Abstract
Objective:
To determine the predictors for early versus later (breastfeeding) transmission of HIV-1.
Methods:
Secondary data analysis was performed on HIV Network for Prevention Trials 012, a completed randomized clinical trial assessing the relative efficacy of nevirapine (NVP) versus zidovudine in reducing mother-to-child transmission (MTCT) of HIV-1. We used Cox regression analysis to assess risk factors for MTCT. The ViroSeq HIV genotyping and a sensitive point mutation assay were used to detect NVP resistance mutations.
Results:
In this subset analyses, 122 of 610 infants were HIV infected, of whom 99 (81.1%) were infected early (first positive polymerase chain reaction ≤56 days). Incidence of MTCT after 56 days was low [0.7% per month (95% confidence interval, CI: 0.4 to 1.0)], but continued through 18 months. In multivariate analyses, early MTCT “factors” included NVP versus zidovudine (hazard ratio (HR) = 0.57, 95% CI: 0.38 to 0.86), pre-entry maternal viral load (VL, HR = 1.76, 95% CI: 1.28 to 2.41), and CD4 cell count (HR = 1.16, 95% CI: 1.05 to 1.28). Maternal VL (6–8 weeks) was associated with late MTCT (HR = 3.66, 95% CI: 1.78 to 7.50), whereas maternal NVP resistance (6–8 weeks) was not.
Conclusions:
Maternal VL was the best predictor of both early and late transmission. Maternal NVP resistance at 6–8 weeks did not predict late transmission.
Keywords: early/late postnatal, MTCT, HIV-1
INTRODUCTION
Globally, pediatric HIV-1 infections remain very high with an estimated 370,000 new infections in 2007. The vast majority (>90%) of HIV-infected children live in resource-limited breastfeeding settings in sub-Saharan Africa, where HIV seroprevalence among pregnant women ranges from 2% to 40%.1,2
Mother-to-child transmission (MTCT) of HIV-1 may occur during pregnancy, at labor/delivery, or after birth through breastfeeding.3-5 Among populations where the norm is to breast-feed into the second year, 30%–50% of all HIV MTCT is estimated to occur through breastfeeding.6,7
Two studies have suggested that a large proportion of breast milk transmission of HIV occurs quite early, by 1–2 months of age.7,8 A number of reports, including a meta-analysis, have reported that there is then a low (<1% per month) but ongoing risk of transmission throughout breast-feeding after the first 6–8 weeks post delivery.9-11
Identified risk factors for transmission during breast-feeding include increased severity of maternal disease, mastitis and breast abscess, mixed infant feeding, maternal serocon-version during lactation, lower maternal CD4 cell count, and higher maternal HIV viral load (VL).12-15 HIV subtypes C and D have also been associated with an increased risk of breast milk transmission compared with subtype A.16 However, there is a paucity of literature addressing specific clinical and laboratory factors associated with early versus late MTCT among breastfeeding women.
We previously reported that single-dose nevirapine (SD NVP) given to the mother in labor and to the infant after birth compared with zidovudine (ZDV) given to the mother in labor and to the infant daily for 1 week after birth was safe and effective for reducing the risk of transmission of HIV-1 from mothers to their newborns (HIV Network for Prevention Trials, HIVNET 012 study).17,18 The 4% late transmission rate (between 6 and 8 weeks and 18 months) seen in the NVP arm was substantially lower than rates attributed to late breastfeeding in other populations, which ranged between 8% and 16%.7,8,19-21
The follow-up of HIVNET 012 infants through 18 months of age provided a unique opportunity to assess risk factors for early and late HIV MTCT among a group of women and infants receiving the SD NVP regimen or an ultra-short course ZDV regimen. The primary aims of this analysis were to (a) assess risk factors associated with early and late HIV-1 MTCT in the HIVNET 012 trial, (b) compare these findings to those from other perinatal HIV prevention trials, and (c) assess whether there were differences in transmission risk in the NVP versus ZDV arms of the trial across subgroups based on CD4 cell count strata, VL strata, or the presence of maternal NVP-resistant HIV variants.
METHODS
Participants and Procedures Used in the HIVNET 012 Trial
The methods used for identification of research participants, determination of eligibility, informed consent, randomization, data collection, and laboratory specimen processing and testing have been described in detail elsewhere.17,18
Both Institutional Review Boards in Uganda and the United States reviewed and approved the HIVNET 012 protocol before implementation. Written informed consent was obtained from all study women.
Maternal demographic and clinical information were collected at pre-entry, delivery, and 7 days and 6–8 weeks after delivery. Laboratory studies included CD4 cell counts, which were done at pre-entry, and complete blood counts, which were done at pre-entry and delivery. VL was determined at pre-entry, delivery, and 7 days and 6–8 weeks post delivery using the HIV-1 RNA polymerase chain reaction (PCR) Roche AMPLICOR MONITOR assay. Before November 1998, assays were performed using the version 1.0 kit with additional primers to enhance the detection of non-B subtypes. After that date, the 1.5 version kit was used.22 Antiretroviral drugs for treatment were not generally available at that time in Uganda, and none of the women received other antiretroviral medication except for the peripartum study drugs.
