Abstract
Circulating levels of vitamin C (ascorbate) are low in patients with sepsis. Parenteral administration of ascorbate raises plasma and tissue concentrations of the vitamin and may decrease morbidity. In animal models of sepsis, intravenous ascorbate injection increases survival and protects several microvascular functions, namely, capillary blood flow, microvascular permeability barrier, and arteriolar responsiveness to vasoconstrictors and vasodilators. The effects of parenteral ascorbate on microvascular function are both rapid and persistent. Ascorbate quickly accumulates in microvascular endothelial cells, scavenges reactive oxygen species, and acts through tetrahydrobiopterin to stimulate nitric oxide production by endothelial nitric oxide synthase. A major reason for the long duration of the improvement in microvascular function is that cells retain high levels of ascorbate, which alter redox-sensitive signaling pathways to diminish septic induction of NADPH oxidase and inducible nitric oxide synthase. These observations are consistent with the hypothesis that microvascular function in sepsis may be improved by parenteral administration of ascorbate as an adjuvant therapy.
Keywords: Arteriole, ascorbic acid, blood flow, capillary, inflammation, microvascular permeability, nitric oxide, peroxynitrite, tetrahydrobiopterin
1. Introduction
Vitamin C (ascorbic acid) dissociates at physiological pH to form ascorbate, the redox state of the vitamin which is found most abundantly in cells [1]. It is well known that ascorbate acts physiologically as a reductant and enzyme cofactor. The purpose of the present review is to examine recent evidence that ascorbate modulates the intracellular mechanisms that cause microvascular dysfunction in critical illnesses such as sepsis.
The clinical syndrome of sepsis is not a single homogeneous disease process but a generic term for a large group of diseases [2]. Sepsis may develop as a consequence of surgery, pneumonia, soft-tissue infection associated with malignancy or peripheral vascular disease, or many other events. Sepsis syndromes range from the systemic inflammatory response syndrome to severe sepsis (acute organ dysfunction secondary to infection) and septic shock (severe sepsis plus hypotension not reversed with fluid resuscitation) [2,3]. These syndromes are the major causes of death in critical care units worldwide. The mainstays of treatment include fluid resuscitation to restore mean circulating filling pressure, antibiotic therapy and source control to remove the sepsis-inducing insult, vasopressor or combined inotropic-vasopressor therapy to prevent shock, institution of glycemic control, prophylaxis for deep vein thrombosis, and stress ulcer prophylaxis to prevent upper gastrointestinal bleeding [3]. Nevertheless, despite best medical and surgical managements, mortality remains high.
In sepsis, patients respond to whole bacteria, bacterial products such as endotoxin [e.g., Escherichia coli lipopolysaccharide (LPS)], and intracellular products released from injured tissues [2]. The responses include changes in microvascular function that comprise: (i) decreased density of perfused capillaries and elevated proportion of nonperfused capillaries; (ii) increased microvascular permeability (i.e., loss of barrier function) that leads to edema formation and hyperdemia; and (iii) arteriolar hyporesponsiveness to vasoconstrictors and vasodilators [4–16]. If these changes occurred only in small, localized regions of injured tissue, they might benefit the patient by lessening hemorrhage from disrupted blood vessels, delivering antimicrobial mediators and phagocytic cells to the site of injury, or preventing dissemination of toxic substances [2]. But the widespread, systemic occurrence of these changes in sepsis is recognized as microvascular dysfunction because it leads to tissue hypoxia, mitochondrial dysfunction, and ATP depletion that precipitate organ failure, even in fluid-resuscitated patients with adequate arterial blood oxygenation and cardiac output [17]. Indeed, microvascular dysfunction is a significant predictor of death, and one-third of severe sepsis patients die of organ failure [10]. The therapeutic efficacy of antibiotics is confounded by the increasing number of infections due to multidrug resistant bacteria. Furthermore, the pathogens that are killed by antibiotics may release large amounts of toxic products (e.g., LPS) that continue to injure the patient [18]. Therefore, septic patients may benefit from adjuvant therapy that targets microvascular dysfunction.
2. Vitamin C levels in critically ill patients and relevant experimental models
Subnormal ascorbate concentrations in plasma and leukocytes are common features of the critically ill in general and of patients with sepsis in particular [19–25]. Furthermore, plasma ascorbate correlates inversely with multiple organ failure [19] and directly with survival [21].
One reason for ascorbate depletion in hospitalized, critically ill patients may be low levels of the vitamin in parenteral nutrition solutions, because of the degradation of ascorbate and dehydroascorbic acid (DHA) that occurs during preparation and storage [26,27]. Another cause of vitamin C depletion is an increased requirement for ascorbate [22,28]. The amount of vitamin C provided in standard parenteral nutrition multivitamin preparations (nominally 200 mg/day) is not adequate to normalize plasma vitamin C levels in critically ill patients, even when administered for 7 days [29]. The basis for the increased requirement may be oxidation of ascorbate by excess reactive oxygen species (ROS). By acting as a ROS scavenger and enzyme cofactor, ascorbate becomes oxidized to ascorbate free radical, which then dismutates to form DHA.
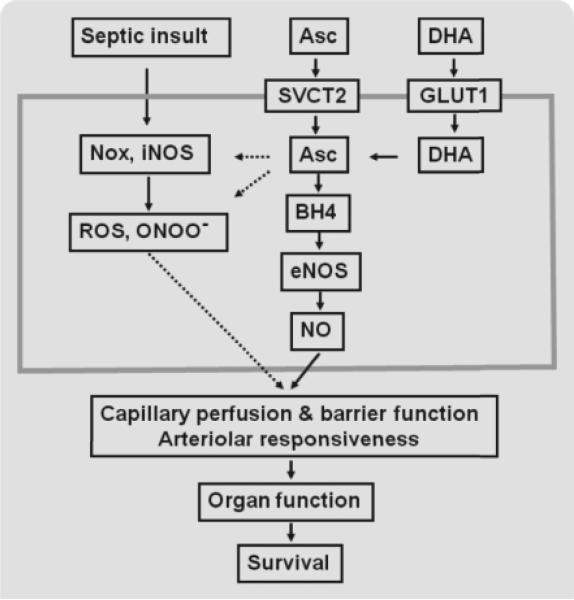
As depicted in Fig. 1, ascorbate is transported into endothelial cells by the specific sodium-dependent vitamin C transporter 2 (SVCT2), while DHA is taken up through facilitative glucose transporters (GLUTs) and then reduced to ascorbate. Ascorbate efflux from endothelial cells can be stimulated by calcium-dependent mechanisms, but these cells normally retain intracellular concentrations of ascorbate that are much higher than the extracellular levels [1,30–33]. Overall, these transport systems cause endothelial cells to rapidly accumulate millimolar levels of ascorbate that either alters intracellular function or is released in regulated ways to the extracellular fluid.
Fig. 1.
Intracellular ascorbate modulates the effects of septic insult on microvascular endothelial cell function. The largest rectangle represents a microvascular endothelial cell, in which arrows with solid lines indicate stimulation and those with dotted lines indicate inhibition. Septic insult increases NADPH oxidase (Nox) and inducible nitric oxide synthase (iNOS) activities, which elevate reactive oxygen species (ROS) and peroxynitrite (ONOO–) levels. ROS and ONOO– impair capillary blood flow, capillary barrier function, and arteriolar responsiveness to vasoconstrictors and vasodilators. Ascorbate (Asc) and dehydroascorbic acid (DHA) enter the cell through sodium-dependent vitamin C transporter 2 (SVCT2) and glucose transporter 1 (GLUT1), respectively, and DHA becomes reduced to Asc. Intracellular Asc rapidly scavenges ROS and ONOO–, while stimulating tetrahydrobiopterin (BH4)-dependent endothelial nitric oxide synthase (eNOS), to increase the local concentration of nitric oxide (NO). Asc also decreases Nox activity, prevents induction of the enzyme's p47phox subunit, and blocks induction of iNOS.
