Abstract
Chronic psychosocial stress exacerbates asthma but the underlying mechanisms remain poorly understood. We hypothesized that psychosocial stress aggravates allergic airway inflammation by altering innate immune cell function. The effects of stress on airway inflammation, lung function and glucocorticoid responsiveness were studied in a novel in vivo murine model of combined social disruption stress and allergic sensitization. The effects of corticosterone were assessed on cytokine profile and glucocorticoid receptor activation in LPS-stimulated spleen cell cultures in vitro.
Airway inflammation resolved 48 hours after a single allergen provocation in sensitized control mice but not in animals that were repeatedly exposed to stress prior to allergen challenge. The enhanced eosinophilic airway inflammation 48 hours after allergen challenge in these mice was associated with increased levels of IL-5, GM-CSF, IgG1, TARC, TNF-α and IL-6 in the airways and a diminished inhibition of these mediators by corticosterone in LPS-stimulated splenocyte cultures in vitro. Stress-induced reduction of the corticosteroid effects paralleled increased p65 expression and a decreased DNA binding capability of the glucocorticoid receptor in vitro. Further, glucocorticoid receptor mRNA and protein expression in the lungs of mice exposed to both stress and allergen was markedly reduced in comparison with that in either condition alone or in naïve mice. Thus, exposure to repeated social stress prior to allergen inhalation enhances and prolongs airway inflammation and alters corticosterone responsiveness. We speculate that these effects were mediated at least in part by impaired glucocorticoid receptor expression and function.
Keywords: Rodent, Allergy, Lung, Inflammation, Th1/Th2 Cells
Introduction
Psychological stress can modulate immune and inflammatory cell function through neural and hormonal pathways that link the autonomic nervous and the immune systems (1–9). Physical and psychological stressors were shown to activate the hypothalamic-pituitary-adrenal axis and increase levels of circulating glucocorticoid hormones. Endogenous circulating glucocorticoids in turn significantly modulate immune cell differentiation, distribution and function and regulate many aspects of the crosstalk between innate and adaptive immune systems (10–12). This mechanism is particularly important in the lung where a pathogen and inflammation-free environment is maintained by a finely orchestrated innate immune system. Stress-induced increase in corticosteroid plasma levels for example, inhibits the interleukin (IL)-12/Interferon (IFN)-γ regulatory axis eliciting an immune suppressed state. Interestingly, in spite of the increased circulating corticosteroid and catecholamine levels, chronic psychosocial stress has been long considered a major cause of exacerbation of allergic airway inflammation (1, 2, 5, 7, 8, 13–16). Experimental studies showed that asthma patients have increased bronchoconstriction after distressful experiences (3), listening to stressful interactions (17) or exposure to asthma-related visual brain-stimulation (13). Asthma is exacerbated in depressed and anxious patients who have low social support (18) and in children during school examinations (19).
Although the underlying mechanisms are not clear, there are indications that altered innate immune cell function may play a role. We have previously reported that LPS-stimulated splenocytes released more tumor necrosis factor (TNF)-α and interleukin (IL)-6 in mice exposed to social stress than in control animals and that these cells were less sensitive to the inhibitory effects of glucocorticoids(20).
Glucocorticoids activate intracellular receptors that are ubiquitously found in most cells (11, 21). The activated cytosolic glucocorticoid receptor translocates to the nucleus and acts as a ligand-dependent transcription factor that modulates the expression of glucocorticoid-responsive genes or modifies the activity of other transcription factors such as NF-κB (10, 21). Recent studies in mice from our laboratories and others suggested that repeated social defeat may disrupt the ability of glucocorticoids to suppress inflammation (22–30). Although the exact mechanism of the impaired glucocorticoid action remains unknown, there was a decreased nuclear translocation of the glucocorticoid receptor in LPS-stimulated splenocytes in vitro, from mice that were repeatedly exposed to social stress (23, 28).
To investigate the effects of psychosocial stress on the allergic airway response, we used a unique murine model that combined social disruption stress (SDR) and sensitization with an allergenic extract of Aspergillus fumigatus (Af). We hypothesized that social stress aggravates allergic airway inflammation by modifying innate immune cell production of cytokines and that this effect is mediated by an altered corticosteroid action.
Materials and methods
Mice
Male CD-1 mice were purchased from Charles River Laboratories (Hollister, CA). Mice that were studied for their stress and allergic responses (aged 6–8 weeks) were allowed to acclimate to their new environment for 1 week prior to experimentation and were maintained on a 12 hr light/dark schedule (lights on at 0600) with food and water available ad libitum. All procedures were approved by the animal use and care committees at the Ohio State University and the University of Pennsylvania.
Social disruption model
Social disruption stress (SDR) has been extensively used and has been previously described by our laboratory and others (26, 30, 31). Briefly, a retired large male breeder mouse (the intruder) was placed into the cage containing an established social hierarchy of 3–5 young male mice (the residents) for a 2 hr confrontation that was repeated on 6 consecutive days. Disrupting an established social hierarchy by introducing an aggressive intruder results in inter male aggression and anxiety-like behavior. The confrontations were continuously monitored and if severe fighting occurred the aggressor was removed. The extent of anxiety that resulted from SDR was assessed using the open field test as described previously (31). Mice in the control groups were left undisturbed with the exception of the handling associated with the vehicle or sensitization injections.
Allergic sensitization and challenge
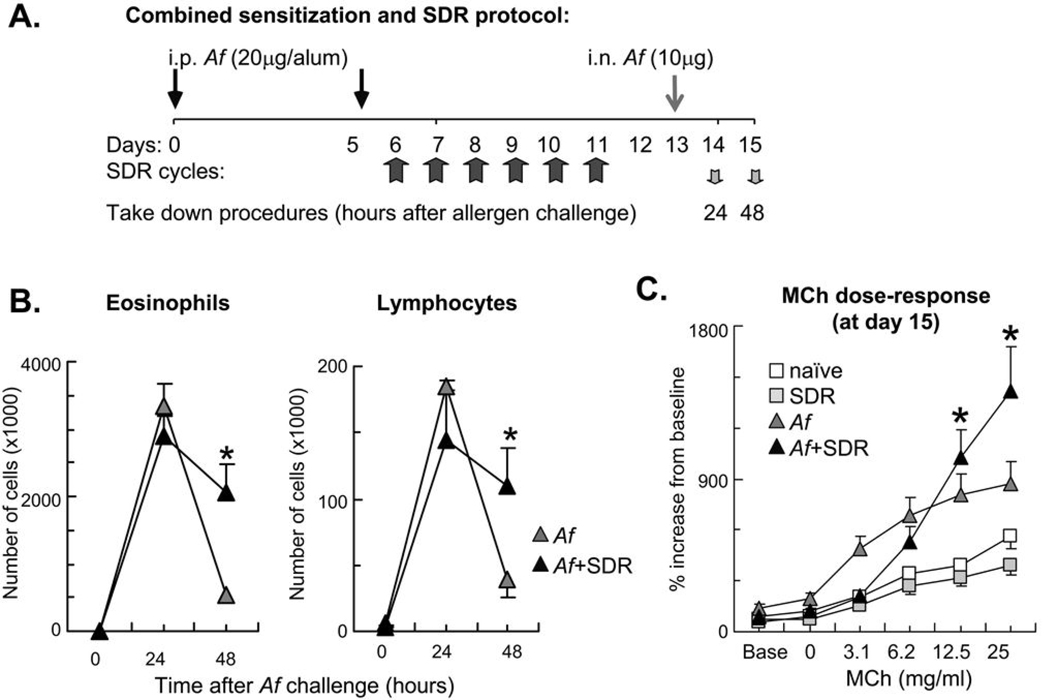
Mice were sensitized by intraperitoneal injection of 20 µg of Aspergillus fumigatus (Af) extract (Hollister-Stier Laboratories LLC, Spokane, WA) and 20 mg of Al(OH)3 (Imject Alum; Pierce, Rockford, IL) in PBS (100 µl) on days 0 and 5 (32–34). Mice assigned to the stress condition were exposed to SDR on days 7–12. On day 13, all sensitized mice were intranasally challenged with 10 µg of Af antigen in PBS (30 µl) and then sacrificed on day 15. The following groups of mice were used: Naïve controls (no stress, no sensitization), SDR (SDR stress, no sensitization), Af (no stress, Af sensitization), and Af+SDR (SDR stress, Af sensitization) (Figure 1A and Figure 2A).
