Abstract
Succinyl-CoA synthetase (SCS) is the only mitochondrial enzyme capable of ATP production via substrate level phosphorylation in the absence of oxygen, but it also plays a key role in the citric acid cycle, ketone metabolism and heme synthesis. Inorganic phosphate (Pi) is a signaling molecule capable of activating oxidative phosphorylation at several sites, including NADH generation and as a substrate for ATP formation. In this study it was shown that Pi-binds porcine heart SCS α-subunit (SCSα) in a non-covalent manner and enhances its enzymatic activity, thereby providing a new target for Pi-activation in mitochondria. Coupling 32P-labeling of intact mitochondria with SDS gel electrophoresis revealed that 32P-labeling of SCSα was enhanced in substrate-depleted mitochondria. Using mitochondrial extracts and purified bacterial SCS (BSCS) it was shown that this enhanced 32P-labeling resulted from a simple binding of 32P, not covalent protein phosphorylation. The ability of SCSα to retain its 32P throughout the SDS denaturing gel process was unique over the entire mitochondrial proteome. In vitro studies also revealed a Pi-induced activation of SCS activity by more than 2-fold when mitochondrial extracts and purified BSCS were incubated with mM concentrations of Pi. Since 32P-binding to SCSα was increased in substrate-depleted mitochondria, where matrix Pi concentration is increased, we conclude that SCS activation by Pi-binding represents another mitochondrial target for the Pi-induced activation of oxidative phosphorylation and anaerobic ATP production in energy-limited mitochondria.
Keywords: Succinyl-CoA Synthetase, Phosphate Activation, Mitochondria, 32P, Proteomics
Protein phosphorylation is an important regulatory mechanism in the cytosol and mitochondrial matrix. 32P-labeling of intact mitochondria, coupled to SDS gel electrophoresis, is a useful technique for identifying phosphoproteins that actively undergo phosphate-exchange in the matrix (1-4). Previous studies from our lab revealed the dynamic nature of the mitochondrial phosphoproteome by exposing intact porcine heart mitochondria to exogenous 32P, which was transported into the matrix and converted to γ-32P-ATP for protein phosphorylation (1;2;4). Naturally, mitochondria must be energized to generate γ-32P-ATP via oxidative phosphorylation. The current work initially sought to screen for the energy-signaling systems within mitochondria by evaluating whether specific changes in 32P-incorporation occurred in substrate-depleted mitochondria, where matrix [ATP], membrane polarization and NADH are low and matrix [inorganic phosphate] and [ADP] are high. As shown here, the most pronounced result of these studies was the enhanced 32P-labeling of the α-subunit of succinyl-CoA synthetase (SCSα) in substrate-depleted mitochondria. The remainder of this work aimed to characterize the mechanism of increased 32P-association to SCSα and evaluate phosphate’s effect on succinyl-CoA synthetase (SCS) activity.
SCS is a Krebs Cycle enzyme that catalyzes substrate-level phosphorylation in the forward direction (5) and replenishes succinyl-CoA for ketone body catabolism (6) and porphyrin biosynthesis (7) in the reverse direction. In mammals two isoforms of SCS have been identified: one specific for ADP/ATP and another specific for GDP/GTP. SCS consists of a highly conserved α subunit and a β subunit that determines nucleotide specificity between ATP and GTP. The αβ complex is required for the SCS reaction to occur in either direction. In highly oxidative tissues, such as heart and skeletal muscle, the ATP-specific β-subunit transcript is more strongly expressed, whereas in tissues serving a biosynthetic role, such as liver, the GTP-specific transcript is preferential (8;9). In some species (i.e., Escherichia coli) SCS is not specific, using either the ATP or GTP (8). SCSα has a well documented histidine phosphorylation site (10-14), but has also been shown to bind phosphate in its de-phosphorylated form as a means of stabilizing the complex (8). Furthermore, SCS is the only enzyme capable of generating ATP in the absence of a proton motive force in the inner membrane, potentially playing a role in maintaining matrix ATP levels under energy-limited conditions, such as transient hypoxia (15-17).
Inorganic phosphate (Pi), a putative cytosolic signaling molecule, plays a multifaceted role in the regulation of mitochondrial metabolism. Pi can alter the free concentration of Mg2+ and Ca2+ ions (18-20), increase mitochondrial volume (21-23), influence the mitochondrial transition pore (24;25), and directly modify the activity of several Krebs cycle dehydrogenases (26-29). In a series of studies on intact mitochondria, Bose et al (30) showed that extra-mitochondrial Pi modulates oxidative phosphorylation at several levels, including substrate oxidation, membrane potential generation and through its well known effect as a substrate for ATP synthesis in Complex V. However, the specific sites of Pi-induced activation for substrate oxidation in mitochondria have not been completely identified (30).
The purpose of this study was to evaluate the mechanism of Pi-association to SCS under energy-limited conditions and to determine if SCS is another Pi-target for activating mitochondrial energetics. To accomplish this task, 32P-labeling of energized and substrate-depleted intact porcine heart mitochondria, protein extracts and purified bacterial SCS (BSCS) was studied with a variety of proteomics approaches. After establishing that simple Pi-binding was responsible for the enhanced 32P-labeling of SCSα in energy-limited mitochondria, the regulatory effect of Pi on SCS activity was determined using in-solution and in-gel assays in mitochondrial homogenates and purified BSCS.
Materials and Methods
Materials
Salts and inorganic phosphate (Pi) were purchased from Sigma (St. Louis, MO). 32P (10 mCi/ml) and γ-32P-ATP (10 mCi/ml) were purchased from PerkinElmer (Boston, MA). Unless otherwise stated, 2D gel electrophoresis reagents, equipment and software were purchased from GE Healthcare (Piscataway, NJ).
Isolation of Porcine Heart Mitochondria
All procedures were performed in accordance with the guidelines described in the Animal Care and Welfare Act (7 U.S.C. 2142 § 13) and approved by the NHLBI Animal Care and Use Committee. Porcine heart mitochondria were isolated as previously described (1). Unless otherwise noted, mitochondria were briefly exposed to 1 mM Pi during the isolation process to prevent matrix Pi depletion. Mitochondrial preparations were tested for viability by measuring the respiratory control ratio (RCR); to be accepted the RCR had to reach at least 8 (31). The mitochondrial protein concentration was determined using the USB Quant Kit (USB Corp., Cleveland, OH).
32P-Labeling of Intact Mitochondria
The standard experimental buffer for studies in this manuscript was Buffer A, composed of 125 mM KCl, 15 mM NaCl, 20 mM HEPES, 1 mM EGTA, 1 mM EDTA, 5 mM MgCl2, at pH 7.1. The pH was titrated separately at room temperature and 37°C. Pi was added as the potassium salt, pre-titrated to pH 7.1. To screen for protein phosphorylation, 32P was added to intact porcine heart mitochondria, as previously described (1). Briefly, mitochondria (1 mg/ml) were incubated with 32P (250 μCi/mg protein) in oxygenated Buffer A for 20 min at 37°C. Energized mitochondria were incubated with 5 mM potassium-glutamate and 5 mM potassium-malate (G/M); substrate-depleted mitochondria were incubated without carbon substrates. Unless otherwise stated, 32P-incorporation was quenched by adding an equal volume of 10% trichloro-acetic acid (TCA). For non-acid experiments, 100% methanol replaced TCA.
32P-Labeling of Mitochondrial Homogenates and Purified SCS
Mitochondria were solubilized to a concentration of 10 mg/ml in Buffer A with 1% dodecyl maltoside detergent and placed on ice for 30 min. Solubilized mitochondria and purified bacterial SCS (BSCS) from E.coli (Megazyme International, Wicklow, Ireland) were incubated with 32P at room temperature for 30 min, under a variety of conditions. Purified BSCS was used to confirm Pi-binding in these studies because it was commercially available at high purity. It is important to note that BSCS α-subunit (BSCSα) displays a large degree of amino acid sequence conservation with mammalian forms (8;32). Prior to the 32P incubation, purified BSCS was diluted with an equal volume of Buffer A and added to 5 μg of bovine serum albumin (BSA) to minimize protein loss during clean-up.
Purification and 32P-Labeling of the Mitochondrial Phosphate Carrier
The mitochondrial phosphate carrier (PiC) was purified from porcine heart mitochondria, as previously described (33). In addition to the PiC, two other bands (identified as the α- and β-subunits of Complex V) were components of the preparation. The PiC was incubated with 32P, as described above for purified SCS.