Inflammatory cytokines (tumor necrosis factor-alpha, interleukin-1beta) inhibit ascorbate uptake in endothelial cell cultures that spontaneously express SVCT2 [34]. This action may deplete intracellular ascorbate from the endothelium during sepsis. A second reason why intracellular ascorbate may be depleted is the poor control of plasma glucose, which leads to episodes of hyperglycemia in septic patients [3]. Acute hyperglycemia causes ascorbate deficiency in endothelial cells and impairs endothelium-dependent vasodilation in healthy human subjects [35]. These effects are consequences of the competitive inhibition by glucose of DHA uptake into endothelial cells, since the impairment of vasodilation can be reversed by intravenous ascorbate (2 g bolus [ref. 36]; 3 mg/min infusion [ref. 37]). A third potential cause of intracellular depletion of ascorbate is that excessive ROS may oxidize ascorbate to DHA and then oxidize the latter irreversibly.
LPS raises ascorbate concentration in the adrenal gland, heart, kidney, and liver [38]. This phenomenon apparently does not require SVCT2, because there is no interaction between the effects of LPS and SVCT2 deficiency (SVCT2+/– heterozygote mice) on ascorbate concentration in these organs [38]. In most cell types that have been studied, the uptake and reduction of extracellular DHA to ascorbate is not impaired by LPS. On the contrary, LPS and nitric oxide donors upregulate the expression of GLUT1 in endothelial cell cultures [39,40]. Septic insults accelerate the rate at which extracellular DHA is taken up and reduced to ascorbate in multiple cell types [38,41] (although not in all, since septic insult inhibits DHA uptake in cultured astrocytes [42]).
Endothelial cells respond to LPS with increased expression of glucose-6-phosphate dehydrogenase, the key enzyme of the pentose cycle (hexose monophosphate shunt) that produces NADPH [43]. Induction by LPS of glucose-6-phosphate dehydrogenase may increase the supply of reducing equivalents from NADPH for conversion of DHA to ascorbate.
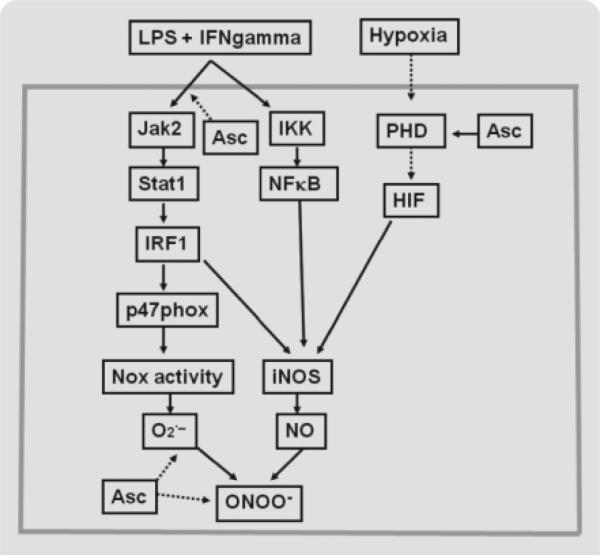
In tissue regions with nonperfused capillaries, hypoxia may inhibit hypoxia-inducible factor (HIF) prolyl-hydroxylase (PHD) and consequently increase the expression of HIFs (Fig. 2). HIF-1 increases the expression of the transporters GLUT1 and GLUT3, glycolytic enzymes, and several genes involved in inflammation [44,45]. Hypoxia stimulates DHA uptake through GLUT1 [46]. The elevated reducing power associated with hypoxia may then increase the capacity for reduction of DHA to ascorbate inside the cells.
Fig. 2.
Intracellular ascorbate (Asc) modulates redox-sensitive signaling pathways in microvascular endothelial cells during sepsis. The largest rectangle represents a microvascular endothelial cell, in which arrows with solid lines indicate stimulation and those with dotted lines indicate inhibition. Lipopolysaccharide (LPS) and cytokines such as interferon-gamma (IFNgamma) stimulate signaling pathways that trigger the expression of NADPH oxidase (Nox) subunit p47phox, inducible nitric oxide synthase (iNOS), and other inflammatory mediators. Hypoxia increases hypoxia-inducible factor 1 (HIF-1), by inhibiting HIF-1 prolyl-hydroxylase (PHD), and thereby induces the expression of sepsis-associated genes. Asc inhibits the activation of Jak2-Stat1-IRF1 pathway and increases the activity of PHD. Asc further regulates local nitric oxide (NO) concentration by scavenging superoxide () and peroxynitrite (ONOO–). IKK; NFκB. Ascorbate does not affect the IKB kinase (IKK) and nuclear factor-κB that also mediate iNos induction in microvascular endothelial cells exposed to LPS and IFN gamma.
3. Clinical trials of vitamin C in critically ill patients
As detailed later, sepsis is associated with increased production of ROS and peroxynitrite that deplete antioxidant molecules and oxidize proteins and lipids. ROS also alter redox-sensitive activation and expression of proteins that alter capillary blood flow distribution, capillary permeability (i.e., capillary barrier function), and arteriolar responsiveness to vasoconstrictors and vasodilators (Figs. 1 and 2). Therefore, patients with sepsis may benefit from adjuvant therapy that prevents the increase of ROS, particularly at intracellular signaling sites. Parenteral ascorbate may be an intervention that confers this benefit.
Administering ascorbate parenterally rather than orally increases its effects on plasma ascorbate concentration and microvascular function [1]. For instance, when oral and intravenous routes of ascorbate administration (500 mg/day for 30 days) are compared in sedentary men, only intravenous ascorbate improves endothelium-dependent arteriolar function as indicated by flow-mediated vasodilation [47].
Parenteral administration of ascorbate may decrease morbidity and mortality in critically ill patients who are septic or at risk of becoming septic. In a randomized, double-blind, placebo-controlled trial with 216 critically ill patients, 28-day mortality was decreased in the patients who received combined ascorbate and vitamin E by intravenous infusion compared with those who did not [48]. A second randomized trial with 595 critically ill surgical patients found that a combination of ascorbate (1,000 mg q8h by intravenous injection) and vitamin E (1,000 IU q8h by naso- or orogastric tube), begun within 24 h of traumatic injury or major surgery, decreased relative risk of pulmonary edema and multiple organ failure [49]. These two trials were not designed to distinguish between the actions of ascorbate and vitamin E. However, a third randomized trial observed decreased morbidity for severely burned patients who received a very high dose of ascorbate (1,584 mg/kg/day) parenterally [50]. Of particular relevance to microvascular barrier function, ascorbate treatment was associated with significant reductions in edema formation, fluid resuscitation volume, and respiratory dysfunction [50].
4. Effects of vitamin C on survival in experimental sepsis
Animal models of sepsis syndromes provide fundamental information about the potential benefit and mechanism of action of ascorbate. Prior depletion of ascorbate decreases survival in mice injected with pathogenic bacteria [51]. Consistently, parenteral administration of ascorbate prevents hypotension and edema in LPS-injected animals [5,6,11] and it improves capillary blood flow, arteriolar responsiveness, arterial blood pressure, liver function, and survival in experimental sepsis [4,12–15,52].
