Abstract
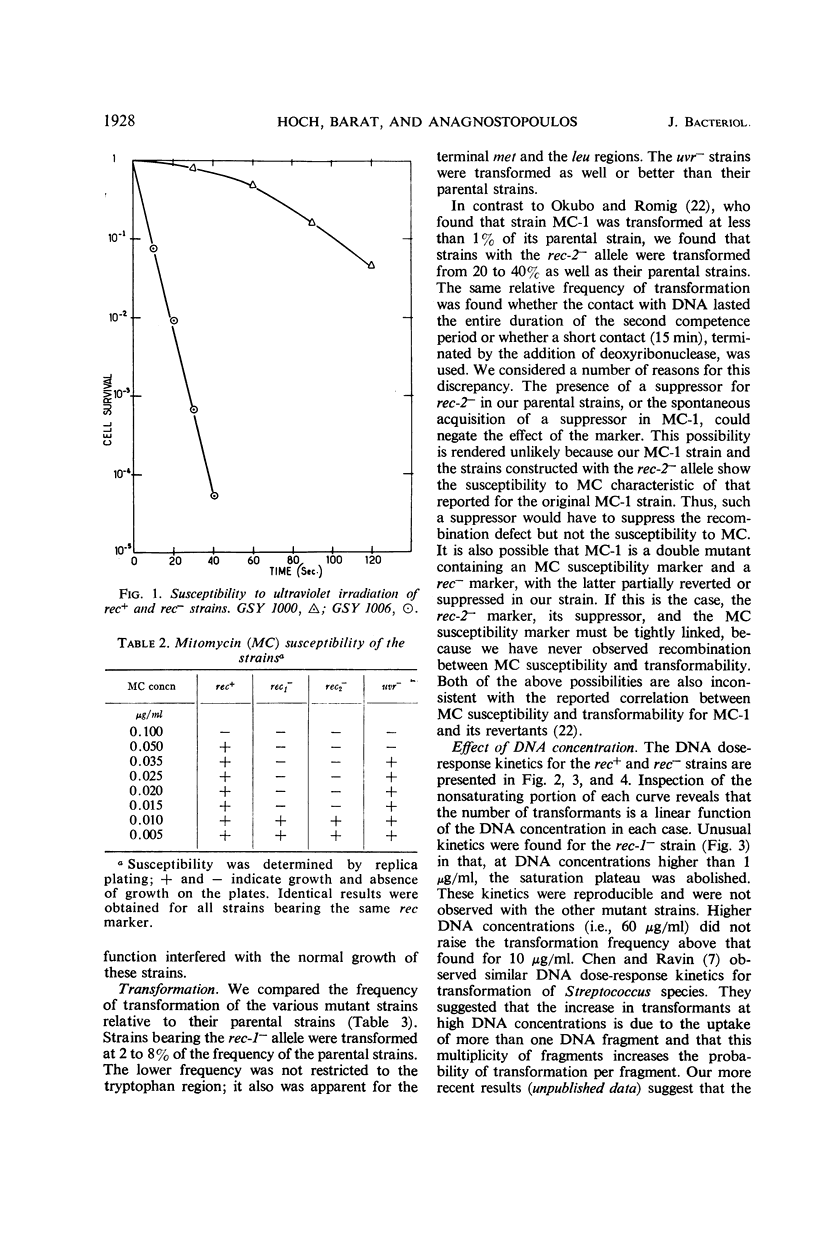
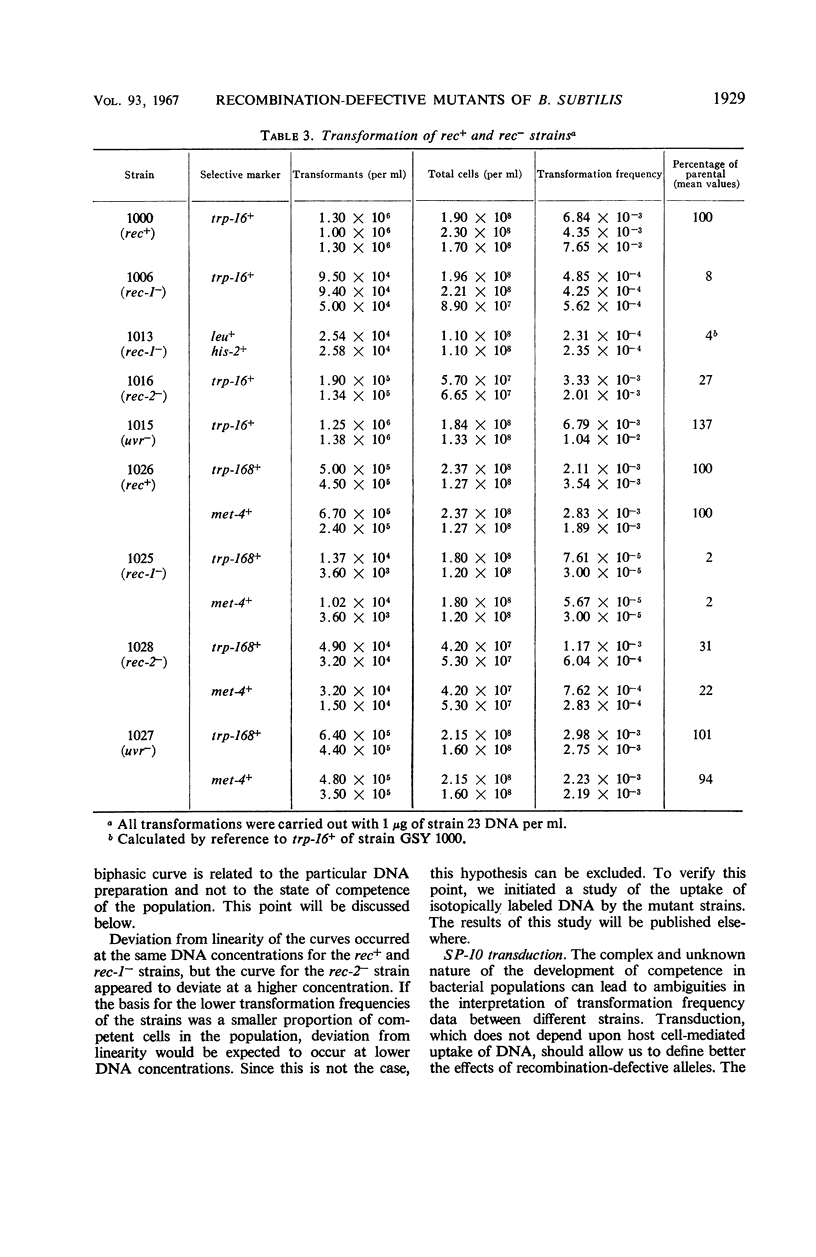
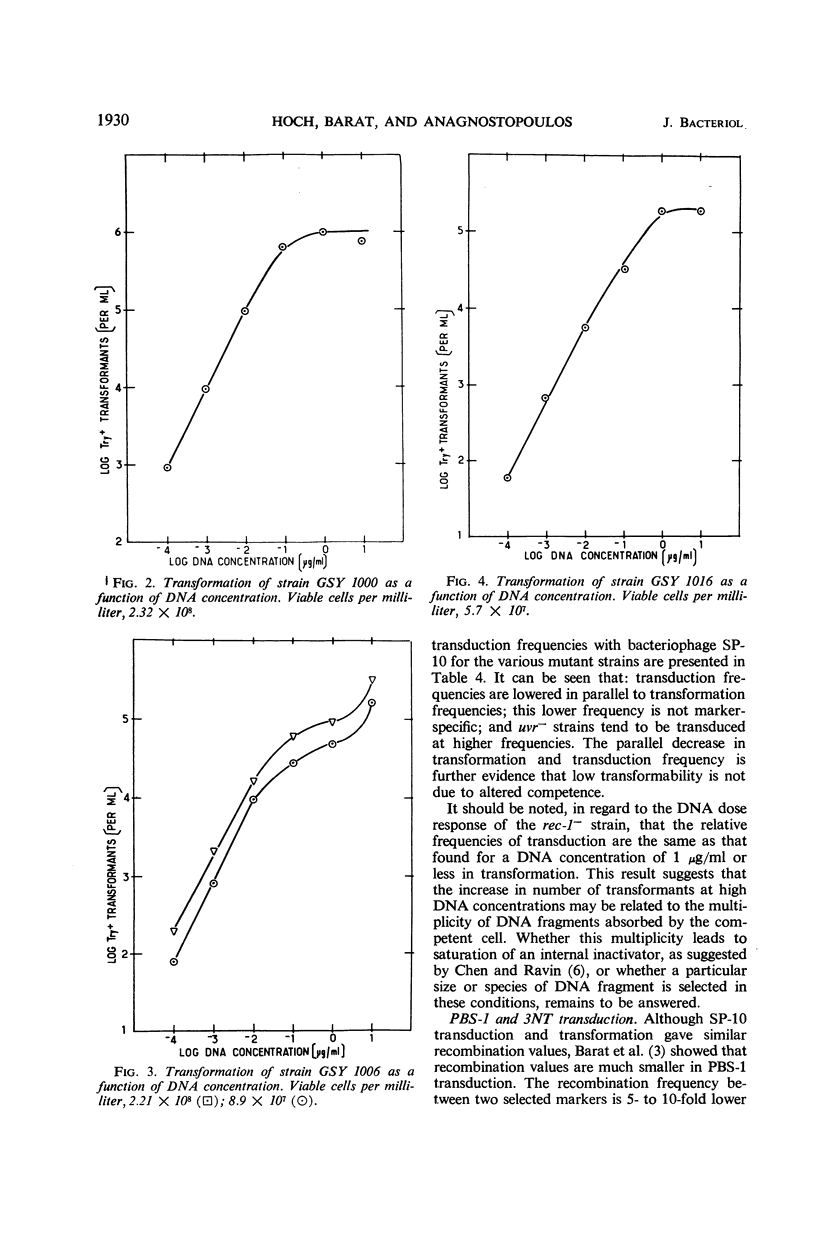
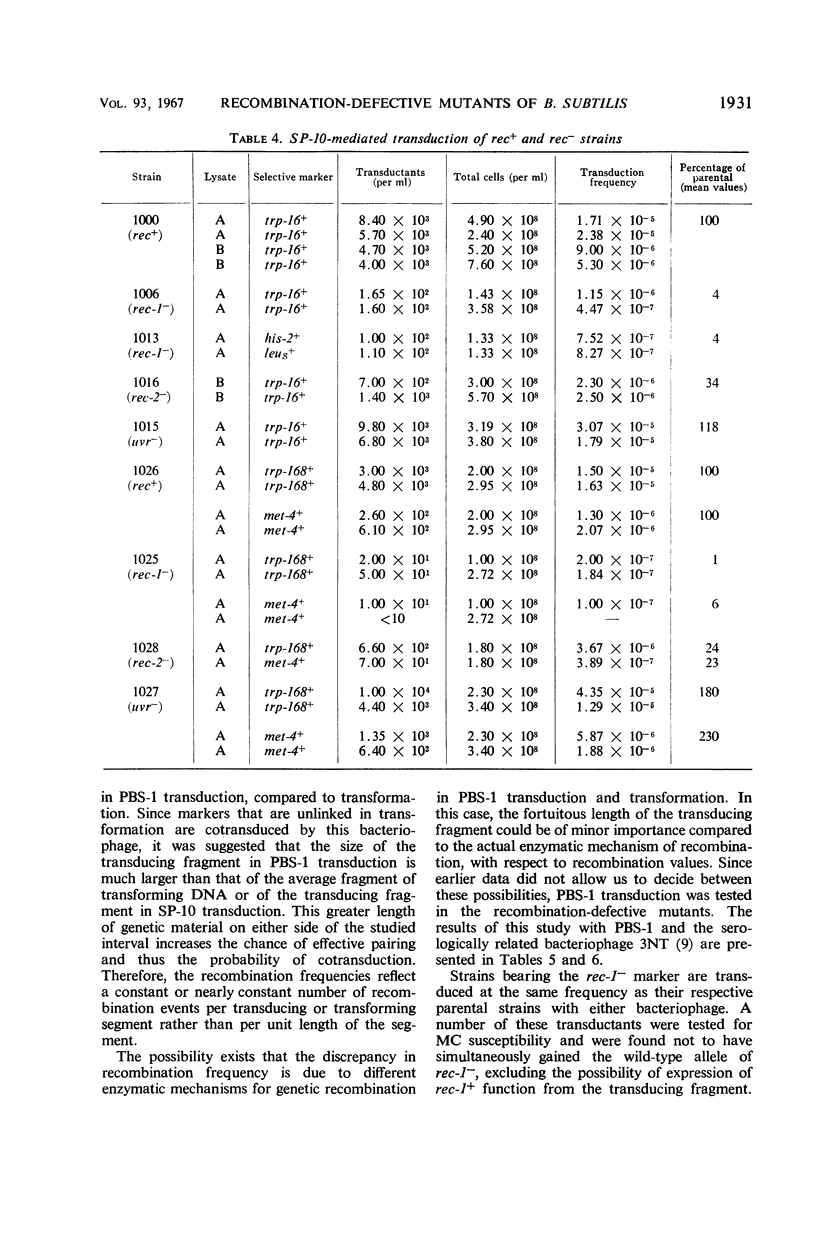
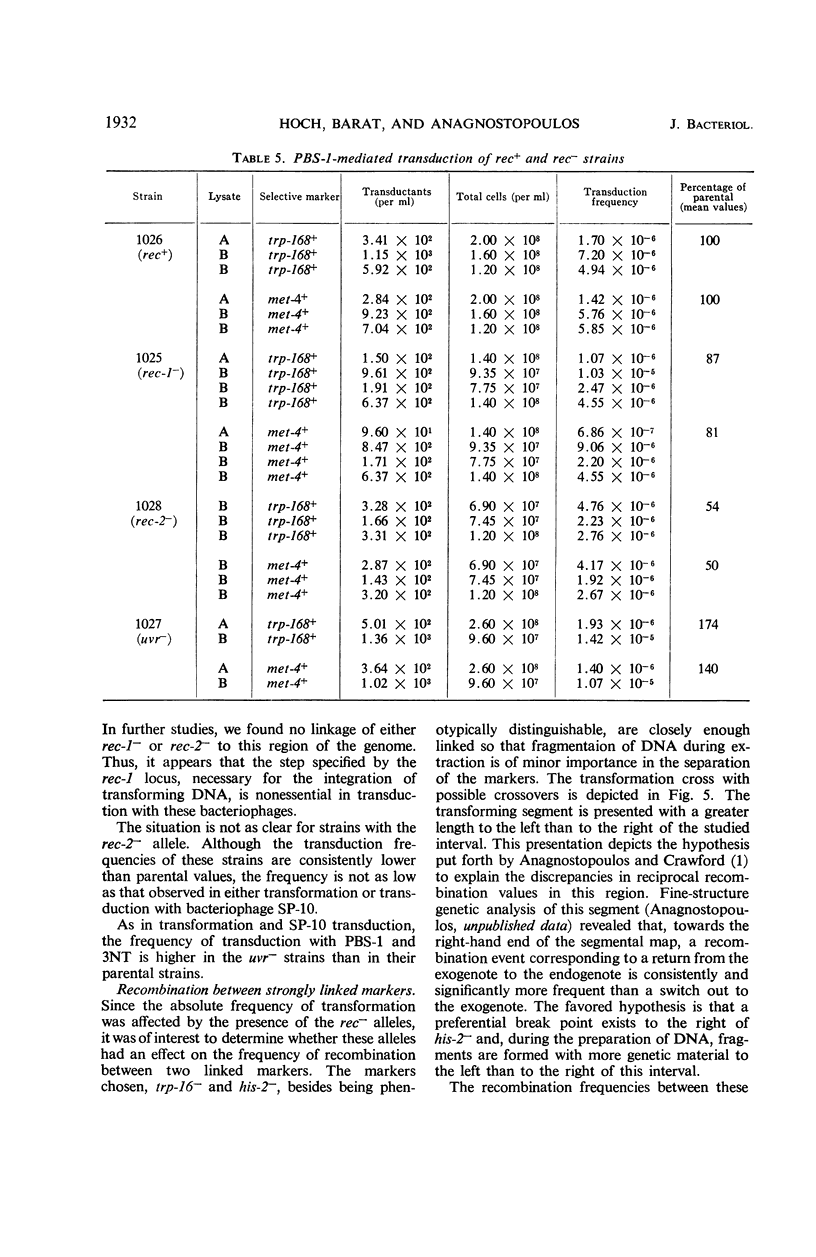
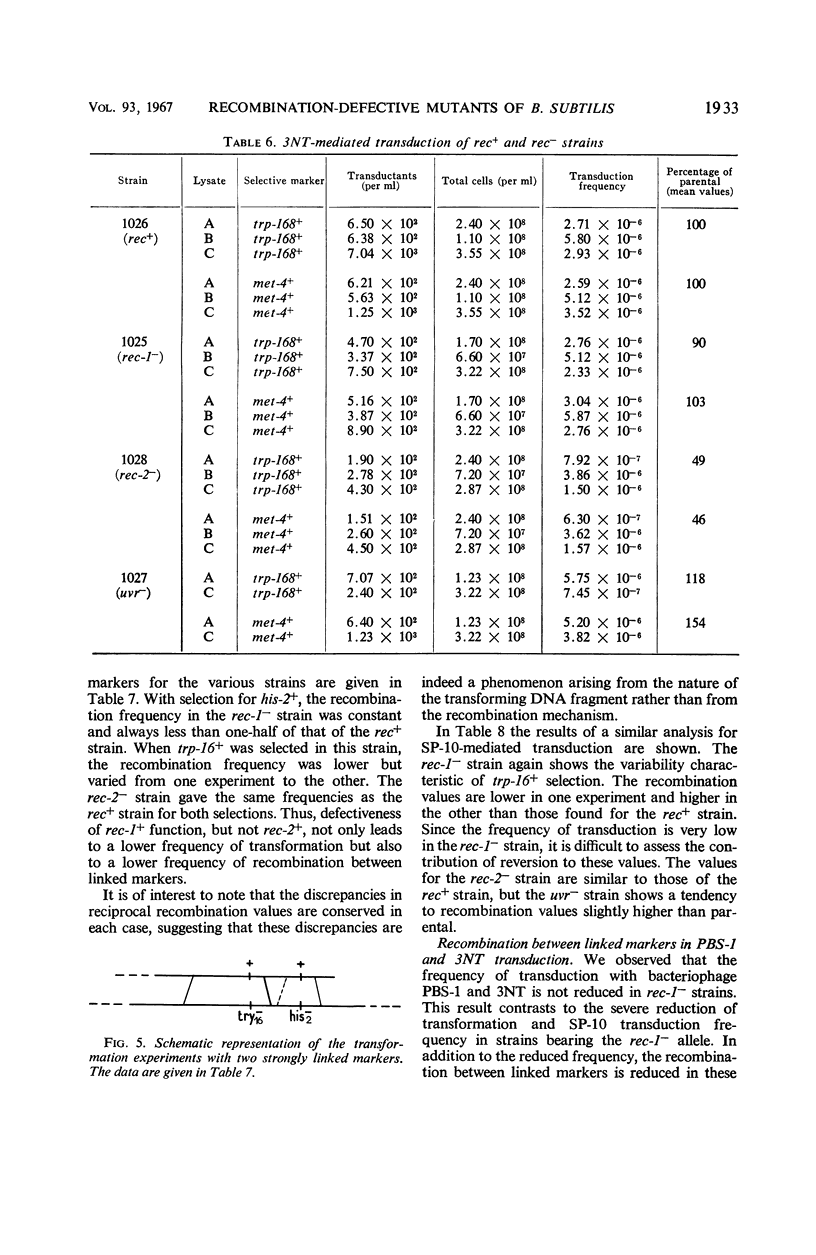
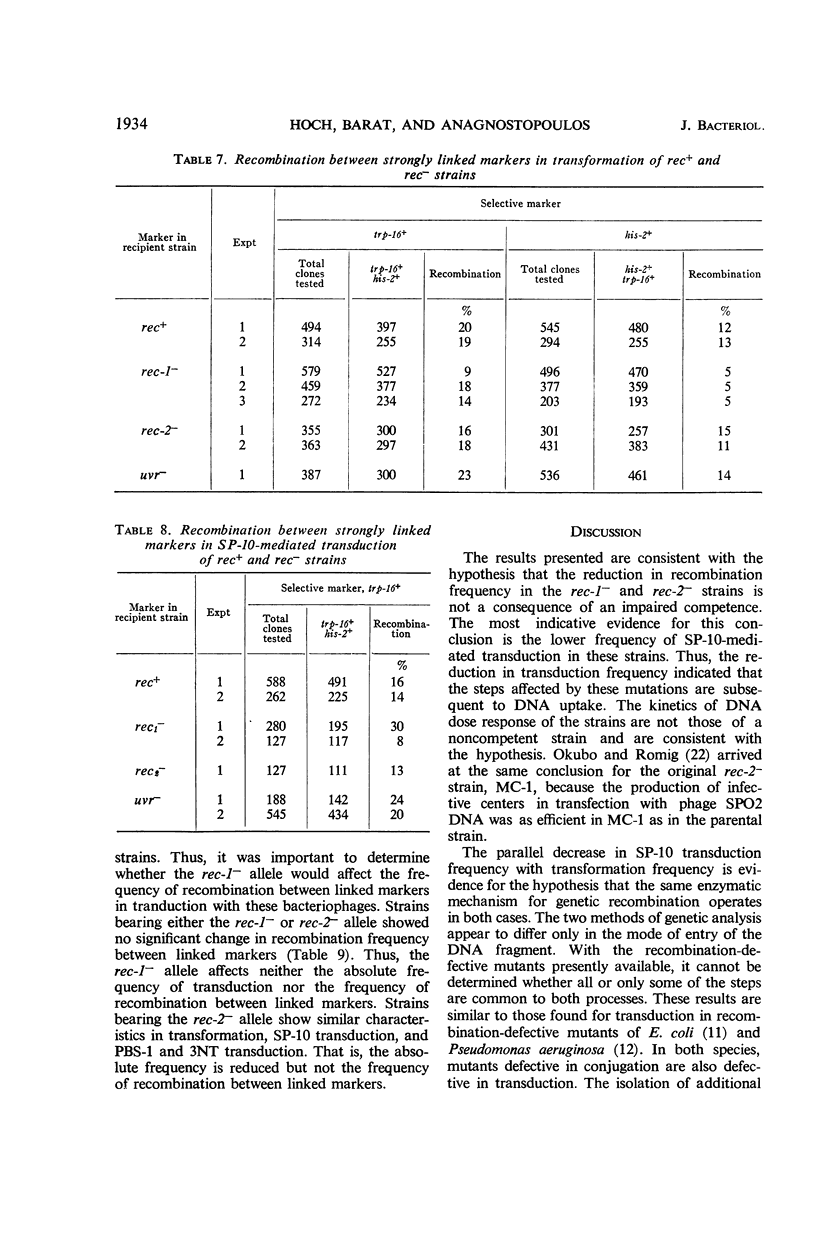
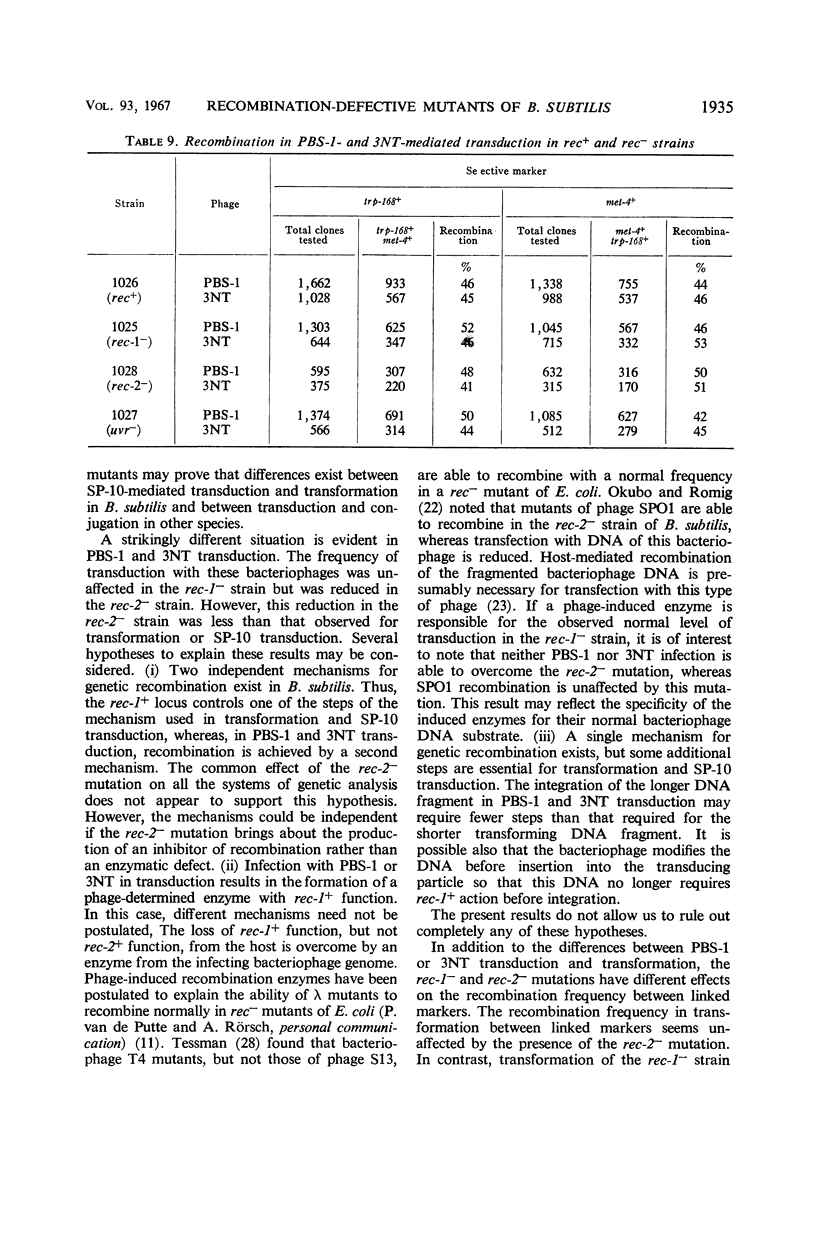
The effects on transformation and transduction of an ultraviolet sensitivity (uvr−) and two ultraviolet sensitivity-recombination deficiency (rec-1− and rec-2−) mutations in isogenic strains of Bacillus subtilis were investigated. Transformation frequency in the rec-1− and rec-2− strains was reduced to approximately 5 and 25%, respectively, of the parental