Abstract
Purpose
To prepare a suspension form of diclofenac and compare the influence of the injected form (suspension versus solution) on the intravitreal pharmacokinetics of diclofenac in Dutch belted pigmented rabbits.
Methods
Diclofenac acid was prepared and characterized in a suspension formulation. Rabbit eyes were injected with either diclofenac sodium solution (0.3 mg) or diclofenac acid suspension (10 mg) prepared in 0.1 mL balanced salt solution. Rabbits were killed at regular time intervals, the eyes enucleated, and drug content quantified in the vitreous humor and retina-choroid tissue by high-performance liquid chromatography. Pharmacokinetic models were developed for both the dosage forms, and simulations were performed for different doses.
Results
Diclofenac acid with an approximate 5-μm particle size exhibited 3.5-fold lower solubility in vitreous humor, when compared with its sodium salt. The estimated settling velocity of the suspension in the vitreous humor was 3 cm/h. After diclofenac sodium salt solution injection, drug levels declined rapidly with no drug levels detectable after 24 hours in the vitreous humor and 4 hours in the RC. Throughout the assessed time course, drug levels were higher in the vitreous. However, sustained, high drug levels were observed in both the vitreous humor and the retina-choroid even on day 21 after diclofenac acid suspension injection, with retina-choroid drug levels being higher beginning at 0.25 hour. The elimination half-life of diclofenac suspension was 24 and 18 days in vitreous and retina-choroid, respectively, compared to 2.9 and 0.9 hours observed with diclofenac sodium. The pharmacokinetic models developed indicated a slow-release distribution or depot compartment for the diclofenac acid suspension in the posterior segment. Simulations indicated the inability of a 10-mg dose of diclofenac sodium solution to sustain drug levels in the vitreous beyond 11 days.
Conclusions
By choosing a less soluble form of a drug such as diclofenac acid, vitreous elimination half-life can be prolonged up to 24 days, potentially resulting in therapeutic levels in the posterior segment tissues for a few months. Higher detectable drug levels in the retina-choroid suggest rapid settling and persistent retention of suspension in retina-choroid tissue.
Anti-inflammatory corticosteroids are being used in the clinical settings for the treatment of age-related macular degeneration (AMD) and diabetic macular edema, which are common causes of vision loss in the United States.1–4 However, due to the high prevalence of side effects associated with the use of corticosteroids,4,5 there is a need to identify other anti-inflammatory agents with a better safety profile. Recent studies have demonstrated the usefulness of nonsteroidal anti-inflammatory drugs (NSAIDs) as an alternative.6–8 NSAIDs are potent cyclooxygenase (COX) inhibitors and anti-inflammatory agents, with potential antiproliferative and antiangiogenic effects as well.9 Also, NSAIDs are not associated with cataract formation or elevated intraocular pressure,10 the main side effects of intravitreal corticosteroids. Since topical administration of NSAIDs does not deliver appreciable drug quantities to the posterior segment,11 intravitreal administration of NSAIDs remains a viable option for treating posterior segment disease. However, repeated intravitreal injections entail a risk of retinal detachment and endophthalmitis. A possible alternative to frequent injections of drug solutions is the development of sustained release systems or molecules with prolonged half-lives to reduce injection-related complications, provide enhanced duration of drug effects, and reduce any high-peak-concentration–related side effects encountered with the solution form of the drug.
Diclofenac, an NSAID, exerts its action by inhibition of both the COX and lipoxygenase pathways.12 Although diclofenac has been used topically in the treatment of inflammatory conditions, recent focus has been on intravitreal delivery (Baranano DE et al. IOVS 2008;49:ARVO E-Abstract 5606).13 Diclofenac sodium was found to be safe and nontoxic up to a dose of 0.3 mg after intravitreal administration. Since diclofenac sodium is a low-molecular-weight drug (MWt: 318.13), when in solution, as is the case with its commercial ophthalmic formulations, it is predicted to disappear rapidly from the vitreous humor, with a short half life of 2.87 hours.14 To maintain safe levels of diclofenac for prolonged periods in the eye, slow-release drug delivery systems such as nanoparticles,15,16 microparticles,9 or implants17 may be useful. Alternatively, the use of a suspension or less soluble form of the drug may be useful in prolonging intravitreal drug delivery. For instance, intravitreal triamcinolone acetonide drug suspension is known to sustain drug levels up to 8 months.18 Keeping the earlier success of triamcinolone acetonide suspension in mind, the objective of this study was to prepare and assess intravitreal drug delivery from a suspension dosage form of diclofenac acid and compare it with diclofenac solution. A pigmented rabbit model was used in this study, because human eyes are pigmented19 and the drug elimination from the vitreous14 and delivery to the back of the eye can be influenced by eye pigmentation.20 Furthermore, pharmacokinetic models were developed in this study to explain the delivery of diclofenac to tissues of the posterior segment from solution and suspension dosage forms.
Methods
Preparation and Characterization of Diclofenac Acid
Diclofenac sodium salt, acetonitrile, and trifluoroacetic acid were purchased from Sigma-Aldrich Co. (St. Louis, MO). Diclofenac acid was prepared by acidification of an aqueous solution of diclofenac sodium. Briefly, 1.59 g of diclofenac sodium salt (Sigma-Aldrich) was dissolved in 300 mL of deionized water. This solution was taken in a stoppered extraction flask, and 100 mL of chloroform was added to the solution. To this solvent mixture, 1 mL of hydrochloric acid (37%) was added, and the mixture was shaken for 15 minutes manually and then allowed to stand for 1 hour. The chloroform layer was collected in a round-bottomed flask, and the solvent was evaporated by using a rotary evaporator for 6 hours. The dried powder (diclofenac acid) was collected and stored in a vacuum desiccator. The yield of diclofenac acid was 1.32 g.
Diclofenac sodium and the prepared diclofenac acid were characterized by Fourier transform infrared spectroscopy (FTIR) and differential scanning calorimetry (DSC). Infrared spectra were acquired with a Fourier transform infrared spectrometer (MB-series; ABB Bomem Quebec City, Quebec, Canada). Samples were prepared by the KBr pelletization method. A 128-scan interferogram was collected for each spectrum in single-beam mode with a resolution of 4 cm–1. A commercial system was used for analyzing samples for the DSC studies (Diamond DSC, equipped with an Intercooler II system for cooling; data analyzed with Pyris 7.0 software; Perkin-Elmer, Boston, MA). Weighed samples (5.5 mg of diclofenac acid; 5.4 mg of diclofenac sodium) were placed in aluminum pans and hermetically sealed. The samples were heated at 10°C/min in aluminum pans under nitrogen purging (20 mL/min). Finally, the particle size of diclofenac suspension was measured in PBS (pH 7.4) by dynamic light-scattering (Nicomp Particle Sizing Systems, Milford, NJ).
Determination of Solubility of Diclofenac Acid and Sodium Salt in PBS and Vitreous Humor
Solubility of diclofenac sodium and diclofenac acid was determined as follows. Briefly, an excess of diclofenac sodium (> 100 mg) or diclofenac acid (> 30 mg) was added to 2 mL of PBS (pH 7.4) or bovine vitreous humor (bovine eyes were obtained from a local abattoir) in capped tubes (in triplicate). The tubes were vortexed for 3 minutes and then kept for shaking at 150 rpm at 37°C for 24 hours. After equilibration, vitreous samples were centrifuged at 10,000g for 20 minutes to separate the excess of insoluble drug. The supernatant was taken and processed as mentioned in the drug extraction section.
Determination of the Settling Rate of Suspension
As particles tend to settle under gravity, the sedimentation rate of diclofenac acid suspension was calculated by using the Stoke's law:
| (1) |
where R is the radius of the particles (in meters), ρ1 is the mass density of particles (kilogram/cubic meter), ρ2 is the mass density of fluid (kilogram/cubic meter), g is the gravitational acceleration (meters per second squared), and μ is the viscosity of the fluid (kilogram/meter/second).