Among the most clinically relevant models of polymicrobial sepsis are cecal ligation and puncture (CLP) and feces injection into peritoneum (FIP). Similar to the changes observed in septic patients, CLP in animals increases oxidative stress markers and decreases ascorbate concentration in plasma and tissue [4,12,14]. Injection of ascorbate (200 mg/kg, i.v.) increases survival in CLP mice [15]. Survival rates at 24 h post-CLP are 9% and 65% in the vehicle-injected and ascorbate-injected mice, respectively. The protective effect is not attributable to inhibition of bacterial replication at the infectious nidus, because the number of bacterial colony-forming units in peritoneal lavage fluid after CLP does not differ between vehicle- and ascorbate-injected mice [15]. In FIP mice, 24-h survival is 19% after saline vehicle injection but 50% after intravenous ascorbate injection (10 mg/kg, i.v.) [13].
5. Capillary perfusion deficit
5.1. Rapid response to ascorbate
Intravenous ascorbate injection may protect several microvascular functions, namely, capillary blood flow, microvascular permeability barrier, and arteriolar responsiveness to vasoconstrictors and vasodilators. Intravenous injection of ascorbate prevents and reverses the maldistribution of blood flow in capillaries of septic models. The effect of parenteral ascorbate is both rapid and persistent. This section discusses the mechanisms underlying the onset of the response to ascorbate.
Systemic inflammation causes stoppage of blood flow in some capillaries. In clinical sepsis, the pattern of capillary blood flow distribution improves in survivors but fails to improve in nonsurvivors [10]. Improved capillary blood flow during fluid resuscitation is associated with prevention of organ failure independently of changes in global hemodynamics [53]. Similar to clinical sepsis, CLP and FIP decrease the density of perfused capillaries and increase the proportion of nonperfused capillaries in skeletal muscles of mice and rats, despite administration of fluid for volume resuscitation to prevent shock [4,12,13].
In critically ill patients, vasodilators transiently increase the proportion of perfused capillaries [54]. Whether vasodilation by ascorbate occurs and is a direct cause of restoration of capillary blood flow in clinical sepsis are not known with certainty. However, no increase in flow velocity (measured as red blood cell velocity) is detectable in capillaries after injection of ascorbate that restores the number of perfused capillaries to normal in septic mouse skeletal muscle [13]. Therefore, the evidence from experimental sepsis studies is that restoration of capillary blood flow is not achieved through a vasodilatory effect of ascorbate. Instead, the reason why blood flow stops in some capillaries may be deficiency of nitric oxide in endothelial cells and platelets. Indeed, nitric oxide appears essential for keeping microvessels patent, and local application of a nitric oxide donor restores capillary blood flow to normal in septic mice (6 h post-FIP) [13].
The decreased availability of nitric oxide inside septic endothelial cells and platelets may be attributable to ROS. Septic insult increases the activity of NADPH oxidases that synthesize ROS in blood vessels and microvascular endothelial cell cultures [16,33,55]. Indeed, NADPH oxidase activity is the principal source for stimulated production of superoxide in microvascular endothelial cells incubated with a septic insult (a combination of LPS and interferon-gamma [IFN-gamma]; LPS + IFNgamma) [33,55]. Accelerated production of superoxide is detectable within 2 h of the cells’ initial exposure to LPS + IFNgamma [33]. NADPH oxidase-derived ROS impair capillary blood flow during sepsis, since either knocking out the gp91phox (Nox2) subunit of NADPH oxidase or pharmacologically inhibiting the enzyme is sufficient to correct the maldistribution of blood flow caused by FIP in mice [13]. ROS oxidize tetrahydrobiopterin, which in its reduced form is a cofactor for enzymatic synthesis of nitric oxide. The loss of tetrahydrobiopterin (due to its oxidation) uncouples endothelial nitric oxide synthase (eNOS) in endothelial cells and platelets, so that this enzyme synthesizes superoxide rather than nitric oxide [56]. Local application of tetrahydrobiopterin restores capillary blood flow during sepsis in wild-type mice but not in eNOS–/– mice [13]. These observations support the hypothesis that tetrahydrobiopterin stimulates eNOS activity to increase nitric oxide production and thus reverses the maldistribution of capillary blood flow in sepsis.
Compared with vehicle injection, bolus intravenous ascorbate injection at 0, 1, 6, or 24 h after the onset of septic insult improves the distribution of capillary blood flow in CLP rat skeletal muscle [4,12]. For example, injection of ascorbate (10 mg/kg) at 6 h after the onset of septic insult reverses the maldistribution of blood flow within 10 min [13]. Ascorbate's rapid improvement of blood flow distribution during sepsis is eNOS-dependent because it occurs in wild-type, neuronal nitric oxide synthase knockout (nNOS–/–) and inducible nitric oxide synthase knockout (iNOS–/–) mice but not in eNOS–/– mice [13]. The stimulatory effect of ascorbate on nitric oxide levels in endothelial cells is attributable to multiple mechanisms. First, as shown in Fig. 1, ascorbate prevents and reverses tetrahydrobiopterin oxidation, increases tetrahydrobiopterin content, and elevates tetrahydrobiopterin-dependent synthesis of nitric oxide by eNOS, which are actions that N-acetylcysteine cannot do [7,57,58]. Second, ascorbate scavenges superoxide and other ROS that otherwise react with nitric oxide [55] (Fig. 2).
Blood flow stoppage in septic capillaries may result from interactions between leukocytes, platelets, and capillary endothelial cells. ROS activate intracellular redox signaling pathways to increase adhesion of leukocytes and platelets to endothelium [59]. Consistent with this fact, platelet adhesion is stimulated and inhibited, respectively, by locally generated superoxide and nitric oxide during experimental sepsis [59]. Endothelial- and platelet-derived ROS also enhance platelet aggregation [60]. It is possible that formation of blood clots in microvessels after platelet adhesion and aggregation may contribute to blood flow stoppage during systemic inflammation. Intravenous injection of 2 g ascorbate enhances the inhibition of platelet aggregation by a nitric oxide donor in patients who are prothrombotic because of chronic heart failure [61]. The mechanism underlying this effect on cell adhesion may involve ascorbate inhibiting the expression and activation of NADPH oxidase, thereby preventing local scarcity of nitric oxide [13,33]. It seems likely that ascorbate has a similar antiaggregation effect in patients who are prothrombotic because of sepsis.
5.2. Persistent response to ascorbate
The capillary perfusion deficit in experimental sepsis can be mitigated for at least 12 and 47 h by ascorbate doses of 10 and 76 mg/kg, respectively [4,12,13]. Thus, microvascular effects of parenteral ascorbate persist for many hours after plasma ascorbate returns to baseline [13]. One reason for the long duration of this effect is that cells retain high concentrations of intracellular ascorbate that persist longer than does extracellular ascorbate [33]. A second reason why the microvascular response to ascorbate endures is that the vitamin alters gene expression, as discussed later.
Cells maintained under standard culture conditions often contain no ascorbate because ascorbate and DHA are either omitted from the medium or inadvertently destroyed during the preparation and storage of culture media and sera. In ascorbate-free microvascular endothelial cells, LPS + INF-gamma rapidly increases the activity of NADPH oxidase [33,55]. Endothelial NADPH oxidase synthesizes intracellular superoxide, which reacts to form other ROS (e.g., dismutation of superoxide produces hydrogen peroxide) that then induce prolonged redox signaling effects [62]. Either LPS + INF-gamma or exogenous hydrogen peroxide stimulates Jak2/Stat1/IRF1 signaling and increases expression of NADPH oxidase subunit proteins [33,55]. Thus, septic insult initiates a feed-forward mechanism to increase NADPH oxidase-derived ROS production. Incubation of microvascular endothelial cells with ascorbate raises intracellular ascorbate concentration and prevents the induction by LPS + IFN-gamma or hydrogen peroxide of endothelial NADPH oxidase activity [33]. Ascorbate also inhibits the induction of the enzyme's p47phox subunit [33]. The latter effect is mediated by the Jak2/Stat1/IRF1 signaling pathway because ascorbate prevents activation of this pathway by LPS + IFNgamma or hydrogen peroxide [33] (Fig. 2). Selectivity is shown by the fact that ascorbate inhibits superoxide synthesis by NADPH oxidase in endothelial cells [33] but not in neutrophil leukocytes [63–66].