Infant demographic and clinical information were collected at birth (age 1–3 days); 7 days; 6–8 weeks; 10 weeks; 14–16 weeks; and at 6, 9, 12, and 18 months. Serum chemistries were done at birth, 7 days, and 6–8 weeks. Complete blood counts were done at birth, 7 days, 6–8 weeks, 14 weeks, 12 months, and 18 months. CD4 cell counts were done at birth, 14 weeks, 12 months, and 18 months. Qualitative plasma HIV-1 RNA PCR assays (as described above) were done at birth, 6–8 weeks, 14 weeks, and 12 months.
If the HIV-1 RNA PCR test was positive, a second confirmatory test was run. Quantitative HIV-1 RNA PCR was done subsequently for all HIV-infected children. At 18 months of age, infants were tested for HIV-1 antibody using an enzyme immunoassay (EIA). If the EIA was reactive, the result was confirmed by HIV-1 Western blot. Diagnosis of HIV infection in infants was based on at least 1 positive qualitative RNA PCR result or a reactive EIA/Western blot.
HIV Subtyping and Resistance Testing
HIV subtypes were determined for women in the NVP arm.23,24 HIV genotyping was performed using the ViroSeq HIV Genotyping System (Celera Diagnostics, Alameda, CA), and the K103N NVP resistance mutation was detected using a sensitive point mutation assay, LigAmp.24,25 Analysis with the LigAmp assay was limited to women with HIV-1 subtypes A and D.
Definitions of Early and Late Transmission
Early transmission (including in utero, intrapartum, and very early postpartum transmission) was defined as the first positive HIV RNA PCR test result obtained either before or at 56 days of age or after 56 days of age, where the midpoint between the last negative and first positive tests was before or at 56 days of age.
Late transmission (presumed to be due to breast milk transmission) was defined as the first positive HIV RNA PCR test result obtained either after 56 days of age among infants who were known to be HIV uninfected at age 56 days or after 56 days of age, where the midpoint between the last negative and first positive tests was after 56 days of age.
Statistical Analysis
Baseline characteristics among women and their infants with early, late, or no transmission were compared using χ2 tests and Wilcoxon rank tests. In survival analysis of early transmission, infants who were uninfected beyond 56 days were censored at day 56. Infants whose midpoint of last negative and first positive test results was on or before 56 days were considered to be HIV infected and the event time was set to the midpoint. Those whose midpoint of last negative and first positive test result was after 56 days were considered as uninfected in regard to early transmission and were censored at the last negative test result. The event time for all other infected infants was set to the age when they first tested positive. In survival analysis of late transmission, all infants not infected by 56 days were included. Analyses to evaluate predictors of both early and late HIV transmission were conducted using univariate and multivariate Cox regression models. In the univariate analyses, factors were considered if they had been associated with HIV transmission in previous studies or were biologically plausible.
Subgroup analyses comparing transmission probabilities across the 2 arms by CD4 cell count and VL categories were based on pre-entry CD4 cell count (<200, 200–349, 350–499, and ≥500 cells/μL) and pre-entry or 6- to 8-week maternal VL (<500, 500–9999, 10,000–49,999, ≥50,000 copies/mL). These analyses were conducted using Kaplan–Meier methods. Breastfeeding HIV incidence rates and the corresponding 95% confidence intervals (CIs) were calculated based on the person-year analysis assuming an underlying Poisson distribution. Infants were censored on the date breastfeeding ceased.
RESULTS
This analysis from HIVNET 012 includes 610 firstborn infants with HIV test results available (Fig. 1). There were 122 HIV-infected infants, of which 99 (81%) had early HIV infection and 23 (19%) were late transmissions. Fifty-six infants (46%) overall had HIV detected at birth (in utero transmissions), 43 (35%) overall were infected within 56 days of life (intrapartum/early breastfeeding transmissions). Of the 610 included in the early transmission study, 114 died or were diagnosed with HIV infection at or before 56 days. Hence, 496 were alive and free from HIV infection after 56 days; 491 of those infants had HIV testing performed after 56 days and were included in the analysis of predictors of late transmission. Among the 5 infants not included in the analysis, 2 died at ages 11 and 12 weeks and 3 were lost to follow-up (2 at 10 weeks and 1 at 4 months). There were 23 HIV infections among the 491 infants included in the late HIV transmission analyses (4.7%). The breastfeeding HIV incidence per infant month of follow-up during the late transmission period was 0.8% (0.3%–1.7%) between 2 and 4 months, 0.6% (0.3%–1.0%) between 4 and 12 months and 0.8% (0.2%–2.3%) between 12 and 18 months. The overall (2–18 months) HIV incidence per infant month was 0.7% (0.4%–1.0%).
FIGURE 1.
HIVNET 012 predictors of early and late transmission.
HIV subtyping was performed for 297 of the 306 women enrolled in the NVP arm. The following subtypes were identified: 156 women (52.5%) had subtype A, 7 (2.4%) had subtype C, 104 (35.0%) had subtype D, and 30 (10.1%) had intersubtype recombinant strains.