The mean particle size of the suspension was determined with a particle size analyzer. Density of diclofenac acid (ρ1) was calculated to be 1.431 ± 0.06 g/cm3 (Advanced Chemistry Development Software ver. 8.14 for Solaris; ACD Laboratories, Toronto, Ontario, Canada). Vitreous viscosity (μ = 6.9 × 10–3 g/cm/s) and density (ρ2 = 1 g/cm3) were obtained from Park et al.21
Intravitreal Pharmacokinetic Study
Animal studies were conducted in collaboration with Stephen Kim at the Emory Eye Center in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Thirteen Dutch belted rabbits were used for the pharmacokinetic study. Both diclofenac salt solution and diclofenac acid suspension were prepared in balances salt solution (BSS; Alcon, Fort Worth, TX). For the solution study, six rabbits were injected with 0.3 mg of diclofenac sodium solution (0.1 mL) in the vitreous of both eyes, and one rabbit was killed at each time point. The time points were 15 minutes and 1, 2, 4, 24, and 48 hours after injection. For the suspension formulation study, seven rabbits were injected with 10 mg diclofenac acid suspension (0.1 mL) in the vitreous of both eyes, and one rabbit was killed at each time point. The time points were 15 minutes; and 6 and 24 hours; and 2, 7, 14, and 21 days. Each eye was enucleated, immediately frozen in liquid nitrogen, and stored at –70°C. The frozen eye was subsequently prepared by carefully isolating the vitreous and the combined retina-choroid (RC) layer.
Extraction of Diclofenac from Tissues for HPLC Analysis
For drug extraction in the diclofenac solution study, a weighed quantity of RC or vitreous humor was added with 10 μL of internal standard (ketorolac tromethamine, 1 mg/mL) and homogenized in 1 mL of PBS (pH 7.4). An aliquot of 0.5 mL of dichloromethane was added to the homogenate and vortexed for 15 minutes followed by centrifugation at 10,000 rpm for 15 minutes. The aqueous layer was separated, and an aliquot of 0.1 mL was injected onto HPLC for analysis. In the case of diclofenac acid, the samples were homogenized in 0.5 mL of PBS (pH 7.4) and the drug was extracted into 1 mL of dichloromethane without an internal standard. The separated organic layer was evaporated under a nitrogen stream, and the residue was reconstituted in 0.2 mL of mobile phase and injected onto HPLC for analysis.
HPLC Conditions for Diclofenac Analysis
An HPLC system including a solvent delivery pump (TM 616; Waters, Milford, MA), a controller (model 600 S; Waters), an autoinjector (model 717 plus; Waters), and a photo diode array detector (PDA; 996, Waters) was used in this study. The mobile phase used for eluting diclofenac was an isocratic mixture of acetonitrile and deionized water (containing 0.1% trifluoroacetic acid; 50:50, vol/vol) pumped at a flow rate of 1.2 mL/min. Diclofenac sodium was eluted on a C-18 column (EC 125/4.6; 100-5; Nucleosil; Varian, Inc., Palo Alto, CA) with a retention time of 6.6 ± 0.12 minutes. Diclofenac acid was eluted with a C-18 column (150 × 4.6 mm; MV 100-5; Microsorb; Varian, Inc.) with a retention time of 4.7 ± 0.3 minutes. Signal linearity was observed between 0.05 and 25 μg/mL (R2 = 0.9997) for diclofenac sodium in PBS and between 0.1 and 25 μg/mL (R2 = 0.9994) for diclofenac acid in acetonitrile.
Pharmacokinetic Analysis, Modeling, and Simulation of Data
Vitreous and RC drug levels were analyzed by noncompartmental analysis (WinNonlin software, ver. 1.5; Scientific Consulting, Inc., Apex, NC). Vitreous data were fit to the bolus intravenous administration model, and RC data were fit to the extravascular input model. Pharmacokinetic modeling of the data were performed using Berkeley Madonna software (ver. 8.3.8; University of California, Berkeley). Modeling was achieved in a forward approach starting with a single compartment. Estimation of model parameters was performed by the curve-fitting procedure, and the integration method used was Runge-Kutta 4. Pharmacokinetic models were developed to simultaneously fit both the vitreous and RC tissue data. Models comparison and selection was based on the goodness-of-fit metric, the second-order Akaike information criterion (AICc).22 AICc was computed using the following equation:
| (2) |
where n is the number of observations, Yi is the observed value, Yi″ is the model predicted value, and p is the number of model parameters. The coefficient of determination (R2) was estimated (Data Analysis Toolpak; Excel 2000; Microsoft, Redmond, WA). The model with lowest AICc was considered the best among the developed models. The models developed for a particular dosage form (solution or suspension) were compared based on the ΔAICc and Akaike weights. ΔAICc is the measure of each model in comparison to the best model (model with lowest AICc) and is calculated using the following equation:
| (3) |
where AICci is the AICc of the model i and lowest AICc is the AICc of the best model. A ΔAICc < 2 indicates substantial evidence for the model, 3 to 7 indicates less support for the model, and >10 indicates that the model is very unlikely.22 Akaike weight is the ratio of ΔAICc for each model relative to the entire set of n models as shown in the equation below:
| (4) |
Akaike weights indicate the probability that the selected model is the best among the entire set of other models.
The pharmacokinetic parameters obtained for diclofenac sodium solution (0.3-mg dose) was used to simulate a profile for higher dose of solution (10 mg) and was compared with the observed profile in the vitreous and retina after 10 mg intravitreal injection of suspension (diclofenac acid). The pharmacokinetic parameters obtained for diclofenac acid suspension (10-mg dose) was used to simulate a profile for a lower dose of suspension (0.3 mg) and was compared with the observed profile for the 0.3-mg solution (diclofenac sodium). Also simulations were performed for other doses including 0.5, 0.75, and 1.5 mg, to predict the vitreous and RC tissue concentrations.
Results
Characterization by FTIR and DSC
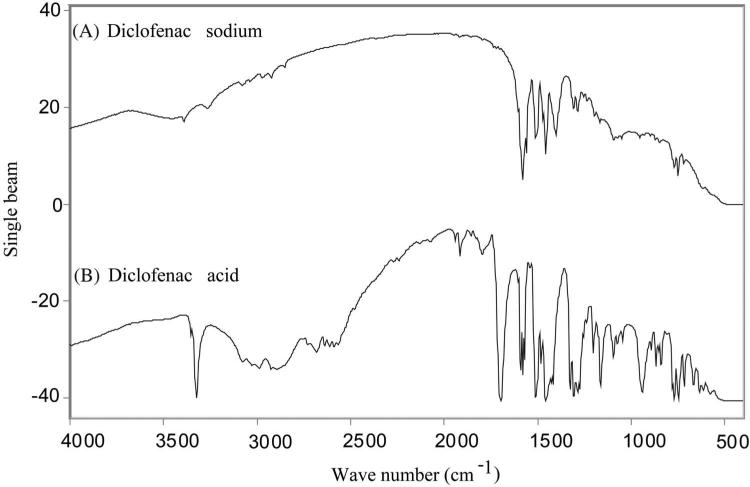
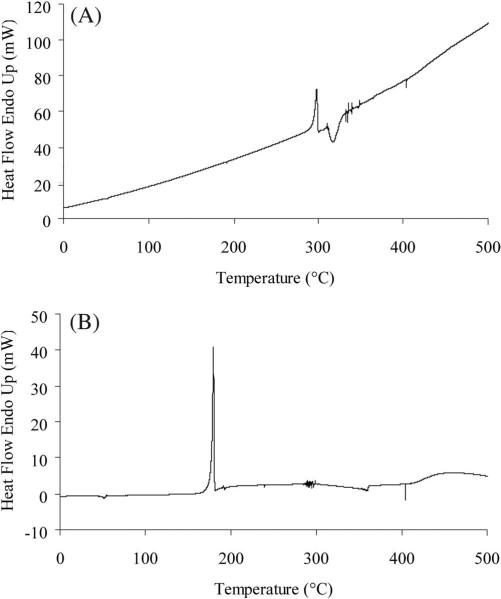
The FTIR spectra of diclofenac sodium and diclofenac acid are shown in Figures 1A and 1B, respectively. The prominent feature of the diclofenac acid spectra in comparison to the diclofenac sodium spectra is a sharp peak at 3323.24 cm–1 corresponding to the O—H stretch of the carboxylic acid. Other confirmation for the formation of free carboxylic groups include a peak at 1694.28 cm–1 corresponding to the C=O stretch and a peak at 1160.30 cm–1 corresponding to the C—O stretch. The DSC curve of diclofenac sodium (Fig. 2A) exhibited a sharp endothermic peak of melting at 298°C (ΔH = 104.794 J/g). However, the endothermic melting peak of diclofenac acid was observed at 179.5°C (ΔH = 108.933 J/g) as shown in Figure 2B, and similar melting points were previously reported for diclofenac acid,23 indicating the material to be diclofenac acid.
