SUMMARY
The dupA gene, encoding a putative tyrosine kinase/dual specificity phosphatase (Dusp), was identified in a screen for Dictyostelium discoideum mutants altered in supporting Legionella pneumophila intracellular replication. The absence of dupA resulted in hyperphosphorylation of ERK1, consistent with the loss of a phosphatase activity, as well as degradation of ERK2. ERK1 hyperphosphorylation mimicked the response of this protein after bacterial challenge of wild type amoebae. Similar to Dusps in higher eukaryotic cells, the amoebal dupA gene was induced after bacterial contact, indicating a response of Dusps that is conserved from amoebae to mammals. A large set of genes was misregulated in the dupA− mutant that largely overlaps with genes responding to L. pneumophila infection. Some of the amoebal genes appear to be involved in a response similar to innate immunity in higher eukaryotes, indicating there was misregulation of a conserved response to bacteria.
INTRODUCTION
Legionella pneumophila is a facultative intracellular bacterium that causes Legionnaires' disease, initiated after inhalation of aerosols from contaminated water supplies (Marston et al., 1994). The ability of L. pneumophila to cause disease is dependent on its ability to modulate the biogenesis of its replication vacuole and grow within host cells. After avoidance of the host cell endocytic pathway, the Legionella-containing vacuole (LCV) matures into an endoplasmic reticulum (ER)-like compartment (Swanson and Isberg, 1995). Formation of the LCV requires the function of the L. pneumophila Dot/Icm type IV secretion system, a 27 protein complex that translocates a large number of protein substrates into the host cell (Nagai et al., 2002). It is believed that amoebae, such as Hartmannella vermiformis and Acanthamoeba castellanii (Henke and Seidel, 1986), maintain the reservoir for bacteria that come in contact with the human population. Replication of the bacteria within amoebae appears to require a similar, although not identical, set of bacterial proteins as those involved in growth within macrophages (Gao et al., 1997; Segal and Shuman, 1999).
Several host proteins have been identified that contribute to L. pneumophila replication. These include factors involved in secretory traffic and ER dynamics, such as vesicle budding factors Sar1 and Arf1 (Kagan et al., 2004) as well as the fusion and tethering factor Rab1 (Kagan et al., 2004). In addition, lowered expression of the amino acid transporter slc1a5 interferes with L. pneumophila growth (Wieland et al., 2005). Most of the studies that have identified these factors have targeted a subset of proteins for analysis. Thus, the total repertoire of host proteins that modulate intracellular growth is unknown.
Dictyostelium discoideum has been used as a model to dissect the amoebal factors that modulate intracellular growth because there are extensive genetic tools available for this organism. D. discoideum is a free-living organism that has both a vegetative amoebal stage and a dormant stage. Using the amoebal stage of the organism, it has been shown that L. pneumophila trafficking to an ER-bound compartment appears identical to that in macrophages and other amoebal species (Fajardo et al., 2004; Hagele et al., 2000; Lu and Clarke, 2005; Solomon et al., 2000). Furthermore, the global transcriptional response of D. discoideum to L. pneumophila also bears some similarity to that observed in higher cells (Farbrother et al., 2006). Various classes of stress response genes are induced in response to the L. pneumophila, with upregulation of genes encoding proteins associated with ubiquitin-dependent degradation processes. A variety of D. discoideum mutants defective in cytoskeletal functions show increased yields of L. pneumophila relative to wild type amoebae (Hagele et al., 2000; Solomon et al., 2000; Weber et al., 2006). Similarly, there is enhanced replication in amoeba having disrupted Nramp1 (Peracino et al., 2006), consistent with this channel acting as an active antimicrobial protein. Analysis of D. discoideum interactions with another intracellular microorganism, Mycobacterium marinum, have uncovered host factors involved replication vacuole maintenance as well as a novel pathway for cell-to-cell spread of the microorganism (Hagedorn et al., 2009; Hagedorn and Soldati, 2007).
In the following study, we performed a non-targeted screen for D. discoideum mutants that have altered ability to support L. pneumophila growth. Among the mutants identified was an insertion in the gene for a putative tyrosine kinase/dual specificity phosphatase, which we call dupA.
RESULTS
Identification of D. discoideum mutants with altered susceptibility to L. pneumophila infection
The strategy for isolation of D. discoideum mutants altered in support of L. pneumophila growth is diagramed in Fig. 1. Individual Restriction Enzyme Mediated Insertion (REMI) strains were challenged with L. pneumophila-GFP at MOI=0.1 for 72 hours (Experimental Procedures). Wells containing the live cells were then visually screened using fluorescence microscopy and quantitive analysis of captured images (Fig. 1). Out of about 7000 original mutants screened, 10 reproducibly yielded Legionella-containing vacuoles (LCVs) that were altered relative to wild type AX4. These alterations were in the size and number of LCVs present as well as the density of bacteria within the LCV, based on quantitation of fluorescent foci of bacterial replication in grabbed images (Figs. 1, 2A). Ten mutants exhibiting a significant change in L. pneumophila growth were chosen for further investigation. Additional mutants with subtle enhancement of growth of L. pneumophila were observed, but due to their mild phenotype, they were not analyzed further.
Figure 1. D. discoideum mutants altered in supporting L. pneumophila intracellular replication.
REMI mutants were plated on optically clear 96 well plates and challenged with L. pneumophila-GFP at MOI = 0.1 (green; Scale Bar: 10μ). Image capture was performed after 72 hrs incubation. The mutations were then rescued, transformed into E. coli, and regenerated by retransforming into AX4 (Experimental Procedures). 1Site of insertion was determined by sequencing the joint between the REMI insertion and the chromosome. 2 Growth alteration was determined by performing 96 hour growth curves, plating for viable colonies.
Figure 2. D. discoideum REMI mutants altered in bacterial growth show a spectrum of phenotypes.
(A) Representative fluorescence micrographs (20X) of REMI mutants after 72 hr. incubation with L. pneumophila. AX4 is the parental wild type strain and RI4, RI11, RI18, RI10, RI6 are REMI mutants. Arrows show representative L. pneumophila phagosomes. (B) REMI mutants alter the number of Legionella-containing vacuoles (LCVs) compared to wild type amoebae. Images (20X) of noted REMI mutants challenged with L. pneumophila-GFP for 72 hrs. were captured as in (A), and fluorescent foci were quantitated (Experimental Procedures). Each fluorescent focus was defined as a single LCV. Shown are the mean ± SD for triplicate infections. (C). REMI mutants show alterations in yield of intracellular L. pneumophila. Images grabbed in panel (B) were used to determine the yield of bacteria. (D). Intracellular growth of wild-type L. pneumophila in REMI mutants. The number of viable bacteria was determined by plating supernatants of lysed D. discoideum onto CYE plates at the denoted times and counting CFU (Experimental Procedures). Plotted is the mean CFU from triplicate samples ± SD.
Representative fluorescence images of 5 mutants incubated with L. pneumophila-GFP for 72 hrs are shown in Fig. 2A. Compared to growth in the wild type AX4 strain, mutant strains RI4 and RI11 contained greater numbers of LCVs (Fig. 2B, quantitative analysis) that were also larger in volume based on increased pixel area (Fig. 2C, quantitative analysis), while mutant strains RI18 and RI10 contained fewer, but larger phagosomes (Fig. 2B,C). In contrast, the F6 mutation in strain RI6 showed fewer and dimmer L. pneumophila phagosomes than those in AX4 cells (Fig. 2A–C). Measurement of intracellular growth rates over a 3 day period showed a correspondence to the visual assays, with the yield of bacteria in the RI6 strain at least 10X lower than AX4 (Fig. 2D).
Identification of sites disrupted by REMI
The integration site of the mutations was determined (Experimental Procedures) and the resulting products were searched versus DictyBase.org ((Chisholm et al., 2006; Eichinger et al., 2005)). Southern blotting confirmed that there was only a single insertion of the plasmid into the genome for each of the mutants (data not shown). Of the six mutants mapped, three showed enhanced intracellular growth and will be detailed elsewhere (Fig. 1; strains RI4, RI11, RI18). Of the three that showed depressed growth, one was a predicted transmembrane protein with a haemaglutinnin repeat (strain RI1). The second (strain RI20) had a mutation in gene predicted to encode a regulator of heterotrimeric G protein signaling (RGS18), resulting in a modest reduction L. pneumophila intracellular growth (Fig. 1).
The third mutant, F6 (strain RI6), had a plasmid insertion site that was mapped to an Orf that predicted to encode a eukaryotic protein kinase (ePK) domain (E = 6.5e-19) and a downstream dual-specificity protein phosphatase (Dsp) domain (E=1e-27; Finn et al., 2008). As the gene predicts two functional domains, the putative protein will be referred to as DupA (dual role protein A) and the strain RI6 REMI insertion mutant called dupA(F6). Interestingly, the mutant also was defective for supporting intracellular growth of Mycobacterium marinum, another intravacuolar pathogen (Supplemental Fig. 1).
