Abstract
Invariant natural killer T cells (iNKT cells) express a restricted T cell antigen receptor (TCR) repertoire and they respond rapidly to glycolipid antigens presented by CD1d. These glycolipid antigens have hexose sugars in α-linkage to two types of lipids that can bind to CD1d. Recent work has shown that the responses of iNKT cells to antigen-bearing microbes can have a profound impact on the development of inflammatory diseases. iNKT cells overcome the limitation of their limited TCR diversity by also responding in a foreign antigen-independent fashion to some infectious agents, similar to NK cells. Recent results demonstrate several mechanisms for the indirect activation of iNKT cells by viruses or TLR ligands, dependent on self-antigen recognition and/or different cytokines produced by antigen presenting cells. The means by which iNKT cells influence other cell types and overall host defense are likewise diverse, illustrating the flexibility and functional diversity of this T lymphocyte sublineage.
Introduction
Many mammals, including rodents and primates, have populations of invariant natural killer T cells (iNKT cells). These cells are bona fide T lymphocytes that express an αβ T cell antigen receptor (TCR) and that are subject to thymus selection, but their immune responses bear a striking resemblance to innate immune cells. Although iNKT cells are reported to have many functions in regulating immune responses, in part through the recognition of self-antigens presented by CD1d, it is our contention that a principal driving force behind the conservation of this population is the detection of microbial infections.
Salient properties of iNKT cells that distinguish them from conventional T lymphocytes include [1–3]: 1) the co-expression of αβ T cell antigen receptors (TCRs) and receptors typically found on NK cells. 2) expression of a semi-invariant TCR, hence their designation as iNKT cells. This TCR is composed of an invariant TCR α chain, encoded by a Vα14-Jα18 rearrangement in mice (Vα14i NKT cells) and a homologous Vα24-Jα18 rearrangement (Vα24i NKT cells) in humans. 3) recognition of glycolipid antigens presented by CD1d, a MHC class I-like antigen presenting molecule that is not highly polymorphic. The specificity of antigen recognition is highly conserved if mouse and human iNKT cells are compared. 4) innate-like immune responses characterized by the ability to produce cytokines such as IL-4 and IFNγ almost immediately after activation. Furthermore, there is no evidence for a memory-like long-term expansion of this population after antigenic stimulation.
We can classify the mechanisms of activation of iNKT cells by microbes into two categories that are summarized in Table 1. First, there is direct activation of the invariant TCR by microbial glycolipids presented by CD1d. Second, there are foreign antigen-independent or indirect mechanisms, which depend on the responses of antigen presenting cells (APC) to microbes. These APC responses include the production of cytokines such as IL-12, and/or the presentation of self-glycolipid antigens by CD1d. The indirect mechanisms allow iNKT cells to respond rapidly to diverse microbes despite a limited TCR diversity. Here we review recent findings on the responses of iNKT cells to microbial infections, with particular emphasis on the responses to bacteria and viruses.
Table 1.
Summary of iNKT cell activation by microorganism
| Type of activation |
Stimuli | Microbial antigen |
Endogenous antigen |
Cytokine | TLR signaling |
Ref |
|---|---|---|---|---|---|---|
| Direct | Sphingomonas | GSL | − | − | − | 7–9 |
| B. burgdorferi | Diacylglycerol | − | − | − | 24 | |
| Indirect |
S. typhimurium LPS |
− | + | IL-12 | n.t | 8, 31 |
|
S. typhimurium LPS |
− | − | IL-12 IL-18 |