Figure 1.
FTIR spectra of (A) diclofenac sodium and (B) diclofenac acid. In the diclofenac acid spectra, the peaks at 1694.28 cm–1 corresponding to the C=O stretch, the peak at 1160.30 cm–1 corresponding to the C—O stretch, and the peak at 3323.24 cm–1 due to the O—H stretch of the carboxylic acid confirmed the formation of free carboxylic acid.
Figure 2.
DSC thermograms of (A) diclofenac sodium and (B) diclofenac acid. The endothermic peak of melting at 298°C of diclofenac sodium was shifted to 179.5°C in diclofenac acid, indicating the formation of free carboxylic group.
Solubility of Diclofenac Acid in PBS (pH 7.4) and Bovine Vitreous Humor and Settling Rate of Suspension
Solubility of diclofenac sodium in PBS was 6.18 ± 0.48 mg/mL, whereas that of diclofenac acid in PBS was 1.37 ± 0.06 mg/mL. A 4.5-fold decrease in the solubility of diclofenac acid was observed when compared with its sodium salt. Solubility of diclofenac sodium in vitreous humor was 6.55 ± 1.40 mg/mL and that of diclofenac acid was found to be 1.87 ± 0.27 mg/mL. This observed solubility was almost similar to that measured in PBS (pH 7.4). This low-solubility diclofenac acid was used to prepare the suspension for intravitreal injection. Based on the particle size of the diclofenac suspension (5.08 ± 1.35 μm mean diameter), the settling rate calculated for the suspension was 3.06 cm/h.
Pharmacokinetic Analysis of Vitreous and RC Tissue Concentrations
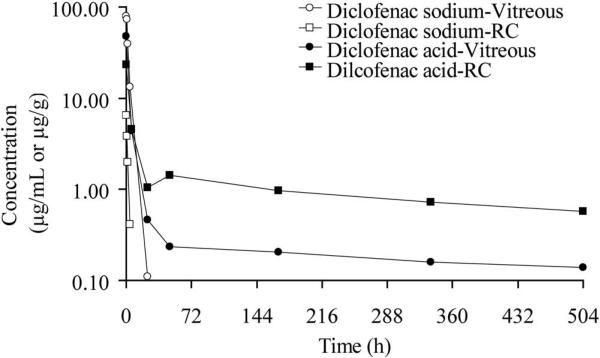
Diclofenac concentrations in the vitreous and RC tissue after intravitreal injection of diclofenac sodium solution and diclofenac acid suspension are shown in Figure 3. The Cmax (peak diclofenac concentration) in the vitreous was 79.90 μg/mL and 6.52 μg/g in RC after intravitreal injection of diclofenac sodium solution. The drug levels were not detectable (below the quantitation limit, 50 ng/mL) after 24 hours in the vitreous and after 4 hours in the RC tissues. In the case of diclofenac acid suspension, the Cmax level in the vitreous was lower (47.71 μg/mL) than the diclofenac sodium solution dose. The drug concentrations in the vitreous declined to 0.46 μg/mL at 24 hours followed by sustained drug levels until the last measured time point (0.14 μg/mL at 504 hours). Although the Cmax of the suspension dosage form was lower for the RC tissue levels (23.15 μg/g) when compared with the vitreous, higher drug levels were observed at all time points after 6 hours when compared with vitreous. The drug levels remained higher in these tissues until the last observed time point (0.57 μg/g at 21 days). Considering diclofenac acid solubility of 1.87 mg/mL in the vitreous humor and an administered dose of 10 mg, a large fraction of the dose would be in suspended particles, which would form the depot to provide the sustained levels observed on day 21.
Figure 3.
Diclofenac drug levels estimated in ocular tissues of Dutch belted male rabbits after intravitreal administration of 0.3 mg diclofenac sodium solution or 10 mg diclofenac acid suspension.
The noncompartmental pharmacokinetic parameters estimated for diclofenac administered in solution and suspension dosage forms are summarized in the Table 1. The AUC0–inf of diclofenac sodium (solution injection) in the vitreous was higher (321.30 μg · h/mL) when compared with the tissue levels (10.57 μg · h/g). The elimination half-life (t½) of the drug from vitreous was 2.85 hours and that from the RC tissues was 0.92 hour. Similar to the t½, the mean residence time (MRT) of the drug in vitreous was higher (2.54 hours) when compared with RC tissue (1.40 hours). However, in the case of diclofenac acid suspension, the AUC0–inf was lower in the vitreous (415.24 μg · h/mL) when compared with the RC tissues (919.82 μg · h/g). A biphasic elimination curve was observed in both vitreous and RC tissues indicating an initial rapid distribution followed by a slower elimination from both the tissues. The elimination half-life was very high in both vitreous (581.39 hours) and RC (436.83 hours). The MRT of the drug after administration as suspension was higher in the RC tissues (546.07 hours) than in the vitreous (431.38 hours). The in vivo mean dissolution (or release) time (MDT), the difference between the MRT for suspension and solution forms, was 429 hours based on vitreous data and 545 hours based on RC data.
Table 1.
Noncompartmental Analysis of Mean Pharmacokinetic Parameters of Vitreous and RC after Intravitreous Injection of Diclofenac Sodium Solution and Diclofenac Acid Suspension in Dutch Belted Rabbits
| Vitreous* |
Retina-Choroid Tissue (RC)† |
|||
|---|---|---|---|---|
| PK Parameter | Diclofenac Sodium Solution (0.3 mg) | Diclofenac Acid Suspension (10 mg) | Diclofenac Sodium Solution (0.3 mg) | Diclofenac Acid Suspension (10 mg) |
| AUC0–24, μg · h/mL or g tissue | 321.30 | 297.81 | 10.02 | 560.61 |
| AUC0-inf, μg · h/mL or g tissue | 321.75 | 415.24 | 10.57 | 919.82 |
| t1/2, h | 2.85 | 581.39 | 0.92 | 436.83 |
| MRT0-inf, h | 2.54 | 431.38 | 1.40 | 546.07 |
| Clearance (mL/h) or clearance/F (mL/g) | 0.9324 | 24.08 | 28.38 | 10.87 |
Noncompartmental analysis of vitreous data was performed using the bolus IV administration model in WinNonlin (Scientific Consulting, Inc., Apex, NC).
Noncompartmental analysis of RC data was performed using extravascular administration model in WinNonlin (Scientific Consulting, Inc.).
