastrand base-pairs) can occur during DNA synthesis, resulting in expansions and contractions via a synthesis-dependent strand annealing (SDSA) model that allows for crossover [73].
In addition to DSBs, many other types of DNA damage can enhance the instability of repetitive sequences that are capable of forming non-B DNA structures. In human colorectal carcinoma cancer RKO cells, mutations due to DNA strand slippage at repetitive sequences were able to reactivate a frame-shift mutated green fluorescent protein (GFP) gene integrated into the chromosome. Treating the cells harboring the tetranucleotide repeat e.g., AAAG(16) repeats within the chromosomally integrated reporter system with N-methyl-N-nitro-N-nitrosoguanidine (MNNG), t-butyl hydrogen peroxide, and UV irradiation (but not γ-irradiation or benzo(a)pyrene diol epoxide), resulted in a significant increase in the number of reactivation mutants compared to cells harboring a reporter gene containing a CA(16) repeat or without a repetitive sequence. In addition, an increase in reactivating frame-shift mutations at the AAAG(16) repeat was observed with increasing doses of UV or MNNG, while control cells did not show any significant changes with increasing doses, suggesting that the (repair of) damage could induce slippage mutations in repeated sequences [74]. However, H2O2 treatment on telomerase-immortalized normal human fibroblasts harboring a plasmid containing G(17), A(17), or CA(17) repeats in the tk-neo gene, reduced mononucleotide repeat slippage-induced reactivation of the reporter neo gene in the (+1) reading frame. No effect was observed when the neo gene was in the (−1) reading frame [75]. These observations suggested that the chemical and/or structural nature of different types of repetitive DNA might be important for the fate of the damaged sequence.
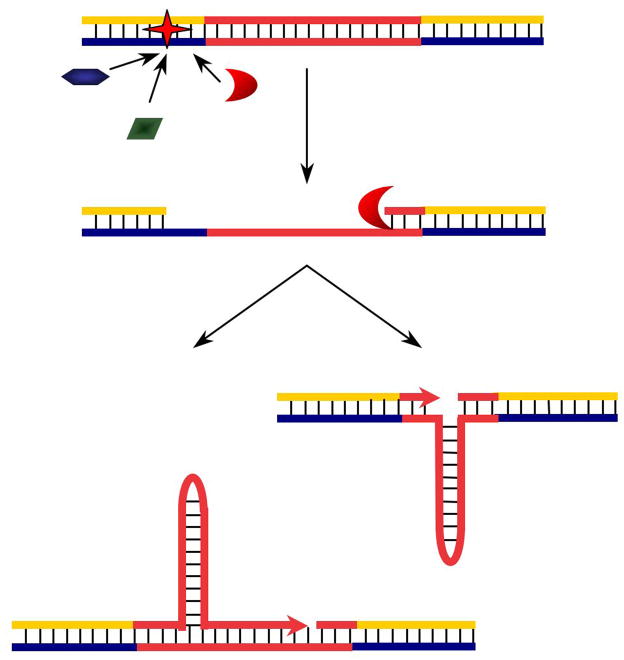
Figure 1 depicts our model of “DNA repair-stimulated non-B DNA structure formation” and subsequent genetic instability. This model provides a plausible explanation for non-B DNA structure induced instability in non-dividing cells, particularly for those mutations caused by intrastrand hairpin formation at simple triplet repeats such as CAG and CCG repeats [76]. Clearly, there are still many questions to be answered to better define this model. For example, based on this model it would be predicted that long single-stranded repetitive sequences where stable non-B structures can form would be more likely to be seen in long patch repair pathways such as nucleotide excision repair (NER), mismatch repair (MMR), long-patch base excision repair (BER) and DSB repair, rather than short-patch BER. However, the monofunctional alkylating agent MNNG, and the oxidative DNA damaging agent t-butyl hydrogen peroxide, induced slippage loops and contraction of AAAG(16) repeats, while γ-irradiation had only a minor effect [74]. Also, oxidative damage that is largely repaired by BER is the predominant type of damage in the brain, where triplet repeat expansion occurs in human disease. And more directly, knocking-out of the base excision repair enzyme, 7,8-dihydro-8-oxoguanine-DNA glycosylase (OGG1), dramatically stabilized CAG repeats in mouse brains, suggesting a critical role for BER in CAG expansion [77]. Thus, the mechanisms involved are more complicated than proposed in the model (Figure 1) and these mechanisms still need to be explored.
Figure 1.
“Excision-repair stimulated hairpin/slippage structure formation” model. In this model, (1) DNA damage (shown as a star) occurs near (or within) a non-B DNA structure-forming sequence (shown in red), recruiting the DNA repair machinery. (2) Processing of the damage by repair enzymes (indicated as various shapes) exposes a single-stranded DNA region within the non-B DNA-forming sequence. (3) Non-B DNA structures (such as a hairpin as shown in the figure) can form at the newly synthesized strand, which causes expansion of the sequence, or at the template strand leading to deletion of the sequence.
DNA STRUCTURE-SPECIFIC CLEAVAGE MODEL IN THE ABSENCE OF DAMAGE
In the “bipartite model” of damage recognition in NER, both DNA distortion and chemical modification are important signals for the damage/distortion recognition proteins (e.g., XPC-RAD23B) to bind DNA lesions [78,79]. Discontinuity and distortion of the DNA double helix induced by lesions are initial signals for recruitment of the DNA damage/distortion recognition proteins to the sites of lesions, as proposed by Yang [80]. XPC-RAD23B can also bind to distorted DNA, such as bubble structures in the absence of a lesion [79]. Further, XPC-RAD23B is not needed for NER if the DNA structure is already sufficiently distorted [81]. During initiation of MMR, MutS heteodimers likely recognize mismatched DNA by the flexible regions of DNA containing both helix distortion and poor base stacking, to distinguish the mismatched DNA as a substrate for MMR processing [82,83]. Recently, by using DNA repair factors fused to the E. coli lac-repressor (LacR) which can be recruited to the chromosomally integrated lac operator sequence (lacO) in NIH-3T3 cells, Soutoglou and Misteli (2008) found that recruitment of Nbs1, MRE11, MDC1, or ATM to the lacO loci activated a DNA damage response in the absence of DNA damage [84].
Formation of non-B DNA structures generally results in discontinuity and distortion of the DNA double helix, either within the structure (e.g., H-DNA and G-DNA) or at the junctions of the non-B and B-forms of DNA (e.g., hairpins/cruciforms and Z-DNA) and may be recognized as “damage” by DNA repair proteins. For example, the Saccharomyces cerevisiae Mre11 protein can bind to G4 DNA with much higher affinity than G-rich DNA in the canonical B-form [85]. Given that recruitment of Mre11 or other DNA repair proteins at non-damaged DNA is sufficient to initiate a DNA damage response [84], it is possible that non-canonical DNA structures may also trigger DNA repair and cellular damage responses in the absence of DNA damage per se.
DNA structure-induced cellular DNA damage responses
Lahiri et al. (2004) [86] determined the effect of expanded CAG repeats on DNA damage responses in a yeast artificial chromosome system. The study was designed to determine if checkpoint deficiency would allow the replication to progress through the repetitive sequence without removing the deleterious structure and result in a higher breakage frequency. Mutation of the checkpoint factors Mec1, Ddc2, Rad9, Rad17, Rad24, or Rad53 gave rise to increased CAG repeat instability, indicating that (the DNA structure formed at) CAG repeats could activate these checkpoint and repair proteins [86]. Interestingly, Chk1-mediated G2 arrest was not required for CAG repeat-induced processing [86]. Long tracts of CAG(175) repeats on plasmids were also recognized as “damage” in E. coli, as evidenced by the induction of an SOS response [87]. However, shorter CAG(25–79) repeats did not appear to induce an SOS response and the SOS system itself did not affect the stability of CAG repeats in E. coli [88]. Intron 21 of the human PKD1 gene contains a 2.5-kb polypurine-polypyrimidine tract that can form an H-DNA structure, and it is thought to contribute to the high mutation rate of the PKD1 gene. This polypurine-polypyrimidine tract in plasmids induced an SOS response and delayed host cell growth, resulting in a dramatic decrease in colony-forming capability. This effect of the polypurine-polypyrimidine tract on host cell colony-forming capability was reduced in a uvrA deficient strain (~100-fold decrease in colony-forming capability versus 500-fold in isogenic wild-type cells), and was almost diminished in uvrB or uvrC deficient strain [89], However, whether or not NER proteins bind directly to polypurine-polypyrimidine tracts is still not known. These data provide evidence for a non-B DNA-structure induced cellular response in the absence of DNA damage.
Structure-induced DNA cleavage and mutagenesis
We speculate that cellular DNA damage responses initiated by non-B DNA structures slow cell cycle progression providing the DNA repair machinery time to remove the “damage”. This process is likely involved in DNA structure-induced cleavage and mutagenesis that has been identified in the absence of DNA replication or exogenous DNA damaging agents (see below). In fact, many types of non-B DNA structures induce mutations that are more complex than simple deletions or insertions within the repeats as proposed in the slippage and misalignment model during replication. For example, in mammalian cells, one half of a palindromic repeat was completely deleted while the other half was maintained (one-sided deletion) [11]. This type of mutation is the result of a breakpoint that occurs at the tip of the hairpin or cruciform structure [11,90,91]. Depending on the lengths of the arms, hairpin structures are processed with different efficiencies in mammalian cells. Short hairpins (≤14 nt) are typically cleaved on the complementary un-structured strand [92,93], and repair on non-palindromic loops of the same size or longer hairpins (~26–40 nt) often occurs at the structured strand and results in deletion of the sequence that forms a loop/hairpin structure [93–97]. Although the substrate DNA containing the inverted repeats was replicated in these studies, it is possible that the cleavage occurred independent of DNA replication [93–97]. Our laboratory has found that H-DNA and Z-DNA structures induced DNA single-strand breaks and DSBs in HeLa cell-free extracts in the absence of DNA replication or DNA damage, and caused large-scale deletions and rearrangements which can not be explained by a slippage/misalignment model ([15,18] and our unpublished results). These data suggest a structure-specific cleavage and DNA strand break model of non-B DNA-induced mutagenesis in vivo.