To confirm the connection between the absence of the dupA gene and increased resistance to L. pneumophila infection, a complete dupA− deletion mutant was constructed (Experimental Procedures). The deletion of the dupA gene was examined by genomic DNA PCR, which showed the predicted deletion, and qRT-PCR which showed that there was no detectable expression of dupA (data not shown). When the ΔdupA− mutant was challenged with L. pneumophila, the phenotype of the deletion mutant appeared very similar to the REMI insertion (Fig. 3B). In each case, there were fewer viable, cell-associated bacteria at early timepoints, followed by a period of growth kinetics similar to wild type. Bacterial replication, however, eventually slowed relative to the wild type AX4 strain. We attempted to isolate a full-length dupA gene to perform complementation analysis, but we were unable to isolate an intact expressed gene. Instead, fragments containing the Dsp domain, the kinase domain, or both were expressed in D. discoideum. None of the partial gene fragments could complement the mutation. Nevertheless, the ability to reconstruct the mutant phenotype using an independent strategy is consistent with depressed intracellular replication of L. pneumophila being the result of dupA deletion.
Figure 3. Reduced intracellular growth and developmental defects in a reconstructed dupA mutant.
(A) DupA protein domains and the insertion site of BsrR. The protein contains a protein kinase (ePK) domain and a dual-specificity protein phosphatase (DSP) domain. (B) Deletion in dupA gene shows depressed L. pneumophila intracellular growth. The values are the mean of CFU from triplicate samples ± SD. (C–G) Developmental defect of RI6(dupA(F6)). (C, D) Micrographs of vegetatively growing AX4 and dupA(F6) cells. (E, F) Multicellular development of (E) AX4 and (F) the dupA(F6) mutant on nitrocellulose membranes. Images were grabbed from a dissecting microscope 30 h. after plating.
DupA is necessary for D. discoideum development
The dupA− mutants displayed slower growth rates in axenic medium than did wild type and grew as microcolonies, instead of spreading evenly on tissue culture plates as seen with AX4 cells (compare Figs. 3C and 3D). When D. discoideum AX4 and dupA(F6) cells were plated on the bacterial lawn of Klebsiella aerogenes AM2515, both strains could form plaques. However, unlike AX4 cells, the dupA(F6) mutant could not form fruiting bodies. To gain insight into the developmental defect of the mutant, a standard development assay was performed on a solid substrate (Experimental Procedures). AX4 cells formed fruiting bodies typical for a wild type strain after 30 hr incubation (Fig. 3E). In contrast, dupA(F6) cells were arrested at the pre- aggregation stage, the initial event in development of fruiting bodies (Fig. 3F).
The absence of DupA leads to increased phosphorylation of Dictyostelium ERK1
The Dsp domain of DupA indicates that DupA may function as a dual specificity phosphatase for a MAP kinase. Dictyostelium has two MAPKs similar to members of the mammalian ERK family: ERK1 and ERK2. Elimination or overexpression of each of these ERK proteins results in developmental and/or chemotactic defects (Segall et al., 1995; Sobko et al., 2002). The similarity of the developmental defects of the dupA− and ERK overexpression prompted us to determine if MAP kinases are possible targets of DupA, by measuring phosphorylation levels of the D. discoideum ERKs.
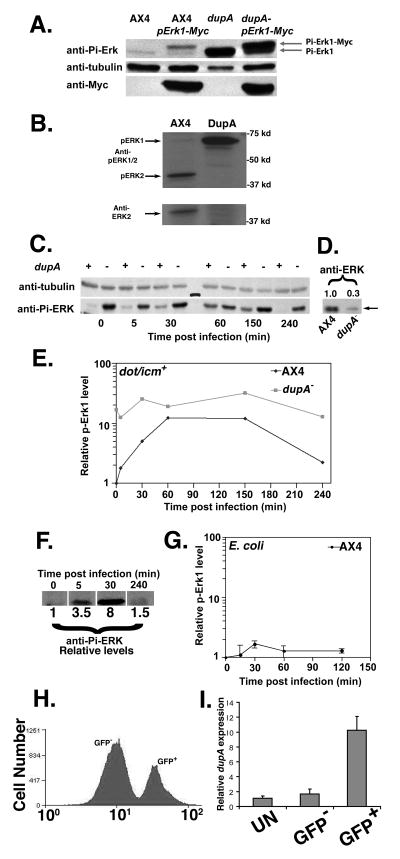
The levels of ERK phosphorylation in dupA− mutant cells were examined by anti-phospho-ERK antibody directed against Thr202/Tyr204 of human p44 MAP kinase (Experimental Procedures; (Maeda et al., 2004)). There were small amounts of a species predicted to comigrate with phospho-ERK1 protein in vegetatively growing AX4 cells (Fig. 4A; AX4), consistent with previous reports (Sobko et al., 2002). In contrast, a much higher level (>15 fold) of phospho-ERK1 was detected in dupA− cells (Fig. 4A; dupA). To confirm that ERK1 was the protein that was highly phosphorylated in dupA− cells, we expressed Myc tagged D. discoideum ERK1 on a plasmid. The Myc-tagged ERK1 showed a phosphorylated band that migrated slightly slower than ERK1(Fig. 4A; AX4 pErk1-Myc) with significantly more phosphorylation of the mycERK1 band in dupA− cells (dupA− pErk1-Myc). Similar results were observed with the ΔdupA strain. In contrast to ERK1, the anti-phospho-ERK reacting band that comigrated with the predicted molecular weight of ERK2 totally disappeared in the dupA− strain (Fig. 4B). The absence of this species apparently resulted from degradation, as stripped blots showed there was a loss of the comigrating band that cross-reacted with anti-human ERK2 (Fig. 4B), in spite of the fact that transcription of erk2 in the dupA− mutant appeared to be normal (P =1; www.ebi.ac.uk/arrayexpress, accession # E-TABM-509).
Figure 4. Control of ERK1 activation by DupA.
(A) ERK1 is hyperphoshosphorylated in the dupA− mutant. Noted strains were transformed with plasmid expressing myc-tagged ERK1 (Experimental Procedures). (B). The absence of DupA results in ERK1 hyperphosphorylation and loss of ERK2. AX4(dupA+) or the dupA(F6) were analyzed by Western blotting with either anti-phophoERK (top) or anti-ERK2 antibodies (bottom; Experimental Procedures) (Maeda et al., 2004). Bottom panel: blot in top panel was stripped and reprobed with anti ERK. (C) ERK1 is phosphorylated in response to L. pneumophila infection. D. discoideum were challenged with L. pneumophila for indicated times and 5×105 cells were analyzed by probing blots with anti-phospho-ERK (Experimental Procedures). (D). Reduced amount of ERK1 antigen in dupA(F6) mutant. Blot in panel C was stripped and reprobed with anti ERK (Experimental Procedures). Numbers above blot denote relative amount of antigen based on densitometry. (E). Disruption of dupA results in high level phosphorylation of ERK1. Bands were quantified by scanning densitometry of Western blots. Data are the mean of two samples. Experiment was performed 3 times. (F). Increase in phospho-ERK1 in response to L. pneumophila dotA3 strain. Relative phosphoERK1 levels determined by denstitometry. (G). Response of AX4 ERK1 phosphorylation to E. coli K12. Amoebae were challenged by E. coli at MOI = 1 and phosphorylation levels determined as in panel E. Data are mean of three samples. (H) Isolation of AX4 population harboring L. pneumophila Lp01-GFP 8 h. post-infection. (I) Total RNA was isolated from each population and qPCR for dupA was performed. Relative dupA gene expression was normalized to uninfected cells (Experimental Procedures). N=3. Values are mean ± S.D.
To investigate whether the hyperactivation of ERK1 observed in the dupA− strain mimics the response of wild type amoebae to bacteria, the phospho-ERK1 level was measured in D. discoideum cells in response to L. pneumophila. ERK1 was phosphorylated rapidly after contact with L. pneumophila (Fig. 4C,E). At 60 min post-infection, maximum phosphorylation of ERK1 was observed, with 12 fold higher activation than prior to infection. The activation of ERK1was transient, as ERK1 phosphorylation returned to low levels by 240 min post-infection (Fig. 4C,E). The dupA− mutant cells, in contrast, showed high levels of ERK1 phosphorylation throughout infection, with only a 2 fold increase approximately 30 min after challenge with bacteria (Fig. 4C,E). Even so, at all timepoints there was still more phospho-ERK1 in the dupA− mutant than observed in the wild type. The elevated level of phospho-ERK1 in the dupA− strain was due to increased phosphorylation of the protein and not due to enhanced expression. Similar to what was observed with ERK2, the dupA− strain showed reduced amounts of 70 kDal antigen that comigrated with the hyperphosphorylated band and cross-reacted with anti-ERK (Fig. 4D). This indicates that the fraction of ERK1 that was phosphorylated relative to the pool of ERK1 present in the dupA− strain was even higher than displayed in Fig. 4E, which was normalized to unrelated protein levels. These results indicate that ERK1 phosphorylation is negatively regulated by DupA and responds to the interaction between L. pneumophila and D. discoideum.
