Abstract
Background
Ischemia and reperfusion (I/R) of tissue provokes an inflammatory process that is highly dependent on circulating natural immunoglobulin M (IgM) and the complement cascade. In mice, a single IgM specificity produced by peritoneal B cells can initiate reperfusion injury. It is unknown whether humans express natural IgM with a similar specificity. It is also unknown whether pathogenic IgM is produced solely from peritoneal B cells or can also be made by circulating B cells.
Methods
Immunodeficient mice lacking endogenous immunoglobulin were used. Mice were reconstituted with normal saline, human serum, or xenografted human peripheral blood lymphocytes (PBLs) and then subjected to tourniquet induced hindlimb ischemia and reperfusion. Serum human IgM and IgG were measured by ELISA. Skeletal muscle was harvested for injury assessment by histology and for immunohistochemistry.
Results
Immunodeficient mice are protected from skeletal muscle injury following hindlimb I/R. Transfer of human serum restores skeletal muscle damage. Rag2/γR -/- mice engrafted with human PBL (huPBL-SCID) have high levels of human IgM. huPBL-SCID mice develop significantly more skeletal muscle injury than control saline treated (p ≤ 0.01) and human serum reconstituted Rag2/γR -/- mice (p ≤ 0.01). Sham treated huPBL-SCID mice have no muscle injury, demonstrating that human lymphocyte engraftment does not cause injury in the absence of ischemia. Deposition of human IgM is seen on injured but not sham injured muscle.
Conclusions
Human serum can initiate murine skeletal muscle ischemia reperfusion injury. Circulating human PBL may be a source of pathogenic IgM. The huPBL-SCID mouse may be a useful model to define the specificity of pathogenic human IgM and to test therapeutics for ischemia-reperfusion injury.
Introduction
Ischemia-reperfusion (I/R) injury describes the phenomenon of worsened tissue damage that occurs during the restoration of blood flow following an ischemic event.(1) Reperfusion injury causes pathology in a range of disease entities, such as myocardial infarction, acute mesenteric ischemia, stroke, trauma, and transplantation.
I/R injury is caused by an autologous inflammatory response which is critically dependent on natural IgM antibody and complement.(2) Mice rendered deficient in complement by targeted gene knockout or soluble inhibitors have mitigated reperfusion injury.(3-8) Loss of immunoglobulin in recombinase activating gene 2 knockout (Rag2 -/-) mice also causes decreased reperfusion injury(3). Adoptive transfer of normal mouse serum or purified mouse IgM into Rag2 -/- mice restores I/R injury to wild type levels(3).
Circulating IgM, or natural antibodies, are primarily derived from a specialized subset of B lymphocytes called B1 cells(9). B1 cells are found in the pleural and peritoneal cavities, where they produce polyreactive natural antibody thought to be important in early defense against infection and autoimmunity(10-12). IgM deposition can be seen during ischemia and precedes complement deposition, suggesting that IgM binding to ischemic tissue triggers subsequent complement activation and inflammation(13).
From a panel of murine peritoneal B cell hybridomas, a single IgM clone, CM22, was identified that restores I/R injury of multiple tissues in Rag2 -/- mice(14-16). Mouse CM22 binds the self-antigen nonmuscle myosin heavy chain type II (NHMCII)(17). Inhibition of CM22 binding ameliorates reperfusion injury, indicating that CM22 binding of NHMCII is critical to subsequent inflammation and tissue injury. From these experiments, a model has emerged to explain the mechanism of complement mediated I/R injury(18). Ischemia leads to the exposure of neoantigens, such as NHMCII, which are recognized by autoreactive natural IgM with subsequent complement activation, inflammation, and tissue damage.
Whether a similar pathway is at work in humans is not known. It is also not known whether IgM with pathogenic I/R activity are produced solely by the peritoneal B1 cell population. Due to their restricted location, peritoneal B1 cells and their antibodies have not been well characterized in humans. The majority of serum IgM is produced by B1 cells. However, peripheral blood B2 cells can generate IgM, and a B1-like peripheral blood population has been described(9). The presence of a peripheral blood derived pathogenic human IgM would facilitate future studies in man.
Recently, mouse models that temporarily or permanently harbor a humanized immune system have been developed for the study of autoimmunity and human infections(19). In one such model, the huPBL-SCID mice, transfer of human peripheral blood lymphocytes into mice with targeted deletions of both the Rag2 gene and interleukin 2 common gamma chain receptor (Rag2/γR -/-) results in temporary engraftment of B and T lymphocytes(20). These mice eventually succumb to graft vs. host disease but have been shown to produce serum human IgM and IgG in the interim(21).