Two Dimensional SDS Gel Electrophoresis
Proteins were cleaned using a standard TCA/acetone protocol and re-suspended in lysis buffer (15 mM Tris-HCl, 7 M urea, 2 M thiourea, and 4% CHAPS (w/v)) to a final concentration of approximately 10 mg/ml. Two-hundred micrograms of sample were mixed with rehydration solution (7 M urea, 2 M thiourea, 4% CHAPS (w/v), 13 mM DTT, 1% (pH 3-11NL or pH 7-11NL) Pharmalyte (v/v), and 2 μl of Destreak reagent) to a final volume of 210 μl and placed on ice for 5 min before loading onto 11 cm Immobiline DryStrip gels (pH 3-11NL or pH 8-11, Sigma-Aldrich, St. Louis, MO). For pH 3-11NL Immobiline DryStrip gels, isoelectric focusing was achieved by active rehydration for 12 hr at 30 V followed by stepwise application of 500 V (1 hr), 1000 V (1 hr), gradient to 6000 V (2 hr) and final step at 7550 V (1.2 hr) for a total of ∼15,000 Vhr (Ettan IPG Phor2). For pH 8-11 Immobiline DryStrip gels, isoelectric focusing was achieved by active rehydration for 12 hr at 30 V followed by stepwise application of 500 V (1 hr), 1000 V (1 hr), gradient to 6000 V (2 hr) and final step at 65,000 V (11 hr) for a total of ∼78,000 Vhr.
Immobiline DryStrip gels were then equilibrated in 5 ml of SDS equilibration solution (50 mM Tris-HCl (pH 8.8), 6 M urea, 30% glycerol, and 2% SDS) for 10 min, first containing 100 mg of DTT and then 250 mg of iodoacetemide. Gel strips were applied to 8-16% Tris-HCl gels (Bio-Rad, Hercules, CA) and electrophoresis was performed in a Criterion Cell (Bio-Rad), with SDS electrophoresis buffer (25 mM Tris-HCl (pH 8.3), 192 mM glycine and 0.2% SDS) for ∼210 Vhr. Gels were stained for 2 hr with Coomassie Blue Solution (50% methanol, 3% phosphoric acid and 0.05% (w/v) Coomassie Blue G-250 (Bio-Rad)), followed by 2 hrs of destaining with 30% methanol and 3% phosphoric acid. For non-acid experiments, acid was omitted from the Coomassie Blue and destain solutions. Gels were dried, exposed to a phosphor-screen for 24 hrs and scanned on a Typhoon 9410 variable mode imager at a resolution of 100 μm, as previously described (1).
One Dimensional SDS Gel Electrophoresis
Various amounts of solubilized porcine heart mitochondria, purified BSCS and purified mitochondrial PiC were labeled with 32P at room temperature for 30 min. To ensure that the 32P-binding to BSCS was not an artifact of recombinant proteins, an equivalent amount of two additional purified recombinant proteins from E. coli (Complex I 30 kDa subunit and Peroxiredoxin 3 (GenWay Biotech, San Diego, CA)) were incubated with 32P at room temperature for 30 min. 32P-labeling was quenched with tricine sample buffer (Bio-Rad Laboratories, Hercules, CA) and run on a 16.5% tricine gel (Bio-Rad) with tricine-SDS electrophoresis buffer (100 mM Tris-HCl (pH 8.3), 100 mM tricine and 0.2% SDS) for ∼190 Vhr. The gel was stained, dried, exposed, and imaged as described above.
Blue-Native PAGE
Solubilized porcine heart mitochondria and purified BSCS were incubated with 32P as described above. The reaction was quenched by adding an equal volume of 2x Native PAGE buffer (Invitrogen, Carlsbad, CA) with 1% dodecyl maltoside. Samples were then processed for blue native (BN)-PAGE as previously described (34), with 100 μg of solubilized mitochondria and 10 μg of BSCS loaded per well. After electrophoresis gels were cut into individual BN-PAGE lanes and dried immediately or run in the second dimension. For 2D BN-PAGE, lanes were incubated in 5 ml of SDS equilibration solution with 100 mg of DTT for 10 min, applied to 10-15% SDS PAGE gels (Nextgen Sciences, Ann Arbor, MI) and sealed with 0.5% agarose containing bromophenol blue. Electrophoresis was performed in an Ettan DALT-12 tank in 20°C SDS electrophoresis buffer for ∼2150 Vhr. Gels were then stained with Coomassie Blue, dried, exposed, and imaged as described above.
Mass Spectrometry Identifications
Protein identifications were obtained in paired experiments from non-radioactive gels. Protein spots were manually picked using a 1.5 mm spot picker plus system (The gel Company, San Francisco, CA). Each protein spot was trypsin digested as previously described (35) and prepared for LC-MS/MS analysis. In descending order peptide digests #7 to #1 were analyzed using an LTQ-Orbitrap XL mass spectrometer (ThermoFisher, San Jose, CA). In each data collection cycle, one full high-resolution FTMS scan (m/z 300-2000) was acquired in the Orbitrap and then the 6 most abundant ions per cycle were fragmented and analyzed in the linear trap. Database searching was performed using the Mascot search engine (Matrix Science, Boston, MA; version 2.2). All MS/MS datasets were searched against the Swiss Prot mammal database (Swiss Institute of Bioinformatics; v14.9; 64199 sequences). Protein modifications were selected as carbamidomethyl (C) (fixed) and oxidation (M) (variable). Up to one missed cleavage was allowed.
Measurement of Succinyl-CoA Synthetase Activity
SCS activity was assayed in the direction of succinyl-CoA formation by measuring the rate of ATP hydrolysis in solubilized porcine heart mitochondria and purified BSCS. The SCS reaction was run in the reverse direction to avoid the substrate effects of Pi in the forward (ATP synthetic) direction. Porcine heart mitochondria were solubilized as described above and 1 mg was incubated with Pi (0 mM to 20 mM) for 30 min at room temperature. Modified from a previously described spectrophotometric method (36), samples were added to 1 ml of assay buffer, containing 50 mM succinate, 10 mM MgCl2, 0.3 mM CoA, 100 mM Tris, pH 7.5, and 15 μg oligomycin B/ml. After incubating for 2 min at 30°C, the assay was started with the addition of 0.5 mM ATP. Reactions were quenched by adding 150 μg of sample to an equal volume of cold 6% perchloric acid at 0, 1, 2.5, 5, 7.5, and 10 min. After 10 min samples were neutralized with 3 M K2CO3, in the presence of pH-indicator solution. ATP content was then measured using a luciferinluciferase kit (Invitrogen, Carlsbad, CA) with a standard curve of ATP. To ensure that ATP consumption was succinate-dependent, a sample without succinate and CoA was assayed; ATP consumption in this background sample was negligible. The activity of purified BSCS was assessed using the same method, with 100 μg of starting material and measuring ATP content in 15 μg of sample at the various time-points.
SCS activity was also assayed using a newly developed BN-PAGE method to quantify ATP and GTP hydrolysis. One hundred micrograms of purified SCS were diluted with 10 μl of Buffer A and incubated with [Pi] ranging from 0 mM to 10 mM for 30 min at room temperature. The reaction was quenched by adding 20 μl of 2x Native PAGE buffer (Invitrogen, Carlsbad, CA) with 1% dodecyl maltoside. Samples were processed for BN-PAGE as described above, with 10 μg of protein loaded per well. Using BN-PAGE is advantageous because it allows the SCS complex to remain in its intact, active form (37). After electrophoresis, gels were incubated in Buffer B [35 mM Tris-HCl, pH 7.5, 14 mM MgSO4 and 270 mM glycine at a pH 7.8] for 30 min at room temperature. Gels were then transferred to Buffer C [35 mM Tris-HCl, pH 7.5, 14 mM MgSO4, 270 mM glycine, 0.2% (w/v) Pb(NO3)2, 0.5 mM CoA, and 20 mM succinate, pH 7.8] with either 8 mM ATP or 8 mM GTP, for 1.5 hr at room temperature. Gels were fixed for 5 min in a solution containing 30% methanol and 3% phosphoric acid, and then imaged against a Coomassie blue colored background with infrared lights and a red filter (Wratten #25, Kodak, Rochester, NY). Images were converted to grayscale and bands were quantified using ImageQuant™ TL Software (GE Healthcare).
Statistical analysis
The data were analyzed using a student’s t-test. A p-value less than 0.01 was considered statistically significant.
