Abstract
Many extracellular stresses cause inhibition of translation initiation by triggering phosphorylation of the initiation factor, eIF-2α. A major protein kinase responsible for this phosphorylation is PKR, a latent kinase which itself needs activation by autophosphorylation. In stressed cells, this activation occurs when PACT, a PKR-binding protein, is phosphorylated and activates PKR. We have previously demonstrated that the presence of specific residues in domain 3 of PACT is necessary for its ability to activate PKR in vivo. Here, we analyzed the biochemical properties of the inactive PACT mutants by assessing their ability to bind and activate PKR in vitro. Among the essential residues, two serines need to be phosphorylated in vivo for PACT’s ability to activate PKR. We substituted those serines with aspartic acids, mimics of phosphoserines, and investigated the properties of the corresponding mutant PACTs. In vitro, they activate PKR more efficiently because they bind to PKR more tightly. These results indicate that stress-induced phosphorylation of specific serine residues in domain 3 of PACT increases its affinity for PKR, which leads to better activation of PKR and resultant eIF-2α phosphorylation.
Keywords: PACT, PKR, Phosphorylation, Stress, Apoptosis
PKR1 is a ubiquitously expressed cytoplasmic dsRNA sensor whose levels are increased by interferon (IFN) production during the mammalian innate immune response (1, 2). The serine/threonine kinase activity of PKR is latent, and requires activation by autophosphorylation. Binding to dsRNA activates PKR, resulting in eukaryotic translation initiation factor eIF-2α phosphorylation, which subsequently decreases the rate of cellular and viral translation initiation (3). In addition to its central role in antiviral activity of IFNs, PKR also has been implicated in transcriptional signal transduction pathways triggered by extracellular stresses, specific cytokines, growth factors, and dsRNA (4). Furthermore, PKR can also regulate apoptosis, cell growth, transformation, and differentiation (reviewed in 5).
Upon binding dsRNA at its two N terminal dsRNA-binding domains (dsRBDs) (6, 7), PKR changes its conformation (8, 9), exposing the ATP-binding site (10, 11), which causes dimerization and autophosphorylation (12). In addition, the two dsRBDs can mediate dsRNA-independent protein–protein interactions with other proteins that carry similar domains (13). One such protein is PACT, whose binding to PKR leads to the activation of PKR in the absence of dsRNA (14). PACT heterodimerizes to PKR through its first two dsRBDs and its third, C-terminal domain (domain 3). In vitro, domain 3 can activate PKR by itself, but in vivo requires either of the other two domains to anchor it strongly to PKR for effective activation and subsequent cellular effects (15). Domain 3 binding causes PKR autophosphorylation by disrupting an intramolecular interaction within PKR that is responsible for keeping PKR in an inactive conformation (16).
In mammalian cells, the biochemical mechanism by which PACT activates PKR occurs only upon application of cellular stresses such as treatment with a low dose of actinomycin D, peroxide, arsenite, thapsigargin, tunicamycin, or growth factor withdrawal (15, 17–19). After exposure of cells to the stress agent, PACT itself becomes phosphorylated, allowing it to associate with increased affinity, which leads to PKR-dependent apoptosis (15, 18, 20, 21). It is likely that a major feature of the stress response may be to regulate PACT-mediated PKR activation, which is dsRNA-independent.
Previously, we used alanine-scanning mutagenesis of PACT domain 3 to identify two serine residues whose phosphorylation was essential for the cellular actions of PACT (21). We proposed a model in which constitutive phosphorylation of one of these residues was required for stress-induced phosphorylation of the other. In this study, we further examined the biochemical properties of these and other key residues of PACT domain 3 for their contributions in binding and activation of PKR in vitro. Our biochemical results are consistent with our model, indicating that phosphorylation of the specific serine residues in the activation domain of PACT are necessary for transmission of the cellular stress response to PKR.
Experimental Procedures
Reagents, Cells, and Antibodies
HT1080 cells were cultured in Dulbeco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum (FBS) and penicillin/streptomycin. Transfections were done with lipofectamine 2000 reagent (Invitrogen). Anti-FLAG monoclonal M2 antibody was from Sigma and anti-PKR monoclonal antibody was from Ribogene. PACT anti-domain 3 peptide antibody (15, 22) was custom produced by Bio-Synthesis, Inc.
Construction of PACT mutants
The generation of PACT mammalian expression constructs and MBP-3 was described previously (15). Overlap extension PCR was used to construct all PACT mutants (23). PACTΔ1 was used as a template to construct each mutant used for mammalian transfection, with the exception of full length S246A and S287A, which used full length PACT as the template. PCR fragments containing the desired PACT mutant were ligated into restriction enzyme-digested pcDNA3. A FLAG-epitope tag was added at the N-terminal coding end of all PACT constructs.
Apoptosis (TUNEL) Assay
HT1080 cells growing on glass coverslips in 6-well dishes were cotransfected with pcDNA3-MBP, pcDNA3-MBP-3, or pcDNA3-FLAG-PACT. At 6 hours after transfection, the cells were treated with 50 ng/ml actinomycin D. Cells were fixed in 4% methanol free formaldehyde 24 hours after transfection. TdT-mediated dUTP Nick-End Labeling (TUNEL) assay using the Dead End Fluorometric TUNEL System (Promega) was performed using manufacturer protocol.
After washing, cells were stained with primary anti-MBP or anti-FLAG antibody and secondary anti-mouse IgG Texas Red conjugate (Molecular Probes) as described (15). The cells were mounted on glass slides in Vectashield with DAPI (4’-6’-diamidino-2-phenylindole) (Vector Laboratories), and examined under a fluorescence microscope.
Expression and purification of PACT domain 3 mutants from E. coli
The protein coding region of domain 3 for each PACT mutant was subcloned into pMALc2x (New England Biolabs) to generate pMAL2cx-domain 3 mutant. This results in an in frame fusion of correct PACT coding sequence to maltose binding protein. MBP fusion proteins were purified by amylose affinity chromatography (15). Purified PACT proteins were stored at –80°C until use.