The fact that the dupA− mutant appears hyperactivated in a fashion that seems to mimic the response to L. pneumophila raises the possibility that phosphorylation of ERK1 is the result of a general pathway for recognition of bacteria. To test this idea, the AX4 wild type strain was challenged either with L. pneumophila dotA−, which is unable to translocate type IV substrates, or with the laboratory strain E. coli K12 (Fig. 4F, G). There was a robust response of ERK1 phosphorylation in AX4 to the dotA− mutant that appeared similar in intensity and kinetics to that observed with wt L. pneumophila (Fig. 4F), a result that mirrors previous observations with mouse bone marrow macrophages (Shin et al., 2008). Challenge with E. coli K12 also caused an increase in ERK1 phosphorylation (Fig. 4G), although the intensity of this response was markedly lower than that observed with L. pneumophila. Therefore, ERK1 appears to be phosphorylated in response to multiple strains of bacteria, but the intensity of this response is not uniform.
Expression of dupA is induced upon L. pneumophila challenge
The defect in intracellular growth exhibited by amoebae deranged for MAPK regulation brings up an important link to the mammalian response to L. pneumophila. In a human phagocytic cell line, the dual specificity phosphatases dusp1, dusp2 and dusp6, which are negative regulators of the MAPK response, are highly induced genes after L. pneumophila challenge (Losick and Isberg, 2006). The phenotype of the dupA− mutant indicates that downmodulation by Dusps may be an evolutionarily conserved response to bacteria. The expression of the dupA gene in response to L. pneumophila, therefore, was carefully examined by analyzing the subset of cells harboring bacteria and comparing them with uninfected cells in the same population. D. discoideum AX4 was incubated at low MOI with L. pneumophila expressing GFP, the infected GFP positive cells were sorted by flow cytometry and subjected to qPCR (Experimental Procedures: Fig. 4H). Consistent with the results from mammalian cells, the dupA gene was induced 10 fold in the infected GFP+ D. discoideum cells compared to cells from uninfected cultured or to the uninfected sorted GFP− population (Fig. 4I). This is consistent with the notion that downmodulation of MAPK activation is a conserved response to L. pneumophila.
Amoebal genes misregulated in absence of DupA are regulated in response to L. pneumophila challenge
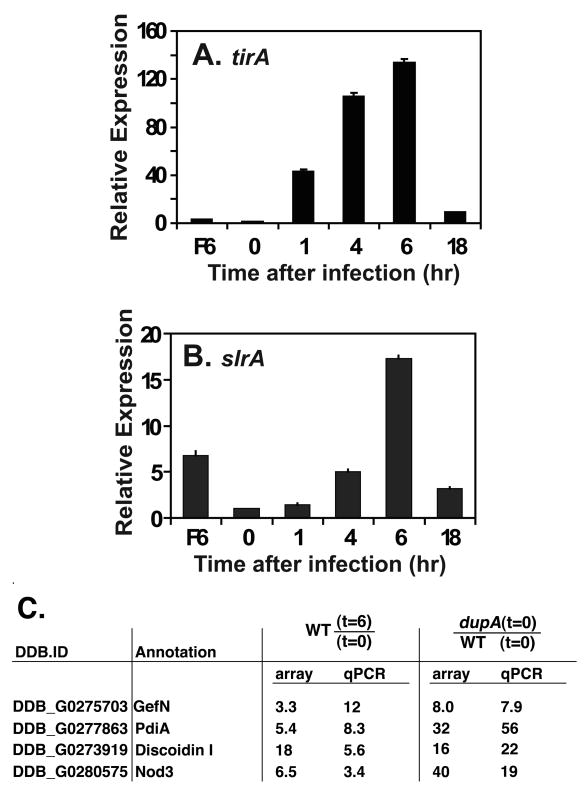
As the absence of DupA caused constitutive activation of a MAP kinase cascade which should control global regulatory circuits, the transcriptional profile of the dupA− mutant was compared to the wild type AX4 in the presence or absence of L. pneumophila (Experimental Procedures). The wild type and dupA− mutant were challenged with L. pneumophila expressing GFP at MOI = 1.0, at 6 h. post-infection cells were collected, and RNA was isolated from infected amoeba as well as uninfected cells otherwise treated identically. The RNA from each sample was used to probe an array of 9320 D. discoideum orf segments, and the four conditions were compared to each other (Experimental Procedures). There were three readily apparent properties of these bacterial challenges: A) In wild type amoebae, there was a large transcriptional response to the presence of L. pneumophila at 6 hrs. post-infection that involved alterations in genes encoding proteins involved in translation and transcriptional regulation (Fig.5; Table 1; Supplemental Tables 1–6); B) a large cadre of genes induced in the wild type amoebae in response to L. pneumophila were overexpressed in the dupA− mutant prior to bacterial infection, while many of the genes that were reduced in expression in response to L. pneumophila were underexpressed in the dupA mutant prior to exposure to bacteria (Fig. 5A; Supplemental Tables 1 and 4); and C) several of the genes that were induced in response to L pneumophila and overexpressed in the dupA− mutant encoded proteins that show similarity to proteins involved in the innate immune response in higher eukaryotes (Table 1; Supplemental Table 8). To confirm the microarray gene expression, qRT-PCR was used to verify that several of the genes upregulated on the arrays including tirA, gefN, pdiA, discoidin I, and nod3 were also strongly induced as measured by qRT-PCR. The amount of altered expression was consistent with that observed on the array (Fig. 6A and C).
Figure 5. Microarray analysis of dupA(F6) mutant compared to L. pneumophila challenged AX4 strain.
(A) Correlation cluster analysis of genes that had altered expression upon L. pneumophila infection or were altered in dupA in the absence of infection. Each condition is represented as the mean from microarrays from three independent infections. Enlarged is a small cluster of genes over-expressed in the dupA− mutant and induced in the infected AX4 cells. Comparison of AX4 to dupA(F6) mutant incubated in absence of bacteria: dupA(t=0)/WT(t=0). Comparison of AX4 incubated after 6 hrs infection compared to incubation in absence of bacteria: WT(t=6)/WT(t=0). Comparison of AX4 to dupA(F6) mutant incubated in presence of bacteria for 6 h.: dupA(t=6)/WT(t=6). dupA(F6) incubated after 6 hrs infection compared to incubation in absence of bacteria: dupA(t=6)/dupA(t=6). (B) A large fraction of the genes upregulated in AX4(WT) after L. pneumophila infection are overexpressed in the dupA(F6) mutant. (C) A large fraction of the genes downregulated in AX4(WT) in response to L. pneumophila are underexpressed in the dupA(F6) mutant. (D) Ribosomal protein genes are both downregulated in AX4(WT) in response to L. pneumophila and underexpressed in dupA(F6). See Supplemental Table 7 for individual data points.
Table 1.
Disruption of dupA results in misregulation of genes that respond to L. pneumophila infection.