The Mre11/Rad50/Nbs1 complex accumulates at DSBs to process the ends of the breakpoints. However, Mre11 also exhibits an incision activity at hairpin/cruciform structures [98]. Although MRN has not been shown to bind to a hairpin/cruciform structure directly, it interacts with BRCA1 which preferentially binds to four-way branched DNA structures (which are similar to cruciforms) [99]. After recruitment of the MRN complex to the cruciform structure, BRCA1 dissociates from the DNA [100], and activates the nuclease activity of the MRN complex. Multiple lines of evidence support an activity of the MRN complex on long (>160 bp) palindromic DNA sequences [9,98,101,102]. In yeast, MRN generates DSBs at or near the inverted repeat at an early stage during pre-meiotic replication, and the Rec12 endonuclease processes hairpin structures to produce DSBs at a later stage, both resulting in meiotic recombination [102]. Spo11, the human ortholog of Rec12 also exhibited nuclease activity at hairpin structures [103,104], and induced DSBs at multiple sites within a CAG(79) repeat, resulting in both expansions and contractions [105]. SbcCD, the bacterial homologs of Rad50 and Mre11, binds to hairpin structures and cleaves at the intact looped strand in the absence of nicks or gaps [106,107]. This breakage can be further extended by ATP-dependent double-stranded DNA exonuclease activity of the SbcCD complex [106]. Deficiency in SbcCD dramatically stabilized a long (571 bp) DNA palindrome, demonstrating the importance of this complex in DNA structure-induced instability [108]. In addition to its activity on cruciform structures, purified yeast Mre11 binds to G4 DNA and forms a stable complex even in the presence of 0.2–1.0 M NaCl [85]. After binding, the intrinsic endonuclease activity of Mre11 cleaved G4 DNA in a Mn2+-dependent manner, at sites different from the cleavage sites observed at single-stranded G-rich DNA [85], suggesting a structure-, but not sequence-specific endonuclease activity on G4 DNA.
As discussed above, the discontinuity and distortion of the DNA helix induced by non-B DNA structures may serve as a signal to recruit MMR or NER proteins and induce repair processes. Single-base or small loops of several nucleotides are efficiently repaired by the MMR mechanism [109]. Larger loop mismatches (12–247 nt) are repaired by an MSH2-independent mechanism with a bias toward using the non-looped strand as the template during repair synthesis, resulting in loss of the loop [94,95]. Kirkpatrick and Petes (1997) reported that repair of 26-base loops in yeast involved both the MMR protein, Msh2 and the NER protein, Rad1 [110]. A 12-base cruciform was repaired with a 2:1 bias toward loop retention, with the bias shifting from loop retention to loop loss with increasing loop size [93,95,111,112]. The human MSH2-MSH3 complex can recognize and bind to cruciform structures formed at perfect inverted repeats, and the affinity is enhanced when the hairpin contains mismatched (e.g. CAG repeats) [113]. Moreover, binding of MSH2-MSH3 to mismatches in the hairpin stem formed at CAG repeats might activate repair mechanisms [114]. The function of the MMR proteins in CAG repeat instability appears to differ among different species. Deficiencies in either mutS, mutL, or mutH greatly reduced large deletion events (greater than 8 repeats) at CAG(180) repeats in E. coli [115], and increased the small deletion and expansion events caused by misalignment [116]. In yeast, loops and cruciform structures were repaired less efficiently than in bacterial and mammalian cells [117], and the absence of Msh2 did not significantly affect the frequency of large deletions at a CAG(50) repeat [118] or large expansions at a CAG(25) repeat [52]. However, yeast deficient in Msh2 did show an increase in the small deletions or additions (of one repeat unit) generated by small loops due to misalignment that are typically repaired by MMR [119]. In mouse models of Huntington’s disease or myotonic dystrophy carrying long CNG repeats, MMR appears to be required for repeat instability, as evidenced by reduced repeat instability in the absence of MSH2 [120], MSH3 [121], or PMS2 [122]. Another mouse model carrying >300 CTG repeats from the human DM1 gene showed that absence of MSH2 shifted the mutations toward deletions rather than expansions, although the mutation frequency was not dramatically altered [123]. In cultured human cells, MSH2 and MSH3 are also involved in the transcription-induced deletion resulting from a CAG(95) repeat [124]. Similar effects of MMR proteins on other types of repeats have also been reported. For example, in E. coli a GC(15) repeat induced both short (<2 bp) and long (>2 bp) deletion events, which are likely caused by two different mechanisms. Deficiency of either mutH, mutL, or mutS enhanced the frequency of 2 bp deletions, indicating a role for MMR in small loop misalignment, but showed no effect on larger deletion events [125].
The bacterial NER protein, UvrA has been shown to bind to bubble and loop regions in duplex DNA [110,126], and is also involved in CAG triplet repeat deletion on plasmids in E. coli [127]. The UvrA protein binds to loops/hairpins of 1–17 CAG repeats on plasmids with a much higher affinity (Kd~15 nM) than to the same sequence in linear DNA [127]. In addition, UvrA binding was able to stimulate subsequent efficient excision on structured CTG repeats [127]. Deficiency of the NER genes, uvrC or uvrD greatly enhanced the instability of a CAG(68) repeat in bacteria, whereas uvrA or uvrB deficiency stabilized CAG tracts [128]. In contrast, Parniewski et al. (1999) reported that uvrA deficiency dramatically increased the instability of CAG repeats, and uvrB deficiency stabilized the CAG tract on plasmids in bacteria [129]. The reason for these discrepancies or why NER factors may have different effects on CAG repeats is not clear, and further studies are warranted. In cultured human cells, knocking-down XPA (as well as MMR proteins, MSH2 or MSH3), but not XPC, significantly reduced the transcription-induced deletion on CAG repeats in a chromosomal context, suggesting that transcription-coupled NER might be involved in CAG repeat instability [124].
In addition to loop or cruciform structures, we found that H-DNA-induced mutagenesis occurs in HeLa cell-free extracts in the absence of replication, suggesting a role for structure-specific cleavage (e.g., DNA repair) activities in inducing mutations in mammalian cells (Wang et al, unpublished results). In MSH2-deficient human Hec59 cells, or XPA-deficient human XP12RO cells, H-DNA-induced mutation frequencies were lower than those found in control cells, implicating MSH2 and XPA in the mutagenic process. However, the plasmids isolated from the MSH2 or XPA-proficient versus deficient cells showed no obvious differences in the amount or location of H-DNA-induced DSBs. These results suggest that while MSH2 and XPA are involved in the structure-induced mutagenesis, MMR or NER pathways are not directly involved in generating the DSBs, consistent with our recent finding that MLH1 deficiency did not affect the H-DNA-induced mutation in human cells (Wang et al, unpublished results). A slightly different result was obtained in E. coli. The H-DNA-forming sequence from the human PKD1 gene induced large deletions in E. coli, and MutS or MutL deficiency dramatically reduced the H-DNA-induced mutation frequency, implicating a role for the MMR pathway in H-DNA metabolism in bacteria [130].
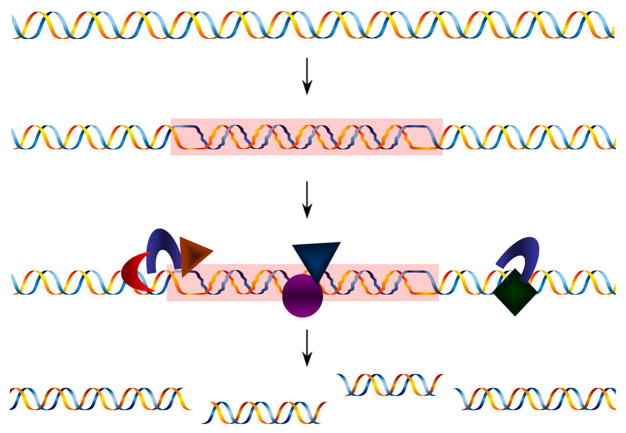
Several proteins have been shown to have nuclease activities on non-B DNA structures. For example, DNA topoisomerase II binds to and cleaves hairpins (e.g. hairpin structure formed at a negatively supercoiled 52-bp palindromic sequence in the human beta-globin gene [131]), but not cruciforms [132]. It cleaves a. Drosophila melanogaster DNA topoisomerase II cuts Z-DNA structures on negatively supercoiled minicircles [133]. DNA topoisomerase II cleavage sites near human immunodeficiency virus integration sites in the human genome consist of Z-DNA-forming sequences and other repetitive sequence elements [134], suggesting that non-B DNA structures are substrates for DNA topoisomerase II. The cruciform structure is similar to the recombination intermediate four-way Holliday junction, such that the Holliday junction resolvases might also have activity on cruciforms formed at inverted repeats. H-DNA and G4 DNA structures expose an unstructured single-stranded DNA region, which may also serve as substrates for single-strand specific nucleases. The studies described above clearly support a model of “DNA structure-specific cleavage” by DNA repair proteins, followed by error-generating repair processes at the breakpoints (Figure 2). If the structured DNA itself is recognized as damage in the genome, this could stimulate gratuitous repair on undamaged DNA, which can occur repeatedly regardless of the replication status until the sequence is mutated and non-B DNA formation is no longer favored.