PKR activation assay in vitro
The kinase activation assay of PKR was performed on PKR purified by monoclonal antibody immobilized on protein G-sepharose (15) or using purified V5-PKR (16). HT1080 cells were treated with 1000U/ml IFN-β for 24 hours and lysed in high salt buffer (20mM Tris-HCl [pH 7.5], 50 mM KCl, 400 mM NaCl, 1% Triton X-100, 0.2 mM PMSF, 100 U/ml aprotinin, 20 % glycerol). HT1080 lysate was mixed with 1µl of PKR monoclonal antibody 71/10 (Ribogene) in high salt buffer and placed on a spinning wheel for 30 min at 4°C. 25µl of protein G-sepharose was added and spun an additional 30 min at 4°C. The protein G-sepharose beads were washed 4 times in 500 µl high salt buffer and 2 times in 500 µl activity buffer (10 mM Tris-HCl [pH 7.5], 50 mM KCl, 2 mM magnesium acetate, 7 mM β-mercaptoethanol, 20% glycerol). The activation assay was performed on PKR in activity buffer containing 1–100 nmol purified PACT or mutant PACT tethered to MBP, 2.5 mM MnCl2 , 0.1 mM ATP, and 10 µCi [γ32-P]ATP for 20 min at 30°C. Labeled protein was analyzed by SDS-PAGE on an 11% resolving gel. Autoradiography was performed at room temperature.
Assay for PACT domain 3 binding to PKR
Purified maltose binding protein (MBP) or MBP tethered to PACT domain 3 (MBP-3) were independently bound (0.5 µg each) to amylose resin (New England Biolabs) in amylose binding buffer (20 mM Tris-HCl [pH 7.5], 10 mM β-mercaptoethanol, 1 mM EDTA, and 10% glycerol). Purified PKR-FLAG protein (1 µg) was added to the resin-bound MBP or MBP-3 in amylose binding buffer, and placed on a rotating wheel for 1 hour at 4°C. After binding, the resin was washed 6 times with 500 µl of amylose binding buffer containing 1% triton X-100. The ability of PKR-FLAG to interact with MBP or MBP-3 was analyzed by Western blotting for FLAG (22). MBP-3 served as the wt PACT positive control.
Expression in mammalian cells and PKR purification
HT1080 cells were transfected in 100 mm culture dishes with 5 µg CMV-PKR (K296R)-FLAG DNA using the lipofectamine 2000 reagent (Invitrogen). At 24 hours after transfection, cells were lysed in high salt buffer (20mM Tris-HCl [pH 7.5], 50 mM KCl, 400 mM NaCl, 1% Triton X-100, 0.2 mM PMSF, 100 U/ml aprotinin, 20 % glycerol) on ice. The cell extract was used to immunoprecipitate PKR-FLAG with anti-FLAG (M2) agarose. The agarose beads were washed 4 times with high salt buffer and twice with low salt buffer (10 mM Tris-HCl [pH 7.5], 50mM KCl, 2 mM magnesium acetate, 100 U/ml aprotinin, 0.2 mM PMSF, 1% Triton X-100, 20% glycerol). PKR-FLAG was eluted with 0.2 mg/ml FLAG peptide (Sigma) in elution buffer (10mM Tris-HCl [ pH 7.5], 10% glycerol). Purified PKR-FLAG constructs were analyzed by Western blotting with anti-FLAG monoclonal antibody (Sigma).
Results
Strong PKR binding is needed for PACT action in vivo
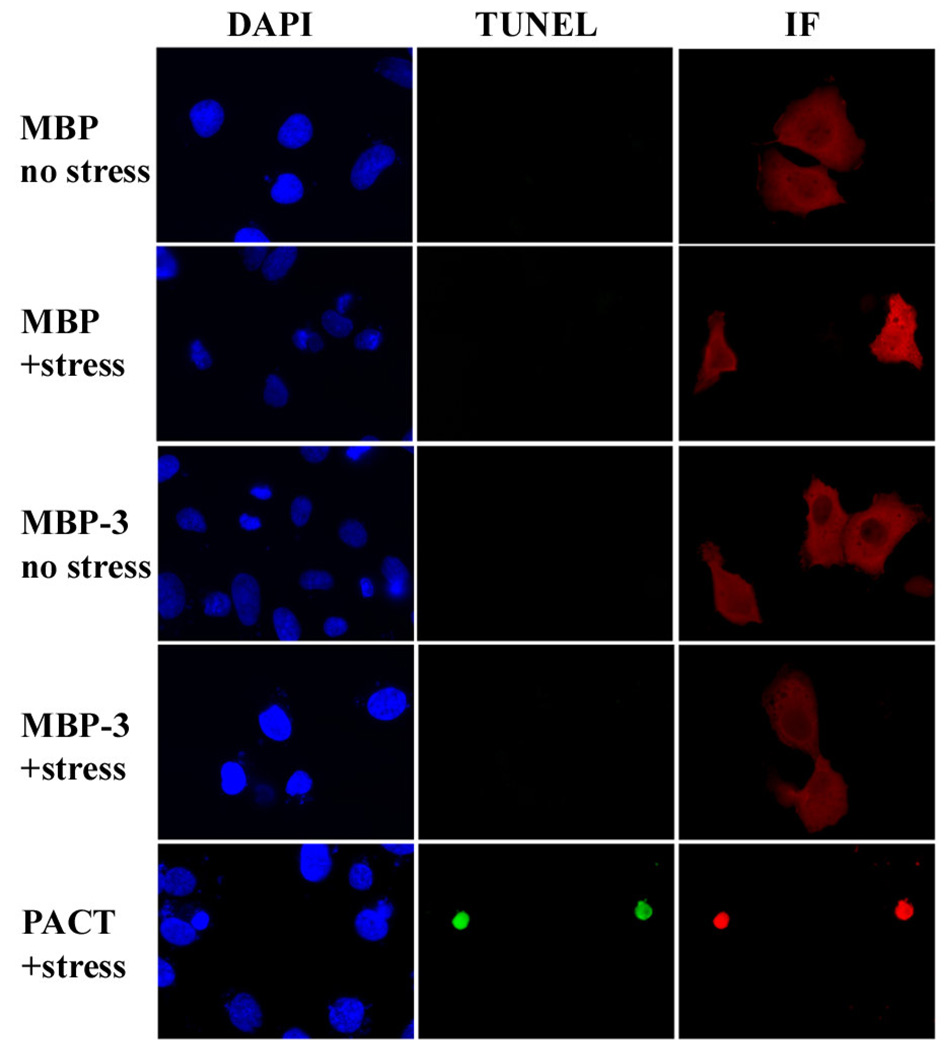
Our previous studies have established that domain 3 of PACT is essential for activating PKR. Purified domain 3, expressed as a fusion protein with maltose-binding protein (MBP-3), binds PKR weakly but activates PKR strongly in vitro (15, 24). In contrast, in order for PACT to activate PKR in vivo, it requires, in addition to its domain 3, either domain 1 or domain 2, both of which can bind to PKR strongly, suggesting that strong PKR binding is needed for PACT-mediated PKR activation in vivo. This conclusion is supported by the fact that PACT domains 1 and 2 can be substituted by the corresponding domains of PKR, which mediate strong homomeric interactions with PKR (15). However, an alternative interpretation of our results has not been ruled out. Domains 1 and 