The prolonged effect of ascorbate on microvascular function may also involve suppression of gene expression, which is dependent on HIF-1 (Fig. 2). Ascorbate acts through the PHD cofactor, iron, to increase the enzyme's activity and thereby inhibit the induction and stabilization of HIF-1alpha by hypoxia [44]. Furthermore, inhibition by ascorbate of endothelial NADPH oxidase [33] and scavenging of oxidants by ascorbate may preserve PHD activity. This is because oxidants, such as NADPH oxidase-derived ROS, inhibit PHD activity [67]. The lowering of HIF-1 levels by ascorbate inhibits expression of HIF-1 sensitive genes, such as GLUT1 and iNOS [14,15,45,55].
Activation of coagulation during sepsis is another potential cause of capillary blood flow impairment, which may be modulated gradually by ascorbate. ROS promote expression of adhesion molecules and tissue factors at the surface of platelets and endothelial cells [60]. Subsequent formation of tissue factors and factor VII complex leads to generation of thrombin that activates NADPH oxidase. This positive feedback mechanism may stimulate formation of microthrombi [60] and its abrogation may be an important mechanism by which ascorbate's improvement of blood flow distribution is sustained long enough to increase survival. Injection of a tissue factor pathway inhibitor increases survival in a CLP model of sepsis [68], which is an effect similar to that achieved by injection of ascorbate [15].
Another potential role for ascorbate is suggested by the observation that superoxide stimulates expression of cell surface intercellular adhesion molecule 1 (ICAM-1) in microvascular endothelial cells [62]. ICAM-1 mediates adhesion of leukocytes to the endothelium and may thereby impair the microcirculation. Since ascorbate inhibits superoxide production in microvascular endothelial cells exposed to septic insult [33], further research is warranted to determine if the vitamin prevents leukocyte plugging of microvessels.
6. Vitamin C and increase in endothelial permeability in sepsis
Increased permeability of the endothelium occurs in multiple organs during sepsis, leading to plasma extravasation and edema formation. This causes respiratory dysfunction, blood volume decrease, and disease progression to septic shock. Parenteral administration of ascorbate decreases edema formation in patients with severe burn injury [50] as well as in burn-injured or LPS-injected animals [5,69,70]. Ascorbate also attenuates the increase in endothelial permeability caused by LPS in vitro [71].
One reason for the loss of barrier function in sepsis may be endothelial cell apoptosis [2]. Therefore, the role of ascorbate in both preventing apoptosis in endothelial cells and stimulating their proliferation may be beneficial [72–75].
Another action of ascorbate on endothelial permeability may involve nitric oxide, superoxide, and peroxynitrite. Basal nitric oxide production by eNOS is necessary for maintenance of the endothelial barrier function (i.e., to keep the endothelium's paracellular permeability to plasma proteins low) [76]. The protective effect of nitric oxide is diminished during the inflammatory response because of simultaneous production of superoxide. Nitric oxide reacts with superoxide to form peroxynitrite, which causes lipid peroxidation, oxidation of sulfhydryl groups, and nitration of tyrosine residues in proteins. In particular, nitration of protein phosphatase type 2 and cytoskeletal proteins by peroxynitrite appears to be a key step in the development of microvascular barrier dysfunction [77,78]. The principal sources of the superoxide are likely endothelial NADPH oxidase and uncoupled eNOS and iNOS. Evidence for the role of iNOS is that genetic or pharmacological interventions that inhibit this enzyme also decrease microvascular leakage in experimental sepsis [79]. By scavenging superoxide, inhibiting protein expression of p47phox and iNOS, and preventing superoxide synthesis by uncoupled eNOS and iNOS, ascorbate decreases the formation of peroxynitrite. Additionally, ascorbate reduces the oxidation products formed by reaction of peroxynitrite with cell proteins [80]. These actions of ascorbate may account for its effectiveness in preventing edema in critically ill patients and experimental models [5,50,69,71].
The mechanism underlying the septic induction of iNOS and its abrogation by ascorbate has been elucidated. The oxidants that arise from NADPH oxidase activity (e.g., hydrogen peroxide formed by dismutation of superoxide) enhance the induction of iNOS in septic blood vessels and endothelial cells [15,33,77]. iNOS synthesizes abundant nitric oxide, which in turn reacts with superoxide, resulting in excessive production of peroxynitrite. Ascorbate prevents the induction of iNOS by septic insults in blood vessels in vivo and endothelial cells in culture [14,15,81]. Ascorbate's suppression of NADPH oxidase mediates, at least in part, this inhibition of iNOS expression [55]. Upon stimulation by LPS + IFNgamma, NADPH oxidase produces ROS that activate the JNK-AP1 and Jak2-IRF1 signaling pathways of iNOS induction, and ascorbate prevents this activation [55].
7. Arteriolar hyporesponsiveness to vasoconstrictors
Hypotension in septic patients may be caused by impairment of myocardial function and by loss of arteriolar responsiveness to vasoconstrictors. Parenteral ascorbate may counter the latter problem, because infusion of ascorbate reverses arteriolar hyporesponsiveness to vasoconstrictors (norepinephrine, angiotensin, vasopressin) in human subjects who have inflammatory disease or have been injected with LPS [9,82].
Comparable results have been obtained in animal models of sepsis. For example, increased heterogeneity of capillary blood flow is followed by the development of arterial hypotension in CLP rats [12]. Arteriolar vasoconstriction and arterial blood pressure responses to norepinephrine and angiotensin II are inhibited in mice at 6 h post-CLP [14,15]. Intravenous ascorbate and iNOS gene deficiency (iNOS–/– mice) are equally effective in preventing the CLP-induced impairment of arteriolar responsiveness [13–15]. Arteriolar responsiveness and arterial blood pressure are higher in CLP rats injected intravenously with ascorbate, compared with those injected with vehicle, when these parameters are measured at 18–24 h postinjection [4,12].
8. Arteriolar hyporesponsiveness to vasodilators
Endothelial cells regulate arteriolar responsiveness to vasodilators through eNOS-derived nitric oxide and prostaglandin endoperoxide H2 synthase-1 (PGHS)-derived prostacyclin [83]. Nitric oxide enters arteriolar smooth muscle cells and activates soluble guanylyl cyclase, thereby raising intracellular cGMP. Prostacyclin stimulates adenylyl cyclase to raise intracellular cAMP. Both cGMP and cAMP then mediate smooth muscle relaxation. However, septic insult increases the production of superoxide, which reacts with nitric oxide to form peroxynitrite that inactivates endothelial PGHS, which can then no longer synthesize prostacyclin. Superoxide and other NADPH oxidase-derived oxidants (i.e., hydrogen peroxide and peroxynitrite) may also decrease the effective cellular level of nitric oxide below that required for guanylyl cyclase activation [83]. Thus arteriolar responsiveness to vasodilators is inhibited by LPS infusion in human subjects and by CLP-induced sepsis in animals [7,8,16].
Infusion of ascorbate or tetrahydrobiopterin prevents inhibition by LPS of endothelium-dependent vasodilation responses (assessed as changes in forearm blood flow) to acetylcholine in healthy human subjects [7,8]. This effect of ascorbate is associated with a marked increase in plasma tetrahydrobiopterin concentration [7]. Ascorbate may maintain normal levels of eNOS-derived nitric oxide and PGHS-derived prostacyclin by suppressing NADPH oxidase expression, scavenging ROS, and enhancing tetrahydrobiopterin levels within the endothelial cells of arterioles.
