Abstract
Schlafen-3 (Slfn-3), a novel gene, has been shown to be a negative regulator of proliferation. The current investigation was undertaken to determine whether Slfn-3 might play a role in regulating cellular differentiation. Butyric acid, a short chain fatty acid, which induced differentiation of intestinal cells as evidenced by increased alkaline phosphatase (ALP) activity in the rat small intestinal IEC-6 cells, also produced a marked increase in Slfn-3 expression. Furthermore, overexpression of Slfn-3 caused stimulation of ALP activity in IEC-6 cells, which was exacerbated by butyrate. On the other hand, downregulation of Slfn-3 by slfn-3-si-RNA greatly attenuated the butyrate mediated induction of differentiation of IEC-6 cells. Additionally, we observed that increased expression of Slfn-3 in colon cancer HCT-116 cells stimulated TGF-β expression and modulated expression of its downstream effectors as evidenced by increased expression of p27kip1 and downregulation of CDK-2. In addition, Slfn-3 increases E-cadherin expression but downregulates β-catenin. In conclusion, our data show that Slfn-3 plays a critical role in regulating intestinal mucosal differentiation. Furthermore our data also show that TGF-β signaling pathway plays an important role in mediating slfn-3 induced differentiation.
Keywords: Schlafen-3, Intestinal Differentiation, Butyrate, Alkaline phosphatase
INTRODUCTION
The mammalian small intestinal mucosa is a single layer of epithelial cells that self renews itself every 4–5 days [1]. The stem cells, located in the crypts of the intestinal mucosa are responsible for orchestrating this process as well as for producing proliferating transit-amplifying/progenitor cells. On the other hand the villi harbor the differentiated specialized cells belonging to one of the following four categories namely: enterocytes, neuroendocrine cells, goblet cells and paneth cells [1; 2]. The process of differentiation into one of the four types is orchestrated by Wnt/β-catenin, Bone morphogenic protein (BMP) and NOTCH pathways mediated through various transcription factors such as krupple like factor(klf)-4, Sox9, elf3 and neurogranin-3 [[1; 3; 4; 5; 6]. This homeostatic process in intestinal differentiation is molecularly well characterized.
We have recently reported that Schlafen-3 (Slfn-3), a novel gene, to be downregulated in the intestinal mucosa during aging Fisher-344 rats [[7]. However, its role in regulating intestinal mucosal homeostasis is unknown. Slfn-3 belongs to murine mutigene family consisting of 10 genes [8]. A unique domain named “Slfn box” along with the adjacent ATP/GTP binding AAA domain is common to all the members in the family. The members are classified into three distinct subtypes based on protein length and functional domain homology namely short, intermediate and long. Slfn-3 belongs to intermediate group [[8; 9; 10]. In addition, members in the intermediate and long family have a highly conserved “SWADL” domain which is defined by a five amino acid sequence (Ser-Trp-Ala-Asp-Leu). Five members in the large subgroup (Schlafen-5, Schlafen-8, Schlafen-9, Schlafen-10 and Schlafen-14) also have a C-terminal sequence motif homologous to the superfamily-I of DNA/RNA helicases, which are known to mediate many different aspects of DNA and RNA metabolism, such as transcription, splicing and translation to RNA degradation [9; 11; 12].
Slfns 1–3 have been reported to possess potent growth inhibitory properties in vitro via downregulation of Cyclin D1 [13]. Slfns plays an important role in maturation of immune cell as evident by decreased thymocyte numbers in transgenic mice expressing Slfn-1 or -8 in the T-cell lineages as well as change in expression of Schlafen gene(s) following infection and/or induction of pro-inflammatory factors such as AP-1 and NF-κB [8; 14]. More interestingly, there is evidence that Slfn expression increases during cellular differentiation of hematopoietic cells [15]. More recently, Slfn-2 induction has been shown to be essential during the process of receptor activator of NF-κβ ligand (RANKL) induced differentiation of monocytes/macrophages to osteoclasts [16]. However, the causal role of increased Slfn expression in induction of differentiation has not been established and more importantly it has not been studied in relation to intestinal mucosal differentiation. Since, Slfn-3 expression decreases with aging in the colonic mucosa and that aging is associated with decreased cellular differentiation in adipocytes [7; 17], we hypothesized that Slfn-3 might play a role in regulating intestinal differentiation. The current investigation was undertaken to test this hypothesis.