2 of PACT and PKR not only mediate strong heteromeric interactions between the two proteins but they also mediate strong homomeric interactions causing homo-dimerization of the two proteins; consequently it remained possible that homo-dimerization of PACT was needed for its ability to activate PKR. The latter possibility could not be ruled out by our in vitro results showing PKR activation by domain 3, because in these assays domain 3 was used as a fusion protein with MBP, and MBP-3 is known to dimerize (16). Although MBP-3 forms dimers, it lacks a strong PKR binding domain (15, 16). Therefore, to distinguish between a need for dimerization and that for strong PKR binding, we tested the ability of MBP-3, in vivo, to activate PKR and cause apoptosis MBP-3 was expressed in mammalian cells that express very little PACT, cells were stressed with a low dose of actinomycin D, and apoptosis was measured by TUNEL assays. Stressed cells expressing MBP-3 did not undergo apoptosis, but, as expected, stressed cells expressing Wt PACT did undergo apoptosis (Fig 1). This result supports our previous conclusion that the strong binding affinity of PACT to PKR is needed to exert its action in vivo (15), and suggests that dimerization of domain 3 alone is not sufficient to activate PKR in vivo.
Figure 1. MBP-3 dimers are not sufficient to induce apoptosis in stressed cells.
HT1080 cells were transfected with each fusion protein or PACT construct and stressed with actinomycin D. Although MBP-3 and wtPACT each can homodimerize and activate PKR, only wt PACT contains a strong PKR-binding domain. Immunofluorescence assays were performed to detect cells expressing protein from the transfected constructs, which were scored for apoptosis using TUNEL assay. For each transfection, panels show independent photographs of the same cells, with different filters, to indicate nuclei (DAPI), cells undergoing apoptosis (TUNEL), and fusion protein or PACT-expressing cells (IF). MBP, maltose binding protein; MBP-3, maltose binding protein fused to PACT domain 3. The TUNEL results are representative of three independent experiments.
In vitro PKR activation properties of PACT domain 3 mutants
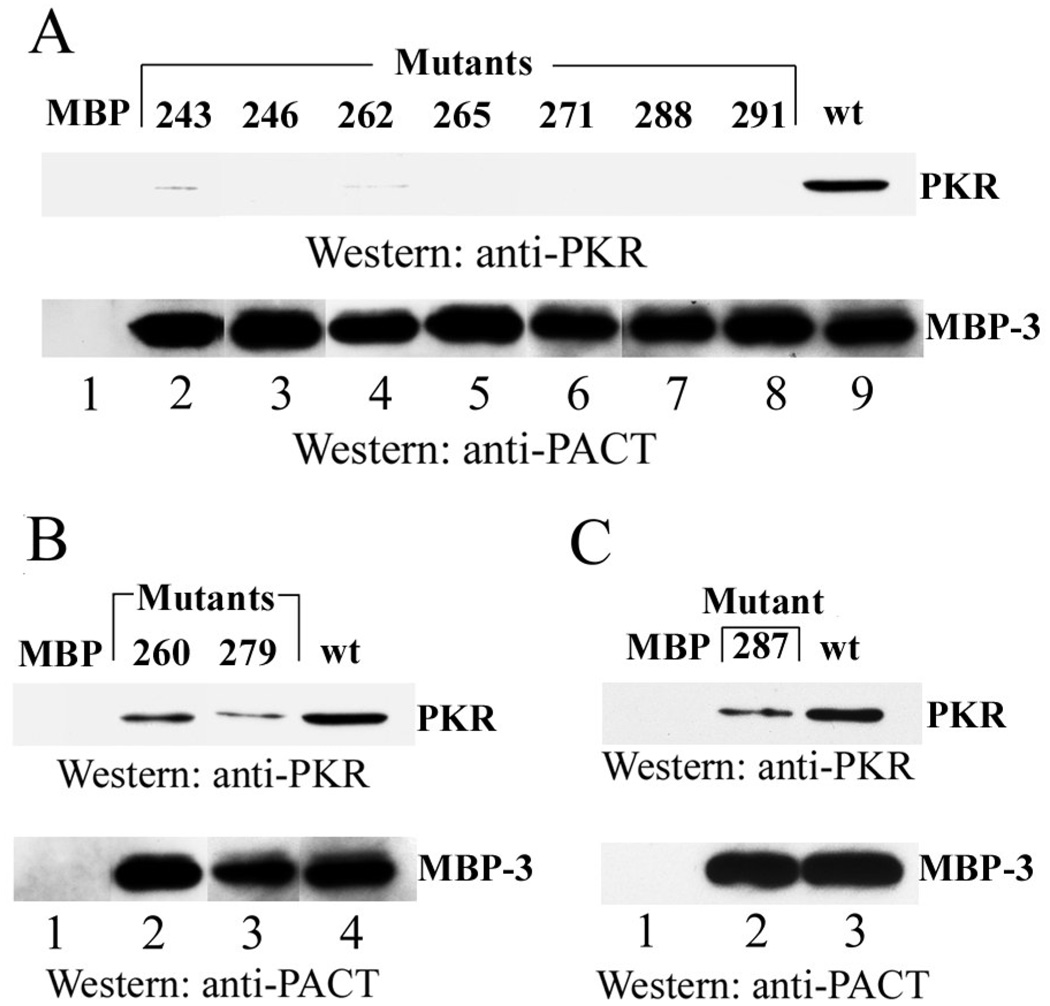
We recently identified the residues within domain 3 that are essential for the ability of PACT to mediate apoptosis in stressed cells. To identify the essential residues, we carried out alanine scanning mutagenesis using a quantitative cell survival assay and identified mutants that have lost the ability to mediate cell killing (21). These mutants shared the same strong PKR binding domain for assay scoring purposes, but each carried within domain 3, a substitution of a single residue with alanine. Ten alanine point mutants lost their activity as measured by the cell survival assay; the inactive mutants were Q243A, S246A, D260A, D262A, S265A, Q271A, S279A, S287A, G288A, and C291A. In the current study, we investigated the biochemical basis of their lack of activity. For this purpose, we used in vitro assays to examine in detail the properties of the inactive mutants. Wt domain 3 and its mutants were expressed as fusion proteins (MBP-3) with maltose-binding protein, purified, and tested for their ability to activate PKR in vitro. In each experiment (Fig 2A through 2E), the activities of increasing amounts (1, 10 and 100nM) of one or more mutant proteins were compared to that of 10nM Wt MBP-3. The Wt protein activated PKR strongly (compare the first and the last lanes in each section) whereas eight mutants failed to activate if at all, even at high concentrations (Fig 2A through 2D). Although it appeared that at higher concentrations, these mutants might be inhibiting the basal auto-phosphorylation of PKR, we verified that this apparent inhibition was non-specific, since MBP alone showed the same effect (data not shown). The other two mutants, D260A, and S279A could partially activate PKR (Fig 2E), but never reached the levels of PKR activation by wt PACT.