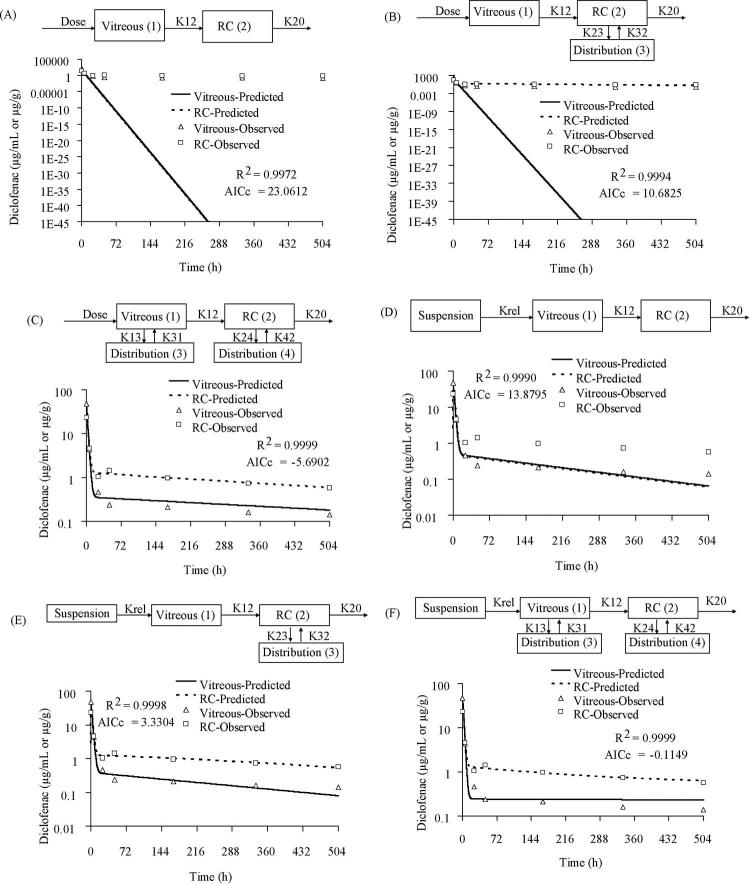
Development of PK Models for Simultaneous Fit of Vitreous and RC Tissue after Intravitreal Injection of Diclofenac Sodium Solution
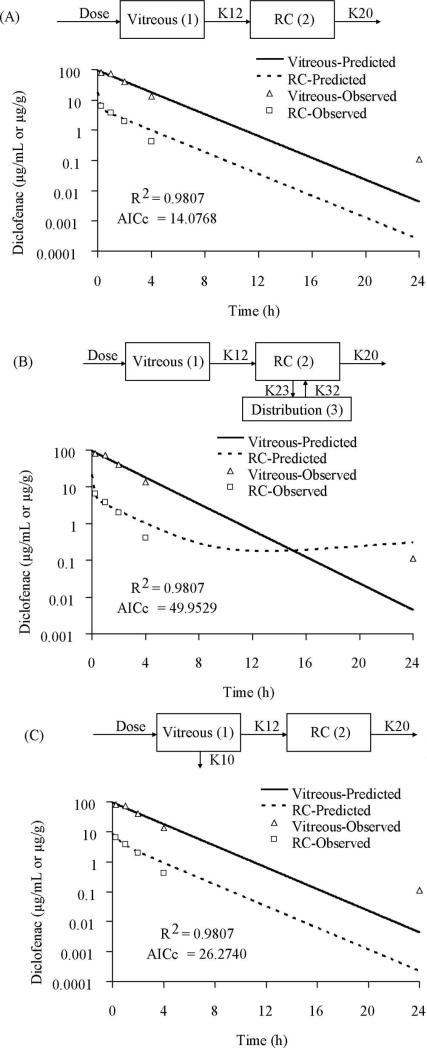
The PK modeling was performed with a forward approach in which two compartments denoting vitreous (compartment 1) and RC tissues (compartment 2) were used, as shown in model 1A (Fig. 4A). The model assumed administration of 0.3 mg diclofenac sodium solution in the vitreous from where it was distributed to RC followed by elimination from the RC tissues. The observed vitreous and tissue data fitted well to this model (R2 = 0.9807; AICc = 14.0768). Addition of a distribution compartment to RC as shown in Figure 4B (model 1B) resulted in poor model fit as indicated by high AICc (R2 = 0.9807; AICc = 49.9529). Also inclusion of a parallel elimination pathway (K10 in model 1C; Fig. 4C) from the vitreous in addition to elimination from RC tissues did not improve the model fit to the observed data (R2 = 0.9807; AICc = 26.2740). None of these models improved the fit when the backflow rate constant (K21) was included, indicating no significant drug redistribution from RC to vitreous.
Figure 4.
The model predicted and observed concentrations of diclofenac sodium in vitreous and RC after intravitreal injection of 0.3 mg solution. (A) Two-compartment model. (B) Two-compartment model with a distribution compartment to RC. (C) Two-compartment model with parallel elimination from the vitreous and RC. The model in (A) was selected as the best-fit model. K12, rate constant for transfer of drug from the vitreous to RC; K20, rate constant for elimination of drug from the RC compartment.
Development of PK Models for Simultaneous Fit of Vitreous and RC Tissue after Intravitreal Injection of Diclofenac Acid Suspension
For modeling the suspension data, a simple model with two compartments denoting vitreous (compartment 1) and RC (compartment 2) was used similar to the solution model. As shown in Figure 5A, the model 2A did not fit the later time points, and the AICc was 23.0612 (R2 = 0.9972). Addition of a distribution compartment to the RC tissues as indicated in model 2B (Fig. 5B), improved the model fit for only RC tissue data (R2 = 0.9994; AICc = 10.6825). Further addition of the distribution compartment to the vitreous as indicated in model 2C (Fig. 5C) improved the overall fit for both the vitreous and RC (R2 = 0.9999; AICc = –5.6902). Addition of a parallel elimination path (K10) from the vitreous to the above models did not fit the data well and also increased the AICc. Hence, in further modeling, parallel elimination from vitreous was not included. None of these models improved the fit when the backflow rate constant (K21) indicating drug redistribution from RC to vitreous was included.
Figure 5.
Model predicted and observed concentrations of diclofenac acid in vitreous and RC after intravitreal injection of a 10-mg suspension. (A) Two-compartment model. (B) Two-compartment model with a distribution compartment to RC. (C) Two-compartment model with distribution compartments to both vitreous and RC. (D) Two-compartment model with Krel, the release/dissolution rate constant for the dissolution of the drug from the suspension. (E) Two-compartment model with a distribution compartment to RC and with Krel.(F) Two-compartment model with distribution compartments to both vitreous and RC and with Krel. (G) Two-compartment model with distribution compartments to both vitreous and RC and with K0, zero-order release rate constant for the dissolution of the drug from the suspension. (H) Two-compartment model with a distribution compartment to RC and with Krel1 and Krel2, two different first-order release rate constants for the dissolution of the drug from the suspension. (I) Two-compartment model with a distribution compartment to RC and with Krel and K0, an initial first-order release rate constant followed by a zero-order release rate constant for the dissolution of the drug from the suspension. (J) Two-compartment model with a distribution compartment to RC and with K0 and Krel. (K) Two-compartment model with distribution compartments in both vitreous and RC and assuming simultaneous bolus and suspension forms of dose with K0. (L) Two-compartment model with a distribution compartment in the RC and assuming the suspension will settle on the RC in 0.25 hour. (M) Two-compartment model with distribution compartments in both vitreous and RC and assuming the suspension will settle in the RC in 0.25 hour. (C) This model was selected as the best fit. K12, rate constant for transfer of drug from the vitreous to RC; K13, rate constant for the transfer of drug from the vitreous to the distribution compartment; K31, rate constant for the transfer of drug from the distribution compartment to the vitreous; K24, rate constant for the transfer of drug from the RC to the distribution compartment; K42, rate constant for the transfer of drug from the distribution compartment to the RC; K20, rate constant for elimination of drug from the RC compartment.
Suspension data were further modeled by using a release constant (Krel) parameter indicating slow dissolution of drug from the suspension. Inclusion of Krel in the model without any distribution compartment for both vitreous and RC tissue (model 2D, Fig. 5D) resulted in moderate fit for both the compartments (R2 = 0.9990; AICc = 13.8795). However, addition of a distribution compartment to the RC tissue as shown in model 2E improved the model fit to both vitreous and tissue data (Fig. 5E, R2 = 0.9998; AICc = 3.3304). Further addition of a distribution compartment to the vitreous as shown in model 2F (Fig. 5F) resulted in improved fit as indicated by high R2 (0.9999) and low AICc (–0.1149). Also, the release from suspension was modeled with a zero-order rate constant as shown in Figure 5G (model 2G, K0 = 0.7362 μg/h). However, this model fit (R2 = 0.9997; AICc = 40.0837) was not better than the previous model, assuming first-order release constants.