Figure 1.
Combination of allergic airway sensitization and SDR caused delayed resolution of the inflammatory airway response and enhancement of allergic airway hyperresponsiveness at high methacholine concentrations. (A): In a combined protocol for allergic sensitization and SDR, mice received intraperitoneal (i.p.) Af in alum on days 0 and 5 followed by repeated SDR for 2 hours on days 6 through 11. Mice received intranasal (i.n.) allergen challenge on day 13 and were sacrificed either 24 or 48 hours later. Control naïve and SDR mice were sacrificed on day 15. (B): Combination of allergic airway sensitization and SDR increased number of airway eosinophils and lymphocytes 48h after Af challenge. BAL differential cell count was evaluated in Giemsa preparations. Naïve and SDR mice did not receive allergen challenge. There was no difference between naïve and SDR mice. (C): Exposure to allergen and stress enhanced airway hyperresponsiveness at high methacholine concentrations. Mice were studied 48 h after a single Af challenge. Mice received increasing doses of inhaled methacholine (MCh). Lung function was assessed using non-invasive plethysmography and the enhanced pause (Penh). There was no difference between naïve and SDR mice. Data are expressed as % changes from baseline. Baseline Penh measurements were as follows: naïve: 0.385±0.050; SDR:0.471±0.028; Af:0.924±0.367; Af+SDR:0.614±0.082 (B–C): Mean±SEM of n=5–8 each; *p<0.05 (Af vs. Af+SDR).
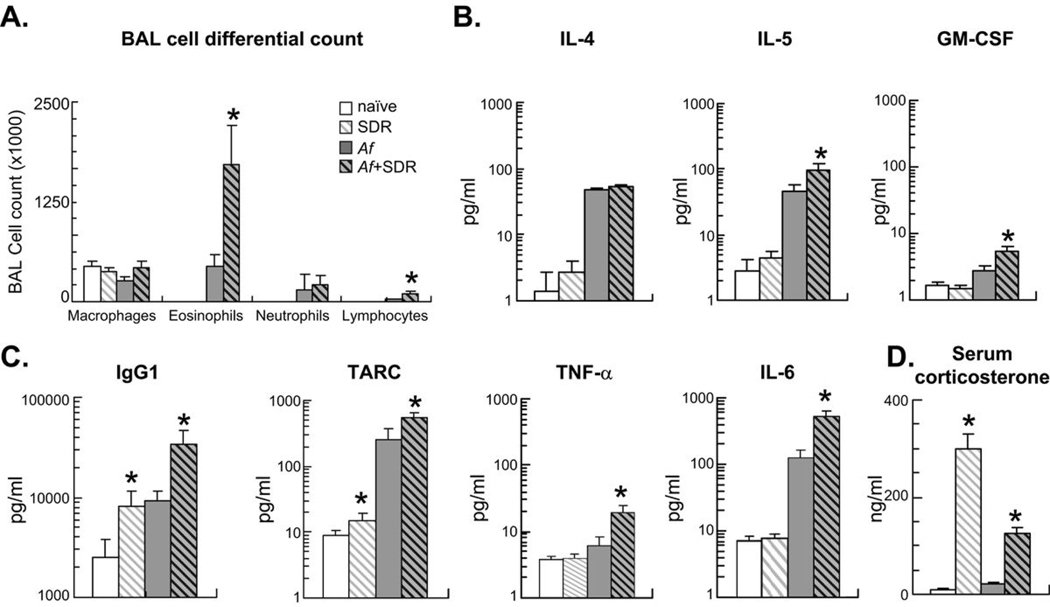
Figure 2.
Mice were sensitized (i.p.) and challenged (i.n.) with Af extract and were also exposed to social stress (SDR) daily between days 7–12 as indicated. Sensitized mice were studied 48 h after a single Af challenge (on day 15). Naïve and SDR mice were also studied on day 15. (A): BAL differential cell count was evaluated in Giemsa preparations. Stress and allergic sensitization enhanced numbers of eosinophils and lymphocytes but not neutrophils and macrophages in the BAL fluid. There was no difference between naïve and SDR mice. (B–C): Chemokine, cytokine and immunoglobulin levels in the BAL were measured in the same groups by SearchLight technology. Increased eosinophilia was paralleled by enhanced levels of the eosinopoietic IL-5 and GM-CSF (B), increased levels of the B-cell derived IgG1 and the innate immune cell-derived TARC, TNF-α and IL-6 (C). (D): Stress significantly increased serum corticosterone levels p<0.01: SDR vs. naïve, Af or Af+SDR. Blood for endocrine measures was taken on day 15. (A–D): (Mean±SEM of n=8 per group) *p<0.05 Naïve vs. SDR and Af vs. Af+SDR; Repeated measure ANOVA followed by Tukey's HSD.
Serum corticosterone radioimmune assay (RIA)
Serum corticosterone was measured in duplicate, using a radioimmunoassay (RIA) kit (ImmuChem Double Antibody corticosterone 125I RIA kit; MP Biomedicals, Orangeburg, NY), per manufacturer’s instructions (27, 30). Blood was collected on Day 15 (Figure 1A) from the retroorbital plexus immediately after euthanasia and serum was stored at −70° C until assay. The minimum sensitivity of the assay was 23.5 ng/ml.
Evaluation of airway hyperresponsiveness
Airway responsiveness to methacholine (MCh) was assessed in conscious, unrestrained, spontaneously breathing mice in a non-invasive whole body plethymograph (Buxco Electronics, Troy, NY) as described previously (32). The airway function of the animal was determined by a dimensionless parameter, Penh, that reflects changes in box pressure from both the inspiration and expiration of the animal, along with the timing comparison of early and late expiration (pause). Each mouse received two baseline readings, followed by saline inhalation. Mice were then exposed to aerosolized MCh at increasing doses of MCh, ranging from 3.1 to 25 mg/ml, for 1 min. Pulmonary function was averaged over the following 4 min period.
BAL analysis for differential cell count and cytokine, chemokine and immunoglobulin levels
Lungs were lavaged with sterile PBS as described previously (32, 34). Briefly, 0.75ml sterile PBS containing protease inhibitors was injected and withdrawn twice through a tracheal canula by a 1ml syringe. This procedure was repeated an additional two times with 1ml PBS each time. The withdrawn lavage volume (approximately 2.5ml) was collected and centrifuged at 400g for 10 min at 4°C. The supernatant was aliquoted and frozen at −80°C until analysis. Total cell number in the BAL was determined by using a Z2 particle counter (Beckman Coulter, Fullerton, CA). Differential cell counts were performed as described previously(34).
Splenocyte survival assay
Glucocorticoid (GC) induced cell death was quantified as described previously (26, 30, 35). A single cell splenocyte suspension was prepared, and triplicate samples were cultured (2.5 × 105 cells per well) in flat-bottom 96-well tissue culture plates. Cells were stimulated with 1 µg/ml LPS and corticosterone (dose range 0.005 – 0.05 µM) for 48 hr at 37°C and 5% CO2. Cell viability was measured with a tetrazolium substrate solution (Cell Titer 96 non-radioactive proliferation kit, Promega), and read at 490 nm by an ELISA plate reader. Cell viability was expressed as % optical density of the no corticosterone control. The cell free supernatant was collected at the end of the 48h culture period and was kept at −80°C pending analysis.
Cytokine, chemokine and immunoglobuline levels
The cell free supernatant from the BAL and splenocyte cultures were investigated without concentration or dilution using SearchLight mouse multiplex arrays. These arrays were performed by the manufacturer (Pierce Biotechnology, Rockford, IL).
Glucocorticoid receptor -DNA binding activity
Splenocytes were cultured in the presence of 1×10−7 M dexamethasone to determine GR binding activity in nuclear extracts. Nuclear extraction was performed as described previously (36, 37). Glucocorticoid receptor (GR) binding activity was assessed by adding 10 µg of nuclear extract to TransAM GR kits according to the manufacturer’s instructions (Active Motif, Carlsbad, CA). The optical densities of the plates were measured at 450 nm and expressed as a percent change from untreated cells.