A recent study demonstrated restoration of intestinal I/R injury in Rag1 -/- mice using purified human IgM(22). We hypothesize that humans harbor natural antibodies that can also induce skeletal muscle I/R injury. In this study, huPBL-SCID mice and human serum reconstituted Rag2 -/- mice were subjected to sham or hindlimb I/R injury. Skeletal muscle was examined for histologic signs of injury and for deposition of human IgM by immunohistochemistry. The purpose of this study was to determine (1) whether human serum can initiate mouse skeletal muscle I/R injury and (2) if pathogenic I/R antibodies are produced by human peripheral blood lymphocytes.
Methods
Mice
Adult male C57BL/6 or Rag2 -/- mice were purchased from Jackson Laboratories. Mice deficient in both the Rag2 and interleukin 2 receptor common gamma chain genes (Rag2/γR -/-) have been previously described(23). Mice were housed in SPF conditions, and all experiments were performed under guidelines of Harvard Medical School Committee on Animals and of the Committee on the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council.
Human peripheral blood lymphocyte and serum
Peripheral blood was obtained from healthy adult donors in sterile vacutainers. Serum was obtained by allowing whole blood to clot overnight at 4 °C, and aliquots were stored at -20 °C until use. In some experiments, pooled whole human serum was used (Sigma). Peripheral blood lymphocytes (PBL) were purified by density gradient centrifugation on Ficoll-Paque (GE Systems).
Mice were injected intravenously with 300 μl of human serum, mouse serum, or sterile 0.9% normal saline. To create huPBL-SCID mice, 3-4 week old male Rag2/γR -/- mice were transplanted with 20 million human PBLs in a total volume of 100-300 μl of sterile PBS intraperitoneally.
ELISA
ELISA for human IgM and IgG was performed as previously described(23). In brief, 96 well ELISA plates (Nunc) were coated overnight at 4 °C with 10 mg/ml of purified polyclonal goat anti-human IgM or IgG (Southern Biotech) in carbonate buffer. Plates were washed with PBS/0.5% Tween, blocked in PBS/10% FCS, and samples were plated in duplicate at multiple dilutions overnight at 4 °C. Mouse peripheral blood was obtained by retro-orbital puncture and serum purified using microcontainer serum separator tubes (Becton Dickinson). Plates were washed in PBS/0.5% Tween, with subsequent overnight 4°C incubations with biotinylated goat anti-human IgM or IgG (Jackson Laboratories) and streptavidin conjugated alkaline phosphatase (Vector Laboratories). Plates were developed with p-nitrophenyl phosphate liquid substrate system (Sigma), and absorbances read at 405 nm.
Hind-limb skeletal muscle ischemia-reperfusion injury
Age-matched, adult (8-10 wk old) male mice were used for IR experiments. Ischemia was induced by placing 2 rubber bands (Latex O-rings) bilaterally above the greater tronchanter of the femur using a McGivney hemorrhoid ligator (Miltex). Following 1-2 hours of ischemia, as specified, reperfusion was initiated by removal of the rubber bands. Prior to reperfusion, all mice received i.v. hydration with 300 μl 0.9% normal saline. Mice were maintained on a heating pad throughout the experiment under pentobarbital anesthesia. Following 3 hours of reperfusion, mice were sacrificed, and bilateral hindlimbs were harvested and fixed in 10% neutral buffered formalin.
Histology and Scoring
Gastrocnemius muscles were dissected and embedded in paraffin. Transverse cross sections of the muscle were H&E stained and examined for histologic signs of injury. Scoring was performed in a blinded fashion, as previously described(24). In brief, fibers were graded on a scale of 0-2, as follows: 0 - no signs of injury; 1 - muscle fiber swelling with loss of uniform staining; 2 - rhabdomyolysis. Five high powered fields were examined for each animal, centering on the area of greatest injury. Injury severity score was calculated as the (# uninjured fibers × 0 + # swollen fibers × 1 + #rhabdomyolysed fibers × 2) / (2 × total number of fibers counted). Statistical significance was calculated using the Wilcoxon test for nonparametric data.
Immunohistochemistry
10 μm thick paraffin sections were rehydrated, and endogenous peroxidase activity was quenched with 0.3% H2O2 for 20 minutes at room temperature. Slides were blocked in 5% normal goat serum (Vector Labs) and subsequently incubated with 1:250 dilution of biotinylated goat anti-human human IgM (Zymed) in PBS overnight at 4 °C. Slides were washed in PBS and developed using Elite Vectastain ABC Kit (Vector Labs) and DAB substrate (Sigma). Slides were counterstained with Gills hematoxylin, dehydrated, and coverslipped (Histomount, National Diagnostics).