Both the vitreous and RC drug profiles in the case of the suspension exhibited a rapid elimination (first phase) followed by a slow elimination (second phase). To explain this varying release profile, models were constructed assuming different release constants for drug release from the suspension. In the first model (Fig. 5H; model 2H), two different first-order release constants were assumed for drug release from the suspension. However, there was not much difference between both the release constants (Krel1 = 0.0031 hour–1; Krel2 = 0.0065 hour–1) and the estimated duration of the Krel1 was 24.99 hours. This model fit both the vitreous and tissue data well with R2 = 0.9998 and AICc = 33.7070. In the next model (model 2I), an initial first-order release (Krel = 0.0031 hour–1) followed by a zero-order release (K0 = 0.076 μg/h) was fit to the data (Fig. 5I). The initial first-order release constant was assumed for the drug solubilized in the injection vehicle, which diffuses faster in the vitreous, and the later zero-order release for the suspension depot formed. The results indicated a good fit of the model to vitreous and RC data (R2 = 0.9998 and AICc = 33.6359). Similar results were obtained for model (Fig. 5J; model 2J) assuming an initial zero-order release (K0 = 0.105 μg/h) followed by a first-order release (Krel = 0.0031 hour –1) of drug from the suspension (R2 = 0.9998 and AICc = 33.6592). Also, model was developed assuming a simultaneous bolus and suspension dose with zero-order drug release from the suspension into the vitreous (Fig. 5K; model 2K). When the suspension was injected into the vitreous, the soluble part of the dose might have been the bolus part and the insoluble depot the suspension part releasing the drug in zero-order fashion. This model fit both the vitreous and RC data; however, the AICc was higher when compared with other models (49.5468). Finally, PK models were developed assuming settling of suspensions. Based on the suspension particle size (5 μm) the calculated settling rate was 3.06 cm/h. In model 2L (Fig. 5L), first-order release was assumed for drug release from suspension to the vitreous and also to RC from 0.25 hour, accounting for the suspension settling. This model also included a distribution compartment to the RC tissues. However, as evident from Figure 5L, poor fit was observed for the vitreous data (R2 = 0.9996; AICc = 35.1318). However, inclusion of a distribution compartment (model 2M; Fig. 5M) improved the model fit to both vitreous and RC tissues (R2 = 0.9998; AICc = 42.8077).
Comparison and Selection of Models
The ΔAICc and Akaike weights of the various models computed for diclofenac sodium solution injection and diclofenac acid suspension are summarized in Table 2. Model 1A with the lowest AICc (14.0768) was the best fit model for the solution data. The delta AICc of other models relative to model 1A were >10, indicating the these models were unlikely.22 Also the high Akaike weight (0.9953) of model 1A clearly indicated the high probability (>99%) that this model was the best among the other models developed for diclofenac sodium solution. The parameter estimates obtained from model 1A are summarized in Table 3.
Table 2.
Delta AICc and Akaike Weights for the Various Pharmacokinetic Models
| Model | Parameters (n) | AICc* | Delta AlCc† | Akaike Weight‡ |
|---|---|---|---|---|
| Diclofenac Sodium Solution Injection | ||||
| 1A | 4 | 14.0768 | 0 | 0.9953 |
| 1B | 6 | 49.9529 | 35.8761 | 0.0000 |
| 1C | 5 | 26.2740 | 12.1972 | 0.0022 |
| Diclofenac Acid Suspension Injection | ||||
| 2A | 4 | 23.0612 | 28.7514 | 5.3 × 10–7 |
| 2B | 6 | 10.6825 | 16.3727 | 0.0003 |
| 2C | 8 | –5.6902 | 0 | 0.9321 |
| Diclofenac Acid Suspension Injection: Models Incorporating Release from Suspension | ||||
| 2D | 5 | 13.8795 | 19.5697 | 5.2 × 10–5 |
| 2E | 7 | 3.3304 | 9.0206 | 0.0102 |
| 2F | 9 | –0.1149 | 5.5753 | 0.0574 |
| 2G | 9 | 40.0837 | 45.7739 | 1.1 × 10–10 |
| 2H | 8 | 33.7070 | 39.3972 | 2.6 × 10–9 |
| 2I | 8 | 33.6359 | 39.3261 | 2.7 × 10–9 |
| 2J | 8 | 33.6592 | 39.3494 | 2.7 × 10–9 |
| 2K | 9 | 49.5468 | 55.2370 | 9.4 × 10–13 |
| 2L | 7 | 35.1318 | 40.8220 | 1.3 × 10–9 |
| 2M | 9 | 42.8077 | 48.4979 | 2.7 × 10–11 |
The models were developed using Berkeley-Madonna software (University of California, Berkeley).
AICc, second-order AIC.
ΔAICc = AICci – lowest AICc.
Akaike weight = e(–ΔAICci/2)/Σ e(–ΔAICc/2).
Table 3.
Final Parameter Estimates for the Best-Fit Pharmacokinetic Models of Diclofenac Sodium and Diclofenac Acid Suspension Injection
| Diclofenac Sodium Solution (0.3 mg) |
Diclofenac Acid Suspension (10 mg) |
||
|---|---|---|---|
| Parameter (units) | Estimate Value | Parameter (units) | Estimate Value |
| K12 (h–1) | 0.4159 | K12 (h–1) | 25.8354 |
| Vvit (mL) | 3.1585 | Vvit (mL) | 188.489 |
| K20 (h–1) | 7.9423 | K20 (h–1) | 12.0709 |
| Vret (g) | 2.1685 | Vret (g) | 59.6416 |
| K13 (h–1) | 0.2889 | ||
| K31 (h–1) | 0.0042 | ||
| K24 (h–1) | 0.7106 | ||
| K42 (h–1) | 0.0174 | ||
The models were developed with Berkeley-Madonna software (University of California, Berkeley). Models 1A and 2C were the best-fit models for diclofenac sodium solution and diclofenac acid suspension, respectively.
The best fit model in the suspension group was model 2C with AICc = –5.6902. The ΔAICc of all other models relative to model 2C were greater than 5, indicating that all these models were unlikely.22 This finding was further confirmed by the high Akaike weight (0.9321) for model 2C, indicating the greater probability (>93%) that this model was the best among other models developed for diclofenac suspension. The next close best-fit model was model 2F (AICc = –0.1149) which included a first-order release constant parameter in addition to the best-fit model (model 2C). Addition of the first-order release constant provided a good fit, but no further improvement in the model fit was observed, and hence the previous model (model 2C) without release constant was selected as the best-fit model for the suspension. All other models assuming sequential or simultaneous first- and zero-order release constants from suspension were of higher AICc because of the large number of parameters involved. The final parameter estimates of the best-fit model (model 2C) are given in Table 3. Among the models with two different release constants (models 2H, 2I, 2J), no difference in the AICc was observed among these three models. However, based on the goodness-of-fit criteria, these models were not better than model 2C.