Results
Two basic objectives were evaluated in this study: to characterize the mechanism of 32P-association to SCSα using SDS gel electrophoresis and to evaluate the functional significance of Pi-binding to SCS.
Effects of Mitochondrial Energetic State on 32P-Labeling
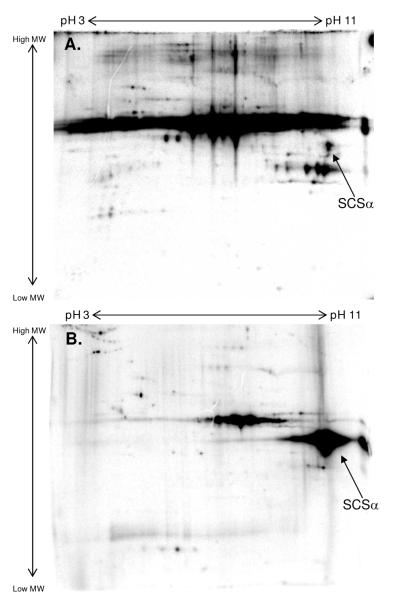
A comparison of porcine heart mitochondrial proteins labeled with 32P under energized and substrate-depleted conditions was evaluated using 2D gel electrophoresis. Substrate-depleted mitochondria represent a low-energy state, meaning that these mitochondria have a membrane potential but are unable to support sustained ATP production. Specifically, these substrate-depleted mitochondria have a membrane potential of about -80 mV and an NADH/NAD ratio of less than 0.10, compared to about -175 mV and 0.65 in mitochondria supplied with 5 mM glutamate and 5 mM malate (G/M). A comparison of 32P-labeled mitochondria under different energetic states is presented in Figure 1. Mitochondria energized with G/M showed 32P-incorporation for hundreds of proteins (Figure 1A). However, substrate-depleted mitochondria revealed decreased 32P-labeling for a majority of proteins, with the exception of SCSα which showed increased 32P-incorporation (Figure 1B). Mass spectrometry was used to identify this protein as SCSα in a paired, non-radioactive study.
Figure 1.
Energy-Sensitive 32P-Association for Succinyl-CoA Synthetase, α-Subunit. Intact porcine heart mitochondria were labeled with 32P under energized conditions with glutamate/malate (A) or substrate-depleted conditions without carbon substrates (B). Proteins were first separated by isoelectric focusing point, over a pH range of 3-11 NL, and then by molecular weight from ∼150 kDa to 10 kDa. The arrow in each panel highlights the 32P-labeling of succinyl-CoA synthetase, α-subunit following 2D SDS gel electrophoresis, where SCSα represents porcine heart mitochondrial succinyl-CoA synthetase, α-subunit.
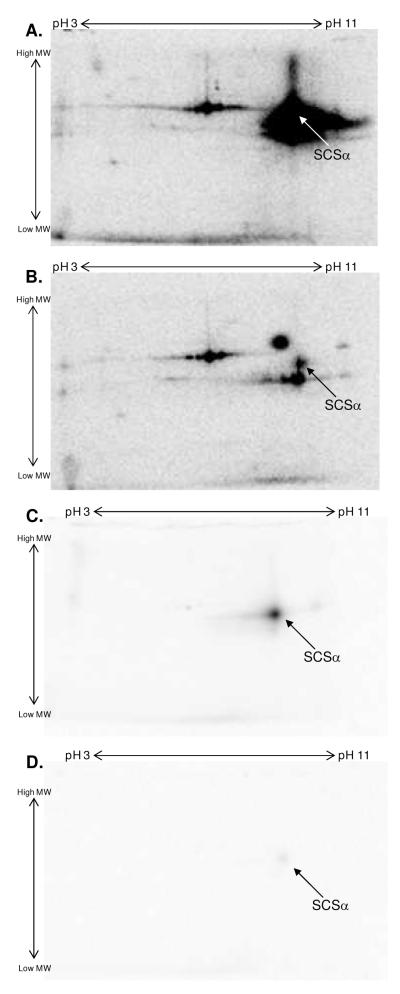
To further evaluate the mechanism of 32P-association to SCSα in substrate-depleted mitochondria, several labeling experiments were conducted with solubilized porcine heart mitochondria protein. To screen for kinase-mediated protein phosphorylation, including auto-phosphorylation, 400 μg of solubilized mitochondria were incubated with 40 μCi γ-32P-ATP for 30 min at room temperature. As observed in the intact 32P-labeling studies described above, intense 32P-incorporation was found in SCSα (Figure 2A). Since commercial preparations of γ-32P-ATP contain a degree of free 32P and γ-32P -ATP is hydrolyzed to ADP and 32Pi in mitochondrial homogenates, it is difficult to determine whether the radio-labeling is dependent on ATP or Pi. To evaluate the source of this binding, solubilized mitochondria were also incubated with a mixture of γ-32P-ATP and 5 mM Pi in a parallel experiment (Figure 2B). In the presence of 5 mM Pi, the 32P-labeling of SCS was decreased, suggesting that a majority of its 32P-incorporation resulted from a simple binding of Pi. Covalent bond formation requires energy. In the absence of an additional energy source, the only way that Pi can participate in this reaction is via a non-covalent binding mechanism. To confirm a non-covalent binding of Pi to SCSα, 400 μg of solubilized mitochondrial protein were incubated with 40 μCi 32P for 30 min at room temperature. As shown in Figure 2C, SCSα was the only mitochondrial protein that bound 32P in a manner that persisted throughout exposure to the denaturing properties of SDS. Furthermore, incubating solubilized mitochondria with a mixture of 40 μCi 32P and 5 mM Pi (Figure 2D) or incubating mitochondria with 40 μCi 32P for 15 min followed by 5 mM Pi for an additional 15 min (results not shown) displaced most of SCSα’s 32P-labeling, consistent with a simple Pi-binding mechanism. It is important to point out that we were unable to exchange out the 32P-bound to SCSα by soaking the SDS containing gel in a 5 mM Pi solution for 3 hrs. This result revealed that the binding of 32P to SCS was labile in the native protein, but extremely tight following denaturation with SDS. This “trapping” of phosphate by the SDS treatment explains why the 32P label can persist for hours in solutions containing essentially zero Pi during the SDS gel process.
Figure 2.
Enhanced 32P-Labeling by Succinyl-CoA Synthetase in Solubilized Mitochondria. Solubilized porcine heart mitochondria incubated with γ-32P-ATP (A) or a mixture of γ-32P-ATP and 5mM Pi (B). The arrow in panels A and B refers to succinyl-CoA synthetase, α-subunit. Solubilized porcine heart mitochondria incubated with 32P (C) or a mixture of 32P and 5mM Pi (D). Proteins were first separated by isoelectric focusing point, over a pH range of 3-11 NL, and then by molecular weight from ∼150 kDa to 10 kDa. In all panels, an arrow is used to indicate the 32P-binding to succinyl-CoA synthetase following 2D SDS gel electrophoresis, where SCSα represents porcine heart mitochondrial succinyl-CoA synthetase, α-subunit.
Confirmation of the Pi-Binding Protein as Succinyl-CoA Synthetase
With an isoelectric focusing point (pI) of ∼9.5 and a molecular weight of ∼35 kDa, SCSα is located in a very complex region of the 2D gel. To confirm the identity of this 32P-binding protein as SCSα, mass spectrometry was performed on a paired, non-radioactive gel that separated proteins across a narrow pH range (pH 8 to 11) (Supplemental Figure 1). Additionally, a stoichiometric amount of purified BSCS (15 μg) was incubated with 40 μCi 32P for 30 min at room temperature. As observed for solubilized porcine heart mitochondria (Figure 3A), purified BSCS also revealed intense 32P-incorporation for the conserved α-subunit (Figure 3B). Notably, purified BSCSα had a slightly lower molecular weight and a more acidic pI than porcine SCSα. These differences were utilized to conduct a mix experiment, where 400 μg of solubilized porcine heart mitochondria and 15 μg of purified BSCS were incubated together with 40 μCi 32P for 30 min at room temperature. Even in this complex mixture (Figure 3C) both forms of SCSα labeled with 32P, underlining the assignment of the porcine protein as SCSα.
Figure 3.