| DDB.ID | Annotation (# of Genes) | dupA 0hours | WT 6hours | dupA 6hours |
|---|---|---|---|---|
| WT 0hours | WT 0hours | dupA 0hours | ||
| Protein Degradation | ||||
| Reduced Expression | ||||
| Various | Papain proteinases (4 genes) | 0.19–0.30 | 0.17–0.32 | 0.67–1.17 |
| DDB_G0290333 | Physarolisin | 0.49 | 0.39 | 0.65 |
| Enhanced Expression | ||||
| DDB_G0278761 | La (Lon) serine protease | 4.43 | 2.93 | 0.75 |
| Various | IBR domain proteins (2 genes) | 2.6–4.3 | 3.6–8.4 | 1.20 |
| DDB_G0267906 | OUT-like cysteine protease | 3.03 | 6.29 | 1.40 |
| Various | Proteosome subunits (2 genes) | 10.70 | 2.0–3.1 | 1.14 |
| DDB_G0288093 | Ring finger proteins (3 genes) | 2.4–2.9 | 3.2–3.6 | 0.92–1.22 |
| DDB_G0269462 | Ubiquitin family member | 2.46 | 3.06 | 1.17 |
| DDB_G0288697 | Ubiquitin E2-4 enzyme | 2.01 | 5.50 | 1.82 |
| Carbon metabolism | ||||
| Reduced Expression | ||||
| DDB_G0288289 | RliF; Beta-xylosidase | 0.49 | 0.18 | 0.26 |
| DDB_G0278275 | Ribulose phosphate-3-epimerase | 0.36 | 0.26 | 0.62 |
| DDB_G0272781 | Phosphomannomutase | 0.46 | 0.32 | 0.74 |
| DDB_G0270018 | Dehydrogenase signature | 0.41 | 0.49 | 0.76 |
| DDB_G0278341 | Citrate lyase b-subunit | 0.19 | 0.47 | 3.07 |
| Enhanced Expression | ||||
| DDB_G0284277 | Dehydrogenase signature (2 genes) | 2.5–2.9 | 5.2–7.7 | 0.64–2.1 |
| Nucleotide metabolism | ||||
| Reduced Expression | ||||
| DDB_G0281551 | GMP synthesis (2 genes) | 0.30–0.34 | 0.14-.30 | 0.23–0.45 |
| DDB_G0280041 | Pyrimidine synthesis (2 genes) | 0.33–0.50 | 0.15–0.21 | 0.32–0.45 |
| DDB_G0277725 | Methylenetetrahydrofolate dehydrogenase | 0.35 | 0.18 | 0.53 |
| DDB_G0288333 | Purine synthesis (3 genes) | 0.27–0.43 | 0.21–0.40 | 0.55–0.81 |
| DDB_G0280567 | CTP synthase | 0.45 | 0.19 | 0.55 |
| Potential Immune response genes/antimicrobial proteins | ||||
| Enhanced Expression | ||||
| DDB_G0280575 | Nod3, Leucine-rich repeat signature | 40.31 | 6.49 | 0.42 |
| DDB_G0289237 | TirA | 2.71 | 4.58 | 0.98 |
| DDB_G0291083 | TirC | 2.30 | 3.99 | 1.18 |
| DDB_G0290971 | TRAF-type zinc finger* | 1.94 | 3.13 | 1.26 |
| DDB_G0271590 | Antibiotic O-methyltransferase | 3.87 | 3.95 | 1.11 |
| DDB_G0289149 | LPS induced TNF alpha Factor* | 2.79 | 1.83 | 1.15 |
| Protein Synthesis | ||||
| Reduced Expression | ||||
| DDB_G0281839 | WD domain, G-beta repeat | 0.43 | 0.20 | 0.77 |
| DDB_G0279387 | Ribosomal proteins (8 genes) | 0.38–0.48 | 0.24–0.41 | 0.23–1.19 |
| Enhanced Expression | ||||
| DDB_G0285725 | Deoxyhypusine synthase Probable | 3.65 | 3.54 | 1.06 |
| DDB_G0283877 | Dihydrouridine synthase (Dus) | 4.03 | 6.72 | 1.65 |
| DDB_G0280703 | EF-1 guanine nucleotide exchange | 8.31 | 17.91 | 1.61 |
| DDB_G0292538 | Elongation factor Tu | 2.40 | 3.60 | 0.85 |
| DDB_G0276493 | Eukaryotic translation initiation factor 6 (EIF-6) | 3.14 | 2.39 | 0.98 |
| DDB_G0275625 | tRNAsynthetases (2 genes) | 2.5–3.9 | 5.0–10.3 | 1.3–1.7 |
| Cell Adhesion/cytoskeleton | ||||
| Reduced Expression | ||||
| DDB_G0285793 | DdCAD-1 putative adhesion molecule | 0.39 | 0.49 | 0.43 |
| Enhanced Expression | ||||
| DDB_G0285981 | von Willebrand factor domain (3 genes) | 2.5–2.6 | 5.5–7.05 | 1.1–1.2 |
| Signal transduction | ||||
| Enhanced Expression | ||||
| DDB_G0284331 | 3′,5′-cyclic-nucleotide phosphodiesterase regA | 11.07 | 2.96 | 0.74 |
| DDB_G0269728 | CIA, Cytosolic Iron-sulfur protein Assembly | 5.18 | 9.39 | 0.97 |
| DDB_G0273533 | Cyclic AMP receptor 1 | 5.71 | 3.77 | 1.41 |
| DDB_G0280339 | Cyclic nucleotide phosphodiesterase inhibitor related | 26.01 | 5.20 | 1.19 |
| DDB_G0275703 | Ras guanine nucleotide exchange factor | 8.04 | 3.27 | 0.90 |
| DDB_G0277863 | Cyclic nucleotide phosphodiesterase inhibitor PdiA | 31.65 | 5.41 | 1.20 |
| DDB_G0287233 | Guanosine polyphosphate phosphohydrolase | 2.33 | 3.35 | 0.95 |
| DDB_G0292160 | G-protein coupled-like receptor, possible | 2.56 | 3.24 | 0.95 |
| Cell cycle | ||||
| Enhanced Expression | ||||
| DDB_G0292758 | Mob1/phocein family cell cycle protein | 2.82 | 3.67 | 0.99 |
| DDB_G0278125 | Regulator of chromosome condensation. | 4.35 | 5.43 | 0.79 |
| DDB_G0293756 | RCC1-containing domain | 2.95 | 3.00 | 0.83 |
| Hydrolases/lipases | ||||
| Reduced Expression | ||||
| DDB_G0282371 | Hyaluronidase | 0.44 | 0.32 | 0.54 |
| DDB_G0293460 | Calcineurin-like phosphoesterase (3 genes) | 0.29–0.45 | 0.35–0.40 | 0.74–1.3 |
| DDB_G0274181 | Glycosyl hydrolases family 25 | 0.27 | 0.41 | 0.91 |
| DDB_G0268064 | Phospholipase/Carboxylesterase | 0.21 | 0.42 | 2.47 |
| DDB_G0293566 | Lysozyme, putative | 0.23 | 0.45 | 1.13 |
| Enhanced Expression | ||||
| DDB_G0290265 | DdFRP1alpha | 2.12 | 7.55 | 0.96 |
| DDB_G0274291 | Similar to Bacteriophage T4 lysozyme | 2.38 | 2.03 | 0.85 |
| DDB_G0290975 | Vegetative specific protein H5 | 6.26 | 2.49 | 0.52 |
| Nucleic Acid Interaction | ||||
| Reduced Expression | ||||
| DDB_G0281293 | Ribonuclease DdI, T2 family | 0.29 | 0.38 | 0.86 |
| between_DDB_G0272334_and_DDB_G0272336 | Ribonuclease, putative | 0.49 | 0.45 | 0.81 |
| Enhanced Expression | ||||
| DDB_G0275469 | Endonuclease V | 5.97 | 12.77 | 1.34 |
| DDB_G0277705 | Ribonuclease HII | 2.89 | 5.03 | 1.16 |
| DDB_G0269630 | IliI, IliK; TatD related Dnases | 3.5–6.3 | 12.0–13.7 | 1.25–1.96 |
| DDB_G0289921 | XRN 5′-3′exonuclease N-terminus | 2.69 | 5.19 | 1.09 |
| DDB_G0284255 | Zinc finger, C2H2 type, nucleic acid binding | 4.37 | 2.53 | 1.49 |
| DDB_G0272048 | NUDIX hydrolase family signature | 2.64 | 5.31 | 1.34 |
| DDB_G0269966 | DEAD box protein DDX1 | 2.01 | 5.20 | 1.19 |
| DDB_G0292618 | HhH-GPD superfamily base excision | 2.16 | 3.63 | 1.15 |
| Transcriptional control | ||||
| Reduced Expression | ||||
| DDB_G0268920 | srfC; putative MADS-box transcription factor | 0.49 | 0.34 | 1.19 |
| Enhanced Expression | ||||
| DDB_G0285057 | Sin3 associated polypeptide p18 | 2.36 | 5.38 | 0.84 |
| DDB_G0289319 | C-myb-like transcription factor | 2.35 | 2.33 | 1.21 |
| DDB_G0293102 | Helix-turn-helix homeodomain | 2.07 | 5.07 | 1.89 |
| DDB_G0286515 | Involucrin repeat, B-box zinc finger | 2.36 | 9.74 | 2.15 |
| DDB_G0284103 | MybZ, homeodomain | 2.41 | 2.23 | 1.01 |
| DDB_G0283917 | NAD-dependent deacetylase sirtuin 2 | 2.04 | 3.16 | 1.51 |
| DDB_G0293590 | NF-X1 type zinc finger | 2.65 | 4.34 | 1.24 |
| DDB_G0293532 | STATc protein | 2.51 | 2.50 | 0.99 |
| Membrane trafficking/lysosomal function | ||||
| Enhanced Expression | ||||
| DDB_G0274391 | Alpha-L-fucosidase precursor | 8.95 | 2.60 | 1.61 |
| DDB_G0291998 | Alpha-N-acetylglucosaminidase | 3.00 | 4.57 | 2.27 |
| DDB_G0288203 | HEAT repeat | 2.84 | 4.20 | 1.21 |
| DDB_G0267440 | LimpC, CD36 | 2.19 | 2.70 | 1.21 |
| DDB_G0275413 | Vacuolar sorting protein 9 (VPS9) | 3.64 | 4.08 | 0.79 |
| DDB_G0289485 | Vacuolin A1 | 2.51 | 5.45 | 1.52 |
| Lipid metabolism | ||||
| Reduced Expression | ||||
| DDB_G0275125 | B-like phospholipase (2 genes) | 0.16–0.19 | 0.34–0.36 | 1.12–1.52 |
| DDB_G0286651 | Saposin-like type B | 0.35 | 0.24 | 0.55 |
| Enhanced Expression | ||||
| DDB_G0273557 | DHHC zinc finger domain | 3.93 | 4.08 | 1.14 |
| DDB_G0284353 | Oxysterol-binding protein | 5.65 | 7.81 | 2.62 |
| DDB_G0272955 | Phytanoyl-CoA dioxygenase (PhyH) | 3.02 | 6.96 | 1.41 |
| DDB_G0268890 | Saposin-like type B, region 2 | 3.50 | 2.43 | 1.18 |
| DDB_G0292668 | Terpenoid cylases/protein prenyltransferase | 4.97 | 2.38 | 1.01 |
| Cell surface proteins of unknown function | ||||
| Reduced Expression | ||||
| DDB_G0289907 | EGF-like domain | 0.22 | 0.23 | 0.93 |
| DDB_G0272434 | EGF domain,thrombomodulin signature | 0.35 | 0.49 | 0.76 |
| DDB_G0286677 | Type III EGF-like signature (2 genes) | 0.26–0.33 | 0.22–0.25 | 0.98–1.43 |
| Enhanced Expression | ||||
| DDB_G0273915 | IliE; Lectin/glucanase superfamily | 18.20 | 22.10 | 1.30 |