Simulation of PK Models of Solution and Suspension Dosage for Different Dose Levels
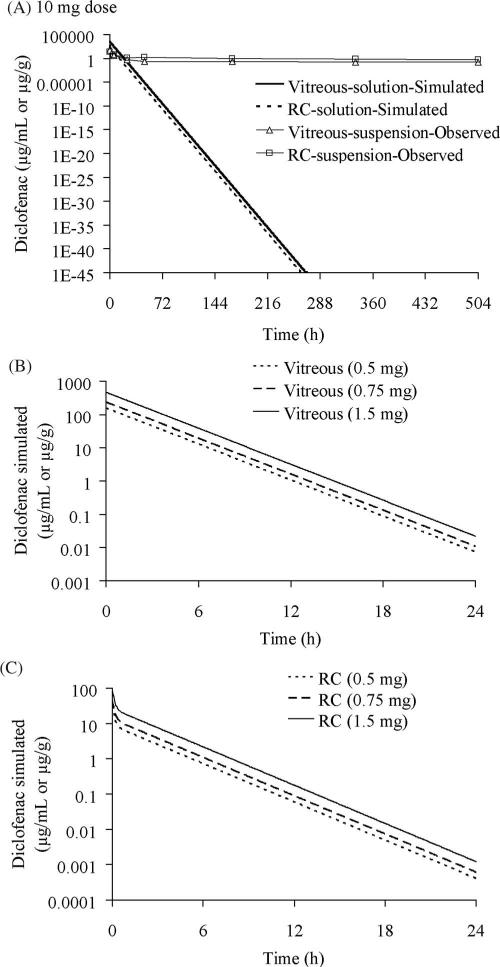
The final parameter estimates obtained from the best-fit model (Table 3; model 1A) for diclofenac sodium solution after 0.3 mg intravitreal injection was used to simulate for higher doses. Figure 6A depicts the simulated drug concentrations in both vitreous and RC tissues for a 10-mg injection of diclofenac sodium as solution. The simulated values indicated rapid clearance of drug with <50 ng/mL (LOQ; limit of quantification) detectable in the vitreous after 26.6 hours and after 19.6 hours in the RC tissues. However, higher concentrations were observed in the vitreous (0.14 μg/mL) and RC (0.57 μg/g) after intravitreal injection of the same dose of diclofenac acid in the suspension dosage form as shown in Figure 6A. The simulated drug levels for the 0.5-mg dose indicated no detectable levels (<50 ng/mL) in the vitreous (Fig. 6B) after 19 hours or in the RC after 12.4 hours (Fig. 6C). Similar results were obtained for simulation of the 0.75-mg dose (20.4 hours in vitreous and 13.4 hours in RC) and the 1.5-mg dose (22 hours in vitreous and 15 hours in RC).
Figure 6.
Simulation of drug levels for diclofenac sodium solution dosage in the vitreous and RC of Dutch belted rabbits by using the final parameters mentioned in Table 3 obtained from model 1A (Fig. 4A). (A) Simulated drug levels for a 10-mg solution of diclofenac sodium and comparison with observed levels for 10 mg diclofenac acid suspension. Simulated (B) vitreous and (C) RC drug levels for diclofenac sodium solutions of 0.5, 0.75, and 1.5 mg.
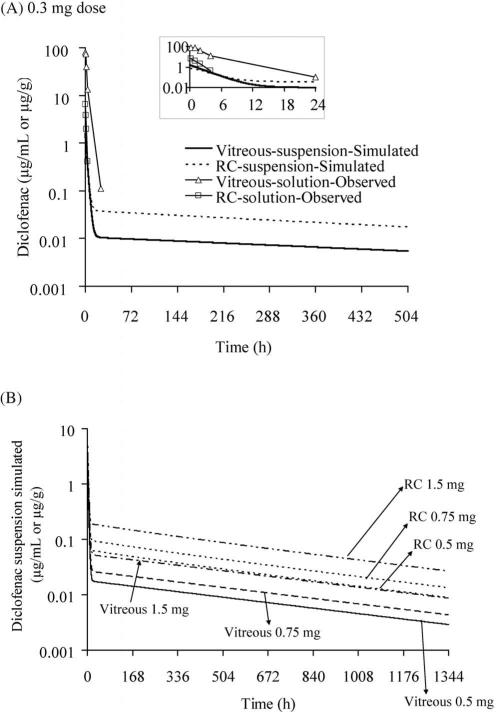
The results of simulations using the final parameters obtained from 10 mg diclofenac acid suspension (Table 3; model 2C) for lower doses were given in Figures 7A and 7B. Simulations result indicated higher drug levels detectable in the retina for the 0.3-mg suspension (> 50 ng/mg until 11 hours), whereas no drug was detected after 4 hours with the 0.3-mg solution dose (Fig. 7A). The simulated drug levels for the other three doses—0.5 mg, 0.75 mg and 1.5 mg (Fig. 7B)—indicated higher detectable drug levels (>50 ng/mg) in the RC tissues at 168, 429, and 909 hours, respectively.
Figure 7.
Simulation of drug levels for diclofenac acid suspension dosage in the vitreous and RC of Dutch belted rabbits by using the final parameters mentioned in Table 3 obtained from model 2C (Fig. 5C). (A) Simulated drug levels for a 0.3-mg suspension dose in comparison with a 0.3-mg observed solution dose. Inset: Simulated drug levels during the initial 24 hours. (B) Simulated vitreous and RC drug levels for suspension dose of 0.5, 0.75, and 1.5 mg.
Discussion
Intravitreal injections are being increasingly used in the treatment of posterior segment eye diseases to provide more efficient drug delivery to the back of the eye when compared with other routes. To maximize therapeutic effect, the injected drug should be retained for longer periods of time (longer half-life) in the vitreous. Diclofenac, an NSAID, is used topically to treat ocular inflammation. Intravitreal injection of diclofenac, however, significantly increases drug delivery to the posterior segment. The intravitreal retention of drugs is affected, not only by their physicochemical properties, but also by the form of the dose administered. In the present study, the influence of the form of the dose on the intravitreal pharmacokinetics of diclofenac was investigated. Pharmacokinetic studies were conducted in rabbits, and mathematical models were developed to fit both the solution (diclofenac salt; 0. 3 mg) and suspension (diclofenac acid; 10 mg) data. Further, final simulations were performed for different doses of diclofenac to predict drug levels in eye tissues.
Less soluble diclofenac acid suspension was prepared from diclofenac sodium salt by an acidification process followed by extraction into an organic solvent. The solvent was evaporated to collect diclofenac acid crystalline powder. Solubility of diclofenac acid was reduced by 4.5-fold in PBS (pH 7.4) and by 3.5-fold in the vitreous humor. The solubility of diclofenac acid in the vitreous humor was 1.87 mg/mL. As discussed in subsequent paragraphs, this difference in the solubility between diclofenac acid and diclofenac sodium resulted in more prolonged drug delivery with the former. Solubility of diclofenac acid was ~100-fold higher in the vitreous humor (and in PBS) at pH 7.4 and 37°C, when compared to its previously reported solubility in deionized water at 25°C (17.8 μg/mL).24 This increased solubility of diclofenac acid in buffer and vitreous humor may be attributable to the ionization of the compound at pH 7.4 (diclofenac acid has a pKa of 4.5). Results obtained in the present study indicate that the solubility measured in PBS (pH 7.4) buffer at 37°C closely resembled the vitreous humor solubility. Application of literature solubility data, which were mostly reported at room temperature or 25°C, for interpreting in vivo results should be viewed with caution, as those data may under- or overpredict the actual solubility of the drug in vivo.
The vitreous elimination half-life of diclofenac administered as solution was faster (2.85 hours) when compared with the suspension (581.39 hours or 24 days). Furthermore, the mean residence time (MRT, the average time a molecule resides in a particular tissue) of diclofenac solution was shorter (2.54 hours) when compared with 431.38 hours or 18 days for the suspension. Similar results were observed for the t½ and MRT in the RC tissues of suspension group when compared with the solution group. The much longer in vivo half-life than that predicted by solubility reduction14 suggests the potential formation of a type of depot that further reduces drug release in vivo compared with that anticipated based on in vitro studies.