Parenteral ascorbate remarkably enhances arteriolar responsiveness to vasodilators in several diseases. For example, when either N-acetylcysteine (48 mg/min) or ascorbate (18 mg/min) is infused intra-arterially in human subjects with essential hypertension, only the ascorbate treatment enhances vasodilation by acetylcholine [58]. Recently, the topic of responsiveness to vasodilators in clinical sepsis has become controversial. Kienbaum et al. [84] reported that the acetylcholine-induced decrease in forearm vascular resistance (forearm blood flow/mean arterial pressure) did not differ between septic patients and controls. However, since the septic patients had lower vascular resistance initially, the decrease in vascular resistance caused by acetylcholine infusion may have been less in these patients. In a study of healthy human subjects before and during experimental endotoxemia, arteriolar hyporesponsiveness to acetylcholine was found 4–6 h after LPS administration, at the time when circulating cytokines are at their highest [8]. Therefore, ascorbate-sensitive arteriolar hyporesponsiveness to vasodilators may vary with time or disease severity during sepsis syndrome progression.
9. Unresolved issues meriting further exploration
There are no studies that compare ascorbate and DHA for efficacy in treating sepsis. Maximal uptake rates are higher for DHA than ascorbate in most mammalian cell types, when studied under glucose-free conditions [1]. But glucose inhibits DHA uptake into most cells, including endothelial cells [35], and hyperglycemia that often occurs in sepsis [3] may decrease the cellular uptake and therapeutic efficacy of administered DHA.
The safety of parenteral ascorbate requires further investigation. A study of intravenous ascorbate in patients with advanced malignancies reported that injection of 1.5 g ascorbate/kg body weight three times weekly is well tolerated [85]. However, ascorbate is metabolized to oxalate, which accumulates as nephrotoxic calcium oxalate crystals (nephrolithiasis) in the kidneys of susceptible individuals, as reported in a recent case study [86]. Another concern is that ascorbate donates electrons to transition metals (e.g., iron), which then catalyze the synthesis of hydrogen peroxide. Repeated intravenous injections of 750–7,500 mg/day of ascorbate for 6 days do not induce pro-oxidant changes in the plasma in healthy volunteers [87]. But in surgical patients, intravenous injection of 2 g ascorbate at 2 h before major surgery increases oxidative modification of plasma lipids in the venous blood samples obtained during the ischemic phase of surgery [88].
10. Conclusion
Further study is needed to determine definitively the safety and efficacy of ascorbate in patients with sepsis. Nevertheless, current evidence supports the hypothesis that microvascular function may be improved in sepsis by parenteral administration of ascorbate as an adjuvant therapy.
Acknowledgement
This work was financially supported by the National Institutes of Health Grant 1R01AT003643-01A2.
References
- 1.Wilson JX. Regulation of vitamin C transport. Annu. Rev. Nutr. 2005;25:105–125. doi: 10.1146/annurev.nutr.25.050304.092647. [DOI] [PubMed] [Google Scholar]
- 2.Marshall JC. Sepsis: rethinking the approach to clinical research. J. Leukoc. Biol. 2008;83:471–482. doi: 10.1189/jlb.0607380. [DOI] [PubMed] [Google Scholar]
- 3.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL, International Surviving Sepsis Campaign Guidelines Committee. American Association of Critical-Care Nurses. American College of Chest Physicians. American College of Emergency Physicians. Canadian Critical Care Society. European Society of Clinical Microbiology and Infectious Diseases. European Society of Intensive Care Medicine. European Respiratory Society. International Sepsis Forum. Japanese Association for Acute Medicine. Japanese Society of Intensive Care Medicine. Society of Critical Care Medicine. Society of Hospital Medicine. Surgical Infection Society. World Federation of Societies of Intensive and Critical Care Medicine Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit. Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 4.Armour J, Tyml K, Lidington D, Wilson JX. Ascorbate prevents microvascular dysfunction in the skeletal muscle of the septic rat. J. Appl. Physiol. 2001;90:795–803. doi: 10.1152/jappl.2001.90.3.795. [DOI] [PubMed] [Google Scholar]
- 5.Dwenger A, Pape HC, Bantel C, Schweitzer G, Krumm K, Grotz M, Lueken B, Funck M, Regel G. Ascorbic acid reduces the endotoxin-induced lung injury in awake sheep. Eur. J. Clin. Invest. 1994;24:229–235. doi: 10.1111/j.1365-2362.1994.tb01079.x. [DOI] [PubMed] [Google Scholar]
- 6.Feng NH, Chu SJ, Wang D, Hsu K, Lin CH, Lin HI. Effects of various antioxidants on endotoxin-induced lung injury and gene expression: mRNA expressions of MnSOD, interleukin-1beta and iNOS. Chin. J. Physiol. 2004;47:111–120. [PubMed] [Google Scholar]
- 7.Mittermayer F, Pleiner J, Schaller G, Zorn S, Namiranian K, Kapiotis S, Bartel G, Wolfrum M, Brugel M, Thiery J, Macallister RJ, Wolzt M. Tetrahydrobiopterin corrects Escherichia coli endotoxin-induced endothelial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H1752–H1757. doi: 10.1152/ajpheart.00057.2005. [DOI] [PubMed] [Google Scholar]
- 8.Pleiner J, Mittermayer F, Schaller G, MacAllister RJ, Wolzt M. High doses of vitamin C reverse Escherichia coli endotoxin-induced hyporeactivity to acetylcholine in the human forearm. Circulation. 2002;106:1460–1464. doi: 10.1161/01.cir.0000030184.70207.ff. [DOI] [PubMed] [Google Scholar]
- 9.Pleiner J, Mittermayer F, Schaller G, Marsik C, MacAllister RJ, Wolzt M. Inflammation-induced vasoconstrictor hyporeactivity is caused by oxidative stress. J. Am. Coll. Cardiol. 2003;42:1656–1662. doi: 10.1016/j.jacc.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit. Care Med. 2004;32:1825–1831. doi: 10.1097/01.ccm.0000138558.16257.3f. [DOI] [PubMed] [Google Scholar]
- 11.Shen KP, Lo YC, Yang RC, Liu HW, Chen IJ, Wu BN. Antioxidant eugenosedin-A protects against lipopolysaccharide-induced hypotension, hyperglycaemia and cytokine immunoreactivity in rats and mice. J. Pharm. Pharmacol. 2005;57:117–125. doi: 10.1211/0022357055137. [DOI] [PubMed] [Google Scholar]
- 12.Tyml K, Li F, Wilson JX. Delayed ascorbate bolus protects against maldistribution of microvascular blood flow in septic rat skeletal muscle. Crit. Care Med. 2005;33:1823–1828. doi: 10.1097/01.ccm.0000172548.34622.de. [DOI] [PubMed] [Google Scholar]