| Transporters | ||||
| Reduced Expression | ||||
| DDB_G0267454 | ADP/ATP translocase, Mitochondrial | 0.43 | 0.18 | 0.27 |
| DDB_G0287461 | ABC transporter AbcG3 | 0.28 | 0.22 | 0.63 |
| DDB_G0270720 | Major Facilitator Superfamily | 0.34 | 0.24 | 0.59 |
| DDB_G0277515 | Permease family | 0.30 | 0.26 | 0.78 |
| DDB_G0279301 | Sodium/calcium exchanger protein | 0.38 | 0.43 | 1.08 |
| Enhanced Expression | ||||
| DDB_G0292986 | ABC transporters (3 genes) | 2.25–2.85 | 2.29–3.17 | 0.73–1.10 |
| DDB_G0274661 | Phosphotransferase membrane protein | 2.21 | 2.73 | 1.39 |
| DDB_G0292830 | sodium, hydrogen exchanger | 6.67 | 2.16 | 1.00 |
| DDB_G0283345 | Sugar transporter ComD | 10.69 | 2.89 | 0.77 |
| Development | ||||
| Reduced Expression | ||||
| DDB_G0281823 | V4-7,vegetative stage specific | 0.34 | 0.09 | 0.26 |
| Enhanced Expression | ||||
| DDB_G0273919 | Discoidin I-like chains (2 genes) | 15.5–17.1 | 17.7–21.7 | 1.31–1.33 |
| DDB_G0290925 | Similar to psiA, inducer of prespore cell division | 17.83 | 2.49 | 0.98 |
| Detoxification | ||||
| Reduced Expression | ||||
| DDB_G0287229 | GMC oxidoreductase (choline dehydrogenase) | 0.24 | 0.40 | 1.38 |
| Enhanced Expression | ||||
| DDB_G0273789 | Dyp-type peroxidase family | 2.65 | 6.81 | 1.53 |
| DDB_G0291127 | 4.0–4.23 | 2.66 | 0.75–0.79 | |
Either wild type D. discoideum AX4 or the dupA(F6) mutant were incubated in the presence (6 hours) or absence (0 hours) of L. pnuemophila in MB medium, RNA was extracted and microarrays were performed (Materials and Methods). Shown are array data from comparisons between: 1) infected AX4 and uninfected AX4 (WT 6 hours/WT 0 hours); 2) uninfected dupA(F6) mutant and infected AX4 (dupA 0 hours/WT 0 hours); 3) infected dupA(F6) and uninfected dupA(F6) (dupA 6 hours/dupA 0 hours). The data are given as ratios of the expression levels of each gene between he two conditions, with the numerator and denominator as shown in the header for each column. Shown are fold ratios.
Figure 6. Upregulation of genes associated with an amoebal innate immune response after incubation with L. pneumophila.
L. pneumophila LP01 was introduced onto monolayers of AX4(WT), and total RNA was prepared from the D. discoideum cells at time points noted. qPCR analysis of the either the tirA (A) or slrA genes (B) was determined from triplicate infections and expression level was plotted relative to uninfected WT. Data are mean ± S.E. Total RNA was also prepared from dupA(F6) in absence of infection and qPCR data are plotted relative to uninfected WT. (C) Verification of array data by qPCR (Experimental Procedures). Data are fold changes determined exactly as in panels 6A and 6B, performing qPCR at timepoints noted in each column. Both qPCR and array data are expressed as ratios at headers of each column.
A. The presence of a large transcriptional response to L. pneumophila
Incubation of the wild type AX4 to L. pneumophila for 6 hrs resulted in altered expression of a large number of genes, many of which have been described in a previous microarray study (Farbrother et al., 2006; Supplemental Tables 3 and 6; threshold of 2X change relative to uninfected; P < 0.05). As observed previously, there was upregulation of tRNA synthetase genes in response to L. pneumophila (Supplemental Table 3). In contrast, almost every ribosomal protein gene was downregulated in response to L. pneumophila (Fig. 5D; Supplemental Table 7). Although these may appear to be counterproductive responses, this phenomenon has some similarity to the E. coli stringent response, in which the charging status of tRNAs modulates the rate of protein synthesis (Goldman and Jakubowski, 1990).
B. Genes misregulated in the dupA− mutant showed altered expression in wild type AX4 after L. pneumophila challenge
In the absence of added bacteria, the dupA− mutant showed altered expression of a large number of genes, many of which were found to be differentially expressed in response to bacterial challenge of the wild type AX4 strain (2-fold cutoff for minimal change; P<0.05; Supplementary Tables 2 and 5). 64% of the genes overexpressed in the uninfected dupA− mutant were upregulated after L. pneumophila was incubated with the wild type amoebae, while 48% of the underexpressed genes were downregulated (Supplementary Tables 3 and 6; Fig. 5A–C). 521 genes that were differentially expressed (p<0.05) when AX4 was challenged by L. pneumophila-challenged AX4 also showed misregulation in the uninfected dupA− mutant when compared to AX4. 244 of these genes were upregulated in both comparisons and 261were downregulated in both comparisons; only 16 genes were significantly upregulated in one comparison and downregulated in the other. This association is far greater than one would expect by chance alone (p <2.2e-16, odds ratio = 1249.6; one-tailed Fisher's exact test (R-Development-Core-Team, 2008).
Table 1 shows a summary of an annotated set of genes that were altered in expression in uninfected dupA− and which were regulated after challenge of the wild type with L. pneumophila. When the dupA− mutant was challenged with L. pneumophila there was very little change in expression of most of the differentially expressed genes (Table 1; Fig. 5A). Key among the misregulated genes in the dupA mutant was an apparent switch in the nature of the protein degradation pathways present in the mutant, with several cysteine and serine proteases underexpressed in the mutant, and enhanced expression of the ubiquitin-proteosome machinery (Table 1; Protein Degradation). These changes predicted the response of the wild type amoebae to L. pneumophila, as the proteasome-ubiquitin pathway genes were induced after bacterial challenge.
C. Hyperexpression of genes similar to immune response genes
Several genes that encode proteins with sequence similarities to host innate immune response proteins found in higher eukaryotes were overexpressed in the dupA mutant and were upregulated in response to L. pneumophila (Table 1). Cosson and Soldati had hypothesized that many of these genes may be involved in the amoebal response to bacteria (Cosson and Soldati, 2008). Most notable, the TIR domain containing protein tirA carries out sensor or adaptor functions in the detection of pathogens, and mutations in this gene were previously shown to confer hypersensitivity of D. discoideum to high multiplicity infection by L. pneumophila (Chen et al., 2007). Another gene associated with this response is slrA, encoding a leucine rich repeat (LRR) protein, but it was not differentially expressed by microarray analysis, perhaps due to weak spotting of this gene on the array. As both these proteins appear to be involved in a hypothesized amoebal innate immune response to pathogens (Chen et al., 2007), tirA and slrA gene expression was carefully monitored in infected wild type D. discoideum using qPCR. AX4 cells were incubated with L. pneumophila-GFP at MOI= 1.0, and 1h, 4h, 6h or 18h after infection, RNA was isolated from sorted cells harboring fluorescent bacteria. Both the tirA and slrA genes were induced shortly after L. pneumophila infection (Fig. 6A and B). In the case of tirA, expression increased to more than 130 fold compared to the uninfected control at 6h post infection. In the uninfected dupA− mutant, there was also overexpression of tirA in the absence of bacteria. A similar pattern of induction was also observed for slrA gene (Fig. 6B). The induction of tirA, slrA, as well as other potential immune response genes (Table 1), and their overexpression in the dupA− mutant suggest that DupA plays an important role in a putative D. discoideum innate immune response by negatively regulating a subset of these genes.