METHODS AND MATERIALS
Cell culture and transfection
Human colon cancer HCT-116 cells and rat small intestinal (IEC-6) cells were obtained from American Type Culture Collection (ATCC, Rockville, MD, USA). They were maintained in tissue culture flasks in a humidified incubator at 37°C in an atmosphere of 95% air and 5% CO2. The HCT-116 cells, maintained in DMEM was supplemented with 10% FBS and 1% antibiotic/antimycotic, whereas for IEC-6 cells the DMEM medium was supplemented with 0.1 Unit/ml bovine insulin. The medium for both cell lines was changed three times a week and the cells were passaged using trypsin/ethylenediamine-tetracetic acid (EDTA). We chose IEC-6 cells for our experiments for the following reasons: (a) IEC-6 cells, although immortal, are derived from normal weanling rats and thus represent normal intestinal mucosal cells and (b) Slfn-3 is a murine protein and IEC-6 represents murine intestinal cells, which is suitable for measurement of changes in intrinsic Slfn-3 expression. The reason for choosing HCT-116 cells was that they are of human origin and devoid of Slfn-3 gene.
In some experiments, the cells were transfected with the vector plasmids pEGFP-N1 (Clonetech, CA) or expression plasmid pEGFP-N1/Slfn-3 using Lipofectamine 2000 according to the manufacturer’s instruction. Generation of pEGFP-N1 and pEGFP-N1/Slfn-3 is described below. For transfection, approximately 0.5~1 × 106 IEC-6 or HCT-116 cells in 2 ml DMEM/10% FBS were plated in the 35 mm tissue culture dishes.After 24 h, the cells were transfected with Lipofectamine-2000 according to the manufacterer’s instruction. Four microgram of plasmid DNA was used in each well, and incubated for 48 h .
Cloning of rat schlafen 3 cDNA and Recombinant plasmid construct
This was carried out as described previously [7]. Briefly, total RNA from rat colonic mucosal cells was reverse transcribed with Slfn-3 sequence specific oligos, 5’-TGGTAGAGCGCTTGCCTAGT-3’, and the Slfn-3 cDNA was amplified using primers designed against the date base sequence of coding region (NM_053687): forward primer, 5’-CTCAAGCTTGGATTTCATCTGGGAAGCAG-3’, reverse primer, 5’-GTGGATCCCTAGGCTCTGGGTTCAGTCCCC-3’. Briefly, 1 µg of purified RNA was reverse transcribed in the presence of 2.5 mM MgCl2, 1× RT-PCR buffer [1 mM dNTPs, 10 mM dithiothreitol, 10 units RNase inhibitor, 1.25 µM Schlafen-3] sequence specific oligo and 15 units Multiscribe Reverse Transcriptase in a final reaction volume of 20 µl. The components were mixed, briefly spun down and incubated at 25°C for 10 min for hybridization. Reactions were carried out at 42°C for 30 minutes in a Gene Amp PCR system 9600 (Perkin-Elmer), and then by cooling to 4°C. The RT reactions were subjected to polymerase chain reaction (PCR) amplification. Five microliter of cDNA products was amplified with 25 µl of PfuUltra hotstart 2× PCR Master Mix (Stratagene, La Jalla, CA), 0.3 µM of upstream and downstream primers in 50 µl reaction volume. Reactions were carried out in the Gene Amp PCR system 9600, first hold for 2 minutes at 95°C for activated PfuUltra Hotstart DNA Polymerase, followed by 40 cycles at 95°C for 30 seconds, 62°C for 60 seconds, 72°C for 2 minutes, and final extension at 72°C for 10 min. The PCR products were separated by electrophoresis in a 1% agarose gel and visualized by ethidium bromide staining. The 1816 bp of target DNA fragments were recovered, digested and cloned into HindIII and BamH I sites of plasmid, pEGFP-N1, (Clontech, CA) to generate Slfn3-GFP protein. The constructs were sequenced and confirmed to contain Rattus norvegicus Schlafen-3.
Growth inhibition assay
Changes in cell growth were analyzed by 3-(4, 5-dimethylthiazol-2yl)-2, 5-diphenyltetrazolium bromide (MTT) assay as described previously [18]. The intensity of the color developed, which is the reflection of number of live cells, was measured at a wavelength of 570 nm. All values were compared to the corresponding controls. All assays were performed with 6 replicates.