A low dose (0.3 mg) of diclofenac sodium solution resulted in higher bioavailability in the vitreous, as indicated by its dose normalized AUC0–inf (1072.5 μg · h/mL/mg dose), when compared with a higher dose (10 mg) of diclofenac acid suspension (41.5 μg · h/mL/mg dose). However, the retinal availability was better with the suspension, possibly due to its rapid settling onto the retina. Perhaps because of similar reasons and the measurement of a largely free form of the drug as opposed to the suspended form in the vitreous, after intravitreal injection of a triamcinolone acetonide (6 mg) suspension in rabbits, low drug levels (14.434 μg/mL) were detected in the vitreous at the first time point.18 With the diclofenac acid suspension, unlike the vitreous, the dose normalized AUC0–inf levels (91.98 μg · h/g tissue/mg dose) were higher in the RC tissues than in the vitreous (41.52 μg · h/mL/mg dose) and also when compared with the dose-normalized AUC0–inf in the RC of diclofenac sodium solution (35.23 μg · h/g tissue/mg dose). Although high initial concentrations were not achieved with the suspension dosage form, a sustained release of the drug was measurable for long periods (21 days in the present study) in both vitreous and RC tissues. This finding can be explained by the fact that administration of a high concentration of a drug in the vitreous (which exceeds the drug solubility by severalfold) forms an intravitreal depot that releases the drug in a sustained fashion. Such a depot formation in the vitreous was reported previously for less soluble drugs like triamcinolone acetonide.25
In case of suspension, the RC drug levels were higher than the vitreous after the first time point (0.25 hour). No literature data are available on the retinal drug levels after intravitreal injection of suspensions in rabbits.14 There are two possible explanations of the observed high RC drug levels. The retina is known to be the major elimination pathway for lipophilic drugs, because of the high partitioning.26 Possibly because of the more lipophilic nature of diclofenac acid compared with its salt form, RC tissue uptake of diclofenac acid was higher than that of diclofenac sodium. Another reason may be the sedimentation of suspension on the inferior retina due to gravity. Suspensions usually are of large size (in micrometers), high-density particles, and tend to settle faster than the solution dosage form. Such translocation of intravitreally injected microparticles (7 μm) to the retina has been observed in earlier reports.27 Particles of size 800 nm are estimated to settle in the vitreous at a rate of 1 mm/h.26 In the present case, the administered dose was 10 mg diclofenac acid with a particle size of ~5 μm. Based on the calculated settling rate of 3.06 cm/h for this particle size, the suspension may have settled almost completely onto the retinal layers by 0.25 hour, assuming a radius of 0.72 cm for the rabbit vitreous.28 Thus, injected suspensions can settle on the retina resulting in higher drug levels in the RC tissues when compared with the vitreous. This result was further confirmed by the higher MRT of the drug in the RC tissues (546.07 hours), which was longer when compared with the vitreous (431.38 hours).
To understand the differences in the pharmacokinetics of the two dosage forms, we developed pharmacokinetic models for both the dosage forms. Unlike the previous studies, the PK models were developed to simultaneously fit the data for vitreous and RC. The solution data were best fit by a simple two-compartment model including vitreous and RC tissues, with elimination from the RC. However, the same model did not fit the suspension data, as indicated by its low R2 and high AICc. Disposition studies with suspension clearly indicated that the suspension dosage form exhibited different pharmacokinetics and showed distribution equilibrium between RC and vitreous at steady state. The inclusion of distribution compartments of both vitreous and RC tissues (model 2C) resulted in the best-fit model to the suspension data. The distribution phase in the vitreous may be the depot of suspended particles, and K31 represents slow release of drug from the particles. The higher volume of distribution (Vd) for suspension in the vitreous also suggests a depot in the vitreous while high Vd for RC suggests a selective tissue binding site in equilibrium with free drug in the RC that is eliminated by K20.
As mentioned, suspensions form a depot and release the drug in a sustained fashion.9,25 Although intravitreal injection delivers the suspension directly into the vitreous, the drug has to be first released from the suspension before it can diffuse into the surrounding vitreous. Inclusion of a drug release constant in model 2C, although fitting the data well, resulted in a model that was no better than model 2C as indicated by the AICc of the two models. Two different absorption processes were shown to exist in drugs administered as suspensions.29 In the present study, it was hypothesized that the drug release from the intravitreal suspension follows two different release rates, as shown in model 2G. For modeling purposes, it was assumed that intravitreal injection of a suspension leads to depot formation. The first step, the initial diffusion of injected vehicle into the vitreous, is expected to result in rapid diffusion of drug present in the solution form along with the vehicle. This initial phase resulting in high initial concentrations of the drug can be associated with a fast release rate (Krel1). Once the vehicle diffuses, the less-soluble, lipophilic drug precipitates in the vitreous forming a depot. Formation of such intravitreal depots for lipophilic drugs like triamcinolone acetonide is widely reported in the literature.25 Drug is released from such a precipitate at a slower rate (Krel2) leading to sustained drug delivery. Models for suspensions were developed based on these two different release rate constants with several variations, including two different first-order release constants or one first-order release constant and one zero-order release constant. Although these models fit the observed vitreous and tissue data well (R2 = 0.9998), the AICc was higher, possibly due to the use of a greater number of parameters (two different release rates and fraction dose). Based on the ΔAICc and Akaike weight values, model 2C remains the best-fit model that describes the observed suspension data well. This model assumes distribution compartments to both vitreous and RC tissues. The distribution compartment to the vitreous could be the suspension core. The suspension injected into the vitreous distributed rapidly (K13 = 0.2889 hour–1, Table 3) into a core compartment from where drug was slowly released (K31 = 0.0042 hour–1, Table 3) into the vitreous. All these models clearly indicate altered pharmacokinetics of drug administered as suspension.
Simulations of suspension at 0.3 mg dose that is comparable to the solution dose assessed in animal studies indicated the superiority of the suspension dosage form in sustaining the drug levels in both RC and vitreous well beyond the 21-day measurement (Fig 7A). Simulation of solution data at a 10-mg dose, which is comparable to the suspension dose assessed in animals, indicated that drug levels decline to zero by day 11 (Fig. 6A). As indicated by additional simulations, retinal levels can be further titrated down with lower doses of suspension (Fig. 7B). Thus, at all doses assessed or simulated, the suspension form maintains prolonged drug delivery compared with the solution form.
One potential concern with drug suspensions or injected solid delivery systems in the vitreous is their potential interference with vision. Maurice26 estimated that 50-nm particles when administered at a dose of 10 mg in 4 mL vitreous, can reduce vision on eye chart by 1 line. Further, he estimated that when 800-nm particles are administered at a dose of 0.2 mg, a similar loss of vision can be anticipated. Thus, the suspensions or any other solid delivery system can interfere with the vision depending on its size and dose administered, especially when it is present in the axis of vision. However, such dosage forms may still be clinically viable, based on the experience with other clinical approaches including off-label administration of triamcinolone acetonide suspension up to 40 mg in the vitreous. This is because, it is the risk-benefit ratio that ultimately matters in the use of a delivery system in the clinical setting. Although there is certain blurring or visual obstruction by an injected solid drug, for the patients whose vision is already severely impaired or those likely to have impaired vision due to a disease condition (e.g., age-related macular degeneration or diabetic retinopathy), the benefit provided by a suspension dosage form as proposed in this study is likely to outweigh the risk of any temporary visual interference.
In this study, we used a drug suspension with a particle diameter of approximately 5 μm. When smaller particles at the same dose as larger particles are used, an increase in the drug dissolution or release rate can be anticipated due to an increase in the particle surface area exposed to the aqueous medium. This, in turn, will increase the clearance or removal of the drug from the vitreous. Thus, larger particles or poorly soluble forms of the drug will prolong the drug release and hence vitreous half-life of the drug to a greater extent when compared with smaller particles. The particle size advantage, however, has to be weighed against its syringeability and potential for causing vision interference. For this reason, micrometer-size particles as developed in this study may be most appropriate.
In summary, diclofenac exhibited altered pharmacokinetics when administered in suspension dosage form, resulting in increased mean residence time and elimination half-life, when compared with the solution form. Intravitreally administered high-dose suspensions (that exceeds the solubility in vitreous humor) settle under gravity on the retina, resulting in high sustained retinal drug levels. However, the safety of such high retinal drug levels remains to be assessed. In case retinal safety is a concern, lower doses of suspensions can be used to maintain prolonged retinal drug levels within the therapeutic window. The pharmacokinetic models developed in this study for solution and suspension dosage forms can potentially be extrapolated to other drugs.
Acknowledgments
Supported in part by National Institutes of Health grant R24 EY017045 and in part by a grant from Pfizer Global Research and Development, Groton, CT (UBK).