DISCUSSION
We have identified a mutant disrupted in the regulation of a large number of amoebal genes that respond to Legionella pneumophila infection. As bacterial replication was reduced in this mutant, the regulatory circuit partially controlled by DupA appears to be an important determinant of the fate of L. pneumophila after it encounters amoebae. Furthermore, some common elements of this amoebal response to pathogens may control the intracellular replication of a broad swath of microorganisms, given that the unrelated Mycobacterium marinum was defective for either uptake or initiation of replication in dupA mutants (Supplementary Fig. 1).
The regulatory circuit controlled by DupA is also evolutionarily conserved. Mutations in dupA resulted in hyper-phosphorylation of the D. discoideum ERK1 protein as well as apparent degradation of ERK2 (Fig.4A and B). The predicted gene product of dupA encodes both a kinase and a dual specificity phosphatase domain (Dsp), making it the most likely candidate to be the direct negative regulator of ERK1 that is missing in the mutant. Although we do not know that the DupA phosphatase domain acts directly on ERK1, there is likely to be at least one important phosphatase activity that is misregulated in the mutant, as a large number of organisms use Dusps as the primary negative regulators of MAP kinases (Camps et al., 2000). Consistent with a function that is similar to Dusps in higher eukaryotes, the DupA gene was shown to be upregulated in the wild type D. discoideum AX4 in response to L. pneumophila infection (Fig. 4I). This is identical to observations in mammalian cells, in which three of the most highly upregulated genes in response to L. pneumophila are MAP kinase phosphatases (Losick and Isberg, 2006).
At least some of the phenotypic changes associated with the dupA− mutant can be attributed to hyperphosphorylation of ERK1. We have analyzed a D. discoideum strain that overproduces a constitutively active MEK1 protein, which causes hyperphosphorylation of ERK1 (Sobko et al., 2002) and has no effect on ERK2 phosphorylation or stability (Supplemental Fig. 2). Intracellular growth of L. pneumophila was mildly reduced in such a strain, but this result is tempered by the fact that ERK1 phosphorylation levels were significantly lower in the constitutively active strain than in the dupA− mutant (Supplemental Fig. 2). Furthermore, we were unable to demonstrate that the phosphorylation phenotype was due to overproduction of MEK1 rather than due to constitutive activity (Supplemental Fig. 2). At any rate, this result is consistent with ERK1 hyperactivation interfering with intracellular growth, but does not eliminate the possibility that loss of ERK2 or an uncharacterized property of the dupA− mutant make additional contributions to this phenotype.
Although in the dupA− mutant there was altered regulation of a large group of proteins that respond to L pneumophila infection, this did not result in an absolute block on intracellular growth. It is not clear that in every instance, the consequence of the altered gene expression is intended to restrict the pathogen. For instance, there appears to be global reduction of ribosomal protein gene expression after L. pneumophila challenge of wild type (Fig. 5C; Supplemental Table 7). This is similar to what was observed in a previous array analysis of D. discoideum challenged with L. pneumophila (Farbrother et al., 2006), but very different from the response of D. discoideum to E. coli (Sillo et al., 2008). Slowing ribosomal protein synthesis in host cells, which probably leads to slowed growth of the amoebae, may be a response to intracellular pathogens that is counterproductive for the amoebae. In support of this point, L. pneumophila growth within D. discoideum is enhanced in suboptimal growth medium or by plating amoebae at high culture densities (Solomon et al., 2000; E. Chen and R. Isberg, unpublished). Furthermore, L. pneumophila encodes several Dot/Icm translocated substrates that interfere with host protein synthesis, possibly blocking the host cell cycle (Belyi et al., 2008; Kubori et al., 2008). Therefore, the dupA− mutation causes a deranged amoebal response to pathogens, with the consequence that the sum of transcriptional activity of the host cell does not efficiently support intracellular growth under the conditions tested.
The MAP kinase signaling pathways in D. discoideum are simplified versions of what is found in mammals, with ERK1 and ERK2 being the only terminal kinases. ERK2 is involved in oscillatory cAMP signaling during development (Segall et al., 1995), while ERK1 controls events associated with chemotaxis and aggregation (Goldberg et al., 2006; Mendoza et al., 2007). In parallel to the work described here, it has been demonstrated that mouse macrophages challenged with L. pneumophila undergo phosphorylation of multiple MAPKs (Shin et al., 2008). Interestingly, the pattern of ERK phosphorylation in macrophages is reminiscent of our observations, as activation of ERK is independent of the dot/icm system. Surprisingly, the response in macrophages also did not require known pattern recognition receptors. Perhaps there is an evolutionarily conserved response to bacterial adhesion that is shared in both amoebae and mammalian cells that modulates microbial interaction. The ability of the pathogen either to manipulate or defend against such host responses in amoebae could be one of the critical selective pressures that also allow bacterial survival and growth in mammalian phagocytic cells.
A number of genes that may encode a primitive amoebal innate immune response showed altered expression in response to L. pneumophila, and many of these are misregulated in the dupA− mutant. Most striking is the altered regulation of the slrA, tirA and tirC genes (Chen et al., 2007; Sillo et al., 2008; O'Neill and Bowie, 2007). It has been previously reported that tirA expression was increased 16 fold in newly identified Sentinel Cells, which are hypothesized to be immune-like cells generated by the slug form of amoebae (Chen et al., 2007). Furthermore, D. discoideum tirA− is more sensitive than wild type to killing after high multiplicity challenge with L. pneumophila, indicating that the protein may promote enhanced cell survival in the presence of bacteria (Chen et al., 2007). Consistent with this hypothesized role, we observed a large increase in expression of both slrA and tirA in amoebae harboring L. pneumophila, and slrA is severely overexpressed in the dupA mutant (Fig. 6). We have found that a tirA− mutant still is able to support L. pneumophila replication with similar efficiency to wild type strains using low MOI challenge with the bacterium (data not shown). However, the requirement of the tirA product for supporting host cell viability in the presence of high doses of pathogen, as well as the regulatory profile of this gene, are properties that are consistent with the protein playing a role in a global response that modulates D. discoideum protection from pathogens.
In conclusion, disrupted DupA function is associated with profound misregulation of a group of genes that respond to association of L. pneumophila with amoebae. That several of the genes upregulated in response to the bacterium had been suggested previously to encode proteins involved in a form of amoebal innate immunity is consistent with their proposed role in the biology of D. discoideum. In fact, this primitive form of immune surveillance may be part of a larger regulatory circuit controlled by ERK1 and DupA. Most intriguing is the possibility that there are important parallels between macrophages and amoebae, the natural host for L. pneumophila, as negative control of host cell MAPKs appears to be a conserved response in these disparate cell types worthy of further investigation.
EXPERIMENTAL PROCEDURES
Bacteria strains, plasmids and media
L. pneumophila Lp01 is proficient for intracellular growth and was grown on AYE liquid medium or CYE agar plate (Berger and Isberg, 1993) (Feeley et al., 1979). The GFP-encoding plasmid pAM239 was described previously (Losick and Isberg, 2006). The L. pneumophila strains were grown to post-exponential phase (A600 = 3.5–4.0), at which time motile bacteria were added to amoebae at the noted multiplicities of infection (MOI). The approximate concentration of bacteria was determined by assuming that A600 = 1.0 is equivalent to 109 bacteria/ml.
Growth assay of L. pneumophila in D. discoideum
D. discoideum strains were routinely grown axenically in HL-5 liquid medium (Sussman, 1987) supplemented with penicillin and streptomycin (100 U/ml; GibcoBRL) at 21.5°C. Amoebae in logarithmic stage were prepared by plating 1 to 1.25 × 105 cells 2 days prior to use in fresh HL-5 medium. Cells were harvested and washed once with phosphate-buffered saline (PBS) and pelleted for 5 min at 200×g, resuspended at 106 cells/ml in MB medium (20 mM MES (2 (N-morpholino) ethanesulfonic acid) (pH=6.9), 0.7% yeast extract, 1.4% BBL thiotone E peptone). 0.5 ml or 0.1 ml were added to each well in 24-well or 96-well plates prior to incubation at 25.5°C for 2 h. to allow adherence (Solomon et al., 2000) before the bacterial infection. Infections with L. pneumophila were performed as described (Solomon et al., 2000).
REMI mutagenesis, mutant mapping and construction of mutants
REMI mutagenesis was performed as described (Kuspa and Loomis, 1992). 1μg of BamHI linearized plasmid pBsrΔBglII (kind gift of Dr. David Knecht, U. Connecticutt, Storrs) and 5units of DpnII were transformed into AX4 cells by two consecutive electroporation pulses (Bio-RAD, USA) at 0.85kv, 25μF, applied 5 seconds apart. Cells were then serially diluted in HL-5 medium to 10 cells per ml and 100μL of cell were plated in tissue culture treated 96-well plate immediately following electroporation. Transformed cells were allowed to recovery for 24 h before selection in HL-5 media containing 5 μg/ml blasticidin S. Drug-resistant cells were collected, counted and plated.