- 13.Tyml K, Li F, Wilson JX. Septic impairment of capillary blood flow requires nicotinamide adenine dinucleotide phosphate oxidase but not nitric oxide synthase and is rapidly reversed by ascorbate through an endothelial nitric oxide synthase-dependent mechanism. Crit. Care Med. 2008;36:2355–2362. doi: 10.1097/CCM.0b013e31818024f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu F, Wilson JX, Tyml K. Ascorbate inhibits iNOS expression and preserves vasoconstrictor responsiveness in skeletal muscle of septic mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R50–R56. doi: 10.1152/ajpregu.00564.2002. [DOI] [PubMed] [Google Scholar]
- 15.Wu F, Wilson JX, Tyml K. Ascorbate protects against impaired arteriolar constriction in sepsis by inhibiting inducible nitric oxide synthase expression. Free Radic. Biol. Med. 2004;37:1282–1289. doi: 10.1016/j.freeradbiomed.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 16.Yu HP, Lui PW, Hwang TL, Yen CH, Lau YT. Propofol improves endothelial dysfunction and attenuates vascular superoxide production in septic rats. Crit. Care Med. 2006;34:453–460. doi: 10.1097/01.ccm.0000198530.68343.21. [DOI] [PubMed] [Google Scholar]
- 17.Carré JE, Singer M. Cellular energetic metabolism in sepsis: the need for a systems approach. Biochim. Biophys. Acta. 2008;1777:763–771. doi: 10.1016/j.bbabio.2008.04.024. [DOI] [PubMed] [Google Scholar]
- 18.Holzheimer RG. Antibiotic induced endotoxin release and clinical sepsis: a review. J. Chemother. 2001;13:159–172. doi: 10.1179/joc.2001.13.Supplement-2.159. [DOI] [PubMed] [Google Scholar]
- 19.Borrelli E, Roux-Lombard P, Grau GE, Girardin E, Ricou B, Dayer J, Suter PM. Plasma concentrations of cytokines, their soluble receptors, and antioxidant vitamins can predict the development of multiple organ failure in patients at risk. Crit. Care Med. 1996;24:392–397. doi: 10.1097/00003246-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Doise JM, Aho LS, Quenot JP, Guilland JC, Zeller M, Vergely C, Aube H, Blettery B, Rochette L. Plasma antioxidant status in septic critically ill patients: a decrease over time. Fundam. Clin. Pharmacol. 2008;22:203–209. doi: 10.1111/j.1472-8206.2008.00573.x. [DOI] [PubMed] [Google Scholar]
- 21.Galley HF, Davies MJ, Webster NR. Ascorbyl radical formation in patients with sepsis: effect of ascorbate loading. Free Radic. Biol. Med. 1996;20:139–143. doi: 10.1016/0891-5849(95)02022-5. [DOI] [PubMed] [Google Scholar]
- 22.Long CL, Maull KI, Krishnan RS, Laws HL, Geiger JW, Borghesi L, Franks W, Lawson TC, Sauberlich HE. Ascorbic acid dynamics in the seriously ill and injured. J. Surg. Res. 2003;109:144–148. doi: 10.1016/s0022-4804(02)00083-5. [DOI] [PubMed] [Google Scholar]
- 23.Metnitz PG, Bartens C, Fischer M, Fridrich P, Steltzer H, Druml W. Antioxidant status in patients with acute respiratory distress syndrome. Intensive Care Med. 1999;25:180–185. doi: 10.1007/s001340050813. [DOI] [PubMed] [Google Scholar]
- 24.Rumelin A, Humbert T, Luhker O, Drescher A, Fauth U. Metabolic clearance of the antioxidant ascorbic acid in surgical patients. J. Surg. Res. 2005;129:46–51. doi: 10.1016/j.jss.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Schorah CJ, Downing C, Piripitsi A, Gallivan L, Al-Hazaa AH, Sanderson MJ, Bodenham A. Total vitamin C, ascorbic acid, and dehydroascorbic acid concentrations in plasma of critically ill patients. Am. J. Clin. Nutr. 1996;63:760–765. doi: 10.1093/ajcn/63.5.760. [DOI] [PubMed] [Google Scholar]
- 26.Dupertuis YM, Ramseyer S, Fathi M, Pichard C. Assessment of ascorbic acid stability in different multilayered parenteral nutrition bags: critical influence of the bag wall material. JPEN J. Parenter. Enteral. Nutr. 2005;29:125–130. doi: 10.1177/0148607105029002125. [DOI] [PubMed] [Google Scholar]
- 27.Knafo L, Chessex P, Rouleau T, Lavoie JC. Association between hydrogen peroxide-dependent byproducts of ascorbic acid and increased hepatic acetyl-CoA carboxylase activity. Clin. Chem. 2005;51:1462–1471. doi: 10.1373/clinchem.2005.050427. [DOI] [PubMed] [Google Scholar]
- 28.Baines M, Shenkin A. Lack of effectiveness of short-term intravenous micronutrient nutrition in restoring plasma antioxidant status after surgery. Clin. Nutr. 2002;21:145–150. doi: 10.1054/clnu.2001.0524. [DOI] [PubMed] [Google Scholar]
- 29.Luo M, Fernandez-Estivariz C, Jones DP, Accardi CR, Alteheld B, Bazargan N, Hao L, Griffith DP, Blumberg JB, Galloway JR, Ziegler TR. Depletion of plasma antioxidants in surgical intensive care unit patients requiring parenteral feeding: effects of parenteral nutrition with or without alanyl-glutamine dipeptide supplementation. Nutrition. 2008;24:37–44. doi: 10.1016/j.nut.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Best KA, Holmes ME, Samson SE, Mwanjewe J, Wilson JX, Dixon SJ, Grover AK. Ascorbate uptake in pig coronary artery endothelial cells. Mol. Cell Biochem. 2005;271:43–49. doi: 10.1007/s11010-005-3442-0. [DOI] [PubMed] [Google Scholar]
- 31.Davis KA, Samson SE, Best K, Mallhi KK, Szewczyk M, Wilson JX, Kwan CY, Grover AK. Ca2+-mediated ascorbate release from coronary artery endothelial cells. Br. J. Pharmacol. 2006a;147:131–139. doi: 10.1038/sj.bjp.0706492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis KA, Samson SE, Wilson JX, Grover AK. Hypotonic shock stimulates ascorbate release from coronary artery endothelial cells by a Ca2+-independent pathway. Eur. J. Pharmacol. 2006b;548:36–44. doi: 10.1016/j.ejphar.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 33.Wu F, Schuster DP, Tyml K, Wilson JX. Ascorbate inhibits NADPH oxidase subunit p47phox expression in microvascular endothelial cells. Free Radic. Biol. Med. 2007;42:124–131. doi: 10.1016/j.freeradbiomed.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 34.Seno T, Inoue N, Matsui K, Ejiri J, Hirata KI, Kawashima S, Yokoyama M. Functional expression of sodium-dependent vitamin C transporter 2 in human endothelial cells. J. Vasc. Res. 2004;41:345–351. doi: 10.1159/000080525. [DOI] [PubMed] [Google Scholar]
- 35.Price KD, Price CSC, Reynolds RD. Hyperglycemia-induced ascorbic acid deficiency promotes endothelial dysfunction and the development of atherosclerosis. Atherosclerosis 2001. 2001;158:1–12. doi: 10.1016/s0021-9150(01)00569-x. [DOI] [PubMed] [Google Scholar]
- 36.Mullan BA, Ennis CN, Fee HJ, Young IS, McCance DR. Pretreatment with intravenous ascorbic acid preserves endothelial function during acute hyperglycaemia (R1). Clin. Exp. Pharmacol. Physiol. 2005;32:340–345. doi: 10.1111/j.1440-1681.2005.04193.x. [DOI] [PubMed] [Google Scholar]
- 37.Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, Boemi M, Giugliano D. Oscillating glucose is more deleterious on endothelial function and oxidative stress than mean glucose in normals and type 2 diabetic patients. Diabetes. 2008;57:1349–1354. doi: 10.2337/db08-0063. [DOI] [PubMed] [Google Scholar]