Footnotes
Disclosure: C. Durairaj, None; S.J. Kim, None; H.F. Edelhauser, None; J.C. Shah, None; U.B. Kompella, Pfizer (F)
References
- 1.Friedman DS, O'Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 2.Kempen JH, O'Colmain BJ, Leske MC, et al. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122:552–563. doi: 10.1001/archopht.122.4.552. [DOI] [PubMed] [Google Scholar]
- 3.Danis RP, Ciulla TA, Pratt LM, Anliker W. Intravitreal triamcinolone acetonide in exudative age-related macular degeneration. Retina. 2000;20:244–250. [PubMed] [Google Scholar]
- 4.Jonas JB, Kreissig I, Sofker A, Degenring RF. Intravitreal injection of triamcinolone for diffuse diabetic macular edema. Arch Ophthalmol. 2003;121:57–61. [PubMed] [Google Scholar]
- 5.Gillies MC, Kuzniarz M, Craig J, Ball M, Luo W, Simpson JM. Intravitreal triamcinolone-induced elevated intraocular pressure is associated with the development of posterior subcapsular cataract. Ophthalmology. 2005;112:139–143. doi: 10.1016/j.ophtha.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Joussen AM, Poulaki V, Mitsiades N, et al. Nonsteroidal anti-inflammatory drugs prevent early diabetic retinopathy via TNF-alpha suppression. FASEB J. 2002;16:438–440. doi: 10.1096/fj.01-0707fje. [DOI] [PubMed] [Google Scholar]
- 7.Flach AJ. Topical nonsteroidal antiinflammatory drugs in ophthalmology. Int Ophthalmol Clin. 2002;42:1–11. doi: 10.1097/00004397-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Monnier Y, Zaric J, Ruegg C. Inhibition of angiogenesis by non-steroidal anti-inflammatory drugs: from the bench to the bedside and back. Curr Drug Targets Inflamm Allergy. 2005;4:31–38. doi: 10.2174/1568010053622975. [DOI] [PubMed] [Google Scholar]
- 9.Amrite AC, Edelhauser HF, Kompella UB. Modeling of corneal and retinal pharmacokinetics after periocular drug administration. Invest Ophthalmol Vis Sci. 2008;49:320–332. doi: 10.1167/iovs.07-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costagliola C, Parmeggiani F, Antinozzi PP, Caccavale A, Cotticelli L, Sebastiani A. The influence of diclofenac ophthalmic solution on the intraocular pressure-lowering effect of topical 0.5% timolol and 0.005% latanoprost in primary open-angle glaucoma patients. Exp Eye Res. 2005;81:610–615. doi: 10.1016/j.exer.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Rabiah PK, Fiscella RG, Tessler HH. Intraocular penetration of periocular ketorolac and efficacy in experimental uveitis. Invest Ophthalmol Vis Sci. 1996;37:613–618. [PubMed] [Google Scholar]
- 12.Kothari HV, Lee WH, Ku EC. An alternate mechanism for regulation of leukotriene production in leukocytes: studies with an anti-inflammatory drug, sodium diclofenac. Biochim Biophys Acta. 1987;921:502–511. doi: 10.1016/0005-2760(87)90078-6. [DOI] [PubMed] [Google Scholar]
- 13.Kim SJ, Adams NA, Toma HS, et al. Safety of intravitreal ketorolac and diclofenac: an electroretinographic and histopathologic study. Retina. 2008;28:595–605. doi: 10.1097/IAE.0b013e31815e98a5. [DOI] [PubMed] [Google Scholar]
- 14.Durairaj C, Shah JC, Senapati S, Kompella UB. Prediction of vitreal half-life based on drug physicochemical properties: quantitative structure-pharmacokinetic relationships (QSPKR). Pharm Res. 2009;26:1236–1260. doi: 10.1007/s11095-008-9728-7. [DOI] [PubMed] [Google Scholar]
- 15.Jani PD, Singh N, Jenkins C, et al. Nanoparticles sustain expression of Flt intraceptors in the cornea and inhibit injury-induced corneal angiogenesis. Invest Ophthalmol Vis Sci. 2007;48:2030–2036. doi: 10.1167/iovs.06-0853. [DOI] [PubMed] [Google Scholar]
- 16.Mo Y, Barnett ME, Takemoto D, Davidson H, Kompella UB. Human serum albumin nanoparticles for efficient delivery of Cu, Zn superoxide dismutase gene. Mol Vis. 2007;13:746–757. [PMC free article] [PubMed] [Google Scholar]
- 17.Aukunuru JV, Sunkara G, Ayalasomayajula SP, DeRuiter J, Clark RC, Kompella UB. A biodegradable injectable implant sustains systemic and ocular delivery of an aldose reductase inhibitor and ameliorates biochemical changes in a galactose-fed rat model for diabetic complications. Pharm Res. 2002;19:278–285. doi: 10.1023/a:1014438800893. [DOI] [PubMed] [Google Scholar]
- 18.Kamppeter BA, Cej A, Jonas JB. Intraocular concentration of triamcinolone acetonide after intravitreal injection in the rabbit eye. Ophthalmology. 2008;115:1372–1375. doi: 10.1016/j.ophtha.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 19.Menon IA, Wakeham DC, Persad SD, Avaria M, Trope GE, Basu PK. Quantitative determination of the melanin contents in ocular tissues from human blue and brown eyes. J Ocul Pharmacol. 1992;8:35–42. doi: 10.1089/jop.1992.8.35. [DOI] [PubMed] [Google Scholar]
- 20.Cheruvu NP, Amrite AC, Kompella UB. Effect of eye pigmentation on transscleral drug delivery. Invest Ophthalmol Vis Sci. 2008;49:333–341. doi: 10.1167/iovs.07-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park J, Bungay PM, Lutz RJ, et al. Evaluation of coupled convective-diffusive transport of drugs administered by intravitreal injection and controlled release implant. J Control Release. 2005;105:279–295. doi: 10.1016/j.jconrel.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Burnham KP, Anderson DR. 2nd ed. Springer; New York: 2002. Model Selection and Multi-model Inference : a Practical Information-Theoretic Approach. p. 500. [Google Scholar]
- 23.Barbato F, Cappello B, La Rotonda MI, Miro A, Quaglia F. Diclofenac β-cyclodextrin binary systems: a study in solution and in the solid state. J Incl Phen Macr Chem. 2003;46:179–185. [Google Scholar]
- 24.Llinas A, Burley JC, Box KJ, Glen RC, Goodman JM. Diclofenac solubility: independent determination of the intrinsic solubility of three crystal forms. J Med Chem. 2007;50:979–983. doi: 10.1021/jm0612970. [DOI] [PubMed] [Google Scholar]
- 25.Kim H, Csaky KG, Gravlin L, et al. Safety and pharmacokinetics of a preservative-free triamcinolone acetonide formulation for intravitreal administration. Retina. 2006;26:523–530. doi: 10.1097/00006982-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Maurice D. Review: practical issues in intravitreal drug delivery. J Ocul Pharmacol Ther. 2001;17:393–401. doi: 10.1089/108076801753162807. [DOI] [PubMed] [Google Scholar]
- 27.Algvere P, Bill A. Drainage of microspheres and RBCs from the vitreous of aphakic and phakic eyes. Arch Ophthalmol. 1979;97:1333–1336. doi: 10.1001/archopht.1979.01020020075018. [DOI] [PubMed] [Google Scholar]
- 28.Ohtori A, Tojo K. In vivo/in vitro correlation of intravitreal delivery of drugs with the help of computer simulation. Biol Pharm Bull. 1994;17:283–290. doi: 10.1248/bpb.17.283. [DOI] [PubMed] [Google Scholar]
- 29.Wagner JG, Nelson E. Kinetic analysis of blood levels and urinary excretion in the absorptive phase after single doses of drug. J Pharm Sci. 1964;53:1392–1403. doi: 10.1002/jps.2600531126. [DOI] [PubMed] [Google Scholar]