Figure 2. PKR activation in vitro by specific PACT domain 3 alanine point mutants.
PACT domain 3 and its mutants were expressed as fusion proteins with MBP and were designated as MBP-3. The effects of 1, 10, and 100 nM concentrations of purified bacterially expressed MBP-3 mutants were tested on PKR activation. Ten nM wt MBP-3 was the positive control. (A) PACT Q243A cannot activate PKR. Lane 1, activity buffer; lane 5, wt MBP-3. (B) PACT D262A or Q271A cannot activate PKR. Lane 1, activity buffer; lane 8, wt MBP-3. (C) PACT S287A cannot activate PKR. Lane 1, activity buffer; lane 5, wt MBP-3. (D) PACT S246A, S265A, G288A, or C291A cannot activate PKR. Lane 1, activity buffer; lane 14, wt MBP-3. (E) PACT D260A or S279A can partially activate PKR. Lane 1, activity buffer; lane 8, wt MBP-3.
PKR binding properties of PACT domain 3 mutants
We next examined whether the failure to properly activate PKR was due to a failure of the mutant MBP-3 proteins to bind PKR. Because domain 3 binds to PKR with a low affinity (15), the binding assays were carried out at a low salt concentration. Using these conditions, we could demonstrate that wt MPB-3, but not MBP, bound to PKR (Lanes 9 and 1, Fig 3A). Seven mutants, 243, 246, 262, 265,271, 288 and 291, that did not activate PKR, could not bind to PKR either (Fig 3A). The two mutants, 260 and 279, which activated PKR partially, could bind to PKR, but not as efficiently as the wt protein (Fig 3B). Interestingly, the mutant S287A, which could not activate PKR at all in vitro (Fig 2A) or in vivo (21), also bound PKR partially (Fig 3C). The in vivo apoptotic activities and the in vitro PKR-activating and binding activities of all domain 3 mutants were quantitated and compared with the properties of the wt protein (Table 1). They were all essentially inactive in vivo, although three mutants, D260A, S279A and S287A had very low, but detectable, cell killing effects. Two of these mutants, D260A and S279A bound and activated PKR partially. All others, except one, were essentially inactive by all three criteria. The S287A mutant was exceptional because it bound to PKR as well as the D260A mutant but did not activate it at all. We chose this mutant and the totally inert mutant, S246A, for further analyses. Because our in vivo experiments were done with PACT, not MBP3, we verified that these two mutations, when introduced individually to full length PACT, inactivated its pro-apoptotic activity. As measured by the quantitative cell survival assay, full length S246A or S287A lost the ability to mediate cell killing in stressed cells (each mutant allowed 100% cell survival).
Figure 3. PKR binding by specific PACT domain 3 alanine point mutants.
Purified PKR-FLAG was added to amylose resin-immobilized MBP fused to domain 3 or domain 3 mutants. The proteins were incubated for 1 hour, and washed extensively with binding buffer. PKR interacting with immobilized MBP-3 was analyzed by Western blotting using FLAG antibody (top panels). Bottom panels show the stripped Western blot reprobed with anti-PACT domain 3 antibody. (A) Domain 3 alanine mutants that cannot bind to PKR. (B) Domain 3 alanine mutants that can partially bind to PKR. (C) Domain 3 mutant S287A binds to PKR.
TABLE 1.