The population baseline characteristics of the women and infants by infection period are summarized in Table 1. Maternal CD4 cell count and VL at pre-entry and 6–8 weeks and maternal age at pre-entry were significantly different for the uninfected and infected cohorts, whereas other demographic characteristics were similar. However, the test for trends across maternal age categories <20, 20–35, and >35 years was not statistically significant (P = 0.22). Breastfeeding practices were also significantly different among the 3 groups, with more mothers of the infected infants choosing to continue breastfeeding.
TABLE 1.
Characteristics of the Study Population by Infection Period
| HIV Infection Period | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Category | Early (n = 99) n/N (%) |
Late (n = 23) n/N (%) |
Uninfected (n = 488) n/N (%) |
Total (n = 610) n/N (%) |
P |
| Mothers | ||||||
| Maternal age | Mean (SE) | 25.4 (0.48) | 26.0 (0.92) | 24.4 (0.19) | 24.6 (0.18) | 0.0237 |
| Marital status | Married | 87/99 (87.9%) | 20/23 (87.0%) | 418/488 (85.7%) | 525/610 (86.1%) | 0.9282 |
| Never married | 10/99 (10.1%) | 3/23 (13.0%) | 60/488 (12.3%) | 73/610 (12.0%) | ||
| D/S/W* | 2/99 (2.0%) | 0/23 (0.0%) | 10/488 (2.0%) | 12/610 (2.0%) | ||
| Education level | No education | 11/99 (11.1%) | 1/23 (4.3%) | 20/483 (4.1%) | 32/605 (5.3%) | 0.0600 |
| Primary | 56/99 (56.6%) | 16/23 (69.6%) | 288/483 (59.6%) | 360/605 (59.5%) | ||
| Above primary | 32/99 (32.3%) | 6/23 (26.1%) | 175/483 (36.2%) | 213/605 (35.2%) | ||
| Caesarian section | Yes | 10/99 (10.1%) | 2/23 (8.7%) | 60/488 (12.3%) | 72/610 (11.8%) | 0.7400 |
| >4 h ROM† | Yes | 13/97 (13.4%) | 5/21 (23.8%) | 65/467 (13.9%) | 83/585 (14.2%) | 0.4331 |
| Prima gravid | Yes | 13/99 (13.1%) | 4/23 (17.4%) | 71/488 (14.5%) | 88/610 (14.4%) | 0.8589 |
| Hemoglobin (g/dL)‡ | Mean (SE) | 10.4 (0.14) | 10.7 (0.23) | 10.6 (0.06) | 10.6 (0.06) | 0.2970 |
| CD4 cell count pre-entry | Mean (SE) | 357 (22.48) | 300 (51.34) | 504 (11.77) | 472 (10.59) | <0.0001 |
| CD4 cell count pre-entry | <200 | 28/98 (28.6%) | 12/23 (52.2%) | 55/486 (11.3%) | 95/607 (15.7%) | <0.0001 |
| 200–349 | 23/98 (23.5%) | 4/23 (17.4%) | 78/486 (16.0%) | 105/607 (17.3%) | ||
| 350–499 | 22/98 (22.4%) | 3/23 (13.0%) | 127/486 (26.1%) | 152/607 (25.0%) | ||
| ≥500 | 25/98 (25.5%) | 4/23 (17.4%) | 226/486 (46.5%) | 255/607 (42.0%) | ||
| Log10 HIV RNA pre-entry | Mean (SE) | 4.7 (0.06) | 4.8 (0.11) | 4.3 (0.04) | 4.4 (0.03) | <0.0001 |
| HIV RNA pre-entry | <500 | 0/98 (0.0%) | 0/20 (0.0%) | 23/465 (4.9%) | 23/583 (3.9%) | <0.0001 |
| 500–9999 | 11/98 (11.2%) | 2/20 (10.0%) | 137/465 (29.5%) | 150/583 (25.7%) | ||
| 10,000–49,999 | 35/98 (35.7%) | 6/20 (30.0%) | 173/465 (37.2%) | 214/583 (36.7%) | ||
| ≥50,000 | 52/98 (53.1%) | 12/20 (60.0%) | 132/465 (28.4%) | 196/583 (33.6%) | ||
| Log10 HIV RNA 6–8 wks | Mean (SE) | — | 5.2 (0.14) | 4.4 (0.03) | 4.5 (0.03) | <0.0001 |
| HIV RNA 6–8 wks | <500 | — | 0/23 (0.0%) | 7/467 (1.5%) | 7/586 (1.2%) | <0.0001 |
| 500–9999 | — | 1/23 (4.3%) | 127/467 (27.2%) | 137/586 (23.4%) | ||
| 10,000–49,999 | — | 5/23 (21.7%) | 155/467 (33.2%) | 184/586 (31.4%) | ||
| ≥50,000 | — | 17/23 (73.9%) | 178/467 (38.1%) | 258/586 (44.0%) | ||
| Infants | ||||||
| Infant sex | Female | 53/99 (53.5%) | 11/23 (47.8%) | 244/488 (50.0%) | 308/610 (50.5%) | 0.7868 |
| Birth weight (g) | Mean (SE) | 3074 (51.61) | 3175 (96.42) | 3150 (19.90) | 3138 (18.37) | 0.2914 |
| Low birth weight | Yes | 10/99 (10.1%) | 1/21 (4.8%) | 23/479 (4.8%) | 34/599 (5.7%) | 0.1143 |
| Infant is a twin | Yes | 3/99 (3.0%) | 0/23 (0.0%) | 8/488 (1.6%) | 11/610 (1.8%) | 0.5122 |
| Breastfeeding at weeks 6–8 | Yes | 89/92 (96.7%) | 21/21 (100.0%) | 451/459 (98.3%) | 561/572 (98.1%) | 0.5057 |
| Breastfeeding at month 12 | Yes | 51/66 (77.3%) | 9/22 (40.9%) | 135/443 (30.5%) | 195/531 (36.7%) | <0.0001 |
| Breastfeeding at month 18 | Yes | 28/58 (48.3%) | 5/21 (23.8%) | 32/433 (7.4%) | 65/512 (12.7%) | <0.0001 |
Divorced, separated, or widowed.