To map the insertion site of plasmid,s we used plasmid rescue or reverse PCR. Plasmid rescue was performed as described (Kuspa and Loomis, 1992). Briefly, DNA from the mutant was digested with ClaI, which does not cut the integrated vector, or with another enzyme determined by Southern blot to generate a fragment of <15kB, circularized by ligation, and transformed into Escherichia coli. Plasmid DNA isolated from E. coli was sequenced commercially with primers: TGAGCGCAACGCAATTAA and CCATTTTTTTTTTTAAAGATTTGATGG that correspond to plasmid sequences. For inverse PCR isolation, 10 μg of genomic DNA from AX4 was treated with restriction enzymes that digest the plasmid close to the end of the insertion position, such as AflIII or NlaIII. The DNA was set up for self-ligation in a volume of 100 μl. One μl of the ligation mix was used for inverse PCR using the 5′ primer CCAATCAATGATAATGATCCTCCC and the 3′primer AAAGTGAATCCTCGACAAG. The resulting sequences were used to BLAST search DictyBase.org (Chisholm et al., 2006; Eichinger et al., 2005).
To knock out the dupA gene, 1kb genomic DNA fragments located upstream, Blasticidin S resistance (BSR) cassette and 1kb downstream of the targeted gene were generated by PCR to transform D. discoideum AX4 cells as described above. The transformants were selected on blasticidin S for 7 days, single cell cloned, and screened for their phenotypes. The correct insertion of the genomic fragment was determined by DNA sequencing.
D. discoideum screen
Single D. discoideum REMI clones in a 96 well microtiter dish were harvested and replated into two 96 well plates having optically clear bottoms (Corning Cat# 3614). D. discoideum mutants were incubated with L. pneumophila - GFP for 72 h. at MOI=0.1 and individual wells containing infected D. discoideum were visually screened using fluorescence microscopy. 3 images were grabbed from each well and inspected later (Fig. 1). Images were analyzed using IP lab software. Segmentation was first applied for each image to separate the target pixels from background pixels. Thresholds for size and brightness of the fluorescence for each LCV were established for AX4 cells and used as a comparison for REMI mutants. Clones that deviated from wild type were retested in triplicate, and were retained for further analysis, as displayed in Fig. 2.
Quantitative PCR
Total RNA was isolated from 107 Dictyostelium cells using the Qiagen RNeasy mini kit (Qiagen Cat.No. 74104) or Trizol (Invitrogen, Carlsbad, California, United States). The first strand of cDNA were reverse transcribed from 2 μg of DNA-free RNA using the SuperscriptTM First-strand Synthesis System (Invitrogen) containing DNase/Rnase-free water and buffer suggested by the manufacturer in a total reaction volume of 30 μl. 2 μl of the resulting cDNA reaction mix was then used for real-time quantitative PCR (qPCR) analysis. Quantitative PCR was performed using the GenAmp5700 system with the SYBR Green PCR reagent (Applied Biosystems). For each quantification, triplicate samples were performed in parallel, quantification results were normalized based on the β-actin control and average values were calculated.
D. discoideum development assay
Briefly, 5×107 of vegetative cells were harvested by centrifugation at 1000 rpm for 5 min at a density = 1–2 × 106 cells/ml and washed twice with PDF buffer (20 mM KCl, 5 mM MgCl2, 20 mM KPO4, pH=6.2). Cells were resuspended in 500μl of PDF buffer and dispersed on nitrocellulose membrane (Osmonics Inc. E04BG04700) on top of a PDF-soaked pad (Life Sciences, P/N66025). Extra PDF buffer (~500 μl) was added to the side of the Petri dish and left on the bench for 5 min. Cells were then developed at 21.5°C in humid container and observed microscopically.
Western blot analysis and antibodies
To avoid potential post-lysis modification or degradation of proteins of interest, 1×106 of D. discoideum cells were harvested and pelleted by centrifugation at 1000rpm at 4°C for 5 min and directly lysed in 100 μl of 1×SDS sample buffer followed by heat denaturation at 95°C for 5 min. Total protein extracts were separated by SDS–PAGE electrophoresis. Proteins were then transferred onto a nitrocellulose membrane followed by blotting and probing. For immunoblot quantification, appropriately nonsaturated film exposures were selected and scanned, and the images were quantified by densitometry using a Kodak ImageStation. Anti-ERK (sc-153) was from Santa Cruz Biotechnology. Anti-phospho-p44/42 MAPK (#9101S) was from Cell Signaling Technology. Anti-tubulin was from Serotech (MCA785). All antibodies were used following manufacture’s instructions for Western blot.
Micoarray analysis
D. discoideum cells were infected with Lp01 at an MOI=1 for 6 h. and compared to D. discoideum in the absence of infection. RNA was then isolated from the cells and prepared for microarray analysis. The RNA samples were prepared by Trizol extraction.
For array analysis, three independent biological experiments were performed infecting wild-type and mutant RI6 (dupA(F6)) with L. pneumophila. 25 μg RNA was extracted and labeled by incorporation of Cy3-or Cy5-conjugated dCTP (GE Healthcare) in a reverse transcription reaction catalyzed by Superscript III (Invitrogen). For each biological replicate, labeled cDNA samples with complementary dyes incorporated were paired so that the comparisons of direct interest were made: WT 0h was paired with WT 6h, WT 0h with mutant 6h, mutant 0h with mutant 6h, and WT 6h with mutant 6h. In this loop design, each sample was labelled twice, once with each dye. In total twelve microarrays were used; four for each biological replicate. The samples thus paired were hybridized to custom DNA microarrays (Bloomfield et al., 2008). Background fluorescence was subtracted from the scanned images (Kooperberg et al., 2002), the data then normalized using the print-tip Loess algorithm, linear models were fitted and the significance of apparent changes in expression was assessed using limma (Smyth, 2004; Smyth et al., 2005). P-values obtained from limma's moderated t statistics were adjusted to control the false discovery rate and genes with adjusted values < 0.05 taken to show significant evidence of differential expression. We filtered the data further to exclude genes with low-magnitude changes using a fold-change cutoff of 2. The array design is available from ArrayExpress (www.ebi.ac.uk/arrayexpress) under the accession A-SGRP-3. The full data for this experiment has been submitted to the same database under the accession E-TABM-509.
Supplementary Material
Acknowledgments
RI is an Investigator of Howard Hughes Medical Institute. GB, JS and AI were supported by the WellcomeTrust (Grant 06724). We thank Kay Jagels and Theresa Feltwell for microarray support, Fredrik Söderbom for help with D. discoideum methods and instructions on performing the aggregation assay and Max Isberg for help with composition of figures. We thank Drs. Vicki Auerbuch Stone, Elizabeth Creasey, Matt Heidtman, Tamara O’Conner, Alex Ensminger and Molly Bergman for review of the text, Dr. Janet Smith for help with anti-P-ERK detection and discussions, Dr. Adam Kuspa for the tirA mutant, and Dr. Richard A. Firtel for the ERK1-Myc and MEK plasmids.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Belyi Y, Tabakova I, Stahl M, Aktories K. Lgt: a family of cytotoxic glucosyltransferases produced by Legionella pneumophila. J Bacteriol. 2008;190:3026–3035. doi: 10.1128/JB.01798-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger KH, Isberg RR. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol. 1993;7:7–19. doi: 10.1111/j.1365-2958.1993.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Bloomfield G, Tanaka Y, Skelton J, Ivens A, Kay RR. Widespread duplications in the genomes of laboratory stocks of Dictyostelium discoideum. Genome Biol. 2008;9:R75. doi: 10.1186/gb-2008-9-4-r75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps M, Nichols A, Arkinstall S. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J. 2000;14:6–16. [PubMed] [Google Scholar]
