Abstract
The replacement of the 7-N atom on guanine (G) with a C-H to give 7-deazaguanine (c7G) alters the electronic properties of the heterocyclic base and eliminates a potential major groove cation binding site, which affects the organization of salts and water in the major groove. This has a destabilizing effect on DNA. We report herein the characterization of DNA oligomers containing 7-(aminomethyl)-7-deazaguanine (1) residues using a variety of spectroscopic and thermodynamic approaches. 1 is an intramolecular model for the major groove binding of cations and basic amino acid residues to G. In contrast to c7G, the tethering of a cation in the major groove using 1 affords DNA that is as, or more, stable than the corresponding unmodified DNA. The stabilization is associated with the folding enthalpy and hydration.
The electrostatics of DNA is generally considered in terms of polyelectrolyte theory that treats DNA as a polyanionic cylinder.1 Even at relatively low salt concentrations, the cation concentration in the vicinity of DNA is estimated to be 1 M, and diffusible cations are treated without regard to high vs. low occupancy sites. Accordingly, the release of electrostricted ions, which is a powerful entropic driving force in the equilibrium binding of molecules to DNA,1 is generally treated as non-sequence specific.
However, there are electrostatic factors associated with the DNA grooves, in addition to the phosphate backbone. NMR studies show that Mg2+ and related divalent cations preferentially bind in the major groove at G runs and appear to bend DNA.2 A compilation of high-resolution structures of DNA shows a consensus for localization of monovalent cations in the major groove within 3.5 Å from the O6- and/or N7-positions of dG.3 The 1.2 Å resolution structure of 5’-d(CGCGAATTCGCG) in the presence of Tl+ clearly indicates that there are high occupancy monovalent cation binding sites in the major groove near G/C regions as well as in the minor groove near A/T rich regions.4
In order to explore the role of groove associated cations on DNA stability, structure and reactivity we previously studied the effects of (i) removing a major groove cation binding site using 7-deazaguanine (c7G)5 and (ii) introducing a tethered cation into the major groove using a 5-(3-aminopropyl)uracil modified base.6 The c7G substitution results in lower thermodynamic stability that is mainly derived from a reduced enthalpy contribution.5a A crystal structure of DNA with c7G shows the predicted loss of a cation from a conserved cation binding site and the NMR indicates a destabilization at the flanking base pair.5 The flexible cationic 3-aminopropyl sidechain shows a complex sequence dependency in its impact on stability and structure that is related to its proximity to a major groove cation binding site.6
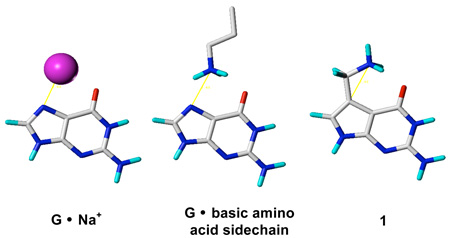
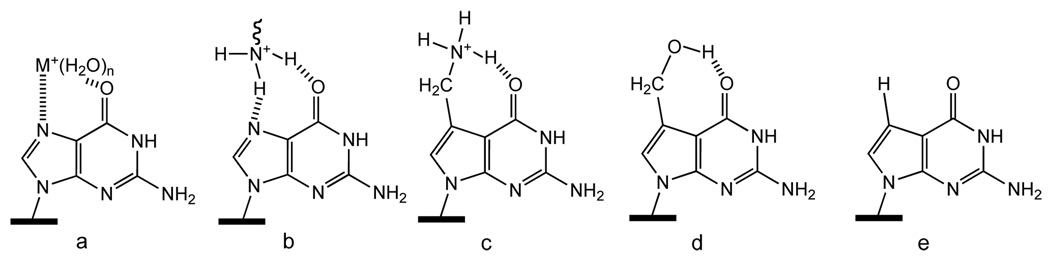
To create a structural model of groove associated cations that more accurately reflects cation distribution and location observed in DNA structures (Figure 1), we synthesized 7-aminomethyl-7-deaza-dG (1), which has a basic primary amine appended onto c7G via a CH2 linker.7 1 recapitulates the location of inorganic cations that are observed near the major groove edge of G: the restricted −NH3+ is approximately 2.6 Å from the major groove face of the G (Figure 1), a distance that is also similar to that seen in crystal structures for cationic groups on basic amino acid residues of DNA binding proteins.8 We also prepared 7-hydroxymethyl-7-deaza-dG (2) as a neutral isostere of 1.7 The spectroscopic and thermodynamic characterization of DNA containing c7G, 1 and 2 are reported.