ROM (rupture of membranes).
An inclusion criteria for the study were hemoglobin ≥7.5 g/dL.
Predictors of Early HIV Transmission
Women in the NVP arm had a significantly lower risk of early transmission than women in the ZDV arm [hazard ratio (HR) = 0.57, 95% CI: 0.38 to 0.85]. Pre-entry maternal log10 VL (HR = 2.11, 95% CI: 1.59 to 2.80 per unit increase) and pre-entry maternal CD4 cell count (HR = 1.24, 95% CI: 1.13 to 1.36 per decrease of 100 cells/mm3) were significantly associated with early transmission, whereas maternal age, duration of labor, prolonged rupture of membranes (>4 hours or not), mode of delivery (caesarean section or not), maternal HIV subtype (D versus A), infant sex, and low birth weight were not.
In a multivariate model, maternal log10 VL (HR = 1.76, 95% CI: 1.28 to 2.41, per unit increase), maternal CD4 cell count (HR = 1.16, 95% CI: 1.05 to 1.28, per decrease of 100 cells/mm3), and NVP compared with ZDV treatment (HR = 0.57, 95% CI: 0.38 to 0.86) remained significant predictors of early HIV transmission (Table 2).
TABLE 2.
Multivariate Cox Models of Risk Factors for HIVNET 012
| Predictors | Hazard Ratio (95% CI) | P |
|---|---|---|
| Early HIV transmission | ||
| Maternal CD4 cell count (pre-entry)* | 1.16 (1.05 to 1.28) | 0.0031 |
| Maternal Log10 HIV RNA (pre-entry)† | 1.76 (1.28 to 2.41) | 0.0004 |
| Treatment (NVP versus ZDV) | 0.57 (0.38 to 0.86) | 0.0075 |
| Late HIV transmission | ||
| Maternal CD4 cell count (pre-entry)* | 1.22 (0.98 to 1.52) | 0.0710 |
| Maternal Log10 HIV RNA (6–8 wks)† | 3.66 (1.78 to 7.50) | 0.0004 |
For a decrease of 100 cells per cubic millimeter.
For every unit increase or an increase of Log10 HIV RNA copies per milliliter.
Predictors of Late HIV Transmission During Breastfeeding
In univariate analysis, statistically significant risk factors included 6–8 weeks postpartum maternal log10 VL (HR = 4.95, 95% CI: 2.56 to 9.60 per unit increase), as well as pre-entry maternal log10 VL (HR = 2.76, 95% CI: 1.51 to 5.04, per unit increase), and pre-entry maternal CD4 cell count (HR = 1.48, 95% CI: 1.19 to 1.84, per decrease of 100 cells/mm3). However, there was no significant difference in risk of late HIV transmission for women in the NVP arm compared with the ZDV arm (HR = 0.69, 95% CI: 0.30 to 1.56). Maternal age, duration of labor, prolonged rupture of membranes (>4 hours or not), mode of delivery (caesarean section or not), maternal HIV subtype (D versus A), infant sex, and low birth weight were not associated with an increased risk of late transmission.
In a multivariate model, 6–8 weeks postpartum maternal log10 VL (HR = 3.66, 95% CI: 1.78 to 7.50, per unit increase) was the strongest predictor of late HIV transmission (Table 2). Pre-entry maternal CD4 cell count had a similar HR for late and early HIV transmission risk, although it was not statistically significant for late transmission (HR = 1.22, 95% CI: 0.98 to 1.52, per decrease of 100 cells/mm3).