Glucocorticoid receptor alpha (GRα) Western blot analysis
50 ug of lung or 25 ug of spleen lysates, were applied to Minigel and run and transferred in semi-dry systems under standard conditions. Anti-GRα (Affinity BioReagents at 0.1 ug/ml) or anti-GAPDH (Sigma at 1:50,000) antibody was added for 1 h at room temperature. Optimal sample concentration and antibody dilutions as well as incubation times were determined by pilot experiments. Secondary antibody (HRP-conjugated goat anti-rabbit antibody, Chemicon, diluted at 1: 100) was added for 1 h at room temperature. For GAPDH Chemicon rabbit anti-mouse HRP was used at 1:10,000. We used the ECL plus Western detection system (Amersham) and Gel-Pro Analyzer 6.0. GR bands were normalized to GAPDH.
Real-time PCR
A relative quantitation of lung glucocorticoid receptor (Nuclear receptor 3c1) mRNA expression was performed by real time PCR using TaqMan(R) Gene Expression Assay (Mm00433832) and TaqMan(R) Universal PCR Master Mix (Applied Biosystems) according to the manufacturer’s instructions. In brief, total RNA (4 samples per time point) was reverse transcribed using Superscript(TM) III First Strand Synthesis System (Invitrogen). Approximately 100 ng of cDNA was used per singleplex PCR reaction in a total volume of 20 µl. Cycling was performed on an ABI SDS-7000 and ABI SDS-7500 respectively with an initial denaturation step at 95°C for 10 min and 40 cycles of 15 sec. at 95°C and 1 min. at 60°C. Every sample was run in triplicate and β-2-microglobin (Mm00437762) was used as endogenous control. The Comparative Ct method was used to analyze the results using ABI PRISM(R) 7900 SDS software. Results are expressed as fold difference (with range incorporating the standard deviation of the ddCt value into the fold difference calculation) relative to the naïve controls.
Results
Social stress inhibited resolution of allergen-induced airway inflammation
Previously we showed that SDR induced significant alterations in the function of splenic leukocytes (26, 30, 31, 38). To investigate whether immune alterations also occur in the lung in response to repeated stress, we combined mouse models of allergen-induced AHR and SDR as illustrated in Figure 1A. The cellular and cytokine profiles in the BAL of mice exposed to Af and SDR were assessed. The behavioral effects of social stress exposure were confirmed using open field testing. SDR markedly reduced the amount of time the mice spent in the center of the open field (an indication of increased anxiety-like behavior) (31), while allergen exposure alone had no effect (data not shown). Af challenge of sensitized mice induced influx of inflammatory cells into the airways that peaked 24 h and partially resolved 48 h after Af exposure. Clearance of eosinophils and lymphocytes from the airways was markedly impaired in mice that were exposed to stress prior to allergen challenge (Figure 1B). Significantly greater numbers of eosinophils and lymphocytes in the BAL fluid were observed in stress and allergen exposed mice in comparison to that obtained with allergen alone (p<0.05, Figure 1B).
We have previously performed extensive kinetic studies on airway function 1, 6, 12, 24, 48, 72 and 96 h after a single Af challenge and found that both baseline Penh and methacholine responsiveness reached a peak 24 h after Af inhalation that was subsequently resolved (32). Although it is possible that stress affects AHR at earlier time points, detailed kinetic studies on the effects of stress on airway physiology exceeded the scope of the current paper. The main goal here was to investigate whether the prolonged airway eosinophilia we observed 48 h after Af was associated with increased AHR. Although Penh in the Af+SDR group at low doses (i.e. 3.1 mg/ml) was lower than in the Af group, there was a significantly enhanced AHR to inhaled methacholine at 12.5 and 25 mg/ml as assessed by whole body plethysmography in the Af+SDR group (p<0.05, Figure 1C).
Despite increased serum corticosterone levels combination of SDR and allergen challenge enhanced airway inflammation 48 hours after allergen challenge
Differential cell counts 48 h after Af challenge showed that stress exposure of Af sensitized and challenged mice significantly enhanced the numbers of eosinophils and lymphocytes but not neutrophils and macrophages in the BAL fluid (Figure 2A). Social stress by itself had no effect on leukocyte trafficking into the BAL fluid since the total and differential cell counts were similar between naïve controls and the SDR mice. The increased eosinophilia paralleled enhanced levels of the T-cell derived eosinopoietic IL-5 and GM-CSF (Figure 2B).
SDR and allergen markedly amplified IgG1 and the chemokine, TARC (Thymus Activated and Regulatory Chemokine) levels in the BAL fluid as compared with that of either exposure alone. It is noteworthy that SDR by itself significantly increased IgG1 and TARC in the BAL without allergen exposure suggesting that stress can skew the immune milieu in the lung through innate mechanisms. For this reason TNF-α and IL-6 that are typically produced by alveolar macrophages and activated dendritic cells were also studied. Indeed both cytokines were consistently elevated in the BAL of mice that received a combination of Af and social stress (Figure 2C) indicating involvement of the innate immune system. Further, SDR by itself elicited proinflammatory changes not only in the lung but also in the spleen of mice. We measured an increased splenic mass and heightened translocation of NF-κB (p65) in the nuclear extract from splenic mononuclear cells together with an increased ability to produce TARC and IgG1 upon LPS stimulation (data not shown).
Proinflammatory mediator release in the SDR mice occurred in the presence of an approximately 30-fold increase of the circulating corticosterone levels (Figure 2D, grey/white hatched bar). While there was less corticosterone in the Af+SDR group (grey/black hatched bar; p<0.05 v.s. SDR), it was still considerably (~15 fold) greater than in the non-stressed mice. Mice in the Af alone group (grey bar) did not have elevated corticosterone indicating that handling and manipulation associated with sensitization and challenge or with the euthanasia did not affect corticosterone release.
Our results raised the possibility that social stress reduces the inhibitory effects of corticosteroids on innate immune function in allergen challenged mice. To address this hypothesis, corticosteroid responsiveness of the innate immune cells was investigated in LPS-stimulated splenocytes.
Stress and allergen exposure reduced the inhibitory effects of corticosterone
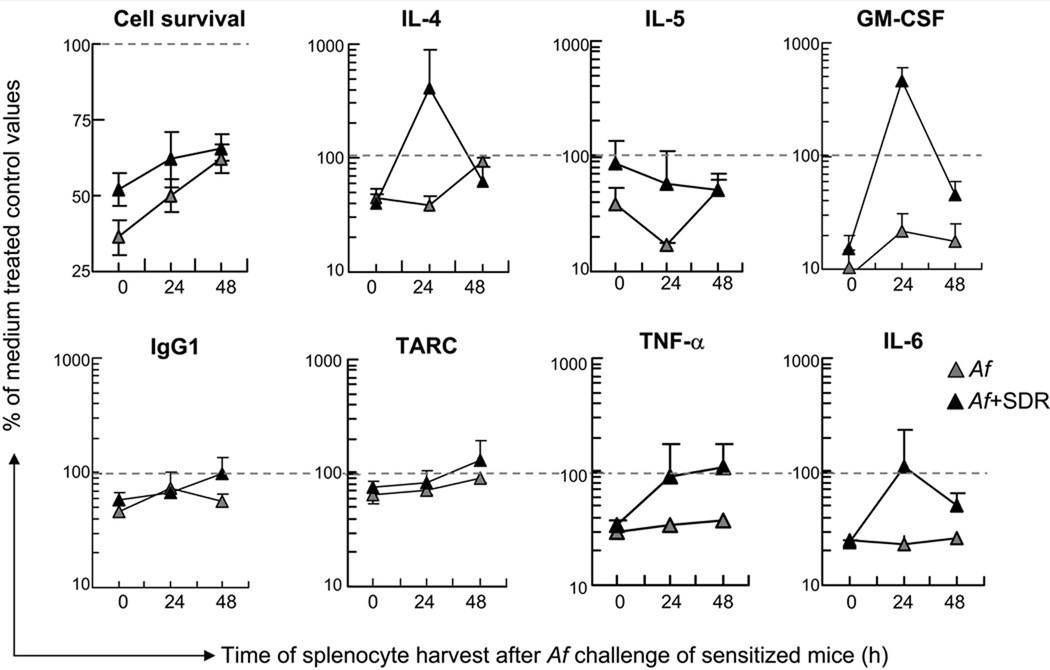
In vitro treatment with corticosterone (5 µM) significantly inhibited survival of cells as well as release of cytokines, chemokines and IgG1 in splenocytes derived from mice before allergen challenge (0 h) as shown in Figure 3 (grey triangles). After Af challenge however, particularly in mice exposed to both stress and Af (black triangles), there was a time-related reversal of the inhibitory effects.