Figure 2.
“DNA structure-specific cleavage” model. A non-B DNA structure formed on non-damaged DNA (shown in the figure as a Z-DNA structure) can be mistakenly recognized as a site of damage. The recruited DNA repair machinery generates breaks within or surrounding the non-B DNA structure, followed by error-generating repair of the breaks. This “structure forming-repair” cycle can occur repeatedly as a futile cycle until the sequence is mutated.
ACCUMULATION OF DNA DAMAGE AT NON-B DNA STRUCTURES
The DNA is constantly being damaged and repaired in living cells. On average, ~19,000 DNA damage incidences occur per cell per day [135], as measured by an alkaline sucrose gradient sedimentation method in human fibroblasts. This number varies among cell type and cellular environment, and the damaged sites are not randomly distributed. The type of DNA sequence motifs to be preferentially attacked depends upon the chemical and/or physical nature of the assaulting agent and the DNA base composition and structure. Some physical characteristics of non-B DNA structures suggest that they may be more susceptible to DNA damage than the canonical B-DNA structure. Although information on the genome-wide distribution of DNA damage and mutation is not available, UV irradiation has been shown to induce a mutation hotspot at a site in the potential hairpin loop of quasi-palindromic sequences in the lacI reporter gene in E. coli [136]. UV irradiation also caused significant chromatin fragmentation at repetitive sequences in mammalian cells, particularly at GT repeats (a Z-DNA-forming sequence) and long stretches of homopurine/homopyrimidine regions (potential H-DNA-forming sequences) [137]. It is not clear whether the accumulated mutations were caused by higher levels of UV damage in the non-B DNA-forming sequences, or due to a lower repair efficiency in the structured regions, or both. In fact, non-B DNA plays an important role in both DNA damage formation and repair efficiency and fidelity, as discussed below.
In canonical B-form DNA the base-pairing between strands is protected within the helix. However, single-stranded regions are exposed in non-B DNA structures such as H-DNA, G4 DNA, the tips of a hairpins or looped structures, and in B–Z junctions of Z-DNA, which are less stable than duplex DNA. Supporting this notion, negatively supercoiled DNA (an important molecular force that drives the formation of non-B DNA structures), and single-stranded DNA were more reactive to 4-Nitroquinoline 1-oxide (4-NQO) than relaxed duplex DNA [138], and B-Z junctions in Z-DNA were hyperreactivity to 4-NQO [139], hydroxylamine, and osmium tetraoxide [140]. Moreover, in a Z-DNA structure, the guanosine nucleotides are in a syn position where the bases are located over the sugar without protection and thus could be more accessible to DNA damaging agents [141]. Diethylpyrocarbonate reacts more strongly with purines in a left-handed Z-DNA structure, and guanines in Z-DNA are more reactive with dimethylsulfate and diethylsulfate [140]. Ionizing radiation preferentially attacks the guanine sites in the Z-DNA structure, while the cytosine sites are more resistant, leading to a characteristic signature damaged pattern of the Z-form that differs from damaged sites seen on B-DNA [142].
In addition, damage occurring to the DNA in a Z conformation is more resistant to processing by DNA repair enzymes. For example, N7-methylguanine, which is typically removed by a DNA glycosylase, was not efficiently repaired when present in Z-DNA, and when the Z-DNA conformation was converted into B-DNA by adding EtBr, the N7-methylguanine became a good substrate for DNA glycosylase [143,144]. O6-methylguanine in alkylated poly(dG-m5dC). poly(dG-m5dC) in the Z conformation was repaired with an efficiency only 10% of that measured on B-DNA [145]. A single nucleotide loop that was otherwise removed efficiently by MMR, was not processed by the repair machinery when present in the stem of a hairpin structure formed at a 33 nt palindromic sequence in S. cerevisiae [112]. It not yet clear how or at which step the non-B DNA structure interferes with the repair process, i.e. the recognition step, recruitment of subsequent repair factors, enzymatic cleavage, excision, DNA synthesis, and/or ligation.
DNA is well organized and packaged into chromatin in vivo, and both DNA damage and repair occur in the context of the chromatin structure. Tertiary chromatin organization in regions of open chromatin is also associated with genetic instability [146–148]. The structures of non-B DNA conformations may interfere with DNA-histone interactions and the non-B DNA regions are less likely to be assembled into nucleosomes. The abnormal positioning or absence of nucleosomes can also change the sensitivity of non-B DNA regions to modification by genotoxic agents. For example, DNA in the Z-form can be refractory to nucleosome formation [149–151], probably because the interaction of the Z-DNA and the positively charged arginines on the histone core are not aligned properly relative to each other [152]. Although the data available are from Z-DNA, it is not hard to imagine that other types of non-B DNA structures may be refractory to nucleosome formation. At an early stage of apoptosis initiated by very low UV doses in rat leukemia cells, the fragmented DNA contained a four-fold excess of repetitive sequences (e.g. H-DNA and Z-DNA-forming sequences), suggesting that these non-B DNA-forming sequences were in an open chromatin structure, rendering them more susceptible to enzymatic cleavage [137].
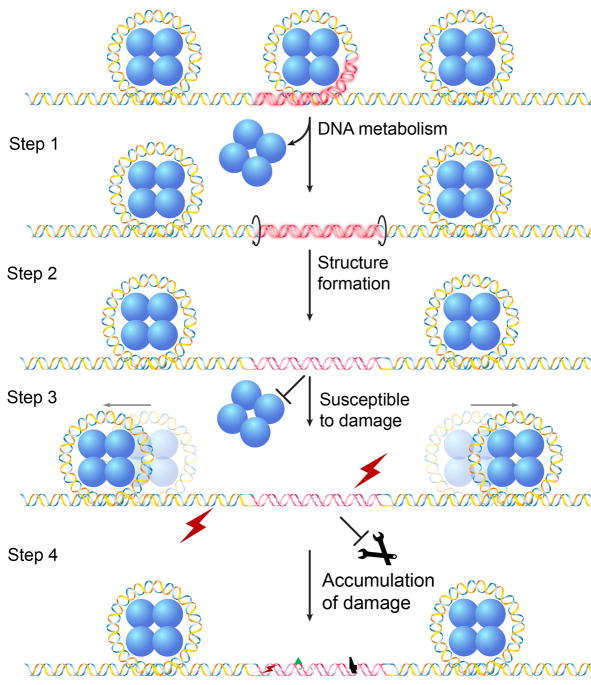
Based on these observations, we propose a model of “accumulation of DNA damage” at non-B DNA structures (Figure 3), where formation of a non-B DNA structure in the chromosome is favored at an open chromatin region, and this structured region is now refractory to nucleosome assembly and remains open. These modified physical characteristics may increase susceptibility of this particular region to DNA damage and/or enzymatic cleavage or DNA damage, resulting in increased localized genetic instability.
Figure 3.
“Accumulation of DNA damage in non-B DNA structures” model. Unwrapping of a non-B DNA-forming sequence (highlighted in red) from the histone core during DNA metabolism, e.g., DNA replication, transcription, or repair (Step 1), facilitates the non-B DNA conformation (Step 2, shown in the figure is a Z-DNA structure). The non-B DNA conformation is more susceptible to DNA damage and the damage in the non-B DNA region is more resistant to repair (Step 3), leading to accumulated damage in this region (Step 4).
TRANSCRIPTION AND RECOMBINATION-MEDIATED NON-B DNA-INDUCED MUTAGENESIS
DNA transcription opens the chromatin structure, unwinds the DNA helix, exposes single-stranded DNA, and generates negative supercoiling behind the translocating polymerase complex. These processes generate conditions that are conducive to the formation of non-B DNA structures. For example, in permeabilized nuclei from mammalian cells, binding of anti-Z-DNA antibodies to fragments of the c-MYC gene near the promoter was observed only when the c-MYC gene was actively transcribed [153–155]. For more information on the role of transcription in repeat instability, see the review by Lin et al in this issue. In collaboration with the Hanawalt group, we recently discovered that T7 RNA polymerase transcription was partially blocked within and downstream of the H-DNA-forming homopurine-homopyrimidine mirror repeat sequence from the human c-MYC promoter region, and within CG(14) Z-DNA-forming sequences [156,157]. Similarly, G4-forming sequences inhibit transcription by T7 RNA polymerase and mammalian RNA polymerase II [158]. The transcription blockage was dependent on negative supercoiling of the template, and was enhanced in multi-round transcription, implicating the non-B DNA structures in the transcription arrest [156,157]. Detailed discussion regarding this topic can be found in the review by Tornaletti et al. in this issue. These findings suggest the possibility that stalled RNA polymerase machinery might recruit and activate transcription-coupled NER at the non-B DNA region, resulting in genetic instability.
Recombination is another DNA metabolic event that can occur in non-dividing cells and is one of the mechanisms implicated in triplet repeat instability. RecA or RecB deficiency led to decreased CAG repeat contraction of up to 70-fold or 1700-fold, respectively [88]. Z-DNA structures are also correlated with recombination hotspots in eukaryotic cells [159,160]. An ~1,000 bp region in the major histocompatibility complex in mice containing several copies of long GT repeats is a recombination hotspot and accounts for up to 2% of recombination on the entire chromosome [161,162]. H-DNA-forming sequences are also mapped at hotspots of recombination, such as sites of unequal sister chromatin exchange in the Cγ2a and Cγ2b heavy chain genes in the MPC-11 mouse myeloma cell line [163]. Although structural features of some non-B DNA conformations might facilitate recombination, such as H- and Z-DNA [164], it is also possible that the non-B DNA related recombination events are induced by DNA breakage within or near the non-B DNA structure, which occurs spontaneously (i.e., the “structure-specific cleavage model”) or induced by DNA damaging factors (i.e., “repair-induced non-B DNA structure-related genetic instability”) [102,165,166].