n.t. | 36•• | |
| E. coli LPS | − | − | IL-12 IL-18 |
n.t | 36•• | |
| CpG | − | + | Type I IFN |
n.t. | 37•• | |
| CpG | − | − | IL-12 | TLR9 | 38•• | |
| MCMV | − | − | IL-12 Type I IFN |
TLR9− |
38••, 47•• |
|
| HSV1 | − | + | Type I IFN |
n.t. | 45•• |
iNKT cells can be activated by TCR stimulation with microbial antigens (direct activation) or with endogenous antigens and/or cytokines (indirect activation). GSL; glycosphingolipid, DAG; diacylglycerol, +: required, −; not required or not involved. n.t.; not tested
Microbial glycolipids recognized by iNKT cells
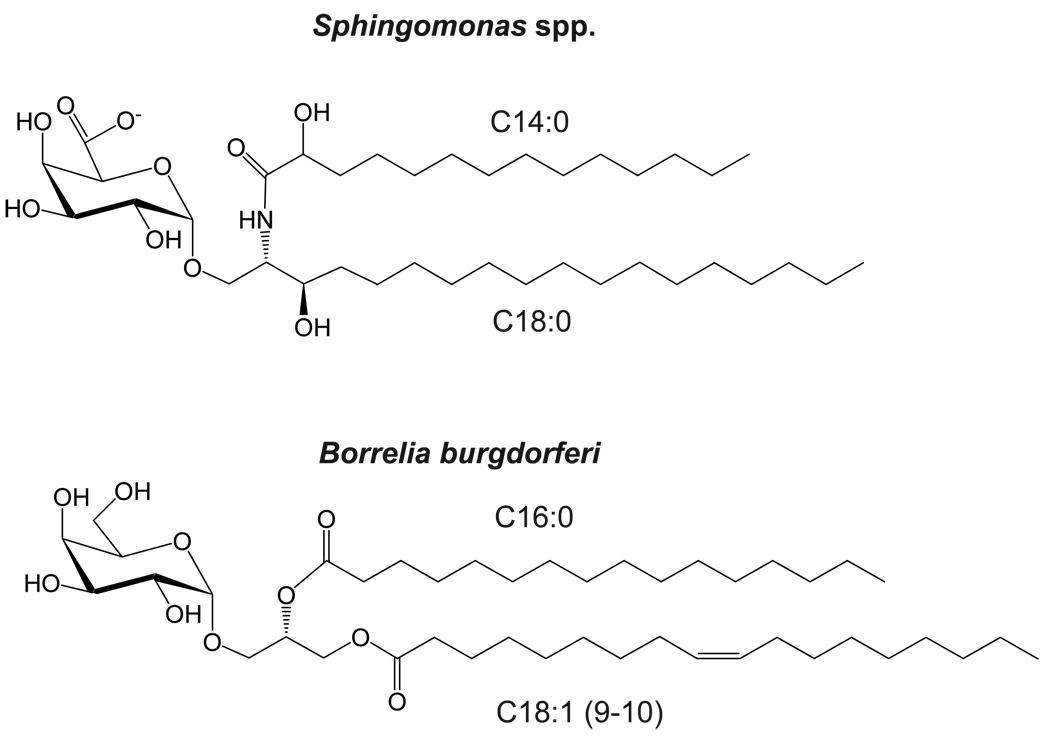
The first antigen identified that is recognized by iNKT cells was α-galactosyl ceramide (αGalCer). This compound was derived from the marine sponge Agelas mauritanius in a screen for a substance that could prevent the metastases of tumors to the mouse liver [4]. It was later shown to act by stimulating Vα14i NKT cells [5]. αGalCer is a glycosphingolipid (GSL), a type of glycolipid with ceramide as the lipid moiety (Figure 1), and which also includes the gangliosides found in many organisms, including mammals. What distinguishes αGalCer from other GSLs is the α-linkage of the 1’ carbon of the galactose to the ceramide lipid, as most of the GSLs in nature have a β linkage. GSLs with the β linkage are not antigenic for iNKT cells.
Figure 1.
In the literature, there are only two types of bacteria that have glycolipid antigens that can activate most iNKT cells, and where iNKT cells are required for optimal host protection or bacterial clearance. This story is rapidly evolving, however, as several additional examples of microbes having iNKT cell antigens were reported at a recent meeting [6].
Recognition of glycosphingolipids
The first example of a microbial glycolipid recognized by iNKT cells is provided by Sphingomonas spp., which are gram-negative bacteria that lack LPS [7–9]. Sphingomonas have abundant GSLs with α-linked sugars, similar to αGalCer [10,11]. Sphingomonas are found in soil and seawater [12], and therefore it is likely that the αGalCer obtained in the original sponge isolate contained these bacteria as the source of the glycolipid antigen. Sphingomonas are unique in having GSLs in their outer membrane.
Although they are not highly pathogenic [13,14], recent work suggests a possible role for Sphingomonas spp. in the causation of primary biliary cirrhosis (PBC), in a mouse model of this disease [15••]. PBC is an autoimmune disease characterized by the presence of anti-mitochondrial antibodies (AMA) and autoimmune attack on the bile ducts [16]. A major target of the AMA is pyruvate dehydrogenase complex E2 (PDC-E2), and there is a striking degree of similarity comparing mammalian PDC-E2 to the sequence in Novosphingobium aromaticivorans, a member of the Sphingomonas genus [17,18]. Injection of N. aromaticivorans into mice caused the CD1d-dependent formation of PDC-E2 antibodies [15••]. The mice eventually also developed a CD1d-dependent inflammation of the liver with characteristics of PBC, including infiltration of the bile ducts, liver hypertrophy and granuloma formation. Although live bacteria were required for chronic disease, the long-term inflammation in the liver was resistant to antibiotic treatment, and the data suggest that autoimmunity dependent on conventional T cells eventually develops in the face of sterile immunity [15••]. Therefore Vα14i NKT cells play a role in the initiating phases rather than in chronic disease. Extrapolating these results to humans, approximately 25% of PBC patients have detectable rDNA from Sphingomonas bacteria in their intestine, as do a similar proportion of the healthy controls [17]. This raises the question as to why only a few people develop PBC when many are exposed N. aromaticivorans.
