The pathogenic relationship between neuromyelitis optica (NMO) and multiple sclerosis (MS) continues to be debated despite mounting evidence that these are distinct entities. 1, 2, 3 NMO-IgG, which targets the water channel protein aquaporin-4 (AQP4), is the first confirmed serum biomarker for any form of central nervous system inflammatory demyelinating disease and reliably distinguishes NMO from MS and other neurological diseases. Four immunopathological patterns (IP I-IV) have been described in early active MS lesions. 4 Some investigators have interpreted humoral MS IP II as having an immunopathogenic link with NMO because both share complement and immunoglobulin deposition and have a greater likelihood than other forms of MS to respond favorably to plasma exchange therapy.4,5 Here we report the NMO-IgG status of a cohort of patients with biopsy-proven early active lesions consistent with MS.
Subjects and Methods
All patients had biopsy-proven CNS inflammatory demyelination consistent with MS (n=85, 43 females), were classified immunohistochemically (IP I-IV), 4 were enrolled into the MS lesion project (NMSS RG 3185), and at last visit were bled to test serum for NMO-IgG (indirect immunofluorescence).
Results
Clinical characteristics of biopsy cohort
Enrolled patients had a spectrum of disease courses (relapsing remitting 57, monophasic 20, primary progressive 0, secondary progressive 7, and uncertain 1). Median age at biopsy was 38 (IQR 27, 46) years, expanded disability status scale at biopsy was 2.0 (IQR 1.0, 3.5) and disease duration prior to biopsy was 1.4 (IQR 0.6, 4.9) months. At last follow-up (median 3.6 years from disease onset), 85% of patients were defined as having definite MS (n=65) or probable MS (N=7). The remaining 13 patients (15%) were classified as a clinically isolated syndrome.
NMO-IgG status of biopsy MS cohort
The distribution of immunopathological patterns for the patients was as follows: IP I (n=15), IP II (n=53), IP III (n=17), IP IV (n=0). All were seronegative for NMO-IgG. Eighteen of 85 patients (22%) had received one or more immunosuppressant medications or plasma-exchange in the 3 month period prior to blood draw (intravenous methylprednisolone 6, prednisone [short term 6, long term 5], methotrexate 1, cyclophosphamide 1, mitoxantrone 2 and plasma exchange 2). A minority (45%) were receiving immunomodulatory medications (interferon beta or glatiramer acetate) in the 3 month period prior to blood draw.
Comment
This study reports lack of detectable NMO-IgG in pathologically confirmed MS patients including those with evidence of humoral MS pathology (IP II) supporting that MS and NMO are distinct clinicopathologic entities. The low percentage of patients receiving immunosuppressant therapy at the time of blood draw minimizes the potential for treatment to have been a determinant of seronegativity. Although immune complex deposition is a characteristic immunopathological finding for both NMO and MS IP II, the distribution of immune complexes is distinct3. In MS IP II lesions, complement is deposited at sites of active myelin destruction with increased AQP4 expression even in regions of immune-complex deposition (Figure, A–B). In active NMO lesions, complement is deposited in a unique rim and rosette-like pattern corresponding to sites of concentrated AQP4 expression on the astrocytic foot process and AQP4 expression is notably absent at sites of immune-complex deposition (Figure, C–D). In vitro, NMO-IgG binds selectively to target cell membranes expressing AQP4 and causes complement activation or rapid down-regulation of AQP4 expression6 implicating a complement-activating AQP4-specific autoantibody in the pathogenesis of NMO lesions. Thus, NMO-IgG is not an epiphenomenon of antibody-mediated pathology, but rather a specific biomarker that reliably distinguishes NMO-related disorders from MS and plausibly allows the classification of NMO as an autoimmune channelopathy selectively targeting the CNS.
Comparison of C9neo and AQP4 immunoreactivity in MS Pattern II and NMO Active Lesions.