Quantitation of PKR binding, activation, and cell surviva
| Mutations | Bindinga | Activationb | Cell Survivalc |
|---|---|---|---|
| Wt | 100 | 100 | 5 |
| Q243A | 9 | 0 | 100 |
| S246A | 0 | 0 | 100 |
| D260A | 63 | 26 | 90 |
| D262A | 2 | 8 | 93 |
| S265A | 0 | 7 | 95 |
| Q271A | 0 | 0 | 95 |
| S279A | 23 | 28 | 88 |
| S287A | 59 | 0 | 88 |
| G288A | 0 | 9 | 93 |
| C291A | 0 | 0 | 95 |
| S246D | 100 | 96 | 2 |
| S287D | 95 | 98 | 2 |
| S246D/S287D | +d | 114 | 7 |
| S246D/S287A | 85 | 0 | ND |
PhosphorImager analysis was done to quantify bands in Fig 3
10nM concentration lanes from Fig 2, Fig 4, and Fig 5. Levels of Wt domain 3 are considered as 100% and the values for the mutants are presented as percentages of that value; these experiments were done with MBP-3.
Stressed cells, from Peters et al., 2006.
see Fig 6. ND=not determined.
Properties of Asp-substitution mutants
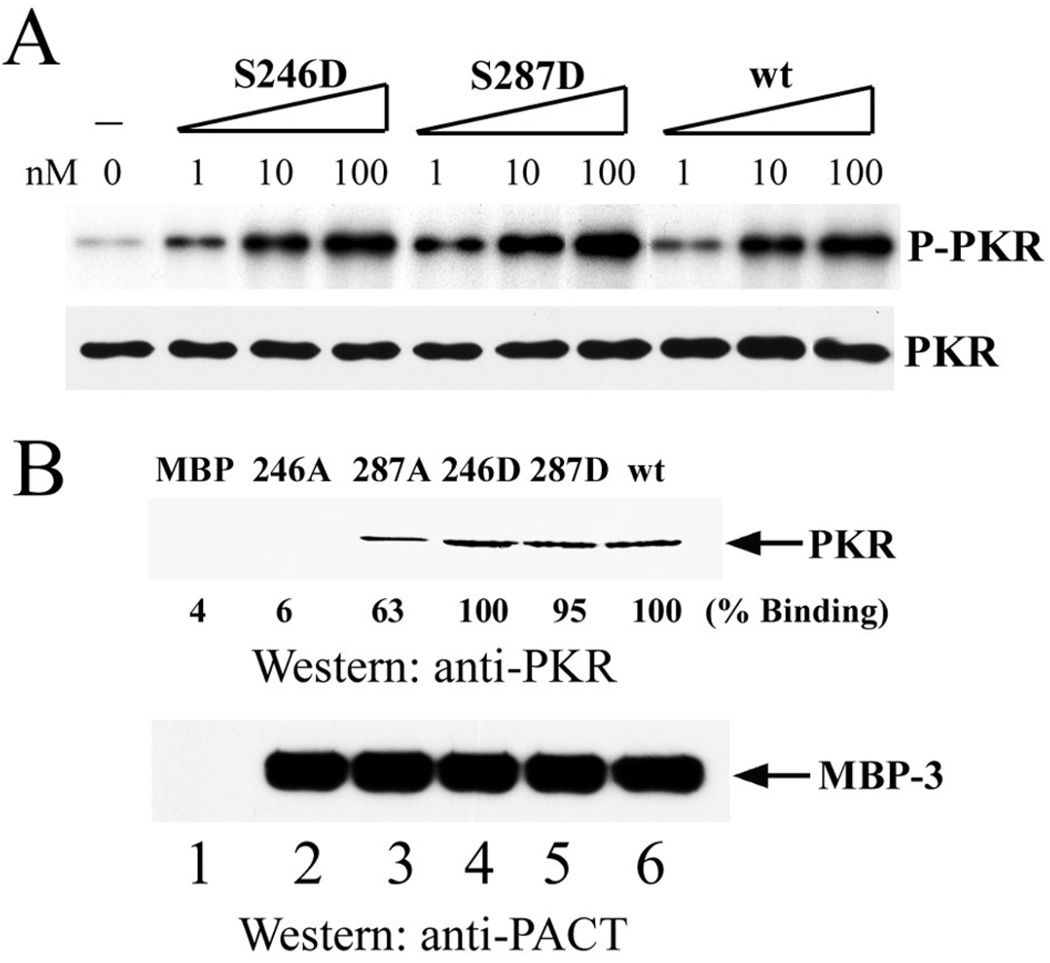
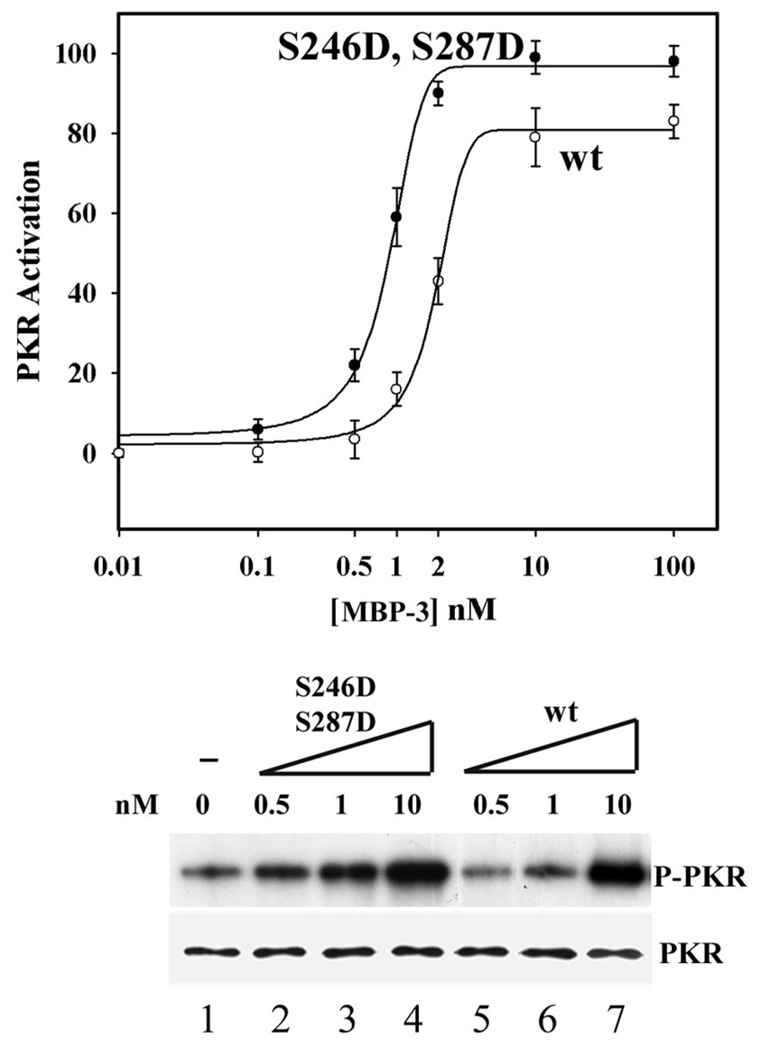
We have previously shown that Ser 246 and Ser 287 of PACT are targets of phosphorylation which is necessary for the ability of PACT to activate PKR in vivo. Moreover, we demonstrated that the substitution of these residues by the phosphoserine mimetic Asp, causes constitutive activation of PACT. To explore the biochemical basis of this process we tested the abilities of the Asp mutants to activate PKR in vitro. As expected from their apoptotic properties (21), the single mutants S246D and S287D mutants activated PKR in vitro as strongly as the wt protein (Fig 4A) and they bound to PKR as efficiently as the wt protein (Fig 4B). When the two mutations were combined, the mutant S246D, S287D (DD mutants), had high apoptotic activity (Table 1). This mutant activated PKR more efficiently than the Wt protein; 50% maximal PKR activation was achieved by about three times less DD mutant protein than the Wt protein (Fig 5). We wondered whether this higher efficiency of PKR activation was due to a higher affinity of the mutant protein for PKR. Indeed, when the strength of the binding between PKR and PACT domain 3 was tested by assaying for their association in the presence of increasing salt concentrations, the mutant protein fared better. As reported before (15), the association between PKR and wt MBP-3 was partially disrupted at 25mM NaCl and completely disrupted at 50mM NaCl. In contrast, the DD mutant MBP3 could bind to PKR even in the presence of 50mM NaCl (Fig 6). These data suggest that like the DD mutant, the corresponding doubly phosphorylated Wt protein can activate PKR better than the unphosphorylated Wt protein because it binds to PKR more strongly. To further solidify this conclusion, we generated the mutant S246D, S287A (DA) and tested its ability to bind and activate PKR. As expected, the DA mutant bound PKR as efficiently as Wt protein (Fig 7A), but could not activate PKR in vitro (Fig 7B).