NVP Resistance and Late Transmission Risk
We assessed the potential impact of NVP resistance on late transmission risk among mothers enrolled in the NVP arm. Fifty-eight of 239 women (24%) who had HIV genotyping results at 6–8 weeks had detectable NVP resistance (detection of one or more NVP resistance mutation using the ViroSeq assay). Ninety of 207 women (43%) who had K103N measured with the more sensitive LigAmp assay at 6–8 weeks had K103N detected above the assay cutoff (>0.5% K103N). Although there was a higher rate of late transmission among women with NVP resistance detected with the ViroSeq system, that difference was not statistically significant (HR = 1.68, 95% CI: 0.42 to 6.72). Similar results were obtained using the LigAmp assay for K103N detection (HR = 4.09, 95% CI 0.82 to 20.24). In 2 separate multivariate models, one using ViroSeq and the other LigAmp assay results, adjusting for pre-entry maternal CD4 cell count and maternal log10 VL at 6–8 weeks, NVP resistance did not predict late transmission (HR = 0.37, 95% CI: 0.07 to 2.04 for ViroSeq and HR = 1.37, 95% CI: 0.24 to 7.88 for LigAmp). However, in both models, maternal log10 VL at 6–8 weeks was strongly predictive of late transmission (HR = 8.87, 95% CI: 2.32 to 33.90 for ViroSeq and HR= 7.60, 95% CI: 1.91 to 30.19 for LigAmp).
Transmission Rates by Subgroups
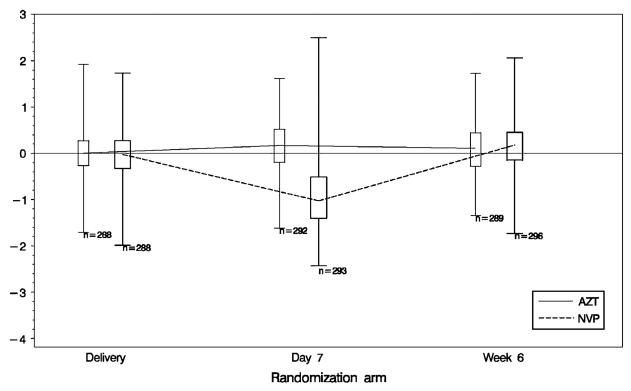
Figure 2 demonstrates the changes in maternal VL from delivery through the first 6–8 weeks postpartum among women randomized to the NVP and ZDV arms. The plasma VL for women in the ZDV arm remained stable throughout, although there was a significant drop in plasma VL by 7 days postpartum for those in the NVP arm, which returned to baseline levels by 6–8 weeks postpartum.
FIGURE 2.
Mothers' log10 HIV RNA change from delivery to week 6 by randomization arm. Box ends represent 25th and 75th percentiles. Center horizontal line is drawn at median. Whisker ends represent 1st and 99th percentiles.
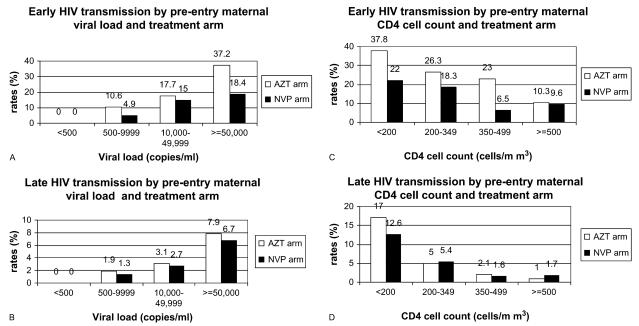
Rates of early and late HIV transmission were directly related to maternal VL and inversely related to maternal CD4 cell count during both early and late transmission periods. However, the transmission probabilities were substantially higher in the early relative to the late period in all VL and CD4 cell count categories (Figs. 3A–D). There were no transmissions among women whose pre-entry VL was <500 copies per milliliter. The lowest maternal pre-entry VL observed among mothers with early HIV transmission occurred in a woman with 4945 HIV-1 RNA copies per milliliter. Similarly, the lowest maternal 6- to 8-week VL associated with late transmission occurred in a woman with 4251 HIV-1 RNA copies per milliliter.
FIGURE 3.
Early and late HIV transmission by pre-entry maternal VL, maternal CD4 cell count, and treatment arm.
The differences in early transmission between women in the NVP and ZDV arms were consistent with an overall 43% relative efficacy across all VLs. However, because of the substantial increase in transmission risk with increase in log10 VL, the absolute differences in early transmission risk by treatment arm were more substantial when the VL was >50,000 RNA copies per milliliter (Fig. 3A).
Figure 3B illustrates the increase in probability of late transmission with higher baseline VL, which cumulatively exceeds 6.5% when the VL was greater than 50,000 copies per milliliter. Late transmission probabilities as high as 12%–17% were observed for mothers with pre-entry CD4 counts less than 200 cells per cubic millimiter (Fig. 3D). Although the observed probabilities of late transmission were slightly lower among women in the NVP arm compared with those in the ZDV arm, the difference was not statistically significant.
DISCUSSION
Analysis of risk factors for early and late HIV transmission in HIVNET 012 revealed a number of important findings. First, most transmissions occurred early. Second, in multivariate analyses, high pre-entry maternal VL, low CD4 cell count, and prophylaxis regimen (ZDV versus NVP) were all predictive of early transmission, whereas only high 6- to 8-week maternal VL predicted late transmission. Third, there was no transmission, either late or early, among women with pre-entry VL < 500 HIV RNA copies per milliliter. Fourth, SD NVP halved the risk of early HIV transmission compared with the ultra-short course ZDV regimen after adjustment for CD4 and VL, including among those mothers with high VL and low CD4 cell count. There was no evidence that maternal NVP resistance at 6–8 weeks was independently associated with risk of late transmission, using either a standard genotyping assay or a sensitive point mutation assay. Finally, there were no significant differences in early or late transmission risk among women with HIV subtypes A and D.