Figure 3.
Combination of stress and allergen challenge attenuated the time-dependent inhibitory effect of corticosterone on splenocytes. Spleens were harvested before (0h), 24 and 48h after Af challenge. Splenocytes were cultured in the presence of LPS (1 µg/ml) and 5 µM of corticosterone for 48 hours. Cell viability (top left panel) was determined using a colorimetric assay. Cytokine, chemokine and immunoglobulin levels in the supernatant were measured by SearchLight technology. Data are presented as the percentage of cell viability or cytokine, chemokine and immunoglobulin concentration of control values measured in the absence of corticosterone, marked by dashed lines (Mean±SEM of n=5–8 per group).
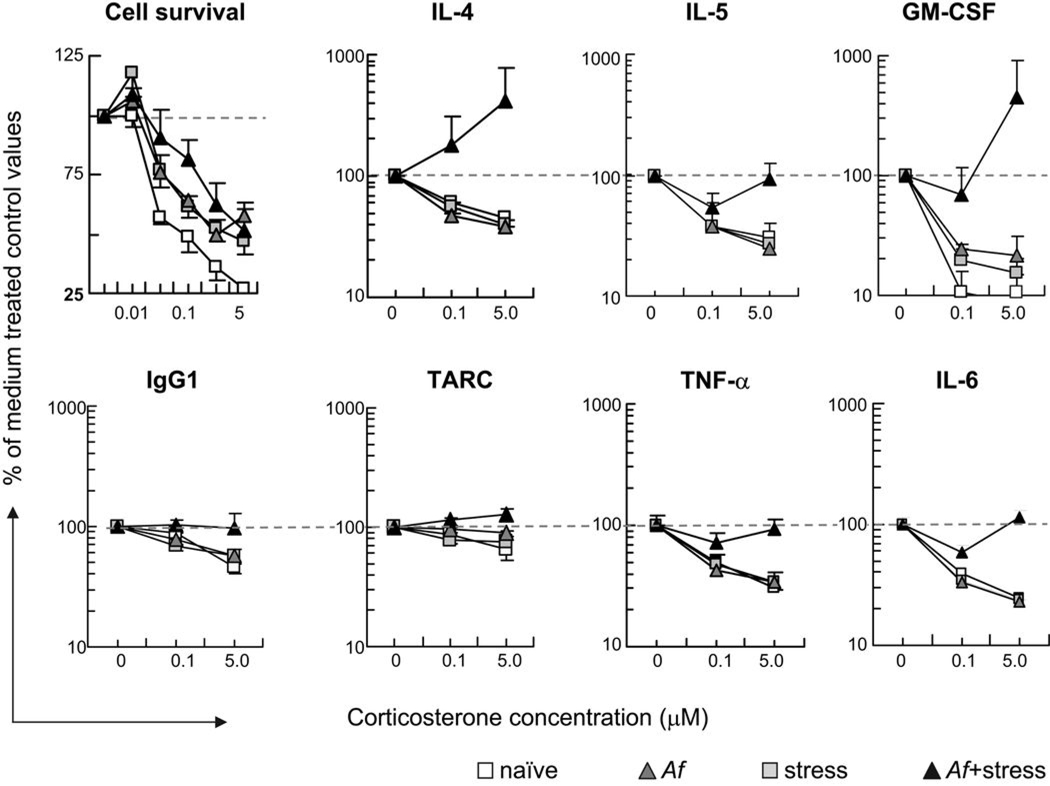
Splenocyte function, including the release of cytokines should normally be significantly inhibited by corticosteroids. Such dose dependent inhibition is clearly shown in Figure 4 in the cells derived from “Af only”-treated mice (grey triangles). However in splenocytes derived from the Af+SDR group the inhibitory effects of corticosterone were completely abolished and on IL-4, GM-CSF and TARC it appeared to be even reversed: instead of inhibition, corticosterone stimulated release of these cytokines in cells from the Af+SDR group. These results supported the hypothesis that corticosteroid responsiveness of immune cells (i.e. susceptibility to inhibition) in the spleen of mice that were subjected to Af+SDR is significantly impaired. The combination of stress and Af appeared synergistic in reversing the inhibitory effects of corticosterone.
Figure 4.
Combination of stress and allergen challenge abolished the dose-dependent inhibitory effects of corticosterone on LPS-stimulated splenocytes. Spleens were harvested from naïve (open squares), or SDR controls (grey squares) and from Af−sensitized mice 24 h after Af challenge (Af, grey triangles, Af+SDR, black triangles). Splenocytes were cultured in the presence of LPS (1 µg/ml) and and 0, 0.01, 0.05, 0.1, 0.5 or 5 µM of corticosterone for 48 hours. Cell viability (top left panel) was determined using a colorimetric assay. Cytokine, chemokine and immunoglobulin levels in the supernatant were measured by SearchLight technology in each groups. Data are presented as the percentage of cell viability or cytokine, chemokine and immunoglobulin concentration of control values measured in the absence of corticosterone, marked by dashed lines (Mean±SEM of n=5–8 per group).
Stress and allergen exposure inhibits the function and expression of the glucocorticoid receptor (GR)
To determine whether the stress-reduced glucocorticoid inhibition was due to an impaired function of the GR, binding to glucocorticoid response elements (GRE) in nuclear fractions from cells of naïve, SDR, Af, and Af+SDR mice was studied. Samples were stimulated with dexamethasone for 2h. The ratio of GRE-bound GR in the nuclear extract of cells was significantly less in the SDR−, Af− or Af+SDR-exposed groups upon dexamethasone stimulation in comparison with the naïve control group as shown in Figure 5A. However, there was no difference between the SDR and the Af+SDR groups. We therefore also studied GR protein expression in splenocytes of the same groups. Using an anti-mouse GRα monoclonal antibody, we found that GR expression in splenocytes of the Af+SDR mice was significantly lower than in the Af group (Figure 5B).
Figure 5.
To study the effects of stress and asthma on function and expression of the glucocorticoid receptor (GR), splenocytes and whole lung tissue was investigated. Naïve mice (white bar) and mice subjected to stress (SDR, hatched bars), allergen (Af, grey bars) or allergen and stress (Af+SDR, grey hatched bars) were studied. On day 15 (48 h after Af challenge) spleens for GR nuclear translocation and lungs for GR mRNA and protein expression were harvested. (A): SDR inhibited nuclear translocation and GRE binding of the GR. Splenocytes were isolated and incubated in the presence of dexamethasone (10-7M). GR nuclear translocation was quantitated by an ELISA based TransAM GR kit with GRE-coated wells. Mean±STDEV of n=3 (duplicate experiments). Densitometric data are corrected for values obtained from HeLa cell positive controls. (B, D): Combination of Af and SDR reduced expression of GR protein. Total protein was isolated from spleens (B) and lung tissue (D). Western blot analysis was performed using an anti mouse GRα monoclonal antibody. Expression was assessed using densitometric analysis and GAPDH as control. Mean±SEM of n=6 (duplicate experiment). (C): Combination of SDR and Af reduced expression of GR mRNA in the lung tissue. Total RNA was isolated from lungs of mice. Reverse transcription and realtime PCR were performed. β2 macroglobulin mRNA was used as control. Data were analyzed using the ΔΔ Ct Method. Mean±SEM of n=4–6 (duplicate experiments) (A–D): *p<0.05 Af vs. Af+SDR
To confirm the effects of Af+SDR on GR expression locally in the lung we studied GR gene activation in the whole lung tissue of mice. Real time PCR showed that Af+SDR significantly inhibited GR mRNA expression in the lung (Figure 5C). Furthermore Western blot analysis of lung extracts from the mice showed that sensitization and challenge with Af significantly increased GR expression compared with the non Af−exposed mice. However, this increase was markedly attenuated in the Af+SDR group (Figure 5D). These data indicated that the impaired resolution of the airway proinflammatory changes 48 h after allergen challenge in stress-exposed mice was associated with a reduction of GR protein expression in the lung.