Confirmation of the Phosphate-Binding Protein as Succinyl-CoA Synthetase. Panel A shows 32P-labeling of solubilized porcine heart mitochondria. Panel B shows 32P-labeling of purified bacterial succinyl-CoA synthetase. Panel C shows 32P-labeling of a mixture of solubilized porcine heart mitochondria and purified bacterial succinyl-CoA synthetase. Proteins were first separated by isoelectric focusing point, over a pH range of 3-11 NL, and then by molecular weight from ∼150 kDa to 10 kDa. In all panels, an arrow is used to indicate the 32P-binding to succinyl-CoA synthetase following 2D SDS gel electrophoresis, where BSCSα represents purified bacterial succinyl-CoA synthetase, α-subunit and SCSα represents porcine heart mitochondrial succinyl-CoA synthetase, α-subunit.
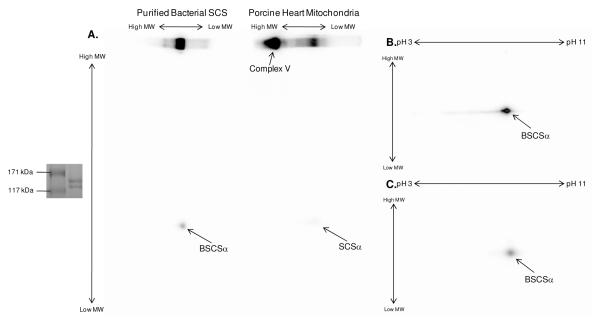
Since the mitochondrial Pi-carrier (PiC) protein binds Pi as a component of its mechanism and has very similar properties to SCSα (molecular weight of ∼35 kDa and pI of ∼9.5) (38-40), there was some concern that the 32P-binding observed in the current study was the result of underlying PiC, and not SCSα. To determine whether the PiC bound 32P in a manner that survived SDS gel electrophoresis, up to 25 μg of purified PiC were incubated with 40 μCi 32P for 30 min at room temperature. As shown in Figure 4, 32P-binding to PiC did not survive 1D or 2D SDS gel electrophoresis. Thus, these results confirmed that SCSα, and not the mitochondrial PiC, was responsible for the enhanced 32P-binding observed in substrate-depleted mitochondria. Furthermore, to ensure that the 32P-association to BSCS was not an artifact of recombinant proteins, purified Complex I 30 kDa subunit and Peroxiredoxin-3 were incubated with 32P under the same conditions; no 32P-binding was observed (results not shown).
Figure 4.
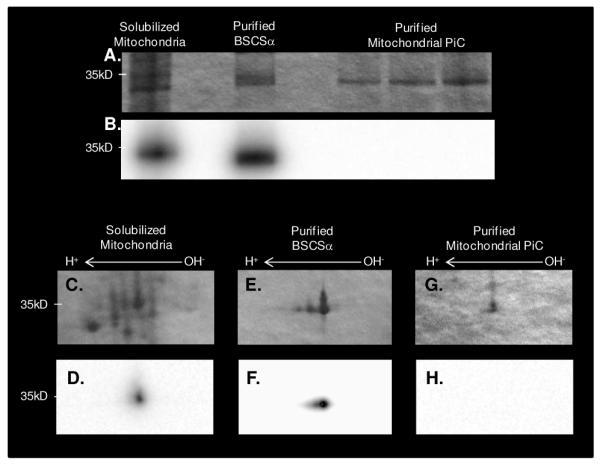
Coomassie Blue Stained and 32P-labeled Solubilized Mitochondria, Purified Succinyl-CoA Synthetase and Purified Mitochondrial Phosphate-Carrier Protein. A 1D gel containing (from left to right) 25μg solubilized porcine heart mitochondria, 1μg purified bacterial succinyl-CoA synthetase, and 1, 2.5, and 5μg of purified mitochondrial phosphate carrier stained with coomassie blue (A) and labeled with 32P (B). Panels C-H contain zoomed in regions of 2D gels containing 400μg solubilized porcine heart mitochondria (C-D), 20mg purified bacterial succinyl-CoA synthetase (E- F), and 25μg purified mitochondrial phosphate carrier (G-H). The top panel is stained with coomassie blue and the bottom panel is labeled with 32P. For 2D gels, proteins were first separated by isoelectric focusing point, over a pH range of 3-11NL, and then by molecular weight from ∼150kD to 10kD; only the relevant region is shown. Purified bacterial succinyl-CoA synthetase, α-subunit is abbreviated by BSCSα and the purified mitochondrial phosphate-carrier protein is abbreviated as PiC.
Effect of SDS and pH on the Extent of 32P-Binding to Succinyl-CoA Synthetase
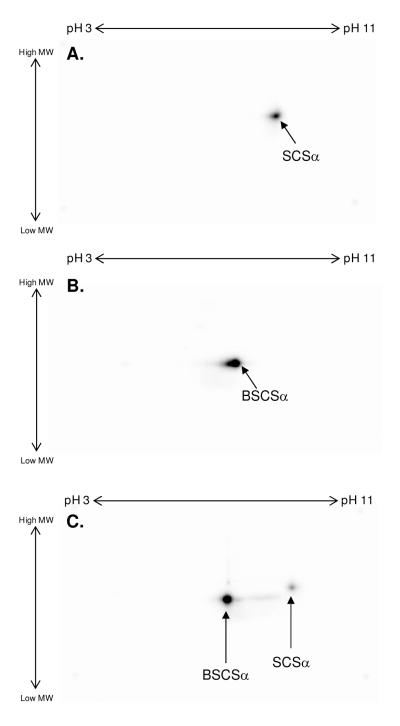
To examine the effect of denaturing agents, such as SDS and acid, on the level of 32P-binding to SCSα two paired experiments were conducted. First, 32P-labeled solubilized porcine heart mitochondria and purified BSCS were run using BN-PAGE, thereby maintaining the SCS protein complex in its intact form. As shown in Figure 5A, the extent of 32P-binding to SCSα was greater when analyzed with BN-PAGE compared to 2D SDS BN-PAGE. Importantly, a strong 32P-association was also observed in the Complex V region of the BN-PAGE gel; however, unlike SCSα, the 32P-association to Complex V was not detected following exposure to SDS via 2D BN-PAGE. This finding is consistent with the results described above, and underlines the uniqueness of SCSα to remain non-covalently bound to 32P throughout the SDS gel process. Next, 32P-binding to SCSα was tested for a pH-sensitivity by precipitating 32P-labeled purified BSCS under acidic (TCA) and neutral (methanol) extraction procedures. In the TCA-treated sample (Figure 5B), 32P-binding was significantly weaker than the neutral-treated sample (Figure 5C). The pH-sensitivity of this non-covalent binding of 32P to SCSα is especially interesting because it has been well-documented that SCSα has an acid labile histidine phosphorylation site (10-14). Taken together these results highlight the difficulty of quantifying 32P-binding by SCSα after denaturing and bring into question the use of pH sensitivity alone in identifying histidine phosphorylation sites.
Figure 5.
Effect of Denaturation on the 32P-Labeling of Succinyl-CoA Synthetase. After incubation with 32P, succinyl-CoA synthetase was run using 1D and subsequent 2D BN-PAGE on purified succinyl-CoA synthetase or total porcine heart mitochondria (A) or was precipitated with methanol (B) or trichloro-acetic acid (C). The insert to the left of Panel A shows that the bacterial succinyl-CoA synthetase complex has a molecular weight of ∼140 kDa, consistent with previous reports (59). Proteins were first separated by either molecular weight with BN-PAGE (A) or isoelectric focusing point (B-C) over a non-linear pH range of 3-11, and then by molecular weight from ∼150kD to 10kD. In all panels, an arrow is used to indicate the 32P-binding to succinyl-CoA synthetase following SDS gel electrophoresis, where BSCSα represents purified bacterial succinyl-CoA synthetase, α-subunit and SCSα represents porcine heart mitochondrial succinyl-CoA synthetase, α-subunit.