SUMMARY
Non-B DNA structures distinguish themselves from the rest of the genome of B-DNA in several aspects, and may recruit and trigger repair cleavage activity in the absence of DNA damage per se. These types of structures occur in more open regions of the chromatin and are more susceptible to DNA damage. In addition, repair of DNA damage can induce the formation of non-B DNA structures, resulting in mutagenesis. Since DNA damage and repair are active processes in living cells regardless of the replication status, models of DNA repair-associated mutagenesis at non-B DNA structures proposed in this review may explain some observations of non-B DNA-induced genetic instability in non-dividing (or slowly dividing) cells such as the brain and muscle. However, many questions remain to be answered, and different types of non-B DNA structures may have unique processing pathways and should be interpreted separately. The enzymes that recognize and/or process each type of non-B DNA structure may differ and the proteins that have been found to be associated with non-B DNA instability might participate in an unexpected way. For example, MSH2 and XPA proteins are both involved in instability of CAG repeats and H-DNA-forming sequences, but this may be through some as yet un-identified functions, evidenced by the finding that knocking down both proteins did not further reduce repeat contraction, suggesting a new role for these proteins in the same pathway [124]. We have found that MSH2 and XPA are involved in H-DNA metabolism but that the DNA structure may not be processed via canonical MMR or NER mechanisms (Wang et al., unpublished results). Future studies to address these questions could lead to a better understanding of the mechanism(s) of non-B DNA-induced genetic instability and related disease development.
Acknowledgments
This work was supported by an NCI grant to K.M.V. (CA093729), an NIEHS Center grant ES07784, and an Odyssey Fellowship (to G.W.) supported by the Odyssey Program and the Theodore N. Law Award for Scientific Achievement at The University of Texas M.D. Anderson Cancer Center.
ABBREVIATIONS
- DSBs
double strand breaks
- NER
nucleotide excision repair
- MMR
mismatch repair
- BER
base excision repair
- DM1
type 1 myotonic dystrophy
References
- 1.Sinden RR. DNA structure and function. San Diego: Academic Press; 1994. p. xxiii.p. 398. [Google Scholar]
- 2.Bacolla A, Wojciechowska M, Kosmider B, Larson JE, Wells RD. The involvement of non-B DNA structures in gross chromosomal rearrangements. DNA Repair (Amst) 2006 doi: 10.1016/j.dnarep.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 3.Wells RD. Non-B DNA conformations, mutagenesis and disease. Trends Biochem Sci. 2007 doi: 10.1016/j.tibs.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Collins J. Instability of palindromic DNA in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):409–416. doi: 10.1101/sqb.1981.045.01.055. [DOI] [PubMed] [Google Scholar]
- 5.Collins J, Volckaert G, Nevers P. Precise and nearly-precise excision of the symmetrical inverted repeats of Tn5; common features of recA-independent deletion events in Escherichia coli. Gene. 1982;19(1):139–146. doi: 10.1016/0378-1119(82)90198-6. [DOI] [PubMed] [Google Scholar]
- 6.Kurahashi H, Emanuel BS. Long AT-rich palindromes and the constitutional t(11;22) breakpoint. Hum Mol Genet. 2001;10(23):2605–2617. doi: 10.1093/hmg/10.23.2605. [DOI] [PubMed] [Google Scholar]
- 7.Edelmann L, Spiteri E, Koren K, et al. AT-rich palindromes mediate the constitutional t(11;22) translocation. Am J Hum Genet. 2001;68(1):1–13. doi: 10.1086/316952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurahashi H, Shaikh TH, Hu P, Roe BA, Emanuel BS, Budarf ML. Regions of genomic instability on 22q11 and 11q23 as the etiology for the recurrent constitutional t(11;22) Hum Mol Genet. 2000;9(11):1665–1670. doi: 10.1093/hmg/9.11.1665. [DOI] [PubMed] [Google Scholar]
- 9.Farah JA, Hartsuiker E, Mizuno K, Ohta K, Smith GR. A 160-bp palindrome is a Rad50. nRad32-dependent mitotic recombination hotspot in Schizosaccharomyces pombe. Genetics. 2002;161(1):461–468. doi: 10.1093/genetics/161.1.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasar F, Jankowski C, Nag DK. Long palindromic sequences induce double-strand breaks during meiosis in yeast. Mol Cell Biol. 2000;20(10):3449–3458. doi: 10.1128/mcb.20.10.3449-3458.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akgun E, Zahn J, Baumes S, et al. Palindrome resolution and recombination in the mammalian germ line. Mol Cell Biol. 1997;17(9):5559–5570. doi: 10.1128/mcb.17.9.5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malfoy B, Rousseau N, Vogt N, Viegas-Pequignot E, Dutrillaux B, Leng M. Nucleotide sequence of an heterochromatic segment recognized by the antibodies to Z-DNA in fixed metaphase chromosomes. Nucleic Acids Res. 1986;14(8):3197–3214. doi: 10.1093/nar/14.8.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnston BH. Generation and detection of Z-DNA. Methods Enzymol. 1992;211:127–158. doi: 10.1016/0076-6879(92)11009-8. [DOI] [PubMed] [Google Scholar]
- 14.Freund AM, Bichara M, Fuchs RP. Z-DNA-forming sequences are spontaneous deletion hot spots. Proc Natl Acad Sci U S A. 1989;86(19):7465–7469. doi: 10.1073/pnas.86.19.7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang G, Christensen LA, Vasquez KM. Z-DNA-forming sequences generate large-scale deletions in mammalian cells. Proc Natl Acad Sci U S A. 2006;103(8):2677–2682. doi: 10.1073/pnas.0511084103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohno M, Fukagawa T, Lee JS, Ikemura T. Triplex-forming DNAs in the human interphase nucleus visualized in situ by polypurine/polypyrimidine DNA probes and antitriplex antibodies. Chromosoma. 2002;111(3):201–213. doi: 10.1007/s00412-002-0198-0. [DOI] [PubMed] [Google Scholar]
- 17.Agazie Y, Burkholder GD, Lee JS. Triplex DNA in the nucleus: Direct binding of triplex-specific antibodies and their effect on transcription, replication and cell growth. Biochem J. 1996;316:461–466. doi: 10.1042/bj3160461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang G, Vasquez KM. Naturally occurring H-DNA-forming sequences are mutagenic in mammalian cells. Proc Natl Acad Sci U S A. 2004;101(37):13448–13453. doi: 10.1073/pnas.0405116101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Djian P. Evolution of simple repeats in DNA and their relation to human disease. Cell. 1998;94(2):155–160. doi: 10.1016/s0092-8674(00)81415-4. [DOI] [PubMed] [Google Scholar]
- 20.Mitas M. Trinucleotide repeats associated with human disease. Nucleic Acids Res. 1997;25(12):2245–2254. doi: 10.1093/nar/25.12.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caskey CT, Pizzuti A, Fu YH, Fenwick RG, Jr, Nelson DL. Triplet repeat mutations in human disease. Science. 1992;256(5058):784–789. doi: 10.1126/science.1589758. [DOI] [PubMed] [Google Scholar]
- 22.Di Prospero NA, Fischbeck KH. Therapeutics development for triplet repeat expansion diseases. Nat Rev Genet. 2005;6(10):756–765. doi: 10.1038/nrg1690. [DOI] [PubMed] [Google Scholar]
- 23.Warren ST, Ashley CT., Jr Triplet repeat expansion mutations: the example of fragile X syndrome. Annual review of neuroscience. 1995;18:77–99. doi: 10.1146/annurev.ne.18.030195.000453. [DOI] [PubMed] [Google Scholar]
- 24.Latha KS, Anitha S, Rao KS, Viswamitra MA. Molecular understanding of aluminum-induced topological changes in (CCG)12 triplet repeats: relevance to neurological disorders. Biochim Biophys Acta. 2002;1588(1):56–64. doi: 10.1016/s0925-4439(02)00133-3. [DOI] [PubMed] [Google Scholar]
- 25.Potaman VN, Oussatcheva EA, Lyubchenko YL, et al. Length-dependent structure formation in Friedreich ataxia (GAA)n*(TTC)n repeats at neutral pH. Nucleic Acids Res. 2004;32(3):1224–1231. doi: 10.1093/nar/gkh274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakamoto N, Chastain PD, Parniewski P, et al. Sticky DNA: self-association properties of long GAA.TTC repeats in R.R. Y triplex structures from Friedreich’s ataxia. Mol Cell. 1999;3(4):465–475. doi: 10.1016/s1097-2765(00)80474-8. [DOI] [PubMed] [Google Scholar]
- 27.Freudenreich CH, Kantrow SM, Zakian VA. Expansion and length-dependent fragility of CTG repeats in yeast. Science. 1998;279(5352):853–856. doi: 10.1126/science.279.5352.853. [DOI] [PubMed] [Google Scholar]
- 28.Parrish JE, Oostra BA, Verkerk AJ, et al. Isolation of a GCC repeat showing expansion in FRAXF, a fragile site distal to FRAXA and FRAXE. Nat Genet. 1994;8(3):229–235. doi: 10.1038/ng1194-229. [DOI] [PubMed] [Google Scholar]