Discussion
This study demonstrates that social stress prolonged the airway inflammatory response to allergen challenge in mice. The enhanced inflammatory changes included methacholine hyperresponsiveness at high concentrations, increased eosinophil and lymphocyte count, and elevated levels of cytokines, chemokines and immunoglobulins reflecting activation of both the innate and adaptive immune system. Importantly, heightened inflammation occurred in the presence of elevated serum corticosterone in mice exposed to social stress and allergen inhalation suggesting a diminished inhibitory action of endogenous corticosteroids. Indeed the combination of stress and allergen exposure profoundly altered the in vitro corticosteroid responsiveness of LPS-stimulated splenocytes and inhibited GR nuclear translocation. Furthermore, social stress significantly diminished GR expression in the spleen as well as in the lung of mice sensitized and challenged with Af. Based on these data we propose that repeated exposure to social stress skews the immune status towards a Th2 type in the airways and predisposes to a persistent and amplified inflammation after allergen challenge. These changes, in part, may be due to endogenous corticosteroid insensitivity, mediated by decreased function and expression of the GR.
Although stress may impair asthma control (1, 2, 5, 7, 8, 13–16) little evidence suggests that stress directly modulates immune function in the disease. Here we describe a unique murine model in which social stress and allergen exposure provides a platform to investigate the psycho-endocrine-immunological axis that regulates airway inflammation. Social disruption, a well characterized psychological stressor, has been used by our laboratory and others to assess the impact of a semi-natural stressor on immune function (35, 39–42). In this model a previously established social hierarchy of a stable cohort of younger male mice is disrupted by a large, aggressive male that confronts and repeatedly defeats the cage residents. This repeated defeat leads to physiological and behavioral responses (collectively referred to as the stress response), such as development of anxiety-like behaviors (31) and glucocorticoid release (22, 43). Endogenous glucocorticoid levels can regulate the development, distribution and function of immune and structural cells (10–12) but the effects of stress-induced glucocorticoid release on the pulmonary immune function have not been studied in detail.
Levels of the immunoglobulin IgG1 and TARC (CCL17), a chemokine produced by dendritic cells and macrophages and responsible for attracting Th2 lymphocytes and activated dendritic cells (44), were enhanced in mice exposed to SDR alone. Both IgG1 (45) and TARC (44) are under the regulation of IL-4/IL-13 and are part of the Th2 immune response. Since these cytokine/chemokine and immunoglobulin changes occurred without the presence of antigenic stimulation, we hypothesized that alterations in innate immune cell function played an important role. TNF-α and IL-6 are prominently involved in allergen-induced inflammation but are also relevant to LPS-stimulated innate immune response. Therefore these cytokines are highly suitable to asses the activation state of innate immune cells both in the lung (in vivo) and in the spleen (in vitro). Activation of dendritic cells and macrophages (46) was confirmed by consistently elevated TNF-α and IL-6 in mice that received Af+SDR.
Because TARC and IgG1 are characteristic players of a Th2-type immune response, we also raised the question whether animals exposed to repeated social stress would have an increased susceptibility to developing allergic airway inflammation (32–34, 47). Indeed combined exposure to allergen and stress significantly enhanced the numbers of eosinophils and lymphocytes in the airways. These cells interact during the allergic airway response through the T lymphocyte-derived IL-4, IL-5 and GM-CSF which are essential for promoting allergen-induced tissue eosinophilia. The genes for these cytokines are located in a cluster of chromosome 5q and are all sensitive to corticosteroid regulation. The enhanced airway eosinophilia 48 h after allergen challenge was paralleled by increased release of the eosinopoietic IL-5 and GM-CSF, as well as IgG1 and TARC in the airways of mice exposed to both stress and allergen challenge.
Although ours is the first study that investigated the effects of SDR, stress-induced enhancement of allergic airway inflammation has been noted in different models. In rats the authors showed an increased cellular influx into inflamed paws and airways in stressed animals (48). In a murine model of ultrasound stress and ovalbumin-induced allergic airway sensitization stressed mice displayed not only increased cell number but also heightened release of IL-4, IL-5 (49), TNFα (50) and eotaxin (51) in comparison with non-stressed mice. Using a different sound stress model on pregnant Balb/c mice, Pincus-Knackstedt et al. showed that maternal stress increased susceptibility of the offspring to allergic sensitization to ovalbumin (52).
The underlying mechanisms of the enhancement of allergic airway inflammation by SDR are unclear. One of the earliest findings in various models of stress has been an acute atrophy of the immune organs and an increased susceptibility to viral or bacterial infections due to the high circulating levels of corticosteroids and a suppressed immune system (53, 54). Stress in the SDR model however was not immunosuppressive. We found increased spleen mass and enhanced NF-κB p65 expression in the nuclear extract of the SDR mice (data not shown), suggesting activation (not suppression) of the innate immune cells and Th2-type immune mediators. How SDR may differ from other models of stress needs further elucidation but there are indications that both the duration and type of stressors applied can alter the effects of corticosteroids on the immune system. For instance, Okuyama and colleagues found that in contrast to acute stress, chronic restraint stress significantly increased the number of eosinophils and lymphocytes as well as the amount of IL-4 and IL-5 released in Balb/c mice in response to allergen challenge indicating that acute and chronic stress may affect the allergic airway response differentially (55). Opposing effects of short- and long-term stress on airway inflammation was also suggested in models of restraint and the forced swim test. Forsythe and colleagues found that the inhibitory effects of short-term stress on airway inflammation were prevented by the glucocorticoid receptor antagonist, RU486, suggesting that acute release of endogenous corticosteroids may attenuate the inflammatory airway response (56). Corticotropin releasing hormone knockout mice in a model of allergic airway sensitization responded with a significantly enhanced cellular inflammation to allergic sensitization compared with wild-type mice (57). Similarly, inhibition of the glucocorticoid receptor with RU-486 enhanced stress-induced eosinophilia in the bone-marrow of allergen sensitized animals in another study. Further, inhibition of glucocorticoid release by metirapon also increased eosinophil numbers suggesting that acute stress-induced endogenous corticosteroids maybe important in attenuating immunological changes (58).
Interestingly, using a psychological stressor, Chida and colleagues more recently showed that pretreatment of mice with RU486 before allergen challenge, completely inhibited stress-induced exacerbation of airway inflammation suggesting that increased levels of circulating corticosterone may be responsible for downregulating glucocorticoid responsiveness in inflammatory cells including eosinophil granulocytes (59). Steroid responsiveness is highly important during the resolution phase of the inflammatory response. For example, eosinophils were shown to have defective caspase-induced apoptosis in corticosteroid resistant asthmatic individuals (60). Thus, low baseline levels of endogenous corticosterone maybe sufficient to facilitate eosinophil clearance while high levels seen in stressed mice may cause a steroid unresponsiveness in these cells. Comparison of our SDR stressor with the non-psychological repeated restraint stress paradigm in our previous studies (using similar duration) also suggested differential effects on the immune system (61, 62). While the HPA axis and the sympathetic nervous systems were similarly activated by the two stressors, repeated restraint resulted in a disruption of the circadian rhythm of the HPA axis and a prolonged elevation of circulating costicosterone with splenic hypotrophy, suppressed mononuclear cell function and cytokine production (61). These responses were restored by blocking the glucocorticoid action with RU486 (63). In comparison, while repeated cycles of SDR elevated circulating corticosterone levels it did not disrupt the circadian rhythm of the HPA axis, caused splenic hypotrophy or suppressed mononuclear cell activation (38). In addition, development of glucocorticoid resistance to inhibition of cytokine production by splenocytes (23) was seen in SDR but not restraint stress (20). Taken together, exposure to sound (49–52) or psychological stressors (59) is more likely to predispose to an allergic immune response than restraint stress. Further, while the acute stress response is immunosuppressive, after a prolonged or repeated stress exposure glucocorticoid unresponsiveness may develop. Our results corroborate these findings and the in vitro splenocyte studies suggest the novel concept that stress and allergen are synergistic in reversing the immunosuppressive action of corticosteroids on pro-inflammatory cytokine production by immune cells.