A related issue is whether there is anything special about the antigen-driven activation of iNKT cells by N. aromaticivorans compared to other Sphingomonas species. Interestingly, a Sphingomonas bacterium can produce more than one type of GSL, and different species differ in their GSLs [10,11,19]. The GSLs can differ with regard to the length and structure of the sphingosine base, as well as the number of carbohydrates. For example, while S. yanoikuyae has GSLs with α-linked monosaccharides containing either galacturonic or glucuronic acid (Figure 1), S. paucimobilis and S. adhaesive also synthesize tetrasaccharide-containing GSLs with the α-linked glucuronic acid further elaborated with three additional sugars [10,11,19]. The oligosaccharide-containing GSLs are weak antigens or not antigenic at all, however, due in part to a failure to efficiently process the complex carbohydrate structures to the antigenic, monosaccharide form [20•,21•]. These data suggest that Sphingomonas spp. may differ in their ability to activate iNKT cells, based in part of the composition of their GSLs. N. aromaticivorans may or may not be exceptional with regard to its ability to activate iNKT cells. Regardless, it is possible that the biosynthesis of certain GSLs, which may compete for CD1d binding but that inhibit iNKT cell activation, may constitute a type of immune evasion mechanism.
Recognition of diacylglycerol glycolipids
The second example of a bacterium having a glycolipid antigen that activates iNKT cells in Borrelia burgdorferi. B. burgdorferi is a pathogen without qualification, as this spirochete is the cause of Lyme disease, the most common vector-borne disease in the United States [22]. Infection with B. burgdorferi results from the bite of Ixodes scapularis ticks, and if not treated promptly with antibiotics, a multisystem inflammatory disorder develops that targets the skin, joints, heart, and nervous system. Mice develop a similar chronic inflammation, with severity dependent on the inbred strain [23]. Interestingly, the glycolipid antigen from B. burgdorferi that activates iNKT cells has a diacylglycerol lipid (Figure 1) rather than a ceramide lipid [24]. What the Sphingomonas and Borrelia antigens have in common, however, is an α-linked hexose sugar, which in the case of B. burgdorferi is a galactose [7–9,24,25]. The glycosylated diacylglycerol antigen for iNKT cells from B. burgdorferi, Borrelia burgdorferi glycolipid II (BbGL-II), is abundant in the spirochete [26]. Mice lacking the Jα18 segment are deficient for Vα14i NKT cells because they cannot form the invariant α chain of the TCR [27]. These mice have reduced spirochete clearance and they are more susceptible to chronic inflammation following B. burgdorferi infection, although the outcome is highly dependent on the mouse strain background [28••, 29••]. In BALB/c mice, the effect of Vα14i NKT cell deficiency was more evident in the joint than in the heart [28••]. Furthermore, there was not a local accumulation of Vα14i NKT cells in the joint tissue, suggesting the cells were acting systemically. By contrast, in C57BL/6 mice, the effect was more prevalent in the heart, and there was evidence for a local accumulation of Vα14i NKT cells and IFNγ in heart tissue [29••].
Indirect recognition of microbes
There are many examples of immune responses influenced by iNKT cells where a foreign antigen recognized by the invariant TCR has not been identified and is presumed not to exist [30]. This includes the responses to some bacteria, such as Salmonella typhimurium [31], but the responses to viral infections perhaps provide the clearest example of iNKT cell activation in the absence of a foreign antigen. The response CD8+ T cell response to several viruses, such as influenza virus, can be augmented by αGalCer [30,32–35]. While this could be important for the development of glycolipid adjuvants, the cytokine storm induced by this highly potent antigen likely does not represent the normal, physiologic role of iNKT cells in anti-viral responses. In this article, we therefore emphasize those studies indicating a role for iNKT cells in the indirect recognition of viruses and other microbes that do not depend on pharmacologic activation of iNKT cells with synthetic glycolipids.