Figure 1.
Models of: (a) cation and (b) basic amino acid sidechain associated with major groove edge of G, (c) tethered -NH3+ in 7-(aminomethyl)-7-deazaguanine (1), (d) 7-(hydroxymethyl)-7-deazaguanine (2) and (e) 7-deazaguanine (c7G) lacking cation binding site.
The sequences of the oligomers studied along with their thermodynamic parameters from UV melting and differential scanning calorimetry (DSC) analyses for unmodified (G), and c7G, 1 and 2 substituted DNA are shown in Table 1. The sequence used (OL-1) was selected because it is self-complementary and forms an intermolecular duplex rather than an intramolecular hairpin. Similar to what was previously observed in another sequence,5a the c7G substitution at G-5 (OL-2) decreases stability relative to OL-1: ΔΔG° = 2.5 and 4.1 kcal/mol at 10 and 100 mM NaCl, respectively. In contrast, the substitution of 1 (OL-3) makes the DNA thermodynamically more stable than OL-1: ΔΔG −2.2 and −1.4 kcal/mole at low and high salt, respectively. Relative to OL-2, the introduction of the tethered NH3+ increases stability by 4.7 and 5.5 kcal/mol at low and high salt, respectively. This translates to each CH2NH3+ appendage increasing the stability by 2.3–2.7 kcal/mol. We attribute the stabilization predominantly to the tethered NH3+ ion since DNA modified with 2 (OL-4) is thermodynamically similar to OL-2 with c7G. A similar trend is observed when c7G, 1 and 2 are introduced at G-3 (Supporting Information).
Table 1.
Thermodynamic Parameters for DNA Formation at 20 °C.a
| OL | sequence | NaClb | T Mc | ΔG°de | ΔH° e | TΔS°e | ΔnNa+f | Δnwf | ΔΔG vs OL-1 or -5 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5’-GAGAGCGCTCTC | 10 | 48.7 | −6.9 | −78.2 | −71.3 | −3.35 ± 0.17 | −41 ± 3 | - |
| 100 | 66.1 | −12.b | −92.0 | −79.b | −3.61 +0.18 | −43 + 4 | |||
| 2 | 5’-GAGA-c7G-CGCTCTC | 10 | 44.7 | −4.4 | −56.3 | −51.9 | −2.14 ± 0.11 | −25 ± 2 | 2.5 |
| 100 | 62.0 | −8.4 | −67.2 | −58.8 | −2.31 ± 0.12 | −27 ± 2 | 4.1 | ||
| 3 | 5’-GAGA-1-CGCTCTC | 10 | 52.0 | −9.1 | −92.9 | −83.8 | −2.86 ± 0.14 | −38 ± 4 | −2.2 |
| 100 | 67.3 | −13.9 | −99.7 | −85.9 | −2.90 ± 0.15 | −36 ± 3 | −1.4 | ||
| 4 | 5’-GAGA-2-CGCTCTC | 10 | 47.2 | −4.6 | −54.b | −49.9 | −1.63 ± 0.10 | −18 ± 2 | 2.3 |
| 100 | 63.9 | −7.6 | −58.2 | −50.6 | −1.52 ± 0.10 | −17 ± 2 | 4.9 | ||
| 5 | 5’-CGCGTTTTCGCG | 10 | 68.4 | −4.4 | −31.0 | −26.6 | −0.26 ± 0.02 | −18 ± 2 | - |
| 6 | 5’-CGCGTTTTC-c7G-CG | 10 | 63.7 | −3.b | −27.0 | −23.5 | −0.21 ± 0.02 | −15 ± 2 | 0.9 |
| 7 | 5’-CGCGTTTTC-1-CG | 10 | 63.0 | −4.0 | −31.3 | −27.3 | 0 | −14 ± 2 | 0.4 |
| 8 | 5’-GGG-1-TTTTCGCG | 10 | 71.3 | −4.3 | −28.9 | −24.6 | 0 | −16 ± 2 | 0.1 |
| 9 | 5’-CGC-1-TTTTC-1-CG | 10 | 65.6 | −3.6 | −27.0 | −23.4 | 0 | −13 ± 1 | 0.8 |
Parameters are measured from UV (TM) and DSC melting curves in 10 mM sodium phosphate buffer (pH 7.0). The observed standard deviations are:TM (± 0.7), ΔHcal (± 3%), ΔG°20 (± 5%), TΔScal (± 3%).