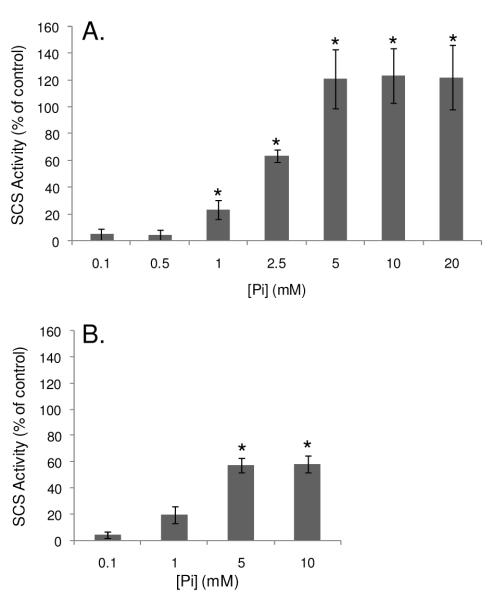
Effect of Pi on Succinyl-CoA Synthetase Activity
To determine the effect of Pi-binding on SCS’s enzymatic activity, the reaction was run backwards and the rate of succinate-dependent ATP hydrolysis was measured in both mitochondrial homogenates and purified BSCS. Since the SCS reaction can be run in both directions (36) and Pi is not a substrate for the reverse reaction, this assay enabled the effect of allosteric Pi-binding to SCS to be determined. As shown in Figure 6, the addition of Pi increased SCS activity in a dose-dependent manner, both in protein homogenates and BSCS. In mitochondrial homogenates an initial increase in SCS activity was observed with the addition of 1 mM Pi and a maximal activity (more than 2-fold of control) was reached with 5 mM Pi (Figure 6A). Since the mitochondrial preparations used for this study included a Pi-loading step, there was some concern that the dynamic range of SCS activity may be limited by a potentially high baseline [Pi]. Thus, SCS activity was also measured in non-Pi-loaded mitochondria. However, no difference in SCS activity was observed between preparations, and therefore these data were pooled. A less pronounced Pi-induced activation of SCS was also observed in purified BSCS (Figure 6B). It is important to note that the baseline Pi level and/or existence of small contaminants in this commercial preparation were unknown.
Figure 6.
Effects of Pi on Succinyl-CoA Synthetase Activity. Dose-response curves for the rate of succinate-dependent ATP hydrolysis in solubilized porcine heart mitochondria (A) and in purified bacterial succinyl-CoA synthetase (B) with various Pi concentrations, as measured in 4 experiments in porcine heart mitochondria and 3 experiments in purified protein. Activity is expressed as a percentage of the control (0mM phosphate condition), where p<0.01 is denoted by *.
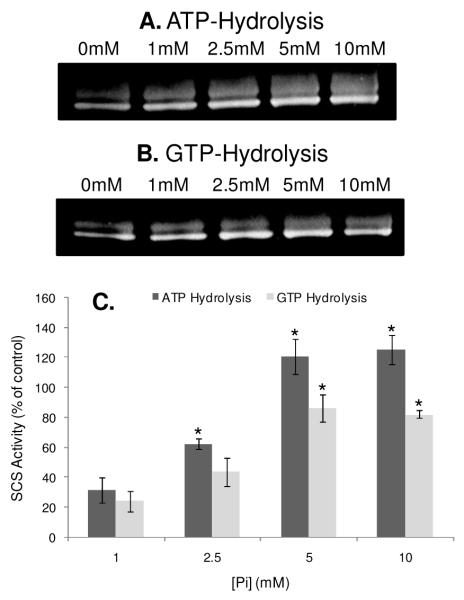
The Pi-induced activation of SCS was also determined with an in-gel assay, which measured the ATP-and GTP-hydrolytic capacity of BSCS (Figure 7). Using this in-gel assay approach, the Pi activation profile for BSCS more closely tracked that of porcine heart mitochondria (Figure 6A), compared to our in-solution studies described above. The results presented in Figure 7C also revealed that the ATP-dependent activity of BSCS was higher than the GTP-dependent activity, implying that the ATP-specific transcript may be more strongly expressed in this purified BSCS enzyme preparation. Since, several studies have shown that the Pi and calcium effects for several mitochondrial proteins are additive (26;30), we also tested for a calcium-induced activation of SCS by incubating solubilized mitochondria and purified BSCS with 0 μM or 1 μM free calcium. Under our experimental conditions, no calcium effect was observed for SCS (results not shown).
Figure 7.
In-Gel Succinyl-CoA Synthetase Activity Assay. The succinate-dependent ATP (A) and GTP (B) hydrolytic activities of purified bacterial succinyl-CoA synthetase pre-treated with different concentrations of phosphate. The increase in succinyl-CoA synthetase activity with phosphate shown in Panel C is quantified relative to the 0mM phosphate condition across 3 experiments for each nucleotide. Activity is expressed as a percentage of the control (0mM phosphate condition), where p<0.01 is denoted by *.
Discussion
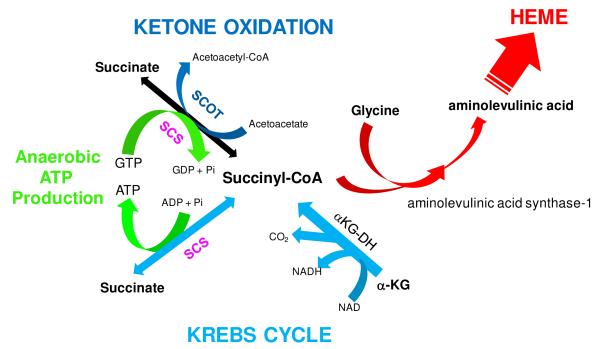
This study demonstrates that one of the sites of Pi regulation in mitochondrial metabolism is SCS. This was shown by monitoring the binding of 32P to SCSα within the intact mitochondrial matrix (Figure 1), solubilized mitochondrial homogenates (Figure 2) and in purified BSCS (Figure 3) as well as by measuring SCS activity in vitro (Figures 6 and 7). The binding of 32P to SCSα in intact mitochondria was dependent on the mitochondrial energetic state, reflecting the concentration of matrix Pi. The ability of SCSα to bind 32P in the absence of an energy source and under the harsh denaturing conditions of SDS, revealed that 32P-labeling in 2D gel studies is not limited to covalent protein modifications. Additionally, the binding of Pi to SCS was associated with an activation of SCS activity both in mitochondrial homogenates and purified BSCS. Taken together, these data reveal that the non-covalent binding of Pi to SCS is regulatory and linked to the energized state of the matrix. The pivotal role that SCS plays in the metabolism of the mitochondria is outlined in Figure 8. SCS plays a crucial role in the citric acid cycle, as the only site of substrate level phosphorylation within mitochondria, and as a component of ketone oxidation and heme synthesis.
Figure 8.
Role of Succinyl-CoA Synthetase in Mitochondrial Energetics and Biosynthesis. Succinyl-CoA synthetase (SCS) is in pink and the pathways associated with the citric acid cycle are shown in light blue; ketone oxidation and succinyl-CoA acetoacetate CoA transferase (SCOT) in dark blue, anaerobic ATP/GTP production in green, and heme synthesis (i.e., porphyrin) in red.
It has previously been shown that Pi can directly activate citric acid cycle dehydrogenases, including α-ketoglutarate dehydrogenase (26), NAD-isocitrate dehydrogenase (27) and malate dehydrogenase (28) as well as several sites of oxidative phosphorylation (30). The current study demonstrated that Pi activates the maximum velocity of SCS in a dose-dependent manner in mitochondrial homogenates and purified BSCS protein. This allosteric effect of Pi is a classical V-type allosteric activation associated with many multi-subunit enzymes, where the complexes are stablized and both the forward and reverse maximum velocities are increased by allosteric factors (41;42). Since Pi has been shown to stablize the SCS complex (8) and increase the Vmax of the reverse reaction, it is reasonable to assume that the forward reaction kinetics are also enhanced by Pi as found in other V-type interactions.
These results suggest that SCS is another target for Pi-activation in mitochondrial energetics. Further support for a Pi-induced activation of SCS is found in the study by Siess et al (29), who determined the effects of Pi on the matrix metabolite concentrations of α-ketoglutarate, succinyl-CoA and malate in liver mitochondria. It was shown that Pi decreased matrix [α-ketoglutarate] and [succinyl-CoA] while increasing [malate]. This metabolite “cross-over” is consistent, but not unique, to an activation of SCS by Pi (29). Also consistent with the activation of SCS in low-energy states is the ∼2.5-fold increase in the SCS product, succinate, during cardiac ischemia (16;43). Collectively, these results provide strong evidence that SCS activity is increased in the presence of mM Pi levels.