Although the mechanisms of such synergistic effects between stress and the allergic immune response need further elucidation, it was previously shown that stress-induced disruption of the glucocorticoid action was associated with a failure of the GR to translocate to the nucleus (23, 28). Here we quantified GR binding to glucocorticoid response elements in the nuclear fractions of splenocytes stimulated with dexamethasone (a steroid that binds both the glucocorticoid and mineralocorticoid receptor with high affinity). Stress by itself significantly inhibited GR binding to glucocorticoid response elements confirming our previous studies (23, 28) but additional Af exposure of the stressed mice did not enhance this effect. We therefore could not explain the synergistic reversal of corticosteroid action we saw on the LPS-stimulated cells from the Af+SDR group. Since constitutive GR expression is essential for an adequate glucocorticoid action we also investigated GR expression both in the spleen and the lung of mice. GR mRNA and protein expression was significantly inhibited in the Af+SDR group in comparison with the Af sensitized control mice. Although there are a number of pathways through which the corticosteroid effects can be changed, we speculate that an impaired nuclear translocation and DNA binding together with reduced GR mRNA and protein expression could be responsible for the observed alterations in corticosteroid responsiveness.
Our results raise a number of intriguing questions. For instance, decreases in GR mRNA could be due to reduced mRNA stability, suppression of promoter activation or both (64). Further, GR expression could be reduced by homologous ligand down-regulation (by GR agonists) in a number of tissues and cell types but interestingly, not in T lymphocytes (64). Although we showed that innate immune cells are important, the cell types that are ultimately responsible for mediating the effects of corticosteroid insensitivity are yet to be identified. Expression of the GR falls under complex transcriptional and post translational regulation that can be significantly modified by inflammatory processes during the allergic airway response (65). Indeed reduced GR expression was found in asthmatic and COPD patients with insensitivity to corticosteroid treatment (66) and is thought to be an important factor in glucocorticoid resistance. Finally, the expression and function of the human GR is somewhat different from that of the mouse. Specifically, the human GRα isoform can mutually transrepress NF-kB, a complex mechanism that could be important in mediating steroid resistance in the inflamed tissue (64, 66). Whether such interaction could be reproduced in mice is currently unclear.
Although previous reports indicated the importance of stress in induction of AHR and airway inflammation in mice (49, 50, 52, 55–59, 67), our study is the first demonstration that social stress enhanced the allergic airway response in association with innate immune cell corticosteroid insensitivity and impaired GR translocation and expression. These findings are significant as they may elucidate potential mechanisms of asthma exacerbation and diminished effectiveness of corticosteroid therapies.
Acknowledgments
This study was supported by: R01AI055593 (AH), ALA/CIA (AH), P30ES013508 (AH and RAP), R01MH46801-15 (JFS) and the Mind, Body, Health Initiative (RAP and JFS)
Contributor Information
Michael Bailey, Department of Oral Biology and Institute for Behavioral Medicine Research, Ohio State University, Columbus, OH.
Sonja Kierstein, Department of Medicine, University of Pennsylvania, Philadelphia, PA.
Satish Sharma, Department of Medicine, University of Pennsylvania, Philadelphia, PA.
Matthew Spaits, Department of Medicine, University of Pennsylvania, Philadelphia, PA.
Steven G. Kinsey, Department of Oral Biology and Institute for Behavioral Medicine Research, Ohio State University, Columbus, OH
Omar Tliba, Department of Medicine, University of Pennsylvania, Philadelphia, PA.
Yassine Amrani, Department of Medicine, University of Pennsylvania, Philadelphia, PA.
John F. Sheridan, Department of Oral Biology and Institute for Behavioral Medicine Research, Ohio State University, Columbus, OH
Reynold A. Panettieri, Department of Medicine, University of Pennsylvania, Philadelphia, PA
Angela Haczku, Department of Medicine, University of Pennsylvania, Philadelphia, PA.
References
- 1.Isenberg SA, Lehrer PM, Hochron S. The effects of suggestion and emotional arousal on pulmonary function in asthma: a review and a hypothesis regarding vagal mediation. Psychosom. Med. 1992;54:192–216. doi: 10.1097/00006842-199203000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Lehrer PM, Isenberg S, Hochron SM. Asthma and emotion: a review. J. Asthma. 1993;30:5–21. doi: 10.3109/02770909309066375. [DOI] [PubMed] [Google Scholar]
- 3.Beggs PJ, Curson PH. An integrated environmental asthma model. Arch. Environ. Health. 1995;50:87–94. doi: 10.1080/00039896.1995.9940884. [DOI] [PubMed] [Google Scholar]
- 4.Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinol. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- 5.Marshall GD, Jr, Agarwal SK. Stress, immune regulation, and immunity: applications for asthma. Allergy Asthma Proc. 2000;21:241–246. doi: 10.2500/108854100778248917. [DOI] [PubMed] [Google Scholar]
- 6.von Hertzen CL. Maternal stress and T-cell differentiation of the developing immune system: possible implications for the development of asthma and atopy. J. Allergy Clin. Immunol. 2002;109:923–928. doi: 10.1067/mai.2002.124776. [DOI] [PubMed] [Google Scholar]
- 7.Miller BD, Wood BL. Emotions and family factors in childhood asthma: psychobiologic mechanisms and pathways of effect. Adv. Psychosom. Med. 2003;24:131–160. doi: 10.1159/000073785. [DOI] [PubMed] [Google Scholar]
- 8.Marshall GD. Neuroendocrine mechanisms of immune dysregulation: applications to allergy and asthma. Ann. Allergy Asthma Immunol. 2004;93:S11–S17. doi: 10.1016/s1081-1206(10)61482-2. [DOI] [PubMed] [Google Scholar]
- 9.Vig RS, Forsythe P, Vliagoftis H. The role of stress in asthma: insight from studies on the effect of acute and chronic stressors in models of airway inflammation. Ann. N. Y. Acad. Sci. 2006;1088:65–77. doi: 10.1196/annals.1366.023. [DOI] [PubMed] [Google Scholar]
- 10.Ashwell JD, King LB, Vacchio MS. Cross-talk between the T cell antigen receptor and the glucocorticoid receptor regulates thymocyte development. Stem Cells. 1996;14:490–500. doi: 10.1002/stem.140490. [DOI] [PubMed] [Google Scholar]
- 11.McEwen BS, Biron CA, Brunson KW, Bulloch K, Chambers WH, Dhabhar FS, Goldfarb RH, Kitson RP, Miller AH, Spencer RL, Weiss JM. The role of adrenocorticoids as modulators of immune function in health and disease: neural, endocrine and immune interactions. Brain Res. Brain Res. Rev. 1997;23:79–133. doi: 10.1016/s0165-0173(96)00012-4. [DOI] [PubMed] [Google Scholar]
- 12.Wilckens T, De Rijk R. Glucocorticoids and immune function: unknown dimensions and new frontiers. Immunol. Today. 1997;18:418–424. doi: 10.1016/s0167-5699(97)01111-0. [DOI] [PubMed] [Google Scholar]