TLR ligands activate iNKT cells
The use of Toll-like receptor (TLR) ligands as a stimulus provides a model for the indirect activation of iNKT cells by infectious agents. Lipopolysaccharide (LPS), a ligand for TLR4 [8,31,36••,37••] and unmethylated CpG oligodeoxynucleotides (CpG ODN), which signal via TLR 9 [37••,38••,39•] activate iNKT cells in vitro and in vivo to produce IFNγ but not IL-4. These TLR ligands activate APC, which then stimulate iNKT cells. Several mechanisms have been reported (Table 1). For LPS, these include the secretion of IL-12 by APC combined with a CD1d-dependence that is strongly suggestive of self-antigen presentation [8,31]. LPS has been reported to alter the synthesis of GSLs in monocytic cell lines [40], which might be related to increased self-antigen presentation—if the self-antigen were in fact a GSL. Furthermore, LPS synergized with IFNγ to increase the level of surface CD1d expression by macrophages [41]. In another study, by contrast, LPS-mediated activation of Vα14i NKT cells required a combination of IL-12 and IL-18 secretion by APC, but not CD1d expression, and therefore presumably did not require antigen presentation by CD1d [36••]. Physiologic levels of IL-12 and IL-18 could activate Vα14i NKT cells in vivo to produce IFNγ and to alter their patrolling behavior in the liver so that they adhered to the liver sinusoidal endothelium [42•]. This cytokine driven response is similar to the cytokine-mediated activation of NK cells, highlighting the innate-like function of Vα14i NKT cells.
Similarly to the LPS response, several mechanisms have been reported for CpG ODN-mediated activation of Vα14i NKT cells. In one report, this depended on type I interferon secretion by APC, and the biosynthesis of a putative GSL self-antigen [37••]. In two subsequent studies, however, this activation event depended on TLR9 and MyD88 sensing of the CpG-ODN by APC, leading to IL-12 secretion, with CD1d expression and self-antigen presentation not playing a major role [38••,39•]. Several reports have documented the activation of Vα24i NKT cells by CpG ODN [43–44,45••]. This required type I interferon secretion by plasmacytoid DC (pDC) that in turn activated myeloid DC to stimulate Vα24i NKT cells. The different outcomes probably relate to differences in the source and dose of the TLR ligand used, as well as the different methods for preparing the APC used in the in vitro studies. Regardless, the divergent results emphasis the existence of multiple pathways that can lead to the indirect activation of iNKT cells.
Viral activation of Vα14i NKT cells
Recent studies have uncovered the mechanism for Vα14i NKT cell activation following infection with mouse cytomegalovirus (MCMV) a well-studied β herpes virus [38••,46,47••]. Vα14i NKT cells produced IFNγ but not IL-4 in vivo by 24–36h after MCMV infection, and this response required TLR9 expression by the APC, and the secretion of IL-12, with a partial dependence on type I interferon secretion. CD1d expression, however, was not required. Therefore, the pathway leading to the stimulation of Vα14i NKT cells by MCMV infection is most similar to the IL-12-dependent pathway described above [38••,47••]. The activation of Vα24i NKT cells by irradiated herpes simplex virus 1 (HSV1) also follows the mechanism outlined for activation by CpG ODN. It requires type I interferon secretion by human pDC, which do not express CD1d, to activate myeloid DC to increase CD1d expression [45••]. Myeloid DC activated the Vα24i NKT cells in a fashion that is partially dependent on CD1d expression, implicating self-antigen recognition in this process.
The consequences of viral activation of iNKT cells for host defense vary depending on the virus and the location of the anti-viral response examined. For example, following infection with lymphocytic choriomeningitis virus (LCMV), Vα14i NKT cells are important for controlling viral replication in the pancreas and liver, but not in the spleen [48••]. The control of LCMV replication depended on the ability of Vα14i NKT cells to recruit pDC to the liver and pancreas, and to activate the pDC to increased secretion of type I interferon to inhibit viral replication. The Vα14i NKT cell-pDC interaction is dependent on OX40 expressed by Vα14i NKT cells interacting with OX40L expressed by pDC. The different outcome in the spleen was attributed to reduced OX40 expression by Vα14i NKT cells there.