- Chisholm RL, Gaudet P, Just EM, Pilcher KE, Fey P, Merchant SN, Kibbe WA. dictyBase, the model organism database for Dictyostelium discoideum. Nucleic Acids Res. 2006;34:D423–427. doi: 10.1093/nar/gkj090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Zhuchenko O, Kuspa A. Immune-like phagocyte activity in the social amoeba. Science. 2007;317:678–681. doi: 10.1126/science.1143991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosson P, Soldati T. Eat, kill or die: when amoeba meets bacteria. Curr Opin Microbiol. 2008;11:271–276. doi: 10.1016/j.mib.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Eichinger L, Pachebat JA, Glockner G, Rajandream MA, Sucgang R, Berriman M, Song J, Olsen R, Szafranski K, Xu Q, et al. The genome of the social amoeba Dictyostelium discoideum. Nature. 2005;435:43–57. doi: 10.1038/nature03481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajardo M, Schleicher M, Noegel A, Bozzaro S, Killinger S, Heuner K, Hacker J, Steinert M. Calnexin, calreticulin and cytoskeleton-associated proteins modulate uptake and growth of Legionella pneumophila in Dictyostelium discoideum. Microbiology. 2004;150:2825–2835. doi: 10.1099/mic.0.27111-0. [DOI] [PubMed] [Google Scholar]
- Farbrother P, Wagner C, Na J, Tunggal B, Morio T, Urushihara H, Tanaka Y, Schleicher M, Steinert M, Eichinger L. Dictyostelium transcriptional host cell response upon infection with Legionella. Cell Microbiol. 2006;8:438–456. doi: 10.1111/j.1462-5822.2005.00633.x. [DOI] [PubMed] [Google Scholar]
- Feeley JC, Gibson RJ, Gorman GW, Langford NC, Rasheed JK, Mackel DC, Baine WB. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J Clin Microbiol. 1979;10:437–441. doi: 10.1128/jcm.10.4.437-441.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, Tate J, Mistry J, Coggill PC, Sammut SJ, Hotz HR, Ceric G, Forslund K, Eddy SR, Sonnhammer EL, Bateman A. The Pfam protein families database. Nucleic Acids Res. 2008;36:D281–288. doi: 10.1093/nar/gkm960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao LY, Harb OS, Abu Kwaik Y. Utilization of similar mechanisms by Legionella pneumophila to parasitize two evolutionarily distant host cells, mammalian macrophages and protozoa. Infect Immun. 1997;65:4738–4746. doi: 10.1128/iai.65.11.4738-4746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM, Manning G, Liu A, Fey P, Pilcher KE, Xu Y, Smith JL. The Dictyostelium kinome--analysis of the protein kinases from a simple model organism. PLoS Genet. 2006;2:e38. doi: 10.1371/journal.pgen.0020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman E, Jakubowski H. Uncharged tRNA, protein synthesis, and the bacterial stringent response. Mol Microbiol. 1990;4:2035–2040. doi: 10.1111/j.1365-2958.1990.tb00563.x. [DOI] [PubMed] [Google Scholar]
- Hagele S, Kohler R, Merkert H, Schleicher M, Hacker J, Steinert M. Dictyostelium discoideum: a new host model system for intracellular pathogens of the genus Legionella. Cell Microbiol. 2000;2:165–171. doi: 10.1046/j.1462-5822.2000.00044.x. [DOI] [PubMed] [Google Scholar]
- Hagedorn M, Rohde KH, Russell DG, Soldati T. Infection by tubercular mycobacteria is spread by nonlytic ejection from their amoeba hosts. Science. 2009;323:1729–1733. doi: 10.1126/science.1169381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn M, Soldati T. Flotillin and RacH modulate the intracellular immunity of Dictyostelium to Mycobacterium marinum infection. Cell Microbiol. 2007;9:2716–2733. doi: 10.1111/j.1462-5822.2007.00993.x. [DOI] [PubMed] [Google Scholar]
- Henke M, Seidel KM. Association between Legionella pneumophila and amoebae in water. Isr J Med Sci. 1986;22:690–695. [PubMed] [Google Scholar]
- Kagan JC, Stein MP, Pypaert M, Roy CR. Legionella subvert the functions of rab1 and sec22b to create a replicative organelle. J Exp Med. 2004;199:1201–1211. doi: 10.1084/jem.20031706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooperberg C, Sipione S, LeBlanc M, Strand AD, Cattaneo E, Olson JM. Evaluating test statistics to select interesting genes in microarray experiments. Hum Mol Genet. 2002;11:2223–2232. doi: 10.1093/hmg/11.19.2223. [DOI] [PubMed] [Google Scholar]
- Kubori T, Hyakutake A, Nagai H. Legionella translocates an E3 ubiquitin ligase that has multiple U-boxes with distinct functions. Mol Microbiol. 2008;67:1307–1319. doi: 10.1111/j.1365-2958.2008.06124.x. [DOI] [PubMed] [Google Scholar]
- Kuspa A, Loomis WF. Tagging developmental genes in Dictyostelium by restriction enzyme-mediated integration of plasmid DNA. Proc Natl Acad Sci USA. 1992;89:8803–8807. doi: 10.1073/pnas.89.18.8803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick VP, Isberg RR. NF-{kappa}B translocation prevents host cell death after low-dose challenge by Legionella pneumophila. J Exp Med. 2006;203:2177–2189. doi: 10.1084/jem.20060766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Clarke M. Dynamic properties of Legionella-containing phagosomes in Dictyostelium amoebae. Cell Microbiol. 2005;7:995–1007. doi: 10.1111/j.1462-5822.2005.00528.x. [DOI] [PubMed] [Google Scholar]
- Maeda M, Lu S, Shaulsky G, Miyazaki Y, Kuwayama H, Tanaka Y, Kuspa A, Loomis WF. Periodic signaling controlled by an oscillatory circuit that includes protein kinases ERK2 and PKA. Science. 2004;304:875–878. doi: 10.1126/science.1094647. [DOI] [PubMed] [Google Scholar]
- Marston BJ, Lipman HB, Breiman RF. Surveillance for Legionnaires' disease. Risk factors for morbidity and mortality. Arch Intern Med. 1994;154:2417–2422. [PubMed] [Google Scholar]
- Mendoza MC, Booth EO, Shaulsky G, Firtel RA. MEK1 and protein phosphatase 4 coordinate Dictyostelium development and chemotaxis. Mol Cell Biol. 2007;27:3817–3827. doi: 10.1128/MCB.02194-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai H, Kagan JC, Zhu X, Kahn RA, Roy CR. A bacterial guanine nucleotide exchange factor activates ARF on Legionella phagosomes. Science. 2002;295:679–682. doi: 10.1126/science.1067025. [DOI] [PubMed] [Google Scholar]
- O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signaling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- Peracino B, Wagner C, Balest A, Balbo A, Pergolizzi B, Noegel AA, Steinert M, Bozzaro S. Function and mechanism of action of Dictyostelium Nramp1 (Slc11a1) in bacterial infection. Traffic. 2006;7:22–38. doi: 10.1111/j.1600-0854.2005.00356.x. [DOI] [PubMed] [Google Scholar]
- Segal G, Shuman HA. Legionella pneumophila utilizes the same genes to multiply within Acanthamoeba castellanii and human macrophages. Infect Immun. 1999;67:2117–2124. doi: 10.1128/iai.67.5.2117-2124.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segall JE, Kuspa A, Shaulsky G, Ecke M, Maeda M, Gaskins C, Firtel RA, Loomis WF. A MAP kinase necessary for receptor-mediated activation of adenylyl cyclase in Dictyostelium. J Cell Biol. 1995;128:405–413. doi: 10.1083/jcb.128.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S, Case CL, Archer KA, Nogueira CV, Kobayashi KS, Flavell RA, Roy CR, Zamboni DS. Type IV secretion-dependent activation of host MAP kinases induces an increased proinflammatory cytokine response to Legionella pneumophila. PLoS Pathog. 2008;4:e1000220. doi: 10.1371/journal.ppat.1000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillo A, Bloomfield G, Balest A, Balbo A, Pergolizzi B, Peracino B, Skelton J, Ivens A, Bozzaro S. Genome-wide transcriptional changes induced by phagocytosis or growth on bacteria in Dictyostelium. BMC Genomics. 2008;9:291. doi: 10.1186/1471-2164-9-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3 doi: 10.2202/1544-6115.1027. Article3. [DOI] [PubMed] [Google Scholar]
- Smyth GK, Michaud J, Scott HS. Use of within-array replicate spots for assessing differential expression in microarray experiments. Bioinformatics. 2005;21:2067–2075. doi: 10.1093/bioinformatics/bti270. [DOI] [PubMed] [Google Scholar]
- Sobko A, Ma H, Firtel RA. Regulated SUMOylation and ubiquitination of DdMEK1 is required for proper chemotaxis. Dev Cell. 2002;2:745–756. doi: 10.1016/s1534-5807(02)00186-7. [DOI] [PubMed] [Google Scholar]
- Solomon JM, Rupper A, Cardelli JA, Isberg RR. Intracellular growth of Legionella pneumophila in Dictyostelium discoideum, a system for genetic analysis of host-pathogen interactions. Infect Immun. 2000;68:2939–2947. doi: 10.1128/iai.68.5.2939-2947.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman M. Cultivation and synchronous morphogenesis of Dictyostelium under controlled experimental conditions. In: Spudich JA, editor. Methods in Cell Biology. Orlando, FL: Ac. Press; 1987. pp. 9–29. [DOI] [PubMed] [Google Scholar]
- Swanson MS, Isberg RR. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect Immun. 1995;63:3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber SS, Ragaz C, Reus K, Nyfeler Y, Hilbi H. Legionella pneumophila exploits PI(4)P to anchor secreted effector proteins to the replicative vacuole. PLoS Pathog. 2006;2:e46. doi: 10.1371/journal.ppat.0020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland H, Ullrich S, Lang F, Neumeister B. Intracellular multiplication of Legionella pneumophila depends on host cell amino acid transporter SLC1A5. Mol Microbiol. 2005;55:1528–1537. doi: 10.1111/j.1365-2958.2005.04490.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.