- 29.Hewett DR, Handt O, Hobson L, et al. FRA10B structure reveals common elements in repeat expansion and chromosomal fragile site genesis. Mol Cell. 1998;1(6):773–781. doi: 10.1016/s1097-2765(00)80077-5. [DOI] [PubMed] [Google Scholar]
- 30.Echlin-Bell DR, Smith LL, Li L, et al. Polymorphisms in the MLL breakpoint cluster region (BCR) Hum Genet. 2003;113(1):80–91. doi: 10.1007/s00439-003-0936-2. [DOI] [PubMed] [Google Scholar]
- 31.Wiener F, Ohno S, Babonits M, et al. Hemizygous interstitial deletion of chromosome 15 (band D) in three translocation-negative murine plasmacytomas. Proc Natl Acad Sci U S A. 1984;81(4):1159–1163. doi: 10.1073/pnas.81.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akasaka T, Akasaka H, Ueda C, et al. Molecular and clinical features of non-Burkitt’s, diffuse large-cell lymphoma of B-cell type associated with the c-MYC/immunoglobulin heavy-chain fusion gene. J Clin Oncol. 2000;18(3):510–518. doi: 10.1200/JCO.2000.18.3.510. [DOI] [PubMed] [Google Scholar]
- 33.Kovalchuk AL, Muller JR, Janz S. Deletional remodeling of c-myc-deregulating chromosomal translocations. Oncogene. 1997;15(19):2369–2377. doi: 10.1038/sj.onc.1201409. [DOI] [PubMed] [Google Scholar]
- 34.Adachi M, Tsujimoto Y. Potential Z-DNA elements surround the breakpoints of chromosome translocation within the 5′ flanking region of bcl-2 gene. Oncogene. 1990;5(11):1653–1657. [PubMed] [Google Scholar]
- 35.Seite P, Leroux D, Hillion J, et al. Molecular analysis of a variant 18;22 translocation in a case of lymphocytic lymphoma. Genes Chromosomes Cancer. 1993;6(1):39–44. doi: 10.1002/gcc.2870060108. [DOI] [PubMed] [Google Scholar]
- 36.Sen D, Gilbert W. A sodium-potassium switch in the formation of four-stranded G4-DNA. Nature. 1990;344(6265):410–414. doi: 10.1038/344410a0. [DOI] [PubMed] [Google Scholar]
- 37.Hoyne PR, Maher LJ., 3rd Functional studies of potential intrastrand triplex elements in the Escherichia coli genome. J Mol Biol. 2002;318(2):373–386. doi: 10.1016/S0022-2836(02)00041-4. [DOI] [PubMed] [Google Scholar]
- 38.Hile SE, Eckert KA. Positive correlation between DNA polymerase alpha-primase pausing and mutagenesis within polypyrimidine/polypurine microsatellite sequences. J Mol Biol. 2004;335(3):745–759. doi: 10.1016/j.jmb.2003.10.075. [DOI] [PubMed] [Google Scholar]
- 39.Rao BS. Pausing of simian virus 40 DNA replication fork movement in vivo by (dG-dA)n. (dT-dC)n tracts. Gene. 1994;140(2):233–237. doi: 10.1016/0378-1119(94)90549-5. [DOI] [PubMed] [Google Scholar]
- 40.Krasilnikova MM, Mirkin SM. Replication stalling at Friedreich’s ataxia (GAA)n repeats in vivo. Mol Cell Biol. 2004;24(6):2286–2295. doi: 10.1128/MCB.24.6.2286-2295.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voineagu I, Narayanan V, Lobachev KS, Mirkin SM. Replication stalling at unstable inverted repeats: interplay between DNA hairpins and fork stabilizing proteins. Proc Natl Acad Sci U S A. 2008;105(29):9936–9941. doi: 10.1073/pnas.0804510105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samadashwily GM, Raca G, Mirkin SM. Trinucleotide repeats affect DNA replication in vivo. Nat Genet. 1997;17(3):298–304. doi: 10.1038/ng1197-298. [DOI] [PubMed] [Google Scholar]
- 43.Martorell L, Monckton DG, Gamez J, et al. Progression of somatic CTG repeat length heterogeneity in the blood cells of myotonic dystrophy patients. Hum Mol Genet. 1998;7(2):307–312. doi: 10.1093/hmg/7.2.307. [DOI] [PubMed] [Google Scholar]
- 44.Wong LJ, Ashizawa T, Monckton DG, Caskey CT, Richards CS. Somatic heterogeneity of the CTG repeat in myotonic dystrophy is age and size dependent. Am J Hum Genet. 1995;56(1):114–122. [PMC free article] [PubMed] [Google Scholar]
- 45.Martorell L, Martinez JM, Carey N, Johnson K, Baiget M. Comparison of CTG repeat length expansion and clinical progression of myotonic dystrophy over a five year period. J Med Genet. 1995;32(8):593–596. doi: 10.1136/jmg.32.8.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wohrle D, Kennerknecht I, Wolf M, Enders H, Schwemmle S, Steinbach P. Heterogeneity of DM kinase repeat expansion in different fetal tissues and further expansion during cell proliferation in vitro: evidence for a casual involvement of methyl-directed DNA mismatch repair in triplet repeat stability. Hum Mol Genet. 1995;4(7):1147–1153. doi: 10.1093/hmg/4.7.1147. [DOI] [PubMed] [Google Scholar]
- 47.Anvret M, Ahlberg G, Grandell U, Hedberg B, Johnson K, Edstrom L. Larger expansions of the CTG repeat in muscle compared to lymphocytes from patients with myotonic dystrophy. Hum Mol Genet. 1993;2(9):1397–1400. doi: 10.1093/hmg/2.9.1397. [DOI] [PubMed] [Google Scholar]
- 48.Thornton CA, Johnson K, Moxley RT., 3rd Myotonic dystrophy patients have larger CTG expansions in skeletal muscle than in leukocytes. Annals of neurology. 1994;35(1):104–107. doi: 10.1002/ana.410350116. [DOI] [PubMed] [Google Scholar]
- 49.Zatz M, Passos-Bueno MR, Cerqueira A, Marie SK, Vainzof M, Pavanello RC. Analysis of the CTG repeat in skeletal muscle of young and adult myotonic dystrophy patients: when does the expansion occur? Hum Mol Genet. 1995;4(3):401–406. doi: 10.1093/hmg/4.3.401. [DOI] [PubMed] [Google Scholar]
- 50.Hartenstine MJ, Goodman MF, Petruska J. Base stacking and even/odd behavior of hairpin loops in DNA triplet repeat slippage and expansion with DNA polymerase. J Biol Chem. 2000;275(24):18382–18390. doi: 10.1074/jbc.275.24.18382. [DOI] [PubMed] [Google Scholar]
- 51.Kang S, Jaworski A, Ohshima K, Wells RD. Expansion and deletion of CTG repeats from human disease genes are determined by the direction of replication in E. coli Nat Genet. 1995;10(2):213–218. doi: 10.1038/ng0695-213. [DOI] [PubMed] [Google Scholar]
- 52.Miret JJ, Pessoa-Brandao L, Lahue RS. Orientation-dependent and sequence-specific expansions of CTG/CAG trinucleotide repeats in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1998;95(21):12438–12443. doi: 10.1073/pnas.95.21.12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trinh TQ, Sinden RR. Preferential DNA secondary structure mutagenesis in the lagging strand of replication in E. coli Nature. 1991;352(6335):544–547. doi: 10.1038/352544a0. [DOI] [PubMed] [Google Scholar]
- 54.Hashem VI, Sinden RR. Duplications between direct repeats stabilized by DNA secondary structure occur preferentially in the leading strand during DNA replication. Mutat Res. 2005;570(2):215–226. doi: 10.1016/j.mrfmmm.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 55.Iyer RR, Wells RD. Expansion and deletion of triplet repeat sequences in Escherichia coli occur on the leading strand of DNA replication. J Biol Chem. 1999;274(6):3865–3877. doi: 10.1074/jbc.274.6.3865. [DOI] [PubMed] [Google Scholar]
- 56.Pelletier R, Krasilnikova MM, Samadashwily GM, Lahue R, Mirkin SM. Replication and expansion of trinucleotide repeats in yeast. Mol Cell Biol. 2003;23(4):1349–1357. doi: 10.1128/MCB.23.4.1349-1357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spiro C, Pelletier R, Rolfsmeier ML, et al. Inhibition of FEN-1 processing by DNA secondary structure at trinucleotide repeats. Mol Cell. 1999;4(6):1079–1085. doi: 10.1016/s1097-2765(00)80236-1. [DOI] [PubMed] [Google Scholar]
- 58.Iyer RR, Pluciennik A, Rosche WA, Sinden RR, Wells RD. DNA polymerase III proofreading mutants enhance the expansion and deletion of triplet repeat sequences in Escherichia coli. J Biol Chem. 2000;275(3):2174–2184. doi: 10.1074/jbc.275.3.2174. [DOI] [PubMed] [Google Scholar]
- 59.Levinson G, Gutman GA. Slipped-strand mispairing: a major mechanism for DNA sequence evolution. Molecular biology and evolution. 1987;4(3):203–221. doi: 10.1093/oxfordjournals.molbev.a040442. [DOI] [PubMed] [Google Scholar]
- 60.Chong SS, McCall AE, Cota J, et al. Gametic and somatic tissue-specific heterogeneity of the expanded SCA1 CAG repeat in spinocerebellar ataxia type 1. Nat Genet. 1995;10(3):344–350. doi: 10.1038/ng0795-344. [DOI] [PubMed] [Google Scholar]
- 61.Telenius H, Kremer B, Goldberg YP, et al. Somatic and gonadal mosaicism of the Huntington disease gene CAG repeat in brain and sperm. Nat Genet. 1994;6(4):409–414. doi: 10.1038/ng0494-409. [DOI] [PubMed] [Google Scholar]
- 62.Hashida H, Goto J, Kurisaki H, Mizusawa H, Kanazawa I. Brain regional differences in the expansion of a CAG repeat in the spinocerebellar ataxias: dentatorubral-pallidoluysian atrophy, Machado-Joseph disease, and spinocerebellar ataxia type 1. Annals of neurology. 1997;41(4):505–511. doi: 10.1002/ana.410410414. [DOI] [PubMed] [Google Scholar]