Western-blot analysis
Western blot analysis was performed essentially according to our standard protocol [18]. Briefly, the cells were solubilized in lysis buffer [50 mM Tris; 100 mM NaCl; 2.5 mM EDTA; 1% Triton X-100; 1% Nonidet P-40; 2.5 mM Na3VO4; 25 µg/ml aprotinin; 25 µg/ml leupeptin; 25 µg/ml pepstatin A; and 1 mM phenylmethylsulfonyl fluoride (PMSF)]). Following clarification at 10,000 × g for 15 min, the supernatant was used for Western-blot analysis. In all analyses, protein concentration, determined by the Bio-Rad Protein Assay kit (Bio-Rad, Hercules, CA), was standardized among the samples. Aliquots of cell lysates containing 50 µg of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Following electrophoresis, proteins were transferred electrophoretically onto supported nitrocellulose membranes (Osmonics, Gloucester, MA). Membranes were incubated for 1 h at room temperature with blocking buffer, TBS-T (20 mM Tris, pH 7.6, 100 nM NaCl, 0.1% Tween-20) and 5 % nonfat dry milk with gentle agitation. After washing the membranes with TBS-T, they were incubated overnight at 4°C in TBS-T buffer containing 5% milk and with one of the primary antibodies (1:1000 dilution. The membranes were washed 3 times with TBS-T, and subsequently incubated with appropriate secondary antibodies (1:5000 dilutions) in TBS-T containing 5% milk for 2 hours at room temperature with gentle agitation. The membranes were washed again with TBS-T, and the protein bands were visualized by enhanced chemiluminescence (ECL) detection system (Amersham). The membranes containing the electrophoresed proteins were exposed to X-Omat film. The membranes were then stripped (2 × for 15 min at 55 °C) in stripping buffer containing 100 mM 2-mercaptoethanol, 2% SDS and 62.5 mM Tris-HCl pH6.7, and reprobed with β-actin as loading control. All Western blots were performed at least three times for each experiment. Densitometric measurements of the scanned bands were performed using the digitized scientific software program UN-SCNAT. Data were normalized to β-actin.
Statistical Analysis
Unless otherwise stated, data were expressed as mean ± SD. Where applicable, the results were compared by using the unpaired, two-tailed Student t-test, as implemented by Excel 2000 (Microsoft Corp., Redmond, WA). P values <0.05 were considered statistically significant.
Results
The primary objective of the current investigation was to determine whether Slfn-3 plays a role in regulating intestinal differentiation. The first set of experiments was designed to examine the causal relationship between Slfn-3 expression and differentiation. To accomplish this goal we used butyric acid, a short chain fatty acid produced as a result of degradation of dietary fiber by colonic microbes, which has been shown to inhibit cell proliferation but induces differentiation of the intestinal mucosal cells [19; 20]. The latter is evident by increased expression and activity of the brush border enzyme, alkaline phosphatase (ALP).
In the current investigation, a dose of 2 mM sodium butyrate (Na-butyrate) was used to induce maturation/differentiation of IEC-6 cells. This dose has been found to cause a 50% inhibition of growth of IEC-6 cells, as determined by MTT assay (data not shown). To examine the effect of butyrate on differentiation of intestinal cells, induction in ALP activity was determined in IEC-6 cells following incubation with 2 mM Na-butyrate for 6-, 12- and 24 h. We observed no significant increase in ALP activity 6 h or 12 h after incubation with 2 mM Na-butyrate, whereas after 24 h there was a significant 30% increase in ALP activity, when compared with the control (Fig. 1A). To determine whether a relationship exists between intestinal differentiation and Slfn-3, IEC-6 cells treated with or without 2 mM Na-butyrate for 6, 12 and 24 h were then analyzed for Slfn-3 expression by Western-blot. Na-butyrate caused no apparent change in Slfn-3 levels after 6 h, but at 12 h there was a robust 3.25 fold induction in Slfn-3 h expression, which returned essentially to the baseline by 24 h (Fig 1B). Taken together, the results show that butyrate that induces intestinal differentiation also stimulates Slfn-3 expression. Furthermore, the observation that stimulation of Slfn-3 expression by butyrate precedes butyrate-mediated induction of ALP activity suggests a role for Slfn-3 in cellular differentiation.