The majority (>80%) of infant infections occurred early, by 56 days of age. About two-thirds of the early infections occurred in utero and about a third occurred from birth to 6–8 weeks, due either to intrapartum or early breast milk transmission. Other studies have shown that a large proportion of breast milk transmission occurs by 1–2 months of age.7,8 These studies indicate an absolute risk of transmission due to breastfeeding of between 2.7% and 4.2% per month in the early period. Providing a biological basis for these findings, investigators in Nairobi found that the median HIV VL in colostrum and early breast milk was higher than the median HIV VL in milk collected >14 days after birth, suggesting that a higher breast milk VL could lead to higher transmission rates in the first weeks of life.13 Our findings of a sharp and significant decrease in maternal plasma VL by 1-week postpartum in the NVP arm suggests a biological plausibility for the efficacy of SD NVP relative to the ultra-short course ZDV in reducing early HIV transmission through a similar reduction in VL in colostrum and early breast milk. Chung et al26 found significant and sustained decreases in VL in breast milk during the first weeks postpartum among women who had received SD NVP versus women who received ZDV. After the first 6–8 weeks postpartum, there is a low ongoing risk of transmission of about 0.6%–0.8% per month, which is consistent with several studies.6-11,27
We found that maternal pre-entry and 6- to 8-week VL were the strongest predictors for early and late transmission, respectively. These results are consistent with findings from other trials in African breastfeeding populations, reinforcing that women with either primary HIV infection or advanced HIV disease are most likely to transmit HIV during breastfeeding.7,19 This supports the hypothesis that interventions aimed at either lowering maternal VL in breast milk or giving prophylaxis to the infant during the breastfeeding period could substantially reduce the risk of transmission.
These analyses demonstrate that SD NVP provides protection against early postpartum transmission in women, regardless of their pre-entry HIV VL or pre-entry CD4 cell count. However, use of SD NVP did not impact on transmission risk after 56 days, which is not surprising, because NVP is not detectable in most women >3 weeks after SD NVP.
The greatest absolute change in risk of early transmission by drug arm (NVP versus ZDV) was seen in women with high VL (>50,000 RNA copies/mL, pre-entry) and among women with advanced HIV disease (CD4 cell count <200 cells/mm3). The relative lack of effect of ZDV prophylaxis compared with NVP in women with high VL in this study is consistent with results from a West African ZDV placebo-controlled trial.19 Late transmission risk was similar among women in the NVP and ZDV arms, regardless of VL and CD4 cell count.
Previously, Eshleman et al found that a subset of women who received SD NVP in HIVNET 012 had detectable NVP resistance mutations at 6–8 weeks postpartum, which persisted at low levels in some women for years.28,29 Emergence and persistence of NVP resistance was highly correlated with HIV subtype (D>A) and pre-NVP maternal VL.28,29 After adjusting for 6- to 8-week maternal VL, we found no statistically significant difference in late transmission risk among women with or without detectable NVP resistance mutations at 6–8 weeks.
There are certain caveats to these analyses. First, it is important to note that this was a hospital-based study, performed in a major Ugandan city where the average age of breastfeeding cessation in HIV-infected women receiving infant feeding counseling was 9 months. Thus, this report may tend to underestimate the risk of postnatal transmission in rural settings, where women may breast-feed longer and where breastfeeding practices may differ from those in this cohort. Also, we did not have information available on factors such as clinical or subclinical mastitis, which have previously been shown to be risk factors for postnatal transmission.30 Our analysis of the late transmission risk may be subject to postrandomization bias, as infants remaining uninfected at 6–8 weeks are more likely to be in the NVP arm, and/or to have mothers with lower VL and higher CD4 cell counts. Finally, our failure to detect an association between HIV subtype or HIV resistance and late transmission as well as other factors may have been limited by the sample size.
CONCLUSIONS
There is a need for more intensive interventions to reduce in utero, intrapartum, and early breastfeeding transmissions. New strategies are focusing on comparing use of the current World Health Organization–recommended short-course AZT in the third trimester plus SD NVP and a tail versus the use of maternal highly active antiretroviral therapy during the antepartum and peripartum period. In addition, because breastfeeding remains critical for infant health and survival in resource-limited settings with high HIV prevalence, there is an urgent need to investigate the efficacy of maternal and infant antiretroviral regimens to protect infants from HIV infection throughout the breastfeeding period. Either maternal highly active antiretroviral therapy or extended infant NVP prophylaxis throughout breastfeeding may help decrease the risk of later HIV transmission among infants in breast-feeding populations.