Figure 4. PKR binding and activation in vitro by domain 3 aspartic acid point mutants.
(A) S246D or S287D each activates PKR. The effects of 1, 10, and 100 nM concentrations of purified bacterially-expressed MBP-3 Asp mutants were tested on PKR activation. wt MBP-3 was the positive control. PKR levels were analyzed by Western blotting using anti-PKR antibody. (B) S246D or S287D each binds to PKR. Procedures are described in Figure 3. PhosphorImager analysis was done to quantify the amount of PKR bound. The amount of PKR binding by wt MBP-3 was considered 100%, and the values for other proteins are presented as percentages of that value.
Figure 5. PKR activation in vitro by the domain 3 double aspartic acid point mutant.
S246D, S287D activates PKR better than wt PACT. The effects of various concentrations of purified S246D, S287D mutant and wt MBP-3 were tested on PKR activation. Each concentration of S246D, S287D or wt MBP-3 was tested in triplicate and graphed as mean values of PKR activation (top panel). Curve fitting was performed by nonlinear regression analysis using the program Sigma Plot 2000 (SPSS Software). Error bars represent standard error of the mean (SEM). Lower panels show a representative experiment using 0.5, 1, and 10nM concentrations of MBP-3 or its mutant. PKR levels were analyzed by Western blotting using anti-PKR antibody.
Figure 6. PKR binding in vitro by the domain 3 double aspartic acid point mutant.
S246D, S287D binds to PKR better than wt MBP-3. Purified PKR-FLAG was added to amylose resin-immobilized MBP fused to wt domain 3 or S246D, S287D domain 3. The proteins were incubated for 1 hour, and washed extensively with binding buffers differing in NaCl concentration. PKR protein that remained bound to resin was analyzed by Western blotting using FLAG antibody and MBP-3 was detected using anti-domain 3 antibody.
Figure 7. The PACT mutant S246D, S287A can bind to PKR, but cannot activate PKR.
(A) S246D, S287A binds to PKR. Procedures are described in Figure 6. (B) S246D, S287A cannot activate PKR. The effect of 100 nM concentrations of purified bacterially expressed MBP-3 or MBP-DA mutant were tested on PKR activation.