The Pi-induced activation of SCS is especially interesting in the functional context of myocardial ischemia or any energy supply-and-demand mismatch that occurs in the heart. The most rapid and large change in cytosolic metabolites that occurs during such an energetic-mismatch in the heart involves cytosolic [Pi] (44). Thus, Pi is an excellent signaling molecule that is capable of activating the energy-conversion processes of the mitochondria as well as potentially supporting non-oxygen dependent substrate-level ATP formation. Under energy-limited conditions, such as myocardial ischemia or hypoxia, ATP production by anaerobic glycolysis can prevent broad cellular injury and maintain energization of the mitochondrial matrix by driving Complex V backwards as an ATPase (45;46). However, during prolonged myocardial ischemia, anaerobic glycolysis is suppressed (47), and therefore, anaerobic pathways of mitochondrial metabolism (i.e., substrate-level phosphorylation via SCS) are triggered to support ATP production and potentially mollify cardiac injury (16;17;43;48). The increased activity of substrate-level phosphorylation via SCS has been suggested as the source of high succinate in the ischemic heart as discussed above. To support this notion, kidney proximal tubules subjected to hypoxia and re-oxygenation demonstrated that anaerobic metabolism of α-ketoglutarate plus aspartate increased the recovery of cellular ATP by intra-mitochondrial substrate-level phosphorylation at the level of SCS (17). Therefore, we speculate that Pi acts as both a substrate and allosteric activator of substrate-level phosphorylation via SCS to generate matrix ATP that may limit ischemically-induced mitochondrial dysfunction. We did not attempt to directly monitor the allosteric effects of Pi on matrix ATP at anoxia because the interpretation of such studies is complicated by the fact that Pi is also a substrate for the forward (ATP synthetic) reaction. Thus, unraveling the different effects of Pi as a substrate and/or allosteric modulator of SCS in intact mitochondria will be difficult.
Direct chemical activity measurements of Pi within the mitochondrial matrix of intact cells or even isolated mitochondria are not currently available. Since the pH gradient is small in heart mitochondria (30), it is likely that the driving force for the inner membrane transport of Pi is generated by a concentration gradient, where matrix [Pi] is much lower than cytosolic [Pi]. Cytosolic [Pi] in the heart increases with oxygen delivery limitations, as discussed above, or near maximum work levels as detected by 31P NMR (49). In skeletal muscle, cytosolic [Pi] increases more proportionally with workload, but also exists at a considerably higher concentration than heart in the resting state (50). Thus, at the current time, the relationship between cytosolic [Pi] and workload with Pi-activity in the mitochondrial matrix is unknown. Furthermore, whether the Pi-induced activation process detected here is involved during normal work transitions in heart and skeletal muscle is also unknown.
Another unique finding of this study was that the non-covalent binding of 32P to SCSα survived the entire SDS gel electrophoresis process. Notably, SCSα was the only mitochondrial protein in this study that bound 32P in a manner that persisted throughout SDS gel electrophoresis. Given the harsh denaturing properties of SDS, it is generally assumed that non-covalent protein modifications do not persist. Thus, like previous reports (51), we initially assumed that 32P-labeling of SCSα resulted from phosphorylation. However, the ability to selectively dilute SCSα’s 32P-incorporation with excess cold Pi, in the absence of a high energy nucleotide, implied that a majority of SCSα’s 32P-association resulted from simple Pi-binding, and not phosphorylation. Furthermore, the labile nature of 32P-binding to SCSα in the native protein (Figure 5), coupled with the inability to exchange out its 32P after SDS gel electrophoresis, suggested that conformational changes to SCSα by SDS trap Pi within the protein. Predictably, this extremely tight, high affinity binding of 32P to SCSα allowed the label to persist throughout the SDS gel electrophoresis process.
The current study also revealed a pH-sensitivity for SCSα’s 32P-binding, as acid-treatment decreased the intensity of labeling. This is likely due to changes in the charge state of phosphate or the SCS binding site as a function of pH. Since mitochondria have a bacterial ancestry, several studies have used acid-lability to screen for histidine phosphorylations in mitochondria. To date, SCSα is one of the few defined histidine phosphorylations in eukaryotes (52). Although the decrease in 32P-labeling may result from alterations to the phosphate ion or conformational changes to SCSα, this pH-sensitivity is important to consider when evaluating differences in the “phosphorylation” of SCSα, since such changes may also result from non-covalent Pi-binding.
The apparent affinity for Pi in the activation of SCS was found to be in the mM range (Figures 6 and 7). However, Pi was found to bind SCSα in the 32P gel studies despite the fact that only tracer amounts of Pi were added in order to keep the specific activity of 32P high. As described above, since 32P-binding to SCSα persists throughout SDS gel electrophoresis, the interaction is one of high affinity, which is inconsistent with the apparent mM affinity revealed in the activity assays. This apparent discrepancy is resolved by two observations. First, as shown in Figure 5A only a small fraction of SCSα's 32P-labeling is resolved upon exposure to SDS in the 2D BN-PAGE studies. Consequently, even if the overall affinity for SCS is the mM region, the high sensitivity of 32P autoradiography detects only a small fraction of the associated Pi in the native protein. Additionally, we found that the 32P could be exchanged out of the native protein but not the SDS treated protein in the gel. These later data suggest that denaturing of the protein traps phosphate in a high affinity site, thereby permitting its detection through the SDS PAGE process. These observations imply that while in-gel 32P-binding studies reflect the association of Pi with SCSα, this approach cannot be used to determine the mole fraction SCSα containing bound Pi.
The current study revealed a specific non-covalent binding of 32P to SCSα in substrate-depleted mitochondria. It is important to point out that the specific activity of 32P in the matrix is predictably different in energized and substrate-depleted mitochondria. When energized with carbon substrates, the matrix phosphorylation potential is high, resulting in a low matrix [Pi] and increasing the potential specific activity of 32P in the matrix Pi pool, thereby increasing the sensitivity of 32P-binding. However, in the substrate-depleted matrix, [Pi] is predictably higher, resulting in a lower matrix 32P specific activity, which decreases the 32P-binding sensitivity. Thus, the enhanced 32P-labeling of SCSα in substrate-depleted mitochondria occurred despite the predicted decrease in specific activity, underlining the fact that Pi association to SCSα is increased in the energy-limited state.
The enhanced 32P-binding to SCSα observed in the substrate-depleted matrix suggests that phosphate binding to SCS could be a marker of compromised energy conversion in mitochondria with high matrix [Pi]. Several 31P-NMR studies have described a large, NMR-invisible Pi-pool in mitochondria (∼60% (53)), believed to result from Pi that is bound to proteins or membranes (53-58). Could the binding of Pi to SCS contribute to this NMR invisible pool of Pi? Our 2D gels estimate SCSα to be at a concentration of 0.27 nmol per mg mitochondria, by comparing the SCSα intensity with Complex IV. Assuming 2 μl of matrix volume per mg, the concentration of SCS is on the order of 0.2 mM, which could be quite significant relative to the low matrix [Pi] in the energized state (see above). The BN-PAGE analysis of the entire mitochondrial proteome also suggests that Complex V could be a significant binding site of matrix Pi under energized conditions (Figure 5A). However, in the energy-limited matrix, [Pi] is well above [SCS], and SCS is therefore not likely to significantly influence the chemical activity of Pi. Thus, the binding of Pi by SCS under energy-limited conditions may primarily serve to activate substrate-level phosphorylation.
In summary, Pi was shown to bind and allosterically enhance SCS activity. In the context of energy supply/utilization mismatched conditions, such as ischemia and hypoxia, the findings presented here suggest that a Pi-induced activation of SCS may anaerobically generate ATP in the matrix in order to minimize mitochondrial dysfunction and enable cellular repair before the onset of irreversible injury. The role of this activation process during normal work transitions in the heart and skeletal muscle is yet to be resolved.
Supplementary Material
ACKNOWLEDGMENT
We gratefully acknowledge Ilsa Rovira for her oversight of the radioisotope studies. We also thank Dr Toren Finkel for the laboratory space.
FUNDING INFORMATION: These studies were funded by the NIH Division of Intramural Research.