ACKNOWLEDGMENTS
We thank all the Ugandan mothers and their babies who volunteered to participate in this trial, all the staff of the MUJHU Research Collaboration for their dedication and commitment in the conduct of the trial, the staff at Family Health International for their support of the study, and the staff at Statistical Center for HIV/AIDS Research and Prevention at the Fred Hutchinson Cancer Research Center for supporting the statistical analyses. This article is dedicated to late Prof Francis Mmiro who focused his career on improving the lives of HIV-infected women and their infants and who was the Uganda Principal Investigator for the HIVNET 012 study. F.A.M., J.B.J., L.A.G., P.M.M., and M.G.F. designed and implemented the HIVNET 012 clinical trial, monitored adverse events, interpreted the data, and wrote this article. C.N. recruited the study participants to the HIVNET 012 clinical trial and contributed to writing the article. A.K.M. and D.D. did the statistical analysis and contributed to writing of this article. S.H.E. and her laboratory carried out the resistance testing; she and J.A. contributed to writing the article.
Supported by (a) the HIV Network for Prevention Trials (HIVNET) and sponsored by the US National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health, Department of Health and Human Services, through contract N01-AI-35173 with Family Health International, contract N01-AI-45200 with Fred Hutchinson Cancer Research Center, and subcontract (N01-AI-35173-417) with Johns Hopkins University, (b) the HIV Prevention Trials Network sponsored by the NIAID, National Institutes of Child Health and Human Development, National Institute on Drug Abuse, National Institute of Mental Health, and Office of AIDS Research, of the National Institutes of Health, Department of Health and Human Services (U01-AI-46745, U01-AI-046702, U01-AI-48054, and U01-AI-068613), and (c) the International Maternal Pediatric Adolescent AIDS Clinical Trials Group sponsored by the NIAID and National Institutes of Child Health and Human Development (U01-AI-068632, U01-AI-069530). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIAID.
Footnotes
A poster presentation was made at the 15th Conference on Retroviruses and Opportunistic Infections (CROI), February 3–6, 2008, Boston, MA.
None of the authors has a major conflict of interest including financial, consultant, or institutional.
REFERENCES
- 1.2008 Report on the Global AIDS Epidemic. UNAIDS. 2008 Available at: http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/2008_Global_report.asp. Accessed December 30, 2009.
- 2.WHO . Expert Consultation on new and emerging evidence on the use of antiretroviral drugs for the prevention of mother-to-child transmission of HIV. Geneva: Nov 17–19, 2008. Available at: http://www.who.int/hiv/topics/mtct/en/. Accessed January 2, 2009. [Google Scholar]
- 3.Kourtis AP, Bulterys M, Nesheim SR, et al. Understanding the timing of HIV transmission from mother to infant. JAMA. 2001;285:709–712. doi: 10.1001/jama.285.6.709. [DOI] [PubMed] [Google Scholar]
- 4.Newell ML. Mechanisms and timing of mother-to-child transmission of HIV-1. AIDS. 1998;12:831–837. doi: 10.1097/00002030-199808000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Dabis F, Ekpini ER. HIV-1/AIDS and maternal and child health in Africa. Lancet. 2002;359:2097–2104. doi: 10.1016/S0140-6736(02)08909-2. [DOI] [PubMed] [Google Scholar]
- 6.De Cock K, Fowler MG, Mercier E, et al. Prevention of mother-to-child HIV transmission in resource- poor countries: translating research into policy and practice. JAMA. 2000;283:1175–1182. doi: 10.1001/jama.283.9.1175. [DOI] [PubMed] [Google Scholar]
- 7.Nduati R, John G, Mbori-Ngacha D, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA. 2000;283:1167–1174. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 8.Moodley D, Moodley J, Coovadia H, et al. A multicenter randomized controlled trial of nevirapine versus a combination of zidovudine and lamivudine to reduce intrapartum and early postpartum mother-to child transmission of human immunodeficiency virus type 1. J Infect Dis. 2003;187:725–735. doi: 10.1086/367898. [DOI] [PubMed] [Google Scholar]
- 9.The BHITS Group Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis. 2004;189:2154–2166. doi: 10.1086/420834. [DOI] [PubMed] [Google Scholar]
- 10.Miotti PG, Taha TE, Kumwenda NI, et al. HIV transmission through breastfeeding: a study in Malawi. JAMA. 1999;282:744–749. doi: 10.1001/jama.282.8.744. [DOI] [PubMed] [Google Scholar]
- 11.Leroy V, Newell ML, Dabis F, et al. International multicentre pooled analysis of late post natal mother-to-child transmission of HIV-1 infection. Lancet. 1998;352:597–600. doi: 10.1016/s0140-6736(98)01419-6. [DOI] [PubMed] [Google Scholar]