- 63.Ansved T, Lundin A, Anvret M. Larger CAG expansions in skeletal muscle compared with lymphocytes in Kennedy disease but not in Huntington disease. Neurology. 1998;51(5):1442–1444. doi: 10.1212/wnl.51.5.1442. [DOI] [PubMed] [Google Scholar]
- 64.Gomes-Pereira M, Fortune MT, Monckton DG. Mouse tissue culture models of unstable triplet repeats: in vitro selection for larger alleles, mutational expansion bias and tissue specificity, but no association with cell division rates. Hum Mol Genet. 2001;10(8):845–854. doi: 10.1093/hmg/10.8.845. [DOI] [PubMed] [Google Scholar]
- 65.Tanaka H, Tapscott SJ, Trask BJ, Yao MC. Short inverted repeats initiate gene amplification through the formation of a large DNA palindrome in mammalian cells. Proc Natl Acad Sci U S A. 2002;99(13):8772–8777. doi: 10.1073/pnas.132275999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Butler DK, Yasuda LE, Yao MC. Induction of large DNA palindrome formation in yeast: implications for gene amplification and genome stability in eukaryotes. Cell. 1996;87(6):1115–1122. doi: 10.1016/s0092-8674(00)81805-x. [DOI] [PubMed] [Google Scholar]
- 67.Narayanan V, Mieczkowski PA, Kim HM, Petes TD, Lobachev KS. The pattern of gene amplification is determined by the chromosomal location of hairpin-capped breaks. Cell. 2006;125(7):1283–1296. doi: 10.1016/j.cell.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 68.VanHulle K, Lemoine FJ, Narayanan V, et al. Inverted DNA repeats channel repair of distant double-strand breaks into chromatid fusions and chromosomal rearrangements. Mol Cell Biol. 2007;27(7):2601–2614. doi: 10.1128/MCB.01740-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marcadier JL, Pearson CE. Fidelity of primate cell repair of a double-strand break within a (CTG).(CAG) tract. Effect of slipped DNA structures. J Biol Chem. 2003;278(36):33848–33856. doi: 10.1074/jbc.M304284200. [DOI] [PubMed] [Google Scholar]
- 70.Hebert ML, Spitz LA, Wells RD. DNA double-strand breaks induce deletion of CTG. CAG repeats in an orientation-dependent manner in Escherichia coli. J Mol Biol. 2004;336(3):655–672. doi: 10.1016/j.jmb.2003.12.038. [DOI] [PubMed] [Google Scholar]
- 71.Hebert ML, Wells RD. Roles of double-strand breaks, nicks, and gaps in stimulating deletions of CTG. CAG repeats by intramolecular DNA repair. J Mol Biol. 2005;353(5):961–979. doi: 10.1016/j.jmb.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 72.Pollard LM, Bourn RL, Bidichandani SI. Repair of DNA double-strand breaks within the (GAA*TTC)n sequence results in frequent deletion of the triplet-repeat sequence. Nucleic Acids Res. 2008;36(2):489–500. [Google Scholar]
- 73.Paques F, Leung WY, Haber JE. Expansions and contractions in a tandem repeat induced by double-strand break repair. Mol Cell Biol. 1998;18(4):2045–2054. doi: 10.1128/mcb.18.4.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Slebos RJ, Oh DS, Umbach DM, Taylor JA. Mutations in tetranucleotide repeats following DNA damage depend on repeat sequence and carcinogenic agent. Cancer Res. 2002;62(21):6052–6060. [PubMed] [Google Scholar]
- 75.Yamada NA, Parker JM, Farber RA. Mutation frequency analysis of mononucleotide and dinucleotide repeats after oxidative stress. Environ Mol Mutagen. 2003;42(2):75–84. doi: 10.1002/em.10179. [DOI] [PubMed] [Google Scholar]
- 76.Mirkin SM. Toward a unified theory for repeat expansions. Nat Struct Mol Biol. 2005;12(8):635–637. doi: 10.1038/nsmb0805-635. [DOI] [PubMed] [Google Scholar]
- 77.Kovtun IV, Liu Y, Bjoras M, Klungland A, Wilson SH, McMurray CT. OGG1 initiates age-dependent CAG trinucleotide expansion in somatic cells. Nature. 2007;447(7143):447–452. doi: 10.1038/nature05778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hess MT, Schwitter U, Petretta M, Giese B, Naegeli H. Bipartite substrate discrimination by human nucleotide excision repair. Proc Natl Acad Sci U S A. 1997;94(13):6664–6669. doi: 10.1073/pnas.94.13.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sugasawa K, Okamoto T, Shimizu Y, Masutani C, Iwai S, Hanaoka F. A multistep damage recognition mechanism for global genomic nucleotide excision repair. Genes Dev. 2001;15(5):507–521. doi: 10.1101/gad.866301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang W. Poor base stacking at DNA lesions may initiate recognition by many repair proteins. DNA Repair (Amst) 2006;5(6):654–666. doi: 10.1016/j.dnarep.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 81.Mu D, Sancar A. Model for XPC-independent transcription-coupled repair of pyrimidine dimers in humans. J Biol Chem. 1997;272(12):7570–7573. doi: 10.1074/jbc.272.12.7570. [DOI] [PubMed] [Google Scholar]
- 82.Antony E, Khubchandani S, Chen S, Hingorani MM. Contribution of Msh2 and Msh6 subunits to the asymmetric ATPase and DNA mismatch binding activities of Saccharomyces cerevisiae Msh2-Msh6 mismatch repair protein. DNA Repair (Amst) 2006;5(2):153–162. doi: 10.1016/j.dnarep.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Natrajan G, Lamers MH, Enzlin JH, Winterwerp HH, Perrakis A, Sixma TK. Structures of Escherichia coli DNA mismatch repair enzyme MutS in complex with different mismatches: a common recognition mode for diverse substrates. Nucleic Acids Res. 2003;31(16):4814–4821. doi: 10.1093/nar/gkg677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Soutoglou E, Misteli T. Activation of the cellular DNA damage response in the absence of DNA lesions. Science. 2008;320(5882):1507–1510. doi: 10.1126/science.1159051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ghosal G, Muniyappa K. Saccharomyces cerevisiae Mre11 is a high-affinity G4 DNA-binding protein and a G-rich DNA-specific endonuclease: implications for replication of telomeric DNA. Nucleic Acids Res. 2005;33(15):4692–4703. doi: 10.1093/nar/gki777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lahiri M, Gustafson TL, Majors ER, Freudenreich CH. Expanded CAG repeats activate the DNA damage checkpoint pathway. Mol Cell. 2004;15(2):287–293. doi: 10.1016/j.molcel.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 87.Majchrzak M, Bowater RP, Staczek P, Parniewski P. SOS repair and DNA supercoiling influence the genetic stability of DNA triplet repeats in Escherichia coli. J Mol Biol. 2006;364(4):612–624. doi: 10.1016/j.jmb.2006.08.093. [DOI] [PubMed] [Google Scholar]
- 88.Hashem VI, Rosche WA, Sinden RR. Genetic recombination destabilizes (CTG)n.(CAG)n repeats in E. coli. Mutat Res. 2004;554(1–2):95–109. doi: 10.1016/j.mrfmmm.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 89.Bacolla A, Jaworski A, Connors TD, Wells RD. Pkd1 unusual DNA conformations are recognized by nucleotide excision repair. J Biol Chem. 2001;276(21):18597–18604. doi: 10.1074/jbc.M100845200. [DOI] [PubMed] [Google Scholar]
- 90.Lewis S, Akgun E, Jasin M. Palindromic DNA and genome stability. Further studies. Ann N Y Acad Sci. 1999;870:45–57. doi: 10.1111/j.1749-6632.1999.tb08864.x. [DOI] [PubMed] [Google Scholar]
- 91.Cunningham LA, Cote AG, Cam-Ozdemir C, Lewis SM. Rapid, stabilizing palindrome rearrangements in somatic cells by the center-break mechanism. Mol Cell Biol. 2003;23(23):8740–8750. doi: 10.1128/MCB.23.23.8740-8750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Deng WP, Nickoloff JA. Mismatch repair of heteroduplex DNA intermediates of extrachromosomal recombination in mammalian cells. Mol Cell Biol. 1994;14(1):400–406. doi: 10.1128/mcb.14.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Taghian DG, Hough H, Nickoloff JA. Biased short tract repair of palindromic loop mismatches in mammalian cells. Genetics. 1998;148(3):1257–1268. doi: 10.1093/genetics/148.3.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weiss U, Wilson JH. Repair of single-stranded loops in heteroduplex DNA transfected into mammalian cells. Proc Natl Acad Sci U S A. 1987;84(6):1619–1623. doi: 10.1073/pnas.84.6.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bill CA, Taghian DG, Duran WA, Nickoloff JA. Repair bias of large loop mismatches during recombination in mammalian cells depends on loop length and structure. Mutat Res. 2001;485(3):255–265. doi: 10.1016/s0921-8777(01)00065-9. [DOI] [PubMed] [Google Scholar]
- 96.Chuang YK, Cheng WC, Goodman SD, et al. Nick-Directed Repair of Palindromic Loop Mismatches in Human Cell Extracts. J Biomed Sci. 2005 doi: 10.1007/s11373-005-7891-y. [DOI] [PubMed] [Google Scholar]