Figure 1.
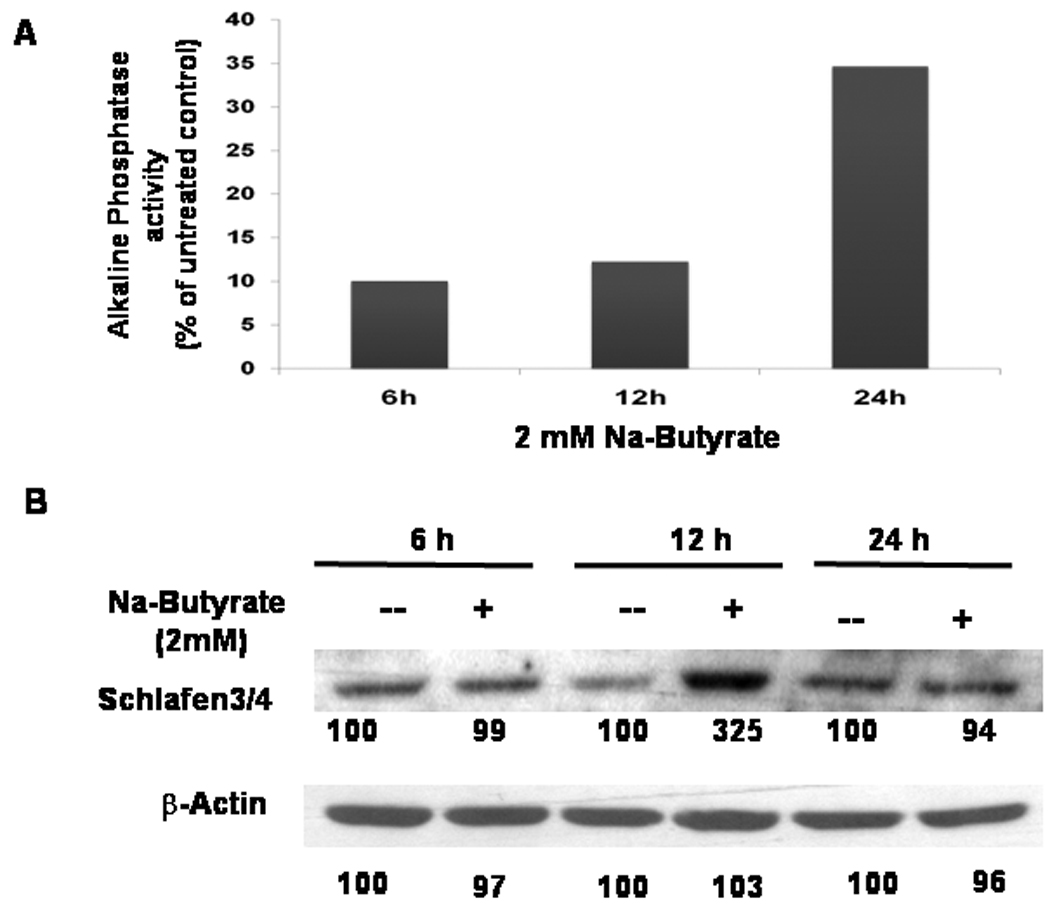
(A)Time related change in ALP in IEC-6 cells treated with butyrate (2mM). The results are expressed as % change in ALP activity compared with untreated control and (B) Time related change in Schlafen-3 expression in IEC-6 cells treated with butyrate (2mM). Cells were incubated with butyrate for 6, 12 and 24 hours. Western blots were probed with anti-Schlafen 3/4 antibody. The number below the bands represent % of the corresponding untreated control.
To further determine the role of Slfn-3 in regulating intestinal differentiation, IEC-6 cells were transiently transfected with Slfn-3 cDNA, control vector or Sfln-3-siRNA. Parental IEC-6 cells served as negative control. Twenty-four hours following transfection, cells were treated with 2 mM Na-butyrate for another 24 h following which they were analyzed for ALP activity. As expected, butyrate caused a modest 30% increase in ALP activity in cells transfected with the control vector, whereas the Slfn-3 transfected cells showed a robust 180% increase in ALP activity in response to butyrate when compared with the parental cells (Fig. 2). In contrast, in IEC-6 cells transfected with Slfn-3 si-RNA demonstrated only a minor 15% increase in ALP activity, compared to the untreated parental cells (Fig 2). The results suggest that induction of Slfn-3 in small intestinal cells is essential for differentiation.