- 13.Rosenkranz MA, Busse WW, Johnstone T, Swenson CA, Crisafi GM, Jackson MM, Bosch JA, Sheridan JF, Davidson RJ. From The Cover: Neural circuitry underlying the interaction between emotion and asthma symptom exacerbation. Proc. Natl. Acad. Sci. U. S. A. 2005;102:13319–13324. doi: 10.1073/pnas.0504365102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agarwal SK, Marshall GD., Jr Stress effects on immunity and its application to clinical immunology. Clin. Exp. Allergy. 2001;31:25–31. [PubMed] [Google Scholar]
- 15.Badoux A, Levy DA. Psychologic symptoms in asthma and chronic urticaria. Ann. Allergy. 1994;72:229–234. [PubMed] [Google Scholar]
- 16.Miller BD. Depression and asthma: a potentially lethal mixture. J. Allergy Clin. Immunol. 1987;80:481–486. doi: 10.1016/0091-6749(87)90080-7. [DOI] [PubMed] [Google Scholar]
- 17.Kolbe J, Garrett J, Vamos M, Rea HH. Influences on trends in asthma morbidity and mortality: the New Zealand experience. Chest. 1994;106:211S–215S. doi: 10.1378/chest.106.4_supplement.211s. [DOI] [PubMed] [Google Scholar]
- 18.Miller BD, Wood BL. Psychophysiologic reactivity in asthmatic children: a cholinergically mediated confluence of pathways. J. Am. Acad. Child Adolesc. Psychiatry. 1994;33:1236–1245. doi: 10.1097/00004583-199411000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Liu LY, Coe CL, Swenson CA, Kelly EA, Kita H, Busse WW. School examinations enhance airway inflammation to antigen challenge. Am. J. Respir. Crit. Care Med. 2002;165:1062–1067. doi: 10.1164/ajrccm.165.8.2109065. [DOI] [PubMed] [Google Scholar]
- 20.Avitsur R, Kavelaars A, Heijnen C, Sheridan JF. Social stress and the regulation of tumor necrosis factor-alpha secretion. Brain Behav. Immun. 2005;19:311–317. doi: 10.1016/j.bbi.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Bantel H, Domschke W, Schulze-Osthoff K, Kaskas B, Gregor M. Abnormal activation of transcription factor NF-kappaB involved in steroid resistance in chronic inflammatoy bowel disease. Am. J. Gastroenterol. 2000;95:1845–1846. doi: 10.1111/j.1572-0241.2000.02143.x. [DOI] [PubMed] [Google Scholar]
- 22.Sheridan JF, Stark JL, Avitsur R, Padgett DA. Social disruption, immunity, and susceptibility to viral infection. Role of glucocorticoid insensitivity and NGF. Ann. N. Y. Acad. Sci. 2000;917:894–905. doi: 10.1111/j.1749-6632.2000.tb05455.x. [DOI] [PubMed] [Google Scholar]
- 23.Quan N, Avitsur R, Stark JL, He L, Lai W, Dhabhar F, Sheridan JF. Molecular mechanisms of glucocorticoid resistance in splenocytes of socially stressed male mice. J. Neuroimmunol. 2003;137:51–58. doi: 10.1016/s0165-5728(03)00042-0. [DOI] [PubMed] [Google Scholar]
- 24.O'Connor KA, Johnson JD, Hammack SE, Brooks LM, Spencer RL, Watkins LR, Maier SF. Inescapable shock induces resistance to the effects of dexamethasone. Psychoneuroendocrinol. 2003;28:481–500. doi: 10.1016/s0306-4530(02)00035-5. [DOI] [PubMed] [Google Scholar]
- 25.Johnson RR, Storts R, Welsh TH, Jr, Welsh CJ, Meagher MW. Social stress alters the severity of acute Theiler's virus infection. J. Neuroimmunol. 2004;148:74–85. doi: 10.1016/j.jneuroim.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Engler H, Engler A, Bailey MT, Sheridan JF. Tissue-specific alterations in the glucocorticoid sensitivity of immune cells following repeated social defeat in mice. J. Neuroimmunol. 2005;163:110–119. doi: 10.1016/j.jneuroim.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Avitsur R, Stark JL, Sheridan JF. Social stress induces glucocorticoid resistance in subordinate animals. Horm. Behav. 2001;39:247–257. doi: 10.1006/hbeh.2001.1653. [DOI] [PubMed] [Google Scholar]
- 28.Quan N, Avitsur R, Stark JL, He L, Shah M, Caligiuri M, Padgett DA, Marucha PT, Sheridan JF. Social stress increases the susceptibility to endotoxic shock. J. Neuroimmunol. 2001;115:36–45. doi: 10.1016/s0165-5728(01)00273-9. [DOI] [PubMed] [Google Scholar]
- 29.Stark JL, Avitsur R, Padgett DA, Campbell KA, Beck FM, Sheridan JF. Social stress induces glucocorticoid resistance in macrophages. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280:R1799–R1805. doi: 10.1152/ajpregu.2001.280.6.R1799. [DOI] [PubMed] [Google Scholar]
- 30.Bailey MT, Avitsur R, Engler H, Padgett DA, Sheridan JF. Physical defeat reduces the sensitivity of murine splenocytes to the suppressive effects of corticosterone. Brain Behav. Immun. 2004;18:416–424. doi: 10.1016/j.bbi.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Kinsey SG, Bailey MT, Sheridan JF, Padgett DA, Avitsur R. Repeated social defeat causes increased anxiety-like behavior and alters splenocyte function in C57BL/6 and CD-1 mice. Brain Behav. Immun. 2007;21:458–466. doi: 10.1016/j.bbi.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haczku A, Atochina EN, Tomer Y, Cao Y, Campbell C, Scanlon ST, Russo SJ, Enhorning G, Beers MF. The late asthmatic response is linked with increased surface tension and reduced surfactant protein B in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2002;283:L755–L765. doi: 10.1152/ajplung.00062.2002. [DOI] [PubMed] [Google Scholar]
- 33.Haczku A, Atochina EN, Tomer Y, Chen H, Scanlon ST, Russo S, Xu J, Panettieri RA, Jr, Beers MF. Aspergillus fumigatus-induced allergic airway inflammation alters surfactant homeostasis and lung function in BALB/c mice. Am. J. Respir. Cell Mol. Biol. 2001;25:45–50. doi: 10.1165/ajrcmb.25.1.4391. [DOI] [PubMed] [Google Scholar]
- 34.Haczku A, Cao Y, Vass G, Kierstein S, Atochina EN, Scanlon ST, Nath P, Li L, Griswold DE, Chung KF, Poulain FR, Hawgood S, Beers MF, Crouch EC. IL-4 and IL-13 form a negative feedback circuit with SP-D in the allergic airway response. J. Immunol. 2006;176:3557–3565. doi: 10.4049/jimmunol.176.6.3557. [DOI] [PubMed] [Google Scholar]
- 35.Avitsur R, Padgett DA, Dhabhar FS, Stark JL, Kramer KA, Engler H, Sheridan JF. Expression of glucocorticoid resistance following social stress requires a second signal. J. Leukoc. Biol. 2003;74:507–513. doi: 10.1189/jlb.0303090. [DOI] [PubMed] [Google Scholar]
- 36.Tliba O, Cidlowski JA, Amrani Y. CD38 expression is insensitive to steroid action in cells treated with tumor necrosis factor-alpha and interferon-gamma by a mechanism involving the up-regulation of the glucocorticoid receptor beta isoform. Mol. Pharmacol. 2006;69:588–596. doi: 10.1124/mol.105.019679. [DOI] [PubMed] [Google Scholar]
- 37.Tliba O, Tliba S, Da Huang C, Hoffman RK, DeLong P, Panettieri RA, Jr, Amrani Y. Tumor necrosis factor alpha modulates airway smooth muscle function via the autocrine action of interferon beta. J. Biol. Chem. 2003;278:50615–50623. doi: 10.1074/jbc.M303680200. [DOI] [PubMed] [Google Scholar]
- 38.Engler H, Bailey MT, Engler A, Sheridan JF. Effects of repeated social stress on leukocyte distribution in bone marrow, peripheral blood and spleen. J. Neuroimmunol. 2004;148:106–115. doi: 10.1016/j.jneuroim.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 39.Avitsur R, Stark JL, Dhabhar FS, Padgett DA, Sheridan JF. Social disruption-induced glucocorticoid resistance: kinetics and site specificity. J. Neuroimmunol. 2002;124:54–61. doi: 10.1016/s0165-5728(02)00010-3. [DOI] [PubMed] [Google Scholar]