- 97.Miller CA, Bill CA, Nickoloff JA. Characterization of palindromic loop mismatch repair tracts in mammalian cells. DNA Repair (Amst) 2004;3(4):421–428. doi: 10.1016/j.dnarep.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 98.Trujillo KM, Sung P. DNA structure-specific nuclease activities in the Saccharomyces cerevisiae Rad50*Mre11 complex. J Biol Chem. 2001;276(38):35458–35464. doi: 10.1074/jbc.M105482200. [DOI] [PubMed] [Google Scholar]
- 99.Paull TT, Cortez D, Bowers B, Elledge SJ, Gellert M. Direct DNA binding by Brca1. Proc Natl Acad Sci U S A. 2001;98(11):6086–6091. doi: 10.1073/pnas.111125998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.De la Torre C, Pincheira J, Lopez-Saez JF. Human syndromes with genomic instability and multiprotein machines that repair DNA double-strand breaks. Histol Histopathol. 2003;18(1):225–243. doi: 10.14670/HH-18.225. [DOI] [PubMed] [Google Scholar]
- 101.Butler DK, Gillespie D, Steele B. Formation of large palindromic DNA by homologous recombination of short inverted repeat sequences in Saccharomyces cerevisiae. Genetics. 2002;161(3):1065–1075. doi: 10.1093/genetics/161.3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Farah JA, Cromie G, Steiner WW, Smith GR. A novel recombination pathway initiated by the Mre11/Rad50/Nbs1 complex eliminates palindromes during meiosis in Schizosaccharomyces pombe. Genetics. 2005;169(3):1261–1274. doi: 10.1534/genetics.104.037515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jankowski C, Nag DK. Most meiotic CAG repeat tract-length alterations in yeast are SPO11 dependent. Mol Genet Genomics. 2002;267(1):64–70. doi: 10.1007/s00438-001-0635-4. [DOI] [PubMed] [Google Scholar]
- 104.Lobachev KS, Gordenin DA, Resnick MA. The Mre11 complex is required for repair of hairpin-capped double-strand breaks and prevention of chromosome rearrangements. Cell. 2002;108(2):183–193. doi: 10.1016/s0092-8674(02)00614-1. [DOI] [PubMed] [Google Scholar]
- 105.Jankowski C, Nasar F, Nag DK. Meiotic instability of CAG repeat tracts occurs by double-strand break repair in yeast. Proc Natl Acad Sci U S A. 2000;97(5):2134–2139. doi: 10.1073/pnas.040460297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Connelly JC, Leach DR. The sbcC and sbcD genes of Escherichia coli encode a nuclease involved in palindrome inviability and genetic recombination. Genes Cells. 1996;1(3):285–291. doi: 10.1046/j.1365-2443.1996.23024.x. [DOI] [PubMed] [Google Scholar]
- 107.Connelly JC, Kirkham LA, Leach DR. The SbcCD nuclease of Escherichia coli is a structural maintenance of chromosomes (SMC) family protein that cleaves hairpin DNA. Proc Natl Acad Sci U S A. 1998;95(14):7969–7974. doi: 10.1073/pnas.95.14.7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chalker AF, Leach DR, Lloyd RG. Escherichia coli sbcC mutants permit stable propagation of DNA replicons containing a long palindrome. Gene. 1988;71(1):201–205. doi: 10.1016/0378-1119(88)90092-3. [DOI] [PubMed] [Google Scholar]
- 109.Duval A, Hamelin R. Genetic instability in human mismatch repair deficient cancers. Ann Genet. 2002;45(2):71–75. doi: 10.1016/s0003-3995(02)01115-2. [DOI] [PubMed] [Google Scholar]
- 110.Kirkpatrick DT, Petes TD. Repair of DNA loops involves DNA-mismatch and nucleotide-excision repair proteins. Nature. 1997;387(6636):929–931. doi: 10.1038/43225. [DOI] [PubMed] [Google Scholar]
- 111.Tran H, Degtyareva N, Gordenin D, Resnick MA. Altered replication and inverted repeats induce mismatch repair-independent recombination between highly diverged DNAs in yeast. Mol Cell Biol. 1997;17(2):1027–1036. doi: 10.1128/mcb.17.2.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nag DK, Kurst A. A 140-bp-long palindromic sequence induces double-strand breaks during meiosis in the yeast Saccharomyces cerevisiae. Genetics. 1997;146(3):835–847. doi: 10.1093/genetics/146.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Owen BA, Yang Z, Lai M, et al. (CAG)(n)-hairpin DNA binds to Msh2-Msh3 and changes properties of mismatch recognition. Nat Struct Mol Biol. 2005;12(8):663–670. doi: 10.1038/nsmb965. [DOI] [PubMed] [Google Scholar]
- 114.Pearson CE, Ewel A, Acharya S, Fishel RA, Sinden RR. Human MSH2 binds to trinucleotide repeat DNA structures associated with neurodegenerative diseases. Hum Mol Genet. 1997;6(7):1117–1123. doi: 10.1093/hmg/6.7.1117. [DOI] [PubMed] [Google Scholar]
- 115.Jaworski A, Rosche WA, Gellibolian R, et al. Mismatch repair in Escherichia coli enhances instability of (CTG)n triplet repeats from human hereditary diseases. Proc Natl Acad Sci U S A. 1995;92(24):11019–11023. doi: 10.1073/pnas.92.24.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Parniewski P, Jaworski A, Wells RD, Bowater RP. Length of CTG. CAG repeats determines the influence of mismatch repair on genetic instability. J Mol Biol. 2000;299(4):865–874. doi: 10.1006/jmbi.2000.3796. [DOI] [PubMed] [Google Scholar]
- 117.Nag DK, Petes TD. Seven-base-pair inverted repeats in DNA form stable hairpins in vivo in Saccharomyces cerevisiae. Genetics. 1991;129(3):669–673. doi: 10.1093/genetics/129.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Miret JJ, Pessoa-Brandao L, Lahue RS. Instability of CAG and CTG trinucleotide repeats in Saccharomyces cerevisiae. Mol Cell Biol. 1997;17(6):3382–3387. doi: 10.1128/mcb.17.6.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schweitzer JK, Livingston DM. Destabilization of CAG trinucleotide repeat tracts by mismatch repair mutations in yeast. Hum Mol Genet. 1997;6(3):349–355. doi: 10.1093/hmg/6.3.349. [DOI] [PubMed] [Google Scholar]
- 120.Manley K, Shirley TL, Flaherty L, Messer A. Msh2 deficiency prevents in vivo somatic instability of the CAG repeat in Huntington disease transgenic mice. Nat Genet. 1999;23(4):471–473. doi: 10.1038/70598. [DOI] [PubMed] [Google Scholar]
- 121.van den Broek WJ, Nelen MR, Wansink DG, et al. Somatic expansion behaviour of the (CTG)n repeat in myotonic dystrophy knock-in mice is differentially affected by Msh3 and Msh6 mismatch-repair proteins. Hum Mol Genet. 2002;11(2):191–198. doi: 10.1093/hmg/11.2.191. [DOI] [PubMed] [Google Scholar]
- 122.Gomes-Pereira M, Fortune MT, Ingram L, McAbney JP, Monckton DG. Pms2 is a genetic enhancer of trinucleotide CAG. CTG repeat somatic mosaicism: implications for the mechanism of triplet repeat expansion. Hum Mol Genet. 2004;13(16):1815–1825. doi: 10.1093/hmg/ddh186. [DOI] [PubMed] [Google Scholar]
- 123.Savouret C, Brisson E, Essers J, et al. CTG repeat instability and size variation timing in DNA repair-deficient mice. Embo J. 2003;22(9):2264–2273. doi: 10.1093/emboj/cdg202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lin Y, Dion V, Wilson JH. Transcription promotes contraction of CAG repeat tracts in human cells. Nat Struct Mol Biol. 2006;13(2):179–180. doi: 10.1038/nsmb1042. [DOI] [PubMed] [Google Scholar]
- 125.Schumacher S, Fuchs RP, Bichara M. Two distinct models account for short and long deletions within sequence repeats in Escherichia coli. J Bacteriol. 1997;179(20):6512–6517. doi: 10.1128/jb.179.20.6512-6517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ahn B, Grossman L. The binding of UvrAB proteins to bubble and loop regions in duplex DNA. J Biol Chem. 1996;271(35):21462–21470. doi: 10.1074/jbc.271.35.21462. [DOI] [PubMed] [Google Scholar]
- 127.Oussatcheva EA, Hashem VI, Zou Y, Sinden RR, Potaman VN. Involvement of the nucleotide excision repair protein UvrA in instability of CAG*CTG repeat sequences in Escherichia coli. J Biol Chem. 2001;276(33):30878–30884. doi: 10.1074/jbc.M104697200. [DOI] [PubMed] [Google Scholar]
- 128.Szwarocka ST, Staczek P, Parniewski P. Chromosomal model for analysis of a long CTG/CAG tract stability in wild-type Escherichia coli and its nucleotide excision repair mutants. Can J Microbiol. 2007;53(7):860–868. doi: 10.1139/W07-047. [DOI] [PubMed] [Google Scholar]
- 129.Parniewski P, Bacolla A, Jaworski A, Wells RD. Nucleotide excision repair affects the stability of long transcribed (CTG*CAG) tracts in an orientation-dependent manner in Escherichia coli. Nucleic Acids Res. 1999;27(2):616–623. doi: 10.1093/nar/27.2.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bacolla A, Jaworski A, Larson JE, et al. Breakpoints of gross deletions coincide with non-B DNA conformations. Proc Natl Acad Sci U S A. 2004;101(39):14162–14167. doi: 10.1073/pnas.0405974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee GE, Kim JH, Chung IK. Topoisomerase II-mediated DNA cleavage on the cruciform structure formed within the 5′ upstream region of the human beta-globin gene. Mol Cells. 1998;8(4):424–430. [PubMed] [Google Scholar]