Figure 2.
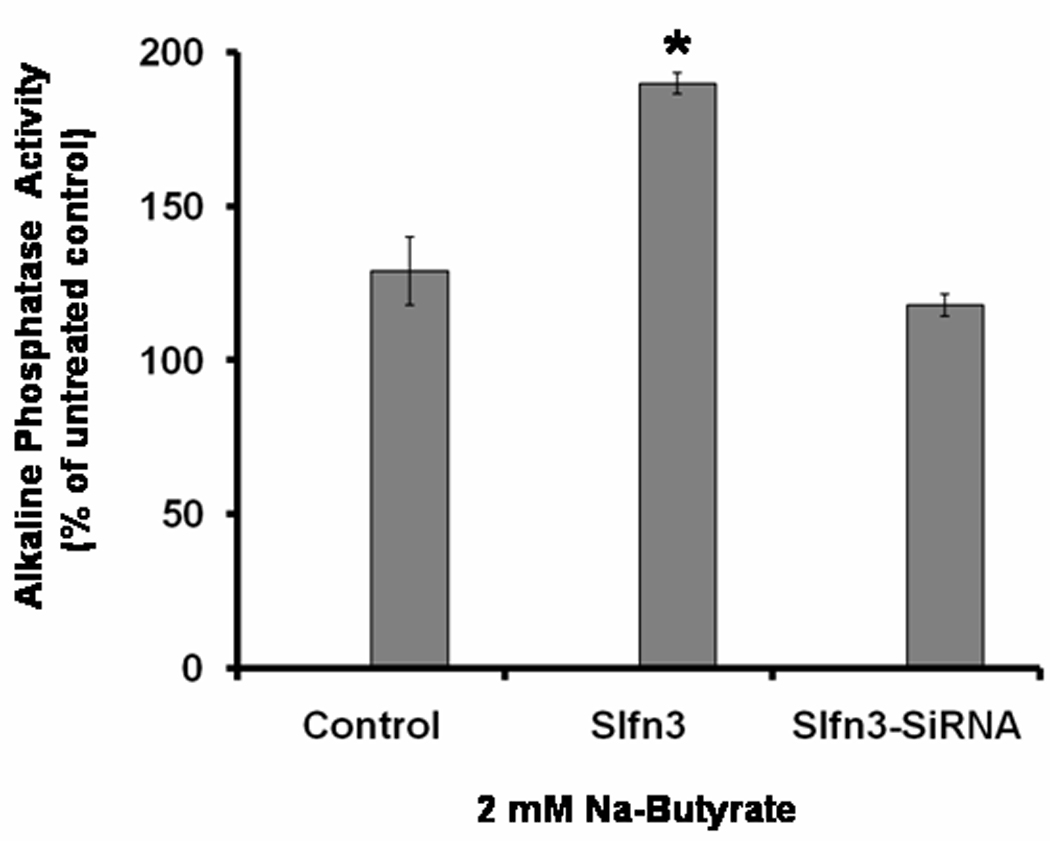
Change in ALP activity following incubation of IEC-6 cells with butyrate (2mM) in cells pretransfected with scramble cDNA, Schlafen-3 cDNA (Slfn3) and Schlafen-3 si-RNA (Slfn3-SiRNA). Data are expressed as % change in ALP activity compared with untreated control.
Although the precise regulatory mechanisms for the Slfn-3-mediated intestinal mucosal differentiation are not fully understood, we examined the possibility whether TGF-β a polypeptide growth factor with pleiotropic effects that affects growth and differentiation [21], might be involved in this process. To test this possibility, human colon cancer HCT-116 cells, which are devoid of Slfn-3 gene, was used. Transfection of Slfn-3 cDNA into HCT-116 cells that induced differentiation, as evidenced by a 40% increase in ALP activity, also augmented TGF-β expression by 55%, when comapred with the corresponding controls (Fig. 3A & B). Increased expression of TGF-β in Slfn-3-transfected HCT-116 cells was accompanied by a concomitant rise in E-cadherin and reduction in β-catenin levels, when compared with the vector-transfected controls (Figs. 3A and B). Changes observed in the expression of E-cadherin and β-catenin in HCT-116 cells following transfection of Slfn-3 gene are compatible with inhibition of cellular growth and induction of differentiation.