The effects of Vα14i NKT cells following intranasal infection with influenza virus, however, are different. In the absence of Vα14i NKT cells, myeloid derived suppressor cells (MDSC) expand in the lung after infection. Vα14i NKT cells prevented this by a mechanism that depended on CD1d expression in the host and the presentation of self-antigen [49••]. It also requires CD40 expressed by the Vα14i NKT cells interacting with CD40L expressed by the MDSC. This interaction leads to a conversion of the myeloid cells from their suppressive function, with increased IL-12 secretion by these cells and decreased production of immune suppressive molecules such as arginase 1 and nitric oxide synthase 2
Conclusions and future perspectives
There is now abundant evidence that iNKT cells can be activated by diverse microbial infections, the limitation of their restricted TCR repertoire is overcome because they can react even in those cases in which a foreign antigen presented by CD1d is not produced. The two general mechanisms for iNKT cell activation are well established, a foreign antigen-dependent mechanism typical of adaptive immunity, and a group of mechanisms independent of foreign antigen that bear a resemblance to the responses of NK cells and other cells of the innate immune system. GSL antigens are found only in Sphingomonas spp., but the discovery of glycosylated diacylglycerol antigens suggests that many other types of bacteria and even protozoan parasites could have glycolipid antigens for the invariant TCR. This is an emerging and rapidly moving area of research. Any infection with antigen-bearing bacteria is almost certain to activate iNKT cells through a combination of the direct and indirect pathways, and the interplay between these two activation pathways has yet to be explored. It must be noted, too, that formal proof that the glycolipid antigen-specific iNKT cell responses are in fact protective has not been obtained. This would require the generation of isogenic microbial strains having and lacking the ability to synthesize the glycolipid antigen in question, a significant challenge considering the abundance of these glycolipids and their likely importance for bacterial viability. Regarding the indirect pathway, identification of the relevant self-antigens clearly is a prerequisite for understanding how microbial infection and cytokines from innate immune cells interface with the synthesis and presentation of self-antigens. The frequency of Vα24i NKT cells varies greatly in the peripheral blood of humans. While genetic differences almost certainly play a role in this variability, the role of infection as a determinant of iNKT cell frequency and responsiveness requires further exploration. However, even at a frequency of 0.1% or less, it is plausible that the rapid and copious cytokine responses by iNKT cells could have a strong influence host defense.
Acknowledgements
Supported by NIH grants AI 71922, AI45053, AI69296.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as
• of special interest
•• of outstanding interest
- 1.Brigl M, Brenner MB. CD1: Antigen Presentation and T Cell Function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 2.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 3.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 4.Morita M, Motoki K, Akimoto K, Natori T, Sakai T, Sawa E, Yamaji K, Koezuka Y, Kobayashi E, Fukushima H. Structure-activity relationship of alpha-galactosylceramides against B16-bearing mice. J Med Chem. 1995;38:2176–2187. doi: 10.1021/jm00012a018. [DOI] [PubMed] [Google Scholar]
- 5.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 6.Burrows PD, Kronenberg M, Taniguchi M. NKT cells turn ten. Nat Immunol. 2009;10:669–671. doi: 10.1038/ni0709-669. [DOI] [PubMed] [Google Scholar]
- 7.Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 8.Mattner J, Debord KL, Ismail N, Goff RD, Cantu C, 3rd, Zhou D, Saint-Mezard P, Wang V, Gao Y, Yin N, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–529. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 9.Sriram V, Du W, Gervay-Hague J, Brutkiewicz RR. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur J Immunol. 2005;35:1692–1701. doi: 10.1002/eji.200526157. [DOI] [PubMed] [Google Scholar]
- 10.Kawahara K, Moll H, Knirel YA, Seydel U, Zahringer U. Structural analysis of two glycosphingolipids from the lipopolysaccharide-lacking bacterium Sphingomonas capsulata. Eur J Biochem. 2000;267:1837–1846. doi: 10.1046/j.1432-1327.2000.01189.x. [DOI] [PubMed] [Google Scholar]
- 11.Kawahara K, Sato N, Tsuge K, Seto Y. Confirmation of the anomeric structure of galacturonic acid in the galacturonosyl-ceramide of Sphingomonas yanoikuyae. Microbiol Immunol. 2006;50:67–71. doi: 10.1111/j.1348-0421.2006.tb03763.x. [DOI] [PubMed] [Google Scholar]
- 12.Neef A, Witzenberger R, Kampfer P. Detection of sphingomonads and in situ identification in activated sludge using 16S rRNA-targeted oligonucleotide probes. J Ind Microbiol Biotechnol. 1999;23:261–267. doi: 10.1038/sj.jim.2900768. [DOI] [PubMed] [Google Scholar]
- 13.Hsueh PR, Teng LJ, Yang PC, Chen YC, Pan HJ, Ho SW, Luh KT. Nosocomial infections caused by Sphingomonas paucimobilis: clinical features and microbiological characteristics. Clin Infect Dis. 1998;26:676–681. doi: 10.1086/514595. [DOI] [PubMed] [Google Scholar]
- 14.Perola O, Nousiainen T, Suomalainen S, Aukee S, Karkkainen UM, Kauppinen J, Ojanen T, Katila ML. Recurrent Sphingomonas paucimobilis -bacteraemia associated with a multi-bacterial water-borne epidemic among neutropenic patients. J Hosp Infect. 2002;50:196–201. doi: 10.1053/jhin.2001.1163. [DOI] [PubMed] [Google Scholar]
- 15. Mattner J, Savage PB, Leung P, Oertelt SS, Wang V, Trivedi O, Scanlon ST, Pendem K, Teyton L, Hart J, et al. Liver autoimmunity triggered by microbial activation of natural killer T cells. Cell Host Microbe. 2008;3:304–315. doi: 10.1016/j.chom.2008.03.009.. This paper shows that infection of mice with N. aromaticivorans can induce the CD1ddependent formation of AMA and a chronic inflammation similar to PBC, implicating antigen recognition of Sphingomonas genus bacteria by Vα14i NKT cells in the pathogenesis of this autoimmune disease.