strains. Normal kinetics of deoxyribonucleic acid dose response in transformation were found for the rec-1+ and rec-2− strains. Biphasic curves were obtained with the rec-1− strains. Transduction frequency with bacteriophage SP-10 decreased parallel to transformation frequency in the rec-1− and rec-2− strains. This result suggests that transformation and SP-10 transduction share a common mechanism for genetic recombination. It also indicates that the reduction in transformation frequency of these strains was not due to altered competence. Transduction frequency with bacteriophage PBS-1 or 3NT, on the contrary, was not diminished in rec-1− strains. This frequency was reduced in rec-2− strains but not as severely as that of transformation or SP-10 transduction. Several hypotheses to interpret these differences are presented. Recombination frequency between linked markers was reduced more than 50% in transformation by the presence of the rec-1− mutation. Linkage was unaffected in the rec-2− strains. Neither the rec-1− nor the rec-2− mutation had an effect on linkage in PBS-1 or 3NT transduction. The uvr− strains were transformed at a frequency equal to or greater than that of the parental strains. These strains were transduced by all bacteriophage systems at frequencies about twofold higher than those of parental strains.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANAGNOSTOPOULOS C., CRAWFORD I. P. Transformation studies on the linkage of markers in the tryptophan pathway in Bacillus subtilis. Proc Natl Acad Sci U S A. 1961 Mar 15;47:378–390. doi: 10.1073/pnas.47.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARAT M., ANAGNOSTOPOULOS C., SCHNEIDER A. M. LINKAGE RELATIONSHIPS OF GENES CONTROLLING ISOLEUCINE, VALINE, AND LEUCINE BIOSYNTHESIS IN BACILLUS SUBTILIS. J Bacteriol. 1965 Aug;90:357–369. doi: 10.1128/jb.90.2.357-369.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSISZAR K., IVANOVICS G. TRANSDUCTION IN BACILLUS SUBTILIS. Acta Microbiol Acad Sci Hung. 1965;12:73–89. [PubMed] [Google Scholar]