Salt concentration in mM.
°C.
Determined at 20 °C.
kcal/mol.
per mol DNA
The origin of the differences in ΔG for the duplexes is derived from the ΔH term for the G-5 substitutions; c7G in OL-2 causes a marked reduction (21.9 and 24.8 kcal/mol at low and high salt, respectively) in enthalpic stabilization, while 1 in OL-3 is enthalpy stabilizing by −14.7 and −7.7 kcal/mol at low and high salt, respectively. DNA with 2 (OL-4) behaves similar to the c7G-modified DNA. At the 3-position in OL-1, the ΔH term for DNA modified with 1 is similar to unmodified DNA and remains higher than for duplexes with c7G and 2, particularly in 100 mM NaCl (Table 1 and Supporting Information).
The differences in the ΔH term can reflect changes in base stacking and/or hydration. Therefore, the effects of the different modifications on cation and water release upon unfolding were examined by TM dependencies on salt concentration and osmolyte molality, respectively.9 There is a correlation between the ΔH term and DNA hydration. OL-1 and OL-3 have similar Δnw values, which are significantly higher than observed for OL-2 and OL-4. Interestingly, the release of cations upon unfolding varied little between OL-1 and OL-3, but was significantly lower for OL-2 and OL-4. (Table 1).
CD spectra provide global DNA conformational information of the different duplexes, and some indication of changes in base stacking by analysis of the relative intensities of the negative band at ∼250 nm.10 The CD spectra of OLs 1–12 are consistent with a B-conformation and there is a correlation between the intensity of the negative bands and the ΔH term at 10 mM NaCl that reflects base stacking for the oligomers (Supporting Information).
In addition to the intermolecular duplex DNA, an intramolecular hairpin with a higher TM (OL-5) was modified with a c7G (OL-6), or a single residue of 1. The introduction of c7G results in a minor destabilization (Table 1), while OL-7 and OL-8 are very similar to the unmodified hairpin. The addition of a second 1 (OL-9) places the two cations as close as 4.0 Å of each other assuming a normal B-conformation. This causes a modest reduction in the thermodynamic parameters. The minimum distance between the tethered NH3+ ions in OL-3 is 8.3 Å. Of note, is that substitution of 1 (OL-7 and OL-8) or two residues (OL-9) into the hairpin removed any TM salt dependency; ΔnNa+ is 0.
In summary, the tethering of cationic and neutral polar functionalities at a distance from the floor of the major groove that mimics the distance observed for diffusible cations and basic amino acid residues is reported. In DNA with a c7G substitution, a conserved cation binding is eliminated and the DNA is thermodynamically destabilized.5 In the current study, it is demonstrated that the insertion of 1 into DNA, which permanently refurbishes a cation that is lost with c7G, increases the stability of DNA relative to that of the natural sequence. Locating a polar hydroxyl group on the major groove edge of c7G does not restore stability. The effect of the tethered cationic modification provides an insight into the stabilizing role that “diffusible” cations associated with high occupancy sites in the major groove play in maintaining DNA structure.