- 16.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–1273. doi: 10.1056/NEJMra043898. [DOI] [PubMed] [Google Scholar]
- 17.Selmi C, Balkwill DL, Invernizzi P, Ansari AA, Coppel RL, Podda M, Leung PS, Kenny TP, Van De Water J, Nantz MH, et al. Patients with primary biliary cirrhosis react against a ubiquitous xenobiotic-metabolizing bacterium. Hepatology. 2003;38:1250–1257. doi: 10.1053/jhep.2003.50446. [DOI] [PubMed] [Google Scholar]
- 18.Padgett KA, Selmi C, Kenny TP, Leung PS, Balkwill DL, Ansari AA, Coppel RL, Gershwin ME. Phylogenetic and immunological definition of four lipoylated proteins from Novosphingobium aromaticivorans, implications for primary biliary cirrhosis. J Autoimmun. 2005;24:209–219. doi: 10.1016/j.jaut.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Kawahara K, Lindner B, Isshiki Y, Jakob K, Knirel YA, Zahringer U. Structural analysis of a new glycosphingolipid from the lipopolysaccharide-lacking bacterium Sphingomonas adhaesiva. Carbohydr Res. 2001;333:87–93. doi: 10.1016/s0008-6215(01)00111-2. [DOI] [PubMed] [Google Scholar]
- 20. Long X, Deng S, Mattner J, Zang Z, Zhou D, McNary N, Goff RD, Teyton L, Bendelac A, Savage PB. Synthesis and evaluation of stimulatory properties of Sphingomonadaceae glycolipids. Nat Chem Biol. 2007;3:559–564. doi: 10.1038/nchembio.2007.19.. This study shows that natural GSL antigens from Sphingomonas spp. Containing oligosaccharide moieties are poorly antigenic because of limitations in the ability of APC to process them to the much more antigenic monosaccharides. This suggests the synthesis of some GSLs could constitute an immune evasion mechanism. Also see 21 •.
- 21.Kinjo Y, Pei B, Bufali S, Raju R, Richardson SK, Imamura M, Fujio M, Wu D, Khurana A, Kawahara K, et al. Natural Sphingomonas glycolipids vary greatly in their ability to activate natural killer T cells. Chem Biol. 2008;15:654–664. doi: 10.1016/j.chembiol.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bacon RM, Kugeler KJ, Mead PS. Surveillance for Lyme disease--United States, 1992–2006. MMWR Surveill Summ. 2008;57:1–9. [PubMed] [Google Scholar]
- 23.Yang L, Weis JH, Eichwald E, Kolbert CP, Persing DH, Weis JJ. Heritable susceptibility to severe Borrelia burgdorferi-induced arthritis is dominant and is associated with persistence of large numbers of spirochetes in tissues. Infect Immun. 1994;62:492–500. doi: 10.1128/iai.62.2.492-500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MR, Zajonc DM, Ben-Menachem G, Ainge GD, Painter GF, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006;7:978–986. doi: 10.1038/ni1380. [DOI] [PubMed] [Google Scholar]
- 25.Zajonc DM, Kronenberg M. CD1 mediated T cell recognition of glycolipids. Curr Opin Struct Biol. 2007;17:521–529. doi: 10.1016/j.sbi.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ben-Menachem G, Kubler-Kielb J, Coxon B, Yergey A, Schneerson R. A newly discovered cholesteryl galactoside from Borrelia burgdorferi. Proc Natl Acad Sci U S A. 2003;100:7913–7918. doi: 10.1073/pnas.1232451100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 28. Tupin E, Benhnia MR, Kinjo Y, Patsey R, Lena CJ, Haller MC, Caimano MJ, Imamura M, Wong CH, Crotty S, et al. NKT cells prevent chronic joint inflammation after infection with Borrelia burgdorferi. Proc Natl Acad Sci U S A. 2008;105:19863–19868. doi: 10.1073/pnas.0810519105.. This study indicates that Vα14i NKT cells are activated after tick-mediated infection of BALB/c mice and that they play an important role in spirochete clearance and the prevention of arthritis. See also 29••.