- 38.Kuo SM, Tan CH, Dragan M, Wilson JX. Endotoxin increases ascorbate recycling and concentration in mouse liver. J. Nutr. 2005;135:2411–2416. doi: 10.1093/jn/135.10.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paik JY, Lee KH, Ko BH, Choe YS, Choi YY, Kim BT. Nitric oxide stimulates 18F-FDG uptake in human endothelial cells through increased hexokinase activity and GLUT1 expression. J. Nucl. Med. 2005;46:365–370. [PubMed] [Google Scholar]
- 40.Spolarics Z, Stein DS, Garcia ZC. Endotoxin stimulates hydrogen peroxide detoxifying activity in rat hepatic endothelial cells. Hepatology. 1996;24:691–696. doi: 10.1002/hep.510240336. [DOI] [PubMed] [Google Scholar]
- 41.May JM, Huang J, Qu ZC. Macrophage uptake and recycling of ascorbic acid: response to activation by lipopolysaccharide. Free Radic. Biol. Med. 2005;39:1449–1459. doi: 10.1016/j.freeradbiomed.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Wilson JX, Dragan M. Sepsis inhibits recycling and glutamate-stimulated export of ascorbate by astrocytes. Free Radic. Biol. Med. 2005;39:990–998. doi: 10.1016/j.freeradbiomed.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 43.Spolarics Z. Endotoxemia, pentose cycle, and the oxidant/antioxidant balance in the hepatic sinusoid. J. Leukoc. Biol. 1998;63:534–541. doi: 10.1002/jlb.63.5.534. [DOI] [PubMed] [Google Scholar]
- 44.Stolze IP, Mole DR, Ratcliffe PJ. Regulation of HIF: prolyl hydroxylases. Novartis Found. Symp. 2006;272:15–25. [PubMed] [Google Scholar]
- 45.Vissers MC, Gunningham SP, Morrison MJ, Dachs GU, Currie MJ. Modulation of hypoxia-inducible factor-1 alpha in cultured primary cells by intracellular ascorbate. Free Radic. Biol. Med. 2007;42:765–772. doi: 10.1016/j.freeradbiomed.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 46.McNulty AL, Stabler TV, Vail TP, McDaniel GE, Kraus VB. Dehydroascorbate transport in human chondrocytes is regulated by hypoxia and is a physiologically relevant source of ascorbic acid in the joint. Arthritis Rheum. 2005;52:2676–2685. doi: 10.1002/art.21254. [DOI] [PubMed] [Google Scholar]
- 47.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J. Physiol. 2004;556:315–324. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crimi E, Liguori A, Condorelli M, Cioffi M, Astuto M, Bontempo P, Pignalosa O, Vietri MT, Molinari AM, Sica V, Della Corte F, Napoli C. The beneficial effects of antioxidant supplementation in enteral feeding in critically ill patients: a prospective, randomized, double-blind, placebo-controlled trial. Anesth. Analg. 2004;99:857–863. doi: 10.1213/01.ANE.0000133144.60584.F6. [DOI] [PubMed] [Google Scholar]
- 49.Nathens AB, Neff MJ, Jurkovich GJ, Klotz P, Farver K, Ruzinski JT, Radella F, Garcia I, Maier RV. Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann. Surg. 2002;236:814–822. doi: 10.1097/00000658-200212000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanaka H, Matsuda T, Miyagantani Y, Yukioka T, Matsuda H, Shimazaki S. Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: a randomized, prospective study. Arch. Surg. 2000;135:326–331. doi: 10.1001/archsurg.135.3.326. [DOI] [PubMed] [Google Scholar]
- 51.Gaut JP, Belaaouaj A, Byun J, Roberts LJ, II, Maeda N, Frei B, Heinecke JW. Vitamin C fails to protect amino acids and lipids from oxidation during acute inflammation. Free Radic. Biol. Med. 2006;40:1494–1501. doi: 10.1016/j.freeradbiomed.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 52.Kim JY, Lee SM. Vitamins C and E protect hepatic cytochrome P450 dysfunction induced by polymicrobial sepsis. Eur. J. Pharmacol. 2006;534:202–209. doi: 10.1016/j.ejphar.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 53.Trzeciak S, McCoy JV, Dellinger RP, Arnold RC, Rizzuto M, Abate NL, Shapiro NI, Parrillo JE, Hollenberg SM, on behalf of the Microcirculatory Alterations in Resuscitation and Shock (MARS) Investigators Early increases in microcirculatory perfusion during protocol-directed resuscitation are associated with reduced multi-organ failure at 24 h in patients with sepsis. Intensive Care Med. 2008;34:2210–2217. doi: 10.1007/s00134-008-1193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spronk PE, Ince C, Gardien MJ, Mathura KR, Oudemans-van Straaten HM, Zandstra DR. Nitroglycerin in septic shock after intravascular volume resuscitation. Lancet. 2002;360:1395–1396. doi: 10.1016/s0140-6736(02)11393-6. [DOI] [PubMed] [Google Scholar]
- 55.Wu F, Tyml K, Wilson JX. iNOS expression requires NADPH oxidase-dependent redox signaling in microvascular endothelial cells. J. Cell. Physiol. 2008;217:207–214. doi: 10.1002/jcp.21495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Invest. 2003;111:1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim HJ, Lee SI, Lee DH, Smith D, Jo H, Schellhorn HE, Boo YC. Ascorbic acid synthesis due to L-gulono-1,4-lactone oxidase expression enhances NO production in endothelial cells. Biochem. Biophys. Res. Commun. 2006;345:1657–1662. doi: 10.1016/j.bbrc.2006.05.090. [DOI] [PubMed] [Google Scholar]
- 58.Schneider MP, Delles C, Schmidt BM, Oehmer S, Schwarz TK, Schmieder RE, John S. Superoxide scavenging effects of N-acetylcysteine and vitamin C in subjects with essential hypertension. Am. J. Hypertens. 2005;18:1111–1117. doi: 10.1016/j.amjhyper.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 59.Tailor A, Cooper D, Granger DN. Platelet-vessel wall interactions in the microcirculation. Microcirculation. 2005;12:275–285. doi: 10.1080/10739680590925691. [DOI] [PubMed] [Google Scholar]
- 60.Herkert O, Djordjevic T, BelAiba RS, Gorlach A. Insights into the redox control of blood coagulation: role of vascular NADPH oxidase-derived reactive oxygen species in the thrombogenic cycle. Antioxid. Redox Signal. 2004;6:765–776. doi: 10.1089/1523086041361695. [DOI] [PubMed] [Google Scholar]
- 61.Ellis GR, Anderson RA, Chirkov YY, Morris-Thurgood J, Jackson SK, Lewis MJ, Horowitz JD, Frenneaux MP. Acute effects of vitamin C on platelet responsiveness to nitric oxide donors and endothelial function in patients with chronic heart failure. J. Cardiovasc. Pharmacol. 2001;37:564–570. doi: 10.1097/00005344-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 62.Li JM, Fan LM, Christie MR, Shah AM. Acute tumor necrosis factor alpha signaling via NADPH oxidase in microvascular endothelial cells: role of p47phox phosphorylation and binding to TRAF4. Mol. Cell. Biol. 2005;25:2320–2330. doi: 10.1128/MCB.25.6.2320-2330.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carr AC, Frei B. Human neutrophils oxidize low-density lipoprotein by a hypochlorous acid-dependent mechanism: the role of vitamin C. J. Biol. Chem. 2002;383:627–636. doi: 10.1515/BC.2002.065. [DOI] [PubMed] [Google Scholar]