Figure 3.
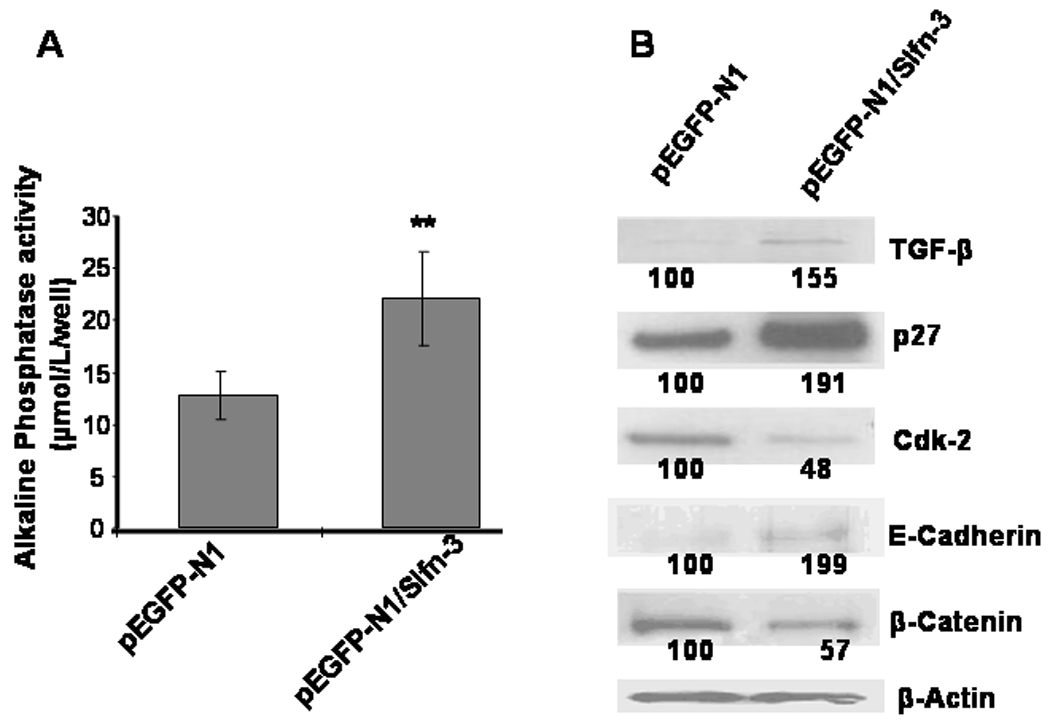
Changes in ALP activity (A) and expression of TGF-β, p27kip1, CDK-2, E-Cadherin and β-catenin (B) following scramble cDNA or Schlafen-3 cDNA (Slfn3) transfection in HCT-116 cells. ** p<0.001 compared to control. The number below the bands represent % of the corresponding untreated control.
To understand the implications of Slfn-3 mediated stimulation of TGF-β in induction of differentiation, we examined downstream regulatory events of the growth factor. Previously, we demonstrated that transfection of colon cancer HCT-116 cells with Slfn-3-cDNA results in cell cycle arrest at G1 phase accompanied by downregulation of cyclin-D1, phopho-Rb and PCNA [7]. As a follow-up we have examined the levels of selected cyclin dependant kinases (Cdks) and their inhibitiors. We observed a 52% reduction in Cdk-2 (Fig. 3B), whereas the levels of CDK-4 showed no apparent change (data not shown). On the other hand, levels of p27kip1, the universal inhibitor of Cdks, were 2-fold increased over the vector transfected control, which may have contributed to the reduction in Cdk2 (Fig. 3B).
Discussion
Butyric acid, a short chain fatty acid, is produced in the human colon by bacterial fermentation of carbohydrates. In addition to serving as a major energy substrate for colonic mucosa, it has important physiologic roles such as inhibition of cell proliferation and induction of differentiation [22]. Therapeutic potential of butyrate in colon cancer has been demonstrated where it causes induction of differentiation as shown by stimulation of brush border enzymes such as ALP,[20]. The our current observation of increased in ALP activity in rat IEC-6 cells in response to Na-butyrate is in agreement with what has been reported earlier. However the fact that we only observed 30–40% induction in IEC-6 cells by Na-butyrate is the probably the result of cell type studied. It has been demonstrated that the extent of ALP activation depends on the dose and cell type and is greater in transformed cancer cells such as caco-2 as opposed to normal intestinal epithelial IEC-6 cells [23].