ABBREVIATIONS
- SCS
succinyl-CoA synthetase
- SCSα
succinyl-CoA synthetase α-subunit
- BSCS
bacterial succinyl-CoA synthetase
- BSCSα
bacterial succinyl-CoA synthetase α-subunit
- Pi
inorganic phosphate
- RCR
respiratory control ratio
- G/M
potassium-glutamate and potassium-malate
- TCA
trichloro-acetic acid
- PiC
mitochondrial phosphate-carrier protein
- IEV
isoelectric variant
- BN-PAGE
blue-native polyacrylamide gel electrophoresis
Footnotes
Supplemental Information Supplemental material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Aponte AM, Phillips D, Harris RA, Blinova K, French S, Johnson DT, Balaban RS. 32P labeling of protein phosphorylation and metabolite association in the mitochondria matrix. Methods Enzymol. 2009;457:63–80. doi: 10.1016/S0076-6879(09)05004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aponte AM, Phillips D, Hopper RK, Johnson DT, Harris RA, Blinova K, Boja ES, French S, Balaban RS. Use of (32)P To Study Dynamics of the Mitochondrial Phosphoproteome. J. Proteome. Res. 2009 doi: 10.1021/pr800913j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bykova NV, Egsgaard H, Moller IM. Identification of 14 new phosphoproteins involved in important plant mitochondrial processes. FEBS Lett. 2003;540:141–146. doi: 10.1016/s0014-5793(03)00250-3. [DOI] [PubMed] [Google Scholar]
- 4.Hopper RK, Carroll S, Aponte AM, Johnson DT, French S, Shen RF, Witzmann FA, Harris RA, Balaban RS. Mitochondrial matrix phosphoproteome: effect of extra mitochondrial calcium. Biochemistry. 2006;45:2524–2536. doi: 10.1021/bi052475e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.KAUFMAN S, GILVARG C, CORI O, OCHOA S. Enzymatic oxidation of alpha-ketoglutarate and coupled phosphorylation. J. Biol. Chem. 1953;203:869–888. [PubMed] [Google Scholar]
- 6.Ottaway JH, McClellan JA, Saunderson CL. Succinic thiokinase and metabolic control. Int. J. Biochem. 1981;13:401–410. doi: 10.1016/0020-711x(81)90111-7. [DOI] [PubMed] [Google Scholar]
- 7.Labbe RF, Kurumada T, Onisawa J. The role of succinyl-CoA synthetase in the control of heme biosynthesis. Biochim. Biophys. Acta. 1965;111:403–415. doi: 10.1016/0304-4165(65)90050-4. [DOI] [PubMed] [Google Scholar]
- 8.Fraser ME, James MN, Bridger WA, Wolodko WT. Phosphorylated and dephosphorylated structures of pig heart, GTP-specific succinyl-CoA synthetase. J. Mol. Biol. 2000;299:1325–1339. doi: 10.1006/jmbi.2000.3807. [DOI] [PubMed] [Google Scholar]
- 9.Johnson JD, Mehus JG, Tews K, Milavetz BI, Lambeth DO. Genetic evidence for the expression of ATP- and GTP-specific succinyl-CoA synthetases in multicellular eucaryotes. J. Biol. Chem. 1998;273:27580–27586. doi: 10.1074/jbc.273.42.27580. [DOI] [PubMed] [Google Scholar]
- 10.BOYER PD, DELUCA M, EBNER KE, Hultquist DE, PETER JB. Identification of phospohistidine in digests from a probable intermediate of oxidative phosphorylation. J. Biol. Chem. 1962;237:C3306–C3308. [PubMed] [Google Scholar]
- 11.Bridger WA. Evidence for two types of subunits in succinyl coenzyme A synthetase. Biochem. Biophys. Res. Commun. 1971;42:948–954. doi: 10.1016/0006-291x(71)90522-5. [DOI] [PubMed] [Google Scholar]
- 12.Leitzmann C, Wu JY, BOYER PD. Subunits, composition, and related properties of succinyl coenzyme A synthetase. Biochemistry. 1970;9:2338–2346. doi: 10.1021/bi00813a018. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell RA, Butler LG, BOYER PD. The association of readily-soluble bound phosphohistidine from mitochondria with succinate thiokinase. Biochem. Biophys. Res. Commun. 1964;16:545–550. doi: 10.1016/0006-291x(64)90190-1. [DOI] [PubMed] [Google Scholar]
- 14.Steiner AW, Smith RA. Endogenous protein phosphorylation in rat brain mitochondria: occurrence of a novel ATP-dependent form of the autophosphorylated enzyme succinyl-CoA synthetase. J. Neurochem. 1981;37:582–593. doi: 10.1111/j.1471-4159.1982.tb12526.x. [DOI] [PubMed] [Google Scholar]
- 15.Holt SJ, Riddle DL. SAGE surveys C. elegans carbohydrate metabolism: evidence for an anaerobic shift in the long-lived dauer larva. Mech. Ageing Dev. 2003;124:779–800. doi: 10.1016/s0047-6374(03)00132-5. [DOI] [PubMed] [Google Scholar]
- 16.Pisarenko OI, Solomatina ES, Ivanov VE, Studneva IM, Kapelko VI, Smirnov VN. On the mechanism of enhanced ATP formation in hypoxic myocardium caused by glutamic acid. Basic Res. Cardiol. 1985;80:126–134. doi: 10.1007/BF01910459. [DOI] [PubMed] [Google Scholar]
- 17.Weinberg JM, Venkatachalam MA, Roeser NF, Nissim I. Mitochondrial dysfunction during hypoxia/reoxygenation and its correction by anaerobic metabolism of citric acid cycle intermediates. Proc. Natl. Acad. Sci. U. S. A. 2000;97:2826–2831. doi: 10.1073/pnas.97.6.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garlid KD. On the mechanism of regulation of the mitochondrial K+/H+ exchanger. J. Biol. Chem. 1980;255:11273–11279. [PubMed] [Google Scholar]
- 19.Gunter TE, Wingrove DE, Banerjee S, Gunter KK. Mechanisms of mitochondrial calcium transport. Adv. Exp. Med Biol. 1988;232:1–14. doi: 10.1007/978-1-4757-0007-7_1. [DOI] [PubMed] [Google Scholar]
- 20.Jung DW, Apel L, Brierley GP. Matrix free Mg2+ changes with metabolic state in isolated heart mitochondria. Biochemistry. 1990;29:4121–4128. doi: 10.1021/bi00469a015. [DOI] [PubMed] [Google Scholar]
- 21.Halestrap AP. Regulation of mitochondrial metabolism through changes in matrix volume. Biochem. Soc. Trans. 1994;22:522–529. doi: 10.1042/bst0220522. [DOI] [PubMed] [Google Scholar]
- 22.Izzard S, Tedeschi H. Ion transport underlying metabolically controlled volume changes of isolated mitochondria. Proc. Natl. Acad. Sci. U. S. A. 1970;67:702–709. doi: 10.1073/pnas.67.2.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Izzard S, Tedeschi H. Characterization of orthophosphate-induced active cation transport in isolated liver mitochondria. Arch. Biochem. Biophys. 1973;154:527–539. doi: 10.1016/0003-9861(73)90005-2. [DOI] [PubMed] [Google Scholar]
- 24.Kushnareva YE, Haley LM, Sokolove PM. The role of low (< or = 1 mM) phosphate concentrations in regulation of mitochondrial permeability: modulation of matrix free Ca2+ concentration. Arch. Biochem. Biophys. 1999;363:155–162. doi: 10.1006/abbi.1998.1039. [DOI] [PubMed] [Google Scholar]
- 25.Basso E, Petronilli V, Forte MA, Bernardi P. Phosphate is essential for inhibition of the mitochondrial permeability transition pore by cyclosporin A and by cyclophilin D ablation. J. Biol. Chem. 2008;283:26307–26311. doi: 10.1074/jbc.C800132200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Zavala JS, Pardo JP, Moreno-Sanchez R. Modulation of 2-oxoglutarate dehydrogenase complex by inorganic phosphate, Mg(2+), and other effectors. Arch. Biochem. Biophys. 2000;379:78–84. doi: 10.1006/abbi.2000.1856. [DOI] [PubMed] [Google Scholar]
- 27.Hansford RG. Some properties of pyruvate and 2-oxoglutarate oxidation by blowfly flight-muscle mitochondria. Biochem. J. 1972;127:271–283. doi: 10.1042/bj1270271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blonde DJ, Kresack EJ, Kosicki GW. The effects of ions and freeze-thawing on supernatant and mitochondrial malate dehydrogenase. Can. J. Biochem. 1967;45:641–650. doi: 10.1139/o67-075. [DOI] [PubMed] [Google Scholar]