- 64.Chatterjee M, Saluja R, Kumar V, Jyoti A, Jain GK, Barthwal MK, Dikshit M. Ascorbate sustains neutrophil NOS expression, catalysis, and oxidative burst. Free Radic. Biol. Med. 2008;45:1084–1093. doi: 10.1016/j.freeradbiomed.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 65.Ellis GR, Anderson RA, Lang D, Blackman DJ, Morris RH, Morris-Thurgood J, McDowell IF, Jackson SK, Lewis MJ, Frenneaux MP. Neutrophil superoxide anion-generating capacity, endothelial function and oxidative stress in chronic heart failure: effects of short- and long-term vitamin C therapy. J. Am. Coll. Cardiol. 2000;36:1474–1482. doi: 10.1016/s0735-1097(00)00916-5. [DOI] [PubMed] [Google Scholar]
- 66.Sharma P, Raghavan SA, Saini R, Dikshit M. Ascorbate-mediated enhancement of reactive oxygen species generation from polymorphonuclear leukocytes: modulatory effect of nitric oxide. J. Leukoc. Biol. 2004;75:1070–1078. doi: 10.1189/jlb.0903415. [DOI] [PubMed] [Google Scholar]
- 67.Goyal P, Weissmann N, Grimminger F, Hegel C, Bader L, Rose F, Fink L, Ghofrani HA, Schermuly RT, Schmidt HH, Seeger W, Hanze J. Upregulation of NAD(P)H oxidase 1 in hypoxia activates hypoxia-inducible factor 1 via increase in reactive oxygen species. Free Radic. Biol. Med. 2004;36:1279–1288. doi: 10.1016/j.freeradbiomed.2004.02.071. [DOI] [PubMed] [Google Scholar]
- 68.Opal SM, Palardy JE, Parejo NA, Creasey AA. The activity of tissue factor pathway inhibitor in experimental models of superantigen-induced shock and polymicrobial intra-abdominal sepsis. Crit. Care Med. 2001;29:13–17. doi: 10.1097/00003246-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 69.Dubick MA, Williams C, Elgjo GI, Kramer GC. High-dose vitamin C infusion reduces fluid requirements in the resuscitation of burn-injured sheep. Shock. 2005;24:139–144. doi: 10.1097/01.shk.0000170355.26060.e6. [DOI] [PubMed] [Google Scholar]
- 70.Sakurai M, Tanaka H, Matsuda T, Goya T, Shimazaki S, Matsuda H. Reduced resuscitation fluid volume for second-degree experimental burns with delayed initiation of vitamin C therapy (beginning 6 h after injury). J. Surg. Res. 1997;73:24–27. doi: 10.1006/jsre.1997.5203. [DOI] [PubMed] [Google Scholar]
- 71.Dimmeler S, Brinkmann S, Neugebauer E. Endotoxin-induced changes of endothelial cell viability and permeability: protective effect of a 21-aminosteroid. Eur. J. Pharmacol. 1995;287:257–261. doi: 10.1016/0014-2999(95)00499-8. [DOI] [PubMed] [Google Scholar]
- 72.Recchioni R, Marcheselli F, Moroni F, Pieri C. Apoptosis in human aortic endothelial cells induced by hyperglycemic condition involves mitochondrial depolarization and is prevented by N-acetyl-l-cysteine. Metabolism. 2002;51:1384–1388. doi: 10.1053/meta.2002.35579. [DOI] [PubMed] [Google Scholar]
- 73.Rössig L, Hoffmann J, Hugel B, Mallat Z, Haase A, Freyssinet JM, Tedgui A, Aicher A, Zeiher AM, Dimmeler S. Vitamin C inhibits endothelial cell apoptosis in congestive heart failure. Circulation. 2001;104:2182–2187. doi: 10.1161/hc4301.098284. [DOI] [PubMed] [Google Scholar]
- 74.Saeed RW, Peng T, Metz CN. Ascorbic acid blocks the growth inhibitory effect of tumor necrosis factor-alpha on endothelial cells. Exp. Cell Biol. (Maywood) 2003;228:855–865. doi: 10.1177/15353702-0322807-12. [DOI] [PubMed] [Google Scholar]
- 75.Schor AM, Schor SL, Allen TD. Effects of culture conditions on the proliferation, morphology and migration of bovine aortic endothelial cells. J. Cell Sci. 1983;62:267–285. doi: 10.1242/jcs.62.1.267. [DOI] [PubMed] [Google Scholar]
- 76.Cirino G, Fiorucci S, Sessa WC. Endothelial nitric oxide synthase: the Cinderella of inflammation? Trends Pharmacol. Sci. 2003;24:91–95. doi: 10.1016/S0165-6147(02)00049-4. [DOI] [PubMed] [Google Scholar]
- 77.Neumann P, Gertzberg N, Vaughan E, Weisbrot J, Woodburn R, Lambert W, Johnson A. Peroxynitrite mediates TNF-alpha-induced endothelial barrier dysfunction and nitration of actin. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;290:L674–L684. doi: 10.1152/ajplung.00391.2005. [DOI] [PubMed] [Google Scholar]
- 78.Wu F, Wilson JX. Peroxynitrate-dependent activation of protein phosphatase type 2A mediates microvascular endothelial barrier dysfunction. Cardiovasc. Res. 2009;81:38–45. doi: 10.1093/cvr/cvn246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hollenberg SM, Guglielmi M, Parrillo JE. Discordance between microvascular permeability and leukocyte dynamics in septic iNOS-deficient mice. Crit. Care. 2007;11:R125. doi: 10.1186/cc6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kirsch M, de Groot H. Ascorbate is a potent antioxidant against peroxynitrite-induced oxidation reactions. Evidence that ascorbate acts by re-reducing substrate radicals produced by peroxynitrite. J. Biol. Chem. 2000;275:16702–16708. doi: 10.1074/jbc.M909228199. [DOI] [PubMed] [Google Scholar]
- 81.Shen KP, Liou SF, Hsieh SL, Chen IJ, Wu BN. Eugenosedin-A amelioration of lipopolysaccharide-induced up-regulation of p38 MAPK, inducible nitric oxide synthase and cyclooxygenase-2. J. Pharm. Pharmacol. 2007;59:879–889. doi: 10.1211/jpp.59.6.0015. [DOI] [PubMed] [Google Scholar]
- 82.Ferlitsch A, Pleiner J, Mittermayer F, Schaller G, Homoncik M, Peck-Radosavljevic M, Wolzt M. Vasoconstrictor hyporeactivity can be reversed by antioxidants in patients with advanced alcoholic cirrhosis of the liver and ascites. Crit. Care Med. 2005;33:2028–2033. doi: 10.1097/01.ccm.0000178173.27923.eb. [DOI] [PubMed] [Google Scholar]
- 83.Frein D, Schildknecht S, Bachschmid M, Ullrich V. Redox regulation: a new challenge for pharmacology. Biochem. Pharmacol. 2005;70:811–823. doi: 10.1016/j.bcp.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 84.Kienbaum P, Prante C, Lehmann N, Sander A, Jalowy A, Peters J. Alterations in forearm vascular reactivity in patients with septic shock. Anaesthesia. 2008;63:121–128. doi: 10.1111/j.1365-2044.2007.05286.x. [DOI] [PubMed] [Google Scholar]
- 85.Hoffer LJ, Levine M, Assouline S, Melnychuk D, Padayatty SJ, Rosadiuk K, Rousseau C, Robitaille L, Miller WH., Jr. Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann. Oncol. 2008 doi: 10.1093/annonc/mdn377. in press. [DOI] [PubMed] [Google Scholar]
- 86.Nasr SH, Kashtanova Y, Levchuk V, Markowitz GS. Secondary oxalosis due to excess vitamin C intake. Kidney Int. 2006;70:1672. doi: 10.1038/sj.ki.5001724. [DOI] [PubMed] [Google Scholar]
- 87.Muhlhofer A, Mrosek S, Schlegel B, Trommer W, Rozario F, Böhles H, Schremmer D, Zoller WG, Biesalski HK. High-dose intravenous vitamin C is not associated with an increase of pro-oxidative biomarkers. Eur. J. Clin. Nutr. 2004;58:1151–1158. doi: 10.1038/sj.ejcn.1601943. [DOI] [PubMed] [Google Scholar]
- 88.Bailey DM, Raman S, McEneny J, Young IS, Parham KL, Hullin DA, Davies B, McKeeman G, McCord JM, Lewis MH. Vitamin C prophylaxis promotes oxidative lipid damage during surgical ischemia-reperfusion. Free Radic. Biol. Med. 2006;40:591–600. doi: 10.1016/j.freeradbiomed.2005.09.024. [DOI] [PubMed] [Google Scholar]