Supplementary Material
Acknowledgment
This work was supported by NIH grant RO1 CA29088. We thank C. Rizzo and A. Kozekova at Vanderbilt University for the synthesis of the DNA oligomers.
Footnotes
Supporting Information. Thermodynamic and CD data are available free (Internet at http://pubs.acs.org).
References
- 1.(a) Manning GS. Quart. Rev. Biophys. 1978;2:179–246. doi: 10.1017/s0033583500002031. [DOI] [PubMed] [Google Scholar]; (b) Record MT, Jr, Anderson CF, Lohman TM. Quart. Rev. Biophys. 1978;2:103–179. doi: 10.1017/s003358350000202x. [DOI] [PubMed] [Google Scholar]; (c) Honig B, Nicholls A. Science. 1995;268:1144–1149. doi: 10.1126/science.7761829. [DOI] [PubMed] [Google Scholar]
- 2.(a) Braunlin WH, Nordenskiöld L, Drakenberg T. Biopolymers. 1991;31:1343–1346. doi: 10.1002/bip.360311111. [DOI] [PubMed] [Google Scholar]; (b) Buckin VA, Kankiya B, Renteperis D, Marky LA. J. Am. Chem. Soc. 1994;116:9423–9429. [Google Scholar]
- 3.Howerton SB, Nagpal A, Williams LD. Biopolymers. 2003;69:87–99. doi: 10.1002/bip.10319. [DOI] [PubMed] [Google Scholar]
- 4.Howerton SB, Sines CC, VanDerveer D, Williams LD. Biochemistry. 2001;40:10023–10031. doi: 10.1021/bi010391+. [DOI] [PubMed] [Google Scholar]
- 5.(a) Ganguly M, Wang F, Kaushik M, Stone MP, Marky LA, Gold B. Nucleic Acids Res. 2007;35:6181–6195. doi: 10.1093/nar/gkm670. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang F, Li F, Ganguly M, Marky LA, Gold B, Egli M, Stone MP. Biochemistry. 2008;47:7147–7157. doi: 10.1021/bi800375m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Soto AM, Kankia BI, Dande P, Gold B, Marky LA. Nucleic Acids Res. 2002;30:3171–3180. doi: 10.1093/nar/gkf430. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Li Z, Huang L, Dande P, Gold B, Stone MP. J. Amer. Chem. Soc. 2002;124:8553–8560. doi: 10.1021/ja0201707. [DOI] [PubMed] [Google Scholar]; (c) Moulaei T, Maehigashi T, Lountos G, Komeda S, Watkins D, Stone M, Marky L, Li J-S, Gold B, Williams LD. Biochemistry. 2004;43:7458–74648. doi: 10.1021/bi050128z. [DOI] [PubMed] [Google Scholar]
- 7.Wang R-W, Gold B. Org. Lett. 2009;11:2465–2468. doi: 10.1021/ol9007537. [DOI] [PubMed] [Google Scholar]
- 8.see Protein Data Bank (http://www.rcsb.org/pdb. e.g., PDB id: 1GU5, 1CF7, 1DP7, 1GCC, 3BPY, 1C7U).
- 9.(a) Spink CH, Chaires JB. Biochemistry. 1999;38:496–508. doi: 10.1021/bi9820154. [DOI] [PubMed] [Google Scholar]; (b) Kaushik MN, Suehl N, Marky LA. Biophys. Chem. 2007;126:154–164. doi: 10.1016/j.bpc.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 10.(a) Tinoco I, Sauer K, Wang JC, Puglisi J. Physical Chemistry: Principles and Applications in Biological Sciences. 4th Ed. Prentice Hall; 2001. [Google Scholar]; (b) Basse WA, Johnson WC., Jr Nucleic Acids Res. 1979;6:797–814. doi: 10.1093/nar/6.2.797. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kypr J, Kejnovska´ I, Rencˇiuk D, Michaela Vorli´cˇkova´ M. Nucleic Acids Res. 2009;37:1713–1725. doi: 10.1093/nar/gkp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.