- Hertman I., Luria S. E. Transduction studies on the role of a rec+ gene in the ultraviolet induction of prophage lambda. J Mol Biol. 1967 Jan 28;23(2):117–133. doi: 10.1016/s0022-2836(67)80021-4. [DOI] [PubMed] [Google Scholar]
- Holloway B. W. Mutants of Pseudomonas aeruginosa with reduced recombination ability. Mutat Res. 1966 Oct;3(5):452–455. doi: 10.1016/0027-5107(66)90055-8. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L. Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics. 1966 Jun;53(6):1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler I. Characteristics of an ultraviolet irradiation sensitive strain of Bacillus subtilis. Biochem Biophys Res Commun. 1965 Nov 22;21(4):384–391. doi: 10.1016/0006-291x(65)90206-8. [DOI] [PubMed] [Google Scholar]
- Mahler I. Effect of mitomycin C on five excision-repair mutants of Bacillus subtilis. Biochem Biophys Res Commun. 1966 Oct 5;25(1):73–79. doi: 10.1016/0006-291x(66)90642-5. [DOI] [PubMed] [Google Scholar]
- Mattern I. E., Zwenk H., Rörsch A. The genetic constitution of the radiation-sensitive mutant Escherichia coli Bs-1. Mutat Res. 1966 Oct;3(5):374–380. doi: 10.1016/0027-5107(66)90047-9. [DOI] [PubMed] [Google Scholar]
- OKUBO S., STRAUSS B., STODOLSKY M. THE POSSIBLE ROLE OF RECOMBINATION IN THE INFECTION OF COMPETENT BACILLUS SUBTILIS BY BACTERIOPHAGE DEOXYRIBONUCLEIC ACID. Virology. 1964 Dec;24:552–562. doi: 10.1016/0042-6822(64)90207-7. [DOI] [PubMed] [Google Scholar]