- 29. Olson CM, Jr, Bates TC, Izadi H, Radolf JD, Huber SA, Boyson JE, Anguita J. Local production of IFN-gamma by invariant NKT cells modulates acute Lyme carditis. J Immunol. 2009;182:3728–3734. doi: 10.4049/jimmunol.0804111.. This study indicates that Vα14i NKT cells are more important for the prevention of carditis than arthritis induced by B. burgdorferi infection of C57BL/6 mice. In this context, they act through local accumulation of Vα14i NKT cells in heart tissue and IFNγ production.
- 30.Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol. 2007;5:405–417. doi: 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- 31.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 32.Ho LP, Denney L, Luhn K, Teoh D, Clelland C, McMichael AJ. Activation of invariant NKT cells enhances the innate immune response and improves the disease course in influenza A virus infection. Eur J Immunol. 2008;38:1913–1922. doi: 10.1002/eji.200738017. [DOI] [PubMed] [Google Scholar]
- 33.Kamijuku H, Nagata Y, Jiang X, Ichinohe T, Tashiro T, Mori K, Taniguchi M, Hase K, Ohno H, Shimaoka T, et al. Mechanism of NKT cell activation by intranasal coadministration of alpha-galactosylceramide, which can induce cross-protection against influenza viruses. Mucosal Immunol. 2008;1:208–218. doi: 10.1038/mi.2008.2. [DOI] [PubMed] [Google Scholar]
- 34.Guillonneau C, Mintern JD, Hubert FX, Hurt AC, Besra GS, Porcelli S, Barr IG, Doherty PC, Godfrey DI, Turner SJ. Combined NKT cell activation and influenza virus vaccination boosts memory CTL generation and protective immunity. Proc Natl Acad Sci U S A. 2009;106:3330–3335. doi: 10.1073/pnas.0813309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tessmer MS, Fatima A, Paget C, Trottein F, Brossay L. NKT cell immune responses to viral infection. Expert Opin Ther Targets. 2009;13:153–162. doi: 10.1517/14712590802653601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol. 2007;178:2706–2713. doi: 10.4049/jimmunol.178.5.2706.. The authors found that Vα14i NKT cells could be activated by a combination of IL-12 and IL-18 synthesized by LPS-stimulated APC, in the absence of CD1d-mediated antigen presentation. This illustrates the ability of Vα14i NKT cells to respond in a fashion similar to NK cells, which are cells of the innate immune system.
- 37. Paget C, Mallevaey T, Speak AO, Torres D, Fontaine J, Sheehan KC, Capron M, Ryffel B, Faveeuw C, Leite de Moraes M, et al. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 2007;27:597–609. doi: 10.1016/j.immuni.2007.08.017.. This study shows that CpG ODN mediated TLR9 stimulation of APC induces IFNγ production by Vα14i NKT cells. This requires APC synthesis of both type I IFN and an endogenous GSL antigen for presentation by CD1d. For a different perspective on CpG ODN-mediated activation of Vα14i NKT cells see 38••. and 39•.
- 38. Tyznik AJ, Tupin E, Nagarajan NA, Her MJ, Benedict CA, Kronenberg M. Cutting edge: the mechanism of invariant NKT cell responses to viral danger signals. J Immunol. 2008;181:4452–4456. doi: 10.4049/jimmunol.181.7.4452.. This study shows that CpG ODN activation of APC stimulates Vα14i NKT cells to produce IFNγ by a mechanism that requires IL-12 secretion by the APC but not antigen presentation by CD1d. MCMV infection activates Vα14i NKT cells by a similar mechanism, requiring TLR9 sensing of virus infection by APC and IL-12 synthesis, but not CD1d expression. Also see 39•.
- 39. Paget C, Bialecki E, Fontaine J, Vendeville C, Mallevaey T, Faveeuw C, Trottein F. Role of invariant NK T lymphocytes in immune responses to CpG oligodeoxynucleotides. J Immunol. 2009;182:1846–1853. doi: 10.4049/jimmunol.0802492.. The CpG-mediated activation of Vα14i NKT cells is also shown here to be dependent on IL-12 synthesis but not CD1d expression by APC. The adjuvant effects of CpG ODN in the anti-tumor and peptide-specific CD8+ T cell responses are shown to be partially dependent on Vα14i NKT cells.