- 29.Siess EA, Kientsch-Engel RI, Fahimi FM, Wieland OH. Possible role of Pi supply in mitochondrial actions of glucagon. Eur. J. Biochem. 1984;141:543–548. doi: 10.1111/j.1432-1033.1984.tb08227.x. [DOI] [PubMed] [Google Scholar]
- 30.Bose S, French S, Evans FJ, Joubert F, Balaban RS. Metabolic network control of oxidative phosphorylation: multiple roles of inorganic phosphate. J. Biol. Chem. 2003;278:39155–39165. doi: 10.1074/jbc.M306409200. [DOI] [PubMed] [Google Scholar]
- 31.Territo PR, French SA, Dunleavy MC, Evans FJ, Balaban RS. Calcium activation of heart mitochondrial oxidative phosphorylation: rapid kinetics of mVO2, NADH, AND light scattering. J. Biol. Chem. 2001;276:2586–2599. doi: 10.1074/jbc.M002923200. [DOI] [PubMed] [Google Scholar]
- 32.Wolodko WT, Fraser ME, James MN, Bridger WA. The crystal structure of succinyl-CoA synthetase from Escherichia coli at 2.5-A resolution. J. Biol. Chem. 1994;269:10883–10890. doi: 10.2210/pdb1scu/pdb. [DOI] [PubMed] [Google Scholar]
- 33.Wohlrab H. Purification of a reconstitutively active mitochondrial phosphate transport protein. J. Biol. Chem. 1980;255:8170–8173. [PubMed] [Google Scholar]
- 34.Blinova K, Levine RL, Boja ES, Griffiths GL, Shi ZD, Ruddy B, Balaban RS. Mitochondrial NADH fluorescence is enhanced by complex I binding. Biochemistry. 2008;47:9636–9645. doi: 10.1021/bi800307y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hellman U, Wernstedt C, Gonez J, Heldin CH. Improvement of an “In-Gel” digestion procedure for the micropreparation of internal protein fragments for amino acid sequencing. Anal. Biochem. 1995;224:451–455. doi: 10.1006/abio.1995.1070. [DOI] [PubMed] [Google Scholar]
- 36.CHA S, PARKS RE., Jr. Succinic thiokinase. I. Purification of the enzyme from pig heart. J. Biol. Chem. 1964;239:1961–1967. [PubMed] [Google Scholar]
- 37.Schagger H, von JG. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal. Biochem. 1991;199:223–231. doi: 10.1016/0003-2697(91)90094-a. [DOI] [PubMed] [Google Scholar]
- 38.Stappen R, Kramer R. Functional properties of the reconstituted phosphate carrier from bovine heart mitochondria: evidence for asymmetric orientation and characterization of three different transport modes. Biochim. Biophys. Acta. 1993;1149:40–48. doi: 10.1016/0005-2736(93)90022-r. [DOI] [PubMed] [Google Scholar]
- 39.Stappen R, Kramer R. Kinetic mechanism of phosphate/phosphate and phosphate/OH- antiports catalyzed by reconstituted phosphate carrier from beef heart mitochondria. J. Biol. Chem. 1994;269:11240–11246. [PubMed] [Google Scholar]
- 40.Wohlrab H, Flowers N. pH gradient-dependent phosphate transport catalyzed by the purified mitochondrial phosphate transport protein. J. Biol. Chem. 1982;257:28–31. [PubMed] [Google Scholar]
- 41.Lara-Lemus R, Calcagno ML. Glucosamine-6-phosphate deaminase from beef kidney is an allosteric system of the V-type. Biochim. Biophys. Acta. 1998;1388:1–9. doi: 10.1016/s0167-4838(98)00141-1. [DOI] [PubMed] [Google Scholar]
- 42.Monod J, WYMAN J, CHANGEUX JP. On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 43.Pisarenko OI. Mechanisms of myocardial protection by amino acids: facts and hypotheses. Clin. Exp. Pharmacol. Physiol. 1996;23:627–633. doi: 10.1111/j.1440-1681.1996.tb01748.x. [DOI] [PubMed] [Google Scholar]
- 44.Balaban RS. Domestication of the cardiac mitochondrion for energy conversion. J. Mol. Cell Cardiol. 2009 doi: 10.1016/j.yjmcc.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nieminen AL, Saylor AK, Tesfai SA, Herman B, Lemasters JJ. Contribution of the mitochondrial permeability transition to lethal injury after exposure of hepatocytes to t-butylhydroperoxide. Biochem. J. 1995;307(Pt 1):99–106. doi: 10.1042/bj3070099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simbula G, Glascott PA, Jr., Akita S, Hoek JB, Farber JL. Two mechanisms by which ATP depletion potentiates induction of the mitochondrial permeability transition. Am. J. Physiol. 1997;273:C479–C488. doi: 10.1152/ajpcell.1997.273.2.C479. [DOI] [PubMed] [Google Scholar]
- 47.Neely JR, Grotyohann LW. Role of glycolytic products in damage to ischemic myocardium. Dissociation of adenosine triphosphate levels and recovery of function of reperfused ischemic hearts. Circ. Res. 1984;55:816–824. doi: 10.1161/01.res.55.6.816. [DOI] [PubMed] [Google Scholar]
- 48.SANADI DR, FLUHARTY AL. On the mechanism of oxidative phosphorylation. VII. The energy-requiring reduction of pyridine nucleotide by succinate and the energy-yielding oxidation of reduced pyridine nucleotide by fumarate. Biochemistry. 1963;2:523–528. doi: 10.1021/bi00903a023. [DOI] [PubMed] [Google Scholar]
- 49.Gard JK, Kichura GM, Ackerman JJ, Eisenberg JD, Billadello JJ, Sobel BE, Gross RW. Quantitative 31P nuclear magnetic resonance analysis of metabolite concentrations in Langendorff-perfused rabbit hearts. Biophys. J. 1985;48:803–813. doi: 10.1016/S0006-3495(85)83839-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vicini P, Kushmerick MJ. Cellular energetics analysis by a mathematical model of energy balance: estimation of parameters in human skeletal muscle. Am. J. Physiol Cell Physiol. 2000;279:C213–C224. doi: 10.1152/ajpcell.2000.279.1.C213. [DOI] [PubMed] [Google Scholar]
- 51.Ferrari S, Moret V, Siliprandi N. Protein phosphorylation in rat liver mitochondria. Mol. Cell Biochem. 1990;97:9–16. doi: 10.1007/BF00231697. [DOI] [PubMed] [Google Scholar]
- 52.Puttick J, Baker EN, Delbaere LT. Histidine phosphorylation in biological systems. Biochim. Biophys. Acta. 2008;1784:100–105. doi: 10.1016/j.bbapap.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 53.Humphrey SM, Garlick PB. NMR-visible ATP and Pi in normoxic and reperfused rat hearts: a quantitative study. Am. J. Physiol. 1991;260:H6–12. doi: 10.1152/ajpheart.1991.260.1.H6. [DOI] [PubMed] [Google Scholar]
- 54.Adler S, Shoubridge E, Radda GK. Estimation of cellular pH gradients with 31P-NMR in intact rabbit renal tubular cells. Am. J. Physiol. 1984;247:C188–C196. doi: 10.1152/ajpcell.1984.247.3.C188. [DOI] [PubMed] [Google Scholar]
- 55.Arnold DL, Matthews PM, Radda GK. Metabolic recovery after exercise and the assessment of mitochondrial function in vivo in human skeletal muscle by means of 31P NMR. Magn Reson. Med. 1984;1:307–315. doi: 10.1002/mrm.1910010303. [DOI] [PubMed] [Google Scholar]
- 56.Bailey IA, Williams SR, Radda GK, Gadian DG. Activity of phosphorylase in total global ischaemia in the rat heart. A phosphorus-31 nuclear-magnetic-resonance study. Biochem. J. 1981;196:171–178. doi: 10.1042/bj1960171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eriksson O, Pollesello P, Saris NE. Effect of phenylephrine on the compartmentation of inorganic phosphate in perfused rat liver during gluconeogenesis and urea synthesis: a 31P-n.m.r.-spectroscopic study. Biochem. J. 1994;298(Pt 1):17–21. doi: 10.1042/bj2980017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hutson SM, Williams GD, Berkich DA, LaNoue KF, Briggs RW. A 31P NMR study of mitochondrial inorganic phosphate visibility: effects of Ca2+, Mn2+, and the pH gradient. Biochemistry. 1992;31:1322–1330. doi: 10.1021/bi00120a007. [DOI] [PubMed] [Google Scholar]
- 59.Buck D, Spencer ME, Guest JR. Primary structure of the succinyl-CoA synthetase of Escherichia coli. Biochemistry. 1985;24:6245–6252. doi: 10.1021/bi00343a031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.