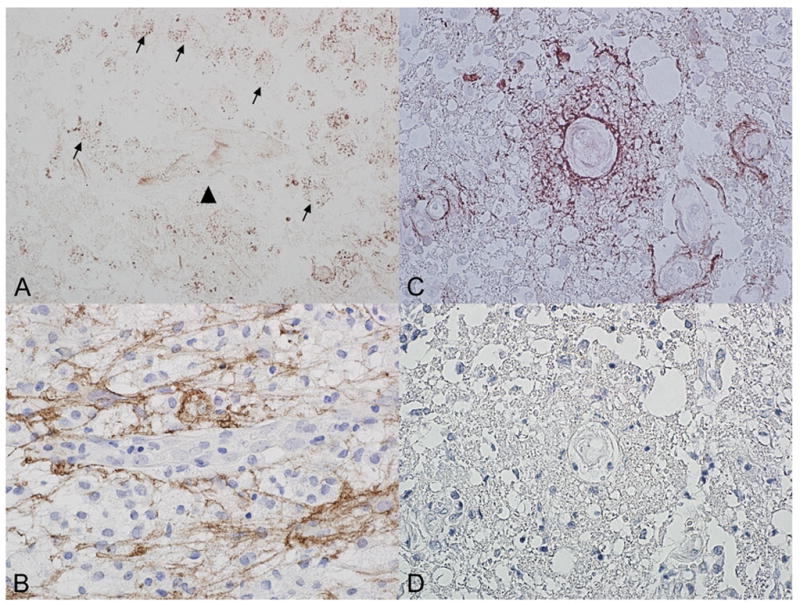
A/B: Pattern II Active MS Brain Lesion: Immunoreactivity for C9neo antigen is noted within myelin laden macrophages (arrow), but absent around blood vessels (arrowhead) (A). This corresponds to a region of increased AQP 4 immunoreactivity, with prominent staining of the cytoplasmic surface and processes of reactive astrocytes (B).
C/D: Active NMO Spinal Cord Lesion. C9neo antigen is deposited in a vasculocentric rim and rosette pattern similar to the normal distribution of AQP4 (C). C9neo reactivity is associated with a complete loss of AQP4 IR within this same region (D) (Immunocytochemistry for C9neu antigen (red) and AQP4 (brown).
Acknowledgments
Supported by grants from NIH RO1-NS049577-01-A2 (CFL), NMSS RG 3185-B-3 (CFL), the Ralph C. Wilson Medical Research Foundation (VAL), the Guthy Jackson Foundation (SJP, NK).
Footnotes
Disclosure statement: All co-authors have seen and agreed with the contents of the manuscript. In accordance with the Bayh Dole Act of 1980and Mayo Foundation policy, Drs. Lennon and Lucchinetti stand to receive royalties for intellectual property related to the AQP4 autoantigen. This intellectual property is licensed to a commercial entity for development of a simple antigen-specific assay to be made available worldwide for patient care. The test will not be exclusive to Mayo Clinic. To date, the authors have received a total of <$10,000 in royalties. Mayo Clinic offers the test as an indirect immunofluorescence assay to aid the diagnosis of NMO, but the authors do not benefit personally from the performance of the test In addition Drs. Pittock, Lennon and Lucchinetti are named inventors on a patent application filed by Mayo Foundation for Medical Education and Research that relates to NMO (aquaporin-4) antibody and its application to functional assays. The remaining authors have no conflicts.
References
- 1.Galetta SL, Bennett J. Neuromyelitis optica is a variant of multiple sclerosis. Arch Neurol. 2007;64(6):901–903. doi: 10.1001/archneur.64.6.901. [DOI] [PubMed] [Google Scholar]
- 2.Compston A. Complexity and heterogeneity in demyelinating disease. Brain. 2007;130:1178–1180. doi: 10.1093/brain/awm092. [DOI] [PubMed] [Google Scholar]
- 3.Roemer SF, Parisi JE, Lennon VA, et al. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain. 2007;130(Pt 5):1194–1205. doi: 10.1093/brain/awl371. [DOI] [PubMed] [Google Scholar]
- 4.Lucchinetti C, Brück W, Parisi J, et al. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 5.Keegan M, König F, McClelland R, et al. Relation between humoral pathological changes in multiple sclerosis and response to therapeutic plasma exchange. Lancet. 2005;366:579–582. doi: 10.1016/S0140-6736(05)67102-4. [DOI] [PubMed] [Google Scholar]
- 6.Hinson SR, Pittock SJ, Lucchinetti CF. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology. 2007;69(24):2221–2231. doi: 10.1212/01.WNL.0000289761.64862.ce. [DOI] [PubMed] [Google Scholar]