Although the mechanism of butyrate induced differentiation is poorly understood, our data for the first time, demonstrate that expression Slfn-3, a novel gene, which we have shown to inhibit intestinal cell proliferation [7]), is stimulated by butyrate. This stimulation is associated with increased activity of ALP, indicating a relationship between the two. Additional support for the role of Slfn-3 in regulating intestinal mucosal differentiation comes from the observation that transfection of intestinal cells with Slfn-3-si-RNA markedly attenuates the butyrate mediated stimulation of differentiation.
Our current data also demonstrate that Slfn-3 causes increased expression of TGF-β, a polypeptide growth factor with pleiotropic effects that affects growth and differentiation [21]. This suggests a role for TGF-β in regulating Slfn-3 mediated differentiation of intestinal cells. This is further supported by the observation that downstream events of TGF-β signaling are also modulated, as evidenced by increased expression of p27kip1 and down regulation of CDK-2. Intestinal differentiation has been shown to require Induction of p27kip1 that inhibits CDK2 activity [24; 25]. Our observation that overexpression of schlafen aguments p27kip1 with concommitent decrease in CDK-2, suggests a possible role for Slfn-3 in intestinal differentiation [24]. Previously it had been demonstrated that butyrate induced differentiation was associated with upregulation of p21waf1 and p27kip1 but the mechanism was not elucidated [26]. Our current data demonstrate that Slfn-3 which is induced by butyrate play a critical role in regulating intestinal differentiation.
In conclusion, our current data demonstrate that butyrate mediated induction of differentiation of intestinal cells is associated with parallel rise in schlafen-3 expression. Downregulation of Slfn-3 by slfn-3 si-RNA greatly attenuates butyrate mediated induction of differentiation. Additionally, TGF-β play an important role in mediating slfn-3 induced differentiation.
Acknowledgement
This work was supported by the NIH/NIA and the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 2.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974;141:537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- 3.Jenny M, Uhl C, Roche C, Duluc I, Guillermin V, Guillemot F, Jensen J, Kedinger M, Gradwohl G. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 2002;21:6338–6347. doi: 10.1093/emboj/cdf649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng AY, Waring P, Ristevski S, Wang C, Wilson T, Pritchard M, Hertzog P, Kola I. Inactivation of the transcription factor Elf3 in mice results in dysmorphogenesis and altered differentiation of intestinal epithelium. Gastroenterology. 2002;122:1455–1466. doi: 10.1053/gast.2002.32990. [DOI] [PubMed] [Google Scholar]
- 5.Blache P, van de Wetering M, Duluc I, Domon C, Berta P, Freund JN, Clevers H, Jay P. SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes. J Cell Biol. 2004;166:37–47. doi: 10.1083/jcb.200311021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katz JP, Perreault N, Goldstein BG, Lee CS, Labosky PA, Yang VW, Kaestner KH. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development. 2002;129:2619–2628. doi: 10.1242/dev.129.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel BB, Yu Y, Du J, Rishi AK, Sarkar FH, Tarca AL, Wali A, Majumdar AP. Schlafen 3, a novel gene, regulates colonic mucosal growth during aging. Am J Physiol Gastrointest Liver Physiol. 2009;296:G955–G962. doi: 10.1152/ajpgi.90726.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwarz DA, Katayama CD, Hedrick SM. Schlafen, a new family of growth regulatory genes that affect thymocyte development. Immunity. 1998;9:657–668. doi: 10.1016/s1074-7613(00)80663-9. [DOI] [PubMed] [Google Scholar]