Discussion
This study complements our previous in vivo analysis of the role of each residue of domain 3 of PACT in its functions. As far as the need of the ten specific residues of domain 3 is concerned, our in vitro and in vivo results are in general agreement (Table 1). The corresponding mutants did not cause apoptosis and most of them did not bind or activate PKR in vitro. But the noted differences were also revealing. The substantial PKR binding of the S287A mutant was not sufficient for PKR activation and the partial activation of PKR by the D260A and S279A mutants was not sufficient for causing appreciable apoptosis. The needs for serine phosphorylation in the in vitro and in vivo assays were less concordant. It seems that such needs are absolute in vivo, whereas in vitro the needs were only quantitative. It is possible that bacterially-expressed PACT and its derivatives were unphosphorylated, because the isoelectric point of bacterially-expressed PACT Δ1 did not change after phosphatase treatment (21). However, they could still activate PKR in vitro, albeit less efficiently than the DD mutant protein. In this context, it should be mentioned that because the in vitro activation assays were done with MBP-3, the domain 3 was not present in its natural context and hence it might have been more accessible to PKR even without phosphorylation. If this line of argument is valid, the in vivo data are more relevant mechanistically. Irrespective of the above caveats, it is clear that, in vivo, the two specific serine residues are targets of phosphorylation and this process is absolutely required for PKR activation and triggering apoptosis. This could be because, as indicated by the results presented here, phosphorylated PACT is a better activator of PKR due to its stronger binding. But it also remains possible that in addition, stress-induced phosphorylation of PACT changes its subcellular location and translocates it to the neighborhood of PKR so that the two proteins are more accessible to each other. Finally, we have examined here the mechanism of PKR activation by PACT in response to only one kind of stress, e.g. that produced by a low dose of actinomycin D. It remains to be seen whether the same mechanism operates for other stresses known to cause PACT phosphorylation and PKR activation. Such stresses include growth factor deprivation and treatment of cells with arsenite, hydrogen peroxide, thapsigargin, and tunicamycin (17–19).
Results presented here and those reported earlier led us to propose a model for PACT phosphorylation and PKR activation (Fig 8). According to this model, phosphorylation of both Ser 246 and Ser 287 is needed to fully activate PACT and their phosphorylation is sequential. Ser 246 is phosphorylated constitutively whereas Ser 287 is phosphorylated in response to stress. Moreover, phosphorylation of Ser 246 is a prerequisite for Ser 287 phosphorylation (Fig 8A). Moreover, we postulate that the doubly phosphorylated protein binds to two sites on PKR thus increasing the affinity between the two proteins. In contrast, the binding of singly phosphorylated PACT to one site is weak. Although the S246D, S287A mutant could bind to PKR in vitro, it could not activate it (Fig 7). In vivo, only the strong binding leads to a conformational change of PKR and its activation (Fig 8B), and hence the need for applying stress to cells. In support of this, a series of coimmunopreciptation experiments showed that more PKR was coimmunoprecipitated with wt PACT after the cells were stressed (21). In contrast, the AA mutant of PACT precipitated only a small amount of PKR even after stress. As expected from our in vitro experiments, the DD mutant of PACT coimmunoprecipitated PKR more efficiently, even without stress. These results demonstrated that phosphorylated wt PACT bound PKR more strongly in vivo, as did the DD mutant both in vivo and in vitro. These results would suggest that in vivo, stress-activated phosphorylation of S287 should make PACT a better activator of PKR and hence a better stimulant of apoptosis. If this were true, the DD mutant should be highly active in cells even without stress. Indeed, PKR activation in vivo, as measured by eIF2α phosphorylation, was equally strong before and after stress, in cells expressing the DD mutant. There was little eIF2α phosphorylation in cells expressing the corresponding AA mutant, even after the application of stress , and the wt protein caused eIF2α phosphorylation only after the cells were stressed. These results regarding PKR activation were completely supported by the apoptotic properties of the mutants. Unlike the wt protein, the DD mutant was equally active, with and without stress, in causing apoptosis whereas the AA mutant was totally inactive in either situation. Interestingly, another mutant, S246A, S287D, was completely inactive indicating that S246 has to be phosphorylated in order for the protein to be active in vivo. Our in vitro results presented here are in concert with the above model. The DA mutant binding to PKR was not sufficient to activate PKR. The phosphomimetic DD mutant bound to PKR more strongly and activated it more efficiently. Nonetheless, unphosphorylated PACT or MBP3, expressed in and purified from bacteria could activate PKR in vitro, albeit less efficiently. This is in contrast to the absolute need of stress-activated phosphorylation of PACT for activating PKR in vivo. This apparent discrepancy can possibly be explained at the biochemical level by the fact that cellular concentrations of both PKR and PACT are much lower than those used in our in vitro assays. The activities of many proteins are known to be increased by phosphorylation, however the unphosphorylated proteins can still function at a higher concentration; PKR activation by PACT apparently uses the same principle to boost the activity in vivo.
Figure 8. Model of PACT domain 3 activation of PKR.
Only the C-terminal domains of PACT and PKR are shown. (A) PACT is constituitively phosphorylated on S246. Upon stress, a stress activated protein kinase (SAPK) phosphorylates S287. The S287 phosphorylation is phospho-S246-dependent. (B) Inactive PKR has two binding sites for domain 3 residues. (C) Domain 3 binds to PKR when PACT is phosphorylated at S246. This binding exposes another binding site on PKR. (D) The phosphorylation of S246 is required for SAPK phosphorylation of S287. (E) Once S246 and S287 are both phosphorylated, domain 3 binding to PKR is increased or stabilized, and it activates PKR.
Acknowledgements
We thank Xinna Li and Mark Kader for PACT mutant construction and technical assistance.
Footnotes
This work was supported by National Institutes of Health grants CA-62220 and CA-68782 to G.C.S.