- Okubo S., Romig W. R. Comparison of ultraviolet sensitivity of Bacillus subtilis bacteriophage SPO2 and its infectious DNA. J Mol Biol. 1965 Nov;14(1):130–142. doi: 10.1016/s0022-2836(65)80235-2. [DOI] [PubMed] [Google Scholar]
- Okubo S., Romig W. R. Impaired transformability of Bacillus subtilis mutant sensitive to mitomycin C and ultraviolet radiation. J Mol Biol. 1966 Feb;15(2):440–454. doi: 10.1016/s0022-2836(66)80120-1. [DOI] [PubMed] [Google Scholar]
- Reiter H., Strauss B. Repair of damage induced by a monofunctional alkylating agent in a transformable, ultraviolet-sensitive strain of Bacillus subtilis. J Mol Biol. 1965 Nov;14(1):179–194. doi: 10.1016/s0022-2836(65)80239-x. [DOI] [PubMed] [Google Scholar]
- Searashi T., Strauss B. Relation of the repair of damage induced by a monofunctional alkylating agent to the repair of damage induced by ultraviolet light in Bacillus subtilis. Biochem Biophys Res Commun. 1965 Sep 22;20(6):680–687. doi: 10.1016/0006-291x(65)90069-0. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIKAWA H., SUEOKA N. Sequential replication of Bacillus subtilis chromosome. I. Comparison of marker frequencies in exponential and stationary growth phases. Proc Natl Acad Sci U S A. 1963 Apr;49:559–566. doi: 10.1073/pnas.49.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]