- 132.Froelich-Ammon SJ, Gale KC, Osheroff N. Site-specific cleavage of a DNA hairpin by topoisomerase II. DNA secondary structure as a determinant of enzyme recognition/cleavage. J Biol Chem. 1994;269(10):7719–7725. [PubMed] [Google Scholar]
- 133.Glikin GC, Jovin TM, Arndt-Jovin DJ. Interactions of Drosophila DNA topoisomerase II with left-handed Z-DNA in supercoiled minicircles. Nucleic Acids Res. 1991;19(25):7139–7144. doi: 10.1093/nar/19.25.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Howard MT, Griffith JD. A cluster of strong topoisomerase II cleavage sites is located near an integrated human immunodeficiency virus. J Mol Biol. 1993;232(4):1060–1068. doi: 10.1006/jmbi.1993.1460. [DOI] [PubMed] [Google Scholar]
- 135.Vilenchik MM, Knudson AG., Jr Inverse radiation dose-rate effects on somatic and germ-line mutations and DNA damage rates. Proc Natl Acad Sci U S A. 2000;97(10):5381–5386. doi: 10.1073/pnas.090099497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Todd PA, Glickman BW. Mutational specificity of UV light in Escherichia coli: indications for a role of DNA secondary structure. Proc Natl Acad Sci U S A. 1982;79(13):4123–4127. doi: 10.1073/pnas.79.13.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Luokkamaki M, Servomaa K, Rytaomaa T. Onset of chromatin fragmentation in chloroma cell apoptosis is highly sensitive to UV and begins at non-B DNA conformation. Int J Radiat Biol. 1993;63(2):207–213. doi: 10.1080/09553009314550271. [DOI] [PubMed] [Google Scholar]
- 138.Panigrahi GB, Walker IG. The N2-guanine adduct but not the C8-guanine or N6-adenine adducts formed by 4-nitroquinoline 1-oxide blocks the 3′-5′ exonuclease action of T4 DNA polymerase. Biochemistry. 1990;29(8):2122–2126. doi: 10.1021/bi00460a023. [DOI] [PubMed] [Google Scholar]
- 139.Rodolfo C, Lanza A, Tornaletti S, Fronza G, Pedrini AM. The ultimate carcinogen of 4-nitroquinoline 1-oxide does not react with Z-DNA and hyperreacts with B-Z junctions. Nucleic Acids Res. 1994;22(3):314–320. doi: 10.1093/nar/22.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Johnston BH, Rich A. Chemical probes of DNA conformation: detection of Z-DNA at nucleotide resolution. Cell. 1985;42(3):713–724. doi: 10.1016/0092-8674(85)90268-5. [DOI] [PubMed] [Google Scholar]
- 141.Zimmerman SB. The three-dimensional structure of DNA. Annu Rev Biochem. 1982;51:395–427. doi: 10.1146/annurev.bi.51.070182.002143. [DOI] [PubMed] [Google Scholar]
- 142.Tartier L, Michalik V, Spotheim-Maurizot M, Rahmouni AR, Sabattier R, Charlier M. Radiolytic signature of Z-DNA. Nucleic Acids Res. 1994;22(25):5565–5570. doi: 10.1093/nar/22.25.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lagravere C, Malfoy B, Leng M, Laval J. Ring-opened alkylated guanine is not repaired in Z-DNA. Nature. 1984;310(5980):798–800. doi: 10.1038/310798a0. [DOI] [PubMed] [Google Scholar]
- 144.Boiteux S, Costa de Oliveira R, Laval J. The Escherichia coli O6-methylguanine-DNA methyltransferase does not repair promutagenic O6-methylguanine residues when present in Z-DNA. J Biol Chem. 1985;260(15):8711–8715. [PubMed] [Google Scholar]
- 145.Boiteux S, Laval F. Repair of O6-methylguanine, by mammalian cell extracts, in alkylated DNA and poly(dG-m5dC) (poly dG-m5dC) in B and Z forms. Carcinogenesis. 1985;6(5):805–807. doi: 10.1093/carcin/6.5.805. [DOI] [PubMed] [Google Scholar]
- 146.Zhang Y, Rowley JD. Chromatin structural elements and chromosomal translocations in leukemia. DNA Repair (Amst) 2006;5(9–10):1282–1297. doi: 10.1016/j.dnarep.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 147.Strick R, Zhang Y, Emmanuel N, Strissel PL. Common chromatin structures at breakpoint cluster regions may lead to chromosomal translocations found in chronic and acute leukemias. Hum Genet. 2006;119(5):479–495. doi: 10.1007/s00439-006-0146-9. [DOI] [PubMed] [Google Scholar]
- 148.Strissel PL, Strick R, Rowley JD, Zeleznik-Le NJ. An in vivo topoisomerase II cleavage site and a DNase I hypersensitive site colocalize near exon 9 in the MLL breakpoint cluster region. Blood. 1998;92(10):3793–3803. [PubMed] [Google Scholar]
- 149.Wong B, Chen S, Kwon JA, Rich A. Characterization of Z-DNA as a nucleosome-boundary element in yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2007 doi: 10.1073/pnas.0611447104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Garner MM, Felsenfeld G. Effect of Z-DNA on nucleosome placement. J Mol Biol. 1987;196(3):581–590. doi: 10.1016/0022-2836(87)90034-9. [DOI] [PubMed] [Google Scholar]
- 151.Casasnovas JM, Azorin F. Supercoiled induced transition to the Z-DNA conformation affects the ability of a d(CG/GC)12 sequence to be organized into nucleosome-cores. Nucleic Acids Res. 1987;15(21):8899–8918. doi: 10.1093/nar/15.21.8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389(6648):251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 153.Wittig B, Dorbic T, Rich A. Transcription is associated with Z-DNA formation in metabolically active permeabilized mammalian cell nuclei. Proc Natl Acad Sci U S A. 1991;88(6):2259–2263. doi: 10.1073/pnas.88.6.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wolfl S, Wittig B, Rich A. Identification of transcriptionally induced Z-DNA segments in the human c-myc gene. Biochim Biophys Acta. 1995;1264(3):294–302. doi: 10.1016/0167-4781(95)00155-7. [DOI] [PubMed] [Google Scholar]
- 155.Wittig B, Wolfl S, Dorbic T, Vahrson W, Rich A. Transcription of human c-myc in permeabilized nuclei is associated with formation of Z-DNA in three discrete regions of the gene. Embo J. 1992;11(12):4653–4663. doi: 10.1002/j.1460-2075.1992.tb05567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Belotserkovskii BP, di Silva E, Tornaletti S, Wang G, Vasquez KM, Hanawalt PC. A triplex-forming sequence from the human c-Myc promoter interferes with DNA transcription. J Biol Chem. 2007 doi: 10.1074/jbc.M704618200. [DOI] [PubMed] [Google Scholar]
- 157.Ditlevson JV, Tornaletti S, Belotserkovskii BP, et al. Inhibitory effect of a short Z-DNA forming sequence on transcription elongation by T7 RNA polymerase. Nucleic Acids Res. 2008;36(10):3163–3170. doi: 10.1093/nar/gkn136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tornaletti S, Park-Snyder S, Hanawalt PC. G4-forming sequences in the non-transcribed DNA strand pose blocks to T7 RNA polymerase and mammalian RNA polymerase II. J Biol Chem. 2008;283(19):12756–12762. doi: 10.1074/jbc.M705003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Blaho JA, Wells RD. Left-handed Z-DNA and genetic recombination. Prog Nucleic Acid Res Mol Biol. 1989;37:107–126. doi: 10.1016/s0079-6603(08)60696-0. [DOI] [PubMed] [Google Scholar]
- 160.Wahls WP, Wallace LJ, Moore PD. The Z-DNA motif d(TG)30 promotes reception of information during gene conversion events while stimulating homologous recombination in human cells in culture. Mol Cell Biol. 1990;10(2):785–793. doi: 10.1128/mcb.10.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kobori JA, Strauss E, Minard K, Hood L. Molecular analysis of the hotspot of recombination in the murine major histocompatibility complex. Science. 1986;234(4773):173–179. doi: 10.1126/science.3018929. [DOI] [PubMed] [Google Scholar]
- 162.Wahls WP. Meiotic recombination hotspots: shaping the genome and insights into hypervariable minisatellite DNA change. Curr Top Dev Biol. 1998;37:37–75. doi: 10.1016/s0070-2153(08)60171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Weinreb A, Collier DA, Birshtein BK, Wells RD. Left-handed Z-DNA and intramolecular triplex formation at the site of an unequal sister chromatid exchange. J Biol Chem. 1990;265(3):1352–1359. [PubMed] [Google Scholar]
- 164.Wang G, Vasquez KM. Z-DNA, an active element in the genome. Front Biosci. 2007;12:4424–4438. doi: 10.2741/2399. [DOI] [PubMed] [Google Scholar]
- 165.Richard GF, Goellner GM, McMurray CT, Haber JE. Recombination-induced CAG trinucleotide repeat expansions in yeast involve the MRE11-RAD50-XRS2 complex. Embo J. 2000;19(10):2381–2390. doi: 10.1093/emboj/19.10.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nag DK, Pata JD, Sironi M, Flood DR, Hart AM. Both conserved and nonconserved regions of Spo11 are essential for meiotic recombination initiation in yeast. Mol Genet Genomics. 2006;276(4):313–321. doi: 10.1007/s00438-006-0143-7. [DOI] [PubMed] [Google Scholar]