- 12.Coutsoudis A, Rollins N. Breast-feeding and HIV transmission: the jury is still out. J Pediatr Gastroenterol Nutr. 2003;36:434–442. doi: 10.1097/00005176-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 13.John GC, Nduati R, Mbori-Ngacha DA, et al. Correlates of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission: Association with maternal plasma HIV-1 RNA load, genital HIV-1 DNA shedding, and breast infections. J Infect Dis. 2001;183:206–212. doi: 10.1086/317918. [DOI] [PubMed] [Google Scholar]
- 14.Semba RD, Kumwenda N, Hoover DR, et al. Human immunodeficiency virus load in breast milk, mastitis and mother-to-child transmission of human immunodeficiency virus type 1. J Infect Dis. 1999;180:93–98. doi: 10.1086/314854. [DOI] [PubMed] [Google Scholar]
- 15.de Martino M, Tovo PA, Tozzi AE, et al. HIV-1 transmission through breast milk: appraisal of risk according to duration of feeding. AIDS. 1992;6:991–997. [PubMed] [Google Scholar]
- 16.Eshleman SH, Hoover DR, Chen S, et al. Resistance after single dose nevirapine prophylaxis emerges in a high proportion of Malawian newborns. AIDS. 2005;19:2167–2169. doi: 10.1097/01.aids.0000194800.43799.94. [DOI] [PubMed] [Google Scholar]
- 17.Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomized trial. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 18.Jackson JB, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18 month follow up of the HIVNET 012 randomized trial. Lancet. 2003;362:859–868. doi: 10.1016/S0140-6736(03)14341-3. [DOI] [PubMed] [Google Scholar]
- 19.Jamieson DJ, Sibailly TS, Sadek R, et al. HIV-1 viral load and other risk factors for mother-to-child transmission of HIV-1 in a breast-feeding population in Cote d'Ivoire. J Acquir Immune Defic Syndr. 2003;34:430–436. doi: 10.1097/00126334-200312010-00011. [DOI] [PubMed] [Google Scholar]
- 20.The Petra Study Team The Petra trial: the efficacy of three short course regimens of zidovudine and lamivudine (3TC) in preventing early and late transmission of HIV-1 from mother-to-child in an African setting: a randomized, double-blind placebo-controlled trial; conducted in South Africa, Tanzania, and Uganda. Lancet. 2002;359:1178–1186. doi: 10.1016/S0140-6736(02)08214-4. [DOI] [PubMed] [Google Scholar]
- 21.Wiktor SZ, Ekpini ER, Karon JM, et al. Short course oral zidovudine for prevention of mother-to-child transmission of HIV-1 in Abidjan, Cote d'Ivoire: a randomized trial. Lancet. 1999;353:781–785. doi: 10.1016/S0140-6736(98)10412-9. [DOI] [PubMed] [Google Scholar]
- 22.Triques K, Coste J, Perret JL, et al. Efficiencies of four versions of the AMPLICOR HIV-1 MONITOR test for quantification of different subtypes of human immunodeficiency virus type 1. J Clin Microbiol. 1999;37:110–116. doi: 10.1128/jcm.37.1.110-116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eshleman SH, Becker-Pergola G, Deseyve M, et al. Impact of HIV-1 subtype on women receiving single dose NVP prophylaxis to prevent HIV-1 vertical transmission (HIVNET 012) J Infect Dis. 2001;184:914–917. doi: 10.1086/323153. [DOI] [PubMed] [Google Scholar]
- 24.Eshleman SH, Guay LA, Mwatha A, et al. Characterization of nevirapine (NVP) resistance mutations in women with subtype A vs. D HIV-1 6-8 weeks after single dose NVP (HIVNET 012) J Acquir Immune Defic Syndr. 2004;35:126–130. doi: 10.1097/00126334-200402010-00004. [DOI] [PubMed] [Google Scholar]
- 25.Flys TS, Chen S, Jones DC, et al. Quantitative analysis of HIV-1 variants with the K103N resistance mutation after single dose nevirapine in women with HIV-1 subtypes A, C and D. J Acquir Immune Defic Syndr. 2006;42:610–613. doi: 10.1097/01.qai.0000221686.67810.20. [DOI] [PubMed] [Google Scholar]
- 26.Chung MH, Kiarie JN, Richardson BA, et al. Breast milk HIV-1 suppression and decreased transmission: a randomized trial comparing HIVNET 012 nevirapine versus short-course zidovudine. AIDS. 2005;19:1415–1422. doi: 10.1097/01.aids.0000181013.70008.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thior I, Lockman S, Smeaton LM, et al. Breastfeeding plus infant zidovudine prophylaxis for 6 months vs. formula feeding plus infant zidovudine for 1 month to reduce mother-to-child HIV transmission in Botswana. A randomized trial: The Mashi Study. JAMA. 2006;296:794–805. doi: 10.1001/jama.296.7.794. [DOI] [PubMed] [Google Scholar]
- 28.Eshleman SH, Mracna M, Guay LA, et al. Selection and fading of resistance mutations in women and infants receiving nevirapine to prevent HIV-1 vertical transmission (HIVNET 012) AIDS. 2001;15:1951–1957. doi: 10.1097/00002030-200110190-00006. [DOI] [PubMed] [Google Scholar]
- 29.Flys TS, Donnell D, Mwatha A, et al. Persistence of K103N-containing HIV-1 variants after single-dose nevirapine for prevention of HIV-1 mother-to-child transmission. J Infect Dis. 2007;195:711–715. doi: 10.1086/511433. [DOI] [PubMed] [Google Scholar]
- 30.Embree JE, Njenga S, Datta P, et al. AIDS. 2000;14:2535–2541. doi: 10.1097/00002030-200011100-00016. [DOI] [PubMed] [Google Scholar]