- 40.De Libero G, Moran AP, Gober HJ, Rossy E, Shamshiev A, Chelnokova O, Mazorra Z, Vendetti S, Sacchi A, Prendergast MM, et al. Bacterial infections promote T cell recognition of self-glycolipids. Immunity. 2005;22:763–772. doi: 10.1016/j.immuni.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 41.Skold M, Xiong X, Illarionov PA, Besra GS, Behar SM. Interplay of cytokines and microbial signals in regulation of CD1d expression and NKT cell activation. J Immunol. 2005;175:3584–3593. doi: 10.4049/jimmunol.175.6.3584. [DOI] [PubMed] [Google Scholar]
- 42. Velazquez P, Cameron TO, Kinjo Y, Nagarajan N, Kronenberg M, Dustin ML. Cutting edge: activation by innate cytokines or microbial antigens can cause arrest of natural killer T cell patrolling of liver sinusoids. J Immunol. 2008;180:2024–2028. doi: 10.4049/jimmunol.180.4.2024.. Previous work showed that Vα14i NKT cells patrol or crawl along the liver sinusoidal endothelial cells, but when activated by antigen, they arrest and adhere to the endothelium. Here the authors demonstrate that physiologic amounts of IL-12 plus IL-18 cause a similar activation in vivo and arrest of Vα14i NKT cell patrolling behavior.
- 43.Marschner A, Rothenfusser S, Hornung V, Prell D, Krug A, Kerkmann M, Wellisch D, Poeck H, Greinacher A, Giese T, et al. CpG ODN enhance antigen-specific NKT cell activation via plasmacytoid dendritic cells. Eur J Immunol. 2005;35:2347–2357. doi: 10.1002/eji.200425721. [DOI] [PubMed] [Google Scholar]
- 44.Montoya CJ, Jie HB, Al-Harthi L, Mulder C, Patino PJ, Rugeles MT, Krieg AM, Landay AL, Wilson SB. Activation of plasmacytoid dendritic cells with TLR9 agonists initiates invariant NKT cell-mediated cross-talk with myeloid dendritic cells. J Immunol. 2006;177:1028–1039. doi: 10.4049/jimmunol.177.2.1028. [DOI] [PubMed] [Google Scholar]
- 45. Raftery MJ, Winau F, Giese T, Kaufmann SH, Schaible UE, Schonrich G. Viral danger signals control CD1d de novo synthesis and NKT cell activation. Eur J Immunol. 2008;38:668–679. doi: 10.1002/eji.200737233.. The authors show that exposure of APC from humans to viral danger signals, including CpG ODN or irradiated HSV1, causes the CD1d-dependent activation of Vα24i NKT cells. This is due to an increase CD1d synthesis by human myeloid DC that is dependent on type I interferon from human pDC, which do not express CD1d.
- 46.Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J, et al. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci U S A. 2004;101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wesley JD, Tessmer MS, Chaukos D, Brossay L. NK cell-like behavior of Valpha14i NK T cells during MCMV infection. PLoS Pathog. 2008;4:e1000106. doi: 10.1371/journal.ppat.1000106.. This paper shows that the response of Vα14i NKT cells to MCMV infection is dependent on IL-12 synthesis by infected APC, and partially upon the synthesis of type I interferon, but it is independent of CD1d antigen presentation. Also see •• 38.
- 48. Diana J, Griseri T, Lagaye S, Beaudoin L, Autrusseau E, Gautron AS, Tomkiewicz C, Herbelin A, Barouki R, von Herrath M, et al. NKT cell-plasmacytoid dendritic cell cooperation via OX40 controls viral infection in a tissue-specific manner. Immunity. 2009;30:289–299. doi: 10.1016/j.immuni.2008.12.017.. Several reports find a role for Vα14i NKT cells in host defense due to IFNγ synthesis. Here the authors report an anti-viral role for Vα14i NKT cells dependent on OX40 expression by Vα14i NKT cells in pancreas and liver. This OX40 interacts with OX40L expressed by pDC, and causes the stimulation of the pDC to secrete anti-viral type I interferon.
- 49. De Santo C, Salio M, Masri SH, Lee LY, Dong T, Speak AO, Porubsky S, Booth S, Veerapen N, Besra GS, et al. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J Clin Invest. 2008;118:4036–4048. doi: 10.1172/JCI36264.. The authors report that following influenza virus infection Vα14i NKT cells prevent the accumulation of MDSC in the lung. The mechanism requires CD40-CD40L interaction and CD1d-mediated activation of the Vα14i NKT cells. The Vα14i NKT cells did not kill the MDSC, but rather the myeloid cells lose suppressive function and secrete more IL-12.