Abbreviations: IFN, interferon; dsRNA, double-stranded RNA; PKR, double-stranded RNA-activated protein kinase R; dsRBD, dsRNA binding domain; PACT, protein activator
References
- 1.Sadler AJ, Williams BR. Structure and function of the protein kinase R. Curr Top Microbiol Immunol. 2007;316:253–292. doi: 10.1007/978-3-540-71329-6_13. [DOI] [PubMed] [Google Scholar]
- 2.Sen GC, Peters GA. Viral stress-inducible genes. Adv Virus Res. 2007;70:233–263. doi: 10.1016/S0065-3527(07)70006-4. [DOI] [PubMed] [Google Scholar]
- 3.García MA, Meurs EF, Esteban M. The dsRNA protein kinase PKR: virus and cell control. Biochimie. 2007;89:799–811. doi: 10.1016/j.biochi.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Williams BR. Signal integration via PKR. Sci STKE. 2001;89:RE2. doi: 10.1126/stke.2001.89.re2. [DOI] [PubMed] [Google Scholar]
- 5.Garcia MA, Gil J, Ventoso I, Guerra S, Domingo E, Rivas C, Esteban M. Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol. Mol. Biol. Rev. 2006;70:1032–1060. doi: 10.1128/MMBR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green SR, Mathews MB. Two RNA-binding motifs in the double-stranded RNA-activated protein kinase. DAI, Genes Dev. 1992;6:2478–2490. doi: 10.1101/gad.6.12b.2478. [DOI] [PubMed] [Google Scholar]
- 7.Patel RC, Sen GC. Identification of the double-stranded RNA-binding domain of the human interferon-inducible protein kinase. J. Biol. Chem. 1992;267:7671–7676. [PubMed] [Google Scholar]
- 8.Nanduri S, Carpick BW, Yang Y, Williams BR, Qin J. Structure of the double-stranded RNA-binding domain of the protein kinase PKR reveals the molecular basis of its dsRNA-mediated activation. EMBO J. 1998;17:5458–5465. doi: 10.1093/emboj/17.18.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nanduri S, Rahman F, Williams BR, Qin J. A dynamically tuned double-stranded RNA binding mechanism for the activation of antiviral kinase PKR. EMBO J. 2000;19:5567–5574. doi: 10.1093/emboj/19.20.5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bischoff JR, Samuel CE. Mechanism of interferon action. The interferon-induced phosphoprotein P1 possesses a double-stranded RNA-dependent ATP-binding site. J. Biol. Chem. 1985;260:8237–8239. [PubMed] [Google Scholar]
- 11.Carpick BW, Graziano V, Schneider D, Maitra RK, Lee X, Williams BRG. Characterization of the solution complex between the interferon-induced double-stranded RNA-activated protein kinase and HIV-I trans-activating region RNA. J. Biol. Chem. 1997;272:9510–9516. doi: 10.1074/jbc.272.14.9510. [DOI] [PubMed] [Google Scholar]
- 12.Romano PR, Garcia-Barrio MT, Zhang X, Wang Q, Taylor DR, Zhang F, Herring C, Mathews MB, Qin J, Hinnebusch AG. Autophosphorylation in the activation loop is required for full kinase activity in vivo of human and yeast eukaryotic initiation factor 2alpha kinases PKR and GCN2. Mol. Cell Biol. 1998;18:2282–2297. doi: 10.1128/mcb.18.4.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel RC, Stanton P, McMillan NM, Williams BR, Sen GC. The interferon-inducible double-stranded RNA-activated protein kinase self-associates in vitro and in vivo. Proc. Natl. Acad. Sci. U.S.A. 1995;92:8283–8287. doi: 10.1073/pnas.92.18.8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel RC, Sen GC. PACT a protein activator of the interferon-induced protein kinase, PKR. EMBO J. 1998;17:4379–4390. doi: 10.1093/emboj/17.15.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters GA, Hartmann R, Qin J, Sen GC. Modular structure of PACT: distinct domains for binding and activating PKR. Mol Cell Biol. 2001;21:1908–1920. doi: 10.1128/MCB.21.6.1908-1920.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li S, Peters GA, Ding K, Zhang X, Qin J, Sen GC. Molecular basis for PKR activation by PACT or dsRNA. Proc. Natl. Acad. Sci. U.S.A. 2006;103:10005–10010. doi: 10.1073/pnas.0602317103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito T, Yang M, May WS. RAX, a cellular activator for double-stranded RNA-dependent protein kinase during stress signaling. J. Biol. Chem. 1999;274:15427–15432. doi: 10.1074/jbc.274.22.15427. [DOI] [PubMed] [Google Scholar]
- 18.Patel CV, Handy I, Goldsmith T, Patel RC. PACT a stress-modulated cellular activator of interferon-induced double-stranded RNA-activated protein kinase, PKR. J. Biol. Chem. 2000;275:37993–37998. doi: 10.1074/jbc.M004762200. [DOI] [PubMed] [Google Scholar]
- 19.Singh M, Fowlkes V, Handy I, Patel CV, Patel RC. Essential role of PACT-mediated PKR activation in tunicamycin-induced apoptosis. J. Mol. Biol. 2009;385:457–468. doi: 10.1016/j.jmb.2008.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen G, Ma C, Bower KA, Ke Z, Luo J. Interaction between RAX and PKR modulates the effect of ethanol on protein synthesis and survival of neurons. J Biol Chem. 2006;281:15909–15915. doi: 10.1074/jbc.M600612200. [DOI] [PubMed] [Google Scholar]
- 21.Peters GA, Li S, Sen GC. Phosphorylation of specific serine residues in the PKR activation domain of PACT is essential for its ability to mediate apoptosis. J. Biol. Chem. 2006;281:35129–35136. doi: 10.1074/jbc.M607714200. [DOI] [PubMed] [Google Scholar]
- 22.Peters GA, Khoo D, Mohr I, Sen GC. Inhibition of PACT-mediated activation of PKR by the herpes simplex virus type 1 Us11 protein. J. Virol. 2002;76:11054–11064. doi: 10.1128/JVI.76.21.11054-11064.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pogulis RJ, Vallejo AN, Pease LR. Trower MK. Vitro Mutagenesis Protocols. Vol. 57. Totowa, N.J.: Humana Press, Inc; 1996. In Vitro Recombination and Mutagenesis by Overlap Extension PCR; pp. 167–176. [DOI] [PubMed] [Google Scholar]
- 24.Huang X, Hutchins B, Patel RC. The C-terminal third conserved motif of the protein activator PACT plays an essential role in the activation of double-stranded-RNA-dependent protein kinase (PKR) Biochem J. 2002;366:175–186. doi: 10.1042/BJ20020204. [DOI] [PMC free article] [PubMed] [Google Scholar]