- 9.Geserick P, Kaiser F, Klemm U, Kaufmann SH, Zerrahn J. Modulation of T cell development and activation by novel members of the Schlafen (slfn) gene family harbouring an RNA helicase-like motif. Int Immunol. 2004;16:1535–1548. doi: 10.1093/intimm/dxh155. [DOI] [PubMed] [Google Scholar]
- 10.Hanson PI, Whiteheart SW. AAA+ proteins: have engine, will work. Nat Rev Mol Cell Biol. 2005;6:519–529. doi: 10.1038/nrm1684. [DOI] [PubMed] [Google Scholar]
- 11.Mackintosh SG, Raney KD. DNA unwinding and protein displacement by superfamily 1 and superfamily 2 helicases. Nucleic Acids Res. 2006;34:4154–4159. doi: 10.1093/nar/gkl501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuller-Pace FV. RNA helicases: modulators of RNA structure. Trends Cell Biol. 1994;4:271–274. doi: 10.1016/0962-8924(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 13.Brady G, Boggan L, Bowie A, O'Neill LA. Schlafen-1 causes a cell cycle arrest by inhibiting induction of cyclin D1. J Biol Chem. 2005;280:30723–30734. doi: 10.1074/jbc.M500435200. [DOI] [PubMed] [Google Scholar]
- 14.Sohn WJ, Kim D, Lee KW, Kim MS, Kwon S, Lee Y, Kim DS, Kwon HJ. Novel transcriptional regulation of the schlafen-2 gene in macrophages in response to TLR-triggered stimulation. Mol Immunol. 2007;44:3273–3282. doi: 10.1016/j.molimm.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Neumann B, Zhao L, Murphy K, Gonda TJ. Subcellular localization of the Schlafen protein family. Biochem Biophys Res Commun. 2008;370:62–66. doi: 10.1016/j.bbrc.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 16.Lee NK, Choi HK, Yoo HJ, Shin J, Lee SY. RANKL-induced schlafen2 is a positive regulator of osteoclastogenesis. Cell Signal. 2008;20:2302–2308. doi: 10.1016/j.cellsig.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Kirkland JL, Hollenberg CH, Gillon WS. Ageing, differentiation, and gene expression in rat epididymal preadipocytes. Biochem Cell Biol. 1993;71:556–561. doi: 10.1139/o93-079. [DOI] [PubMed] [Google Scholar]
- 18.Patel BB, Sengupta R, Qazi S, Vachhani H, Yu Y, Rishi AK, Majumdar AP. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int J Cancer. 2008;122:267–273. doi: 10.1002/ijc.23097. [DOI] [PubMed] [Google Scholar]
- 19.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung YS, Song IS, Erickson RH, Sleisenger MH, Kim YS. Effect of growth and sodium butyrate on brush border membrane-associated hydrolases in human colorectal cancer cell lines. Cancer Res. 1985;45:2976–2982. [PubMed] [Google Scholar]
- 21.Massague J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- 22.Siavoshian S, Segain JP, Kornprobst M, Bonnet C, Cherbut C, Galmiche JP, Blottiere HM. Butyrate and trichostatin A effects on the proliferation/differentiation of human intestinal epithelial cells: induction of cyclin D3 and p21 expression. Gut. 2000;46:507–514. doi: 10.1136/gut.46.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukushima K, Sasaki I, Hasegawa H, Takahashi K, Naito H, Funayama Y, Matsuno S. Sodium butyrate-induced liver-type alkaline phosphatase activity in a small intestinal epithelial cell line, IEC6. Dig Dis Sci. 1998;43:1116–1123. doi: 10.1023/a:1018807524075. [DOI] [PubMed] [Google Scholar]
- 24.Deschenes C, Vezina A, Beaulieu JF, Rivard N. Role of p27(Kip1) in human intestinal cell differentiation. Gastroenterology. 2001;120:423–438. doi: 10.1053/gast.2001.21199. [DOI] [PubMed] [Google Scholar]
- 25.Lecanda J, Parekh TV, Gama P, Lin K, Liarski V, Uretsky S, Mittal K, Gold LI. Transforming growth factor-beta, estrogen, and progesterone converge on the regulation of p27Kip1 in the normal and malignant endometrium. Cancer Res. 2007;67:1007–1018. doi: 10.1158/0008-5472.CAN-06-0235. [DOI] [PubMed] [Google Scholar]
- 26.Litvak DA, Evers BM, Hwang KO, Hellmich MR, Ko TC, Townsend CM., Jr Butyrate-induced differentiation of Caco-2 cells is associated with apoptosis and early induction of p21Waf1/Cip1 and p27Kip1. Surgery. 1998;124:161–169. discussion 169-70. [PubMed] [Google Scholar]