- 40.Avitsur R, Stark JL, Dhabhar FS, Sheridan JF. Social stress alters splenocyte phenotype and function. J. Neuroimmunol. 2002;132:66–71. doi: 10.1016/s0165-5728(02)00310-7. [DOI] [PubMed] [Google Scholar]
- 41.Eells JB, Misler JA, Nikodem VM. Early postnatal isolation reduces dopamine levels, elevates dopamine turnover and specifically disrupts prepulse inhibition in Nurr1-null heterozygous mice. Neuroscience. 2006;140:1117–1126. doi: 10.1016/j.neuroscience.2005.12.065. [DOI] [PubMed] [Google Scholar]
- 42.Sonoda J, Chida Y, Sudo N, Kubo C. Social disruption stress exacerbates alpha-galactosylceramide-induced hepatitis in mice. Neuroimmunomodul. 2005;12:375–379. doi: 10.1159/000091131. [DOI] [PubMed] [Google Scholar]
- 43.Sheridan JF, Padgett DA, Avitsur R, Marucha PT. Experimental models of stress and wound healing. World J. Surg. 2004;28:327–330. doi: 10.1007/s00268-003-7404-y. [DOI] [PubMed] [Google Scholar]
- 44.Berin MC, Eckmann L, Broide DH, Kagnoff MF. Regulated production of the T helper 2-type T-cell chemoattractant TARC by human bronchial epithelial cells in vitro and in human lung xenografts. Am. J. Respir. Cell Mol. Biol. 2001;24:382–389. doi: 10.1165/ajrcmb.24.4.4360. [DOI] [PubMed] [Google Scholar]
- 45.Stevens TL, Bossie A, Sanders VM, Fernandez-Botran R, Coffman RL, Mosmann TR, Vitetta ES. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988;334:255–258. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- 46.Penna G, Vulcano M, Sozzani S, Adorini L. Differential migration behavior and chemokine production by myeloid and plasmacytoid dendritic cells. Human immunol. 2002;63:1164–1171. doi: 10.1016/s0198-8859(02)00755-3. [DOI] [PubMed] [Google Scholar]
- 47.Atochina EN, Beers MF, Tomer Y, Scanlon ST, Russo SJ, Panettieri RA, Jr, Haczku A. Attenuated allergic airway hyperresponsiveness in C57BL/6 mice is associated with enhanced surfactant protein (SP)-D production following allergic sensitization. Respir. Res. 2003;4:15. doi: 10.1186/1465-9921-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Datti F, Datti M, Antunes E, Teixeira NA. Influence of chronic unpredictable stress on the allergic responses in rats. Physiol. Behav. 2002;77:79–83. doi: 10.1016/s0031-9384(02)00811-9. [DOI] [PubMed] [Google Scholar]
- 49.Joachim RA, Quarcoo D, Arck PC, Herz U, Renz H, Klapp BF. Stress enhances airway reactivity and airway inflammation in an animal model of allergic bronchial asthma. Psychosom. Med. 2003;65:811–815. doi: 10.1097/01.psy.0000088582.50468.a3. [DOI] [PubMed] [Google Scholar]
- 50.Joachim RA, Sagach V, Quarcoo D, Dinh T, Arck PC, Klapp BF. Upregulation of tumor necrosis factor-alpha by stress and substance p in a murine model of allergic airway inflammation. Neuroimmunomodul. 2006;13:43–50. doi: 10.1159/000094394. [DOI] [PubMed] [Google Scholar]
- 51.Joachim RA, Sagach V, Quarcoo D, Dinh QT, Arck PC, Klapp BF. Effect of stress on eotaxin and expression of adhesion molecules in a murine model of allergic airway inflammation. J. Neuroimmunol. 2007;182:55–62. doi: 10.1016/j.jneuroim.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 52.Pincus-Knackstedt MK, Joachim RA, Blois SM, Douglas AJ, Orsal AS, Klapp BF, Wahn U, Hamelmann E, Arck PC. Prenatal stress enhances susceptibility of murine adult offspring toward airway inflammation. J. Immunol. 2006;177:8484–8492. doi: 10.4049/jimmunol.177.12.8484. [DOI] [PubMed] [Google Scholar]
- 53.Selye H. A syndrome produced by diverse nocuous agents. Nature. 1936;138:32. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- 54.Selye H. A syndrome produced by diverse nocuous agents. 1936. J. Neuropsychiatry Clin. Neurosci. 1998;10:230–231. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- 55.Okuyama K, Ohwada K, Sakurada S, Sato N, Sora I, Tamura G, Takayanagi M, Ohno I. The distinctive effects of acute and chronic psychological stress on airway inflammation in a murine model of allergic asthma. Allergol. Int. 2007;56:29–35. doi: 10.2332/allergolint.O-06-435. [DOI] [PubMed] [Google Scholar]
- 56.Forsythe P, Ebeling C, Gordon JR, Befus AD, Vliagoftis H. Opposing effects of short- and long-term stress on airway inflammation. Am. J. Respir. Crit. Care Med. 2004;169:220–226. doi: 10.1164/rccm.200307-979OC. [DOI] [PubMed] [Google Scholar]
- 57.Silverman ES, Breault DT, Vallone J, Subramanian S, Yilmaz AD, Mathew S, Subramaniam V, Tantisira K, Pacak K, Weiss ST, Majzoub JA. Corticotropin-releasing hormone deficiency increases allergen-induced airway inflammation in a mouse model of asthma. J. Allergy Clin. Immunol. 2004;114:747–754. doi: 10.1016/j.jaci.2004.06.055. [DOI] [PubMed] [Google Scholar]
- 58.Georen S, Ahnblad P, Stjarne P, Wikstrom AC, Stierna P. Significance of endogenous glucocorticoid sensitivity for airway eosinophilia in a murine model of allergy. Acta Oto-laryng. 2005;125:378–385. doi: 10.1080/00016480410025261. [DOI] [PubMed] [Google Scholar]
- 59.Chida Y, Sudo N, Sonoda J, Hiramoto T, Kubo C. Early-life psychological stress exacerbates adult mouse asthma via the hypothalamus-pituitary-adrenal axis. Am. J. Respir. Crit. Care Med. 2007;175:316–322. doi: 10.1164/rccm.200607-898OC. [DOI] [PubMed] [Google Scholar]
- 60.Walsh GM, Sexton DW, Blaylock MG. Corticosteroids, eosinophils and bronchial epithelial cells: new insights into the resolution of inflammation in asthma. J. Endocrinol. 2003;178:37–43. doi: 10.1677/joe.0.1780037. [DOI] [PubMed] [Google Scholar]
- 61.Hermann G, Beck FM, Tovar CA, Malarkey WB, Allen C, Sheridan JF. Stress-induced changes attributable to the sympathetic nervous system during experimental influenza viral infection in DBA/2 inbred mouse strain. J. Neuroimmunol. 1994;53:173–180. doi: 10.1016/0165-5728(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 62.Dobbs CM, Feng N, Beck FM, Sheridan JF. Neuroendocrine regulation of cytokine production during experimental influenza viral infection: effects of restraint stress-induced elevation in endogenous corticosterone. J. Immunol. 1996;157:1870–1877. [PubMed] [Google Scholar]
- 63.Hermann G, Beck FM, Sheridan JF. Stress-induced glucocorticoid response modulates mononuclear cell trafficking during an experimental influenza viral infection. J. Neuroimmunol. 1995;56:179–186. doi: 10.1016/0165-5728(94)00145-e. [DOI] [PubMed] [Google Scholar]
- 64.Schaaf MJ, Cidlowski JA. Molecular mechanisms of glucocorticoid action and resistance. J. Steroid Biochem. Mol. Biol. 2002;83:37–48. doi: 10.1016/s0960-0760(02)00263-7. [DOI] [PubMed] [Google Scholar]
- 65.Pujols L, Mullol J, Picado C. Alpha and beta glucocorticoid receptors: relevance in airway diseases. Curr. Allergy Asthma Rep. 2007;7:93–99. doi: 10.1007/s11882-007-0005-3. [DOI] [PubMed] [Google Scholar]
- 66.Adcock IM, Barnes PJ. Molecular mechanisms of corticosteroid resistance. Chest. 2008;134:394–401. doi: 10.1378/chest.08-0440. [DOI] [PubMed] [Google Scholar]
- 67.Joachim RA, Sagach V, Quarcoo D, Dinh QT, Arck PC, Klapp BF. Neurokinin-1 receptor mediates stress-exacerbated allergic airway inflammation and airway hyperresponsiveness in mice. Psychosom. Med. 2004;66:564–571. doi: 10.1097/01.psy.0000132878.08780.93. [DOI] [PubMed] [Google Scholar]