Results
Human serum restores skeletal muscle I/R injury
Rag2 -/- mice were injected intravenously with normal human serum or an equal volume of 0.9% normal saline. Mice subsequently underwent two hours of tourniquet induced hindlimb ischemia followed by three hours of reperfusion. Sham mice underwent anesthesia but did not undergo ischemia. Figure 1 shows H&E cross sections representative of areas of skeletal muscle damage. Saline injected Rag2 -/- mice have mild injury with focal areas of edema and muscle fiber swelling (Figure 1a). In contrast, normal human serum (NHS) reconstituted Rag2 -/- mice had significant muscle damage, with edema, muscle fiber swelling, and occasional rhabdomyolysis (Figure 1c). Sham treated NHS treated Rag2 -/- mice did not have any evidence of muscle injury, demonstrating human serum did not directly cause muscle injury in the absence of an ischemic trigger (Figure 1b). Transfer of NHS resulted in increased histologic injury severity score compared to saline injection (p ≤ 0.05, Figure 4).
Figure 1.
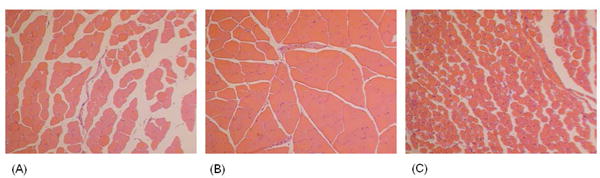
Human sera restores skeletal muscle I/R injury in antibody deficient mice. Transverse cross sections of gastrocnemius muscle from (A) saline injected RAG2 -/- mice, I/R treated, (B) Rag2 -/- mice injected with NHS, sham treated, and (C) Rag2 -/- injected with NHS, I/R treated. Sections were H&E stained. 100× fields of areas of representative of greatest injury are shown.
Figure 4.
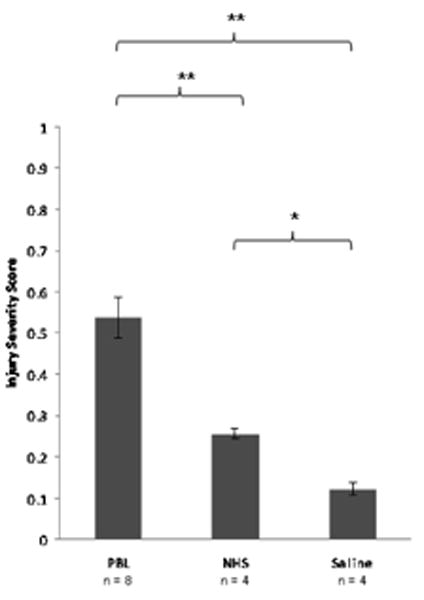
Human PBL engraftment in antibody deficient mice causes greater skeletal muscle injury than transfer of serum. Injury severity score (ISS) of gastrocnemius muscle in immunodeficient mice treated with saline, human serum, and human PBL. Four to eight mice per group were scored in a blinded fashion. Error bars represent SEM. * p ≤ 0.05 ** p ≤ 0.01 by the Wilcoxon test.
High levels of serum IgM in huPBL-SCID mice
Rag2/γR -/- mice were transplanted with 20 million human peripheral blood lymphocytes by intraperitoneal injection. Mice were bled two weeks post PBL transfer, and serum levels of human IgM and IgG were measured by ELISA (Figure 2). Control Rag2/γR -/- mice had no detectable levels of human IgM or IgG, as expected. huPBL-SCID mice had high levels of serum human IgM and IgG. These concentrations were equal to those found in a healthy human donor. Efficiency of transformation as measured by positive serum titers of human IgM and IgG was 100% using this method of engraftment, from multiple donors.
Figure 2.
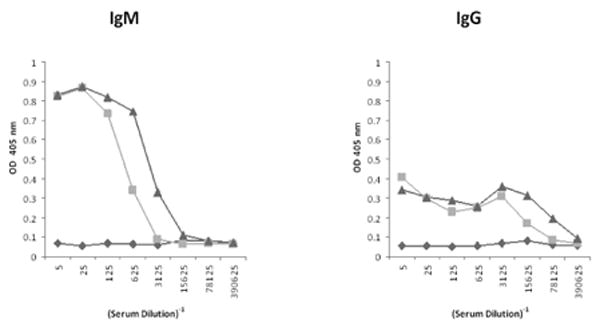
High serum levels of IgM and IgG in huPBL-SCID mice. Rag2/γR -/- mice were xenotransplanted with 20 million human PBL (squares). Two weeks later, mouse serum was tested by ELISA for IgM and IgG. Serum from an untreated Rag2/γR -/- mice (diamonds) and a healthy human donor (triangles) are shown for comparison.
Restoration of skeletal muscle IR injury in huPBL-SCID mice
huPBL-SCID mice and Rag2/γR -/- control mice were subjected to hindlimb I/R injury. In initial experiments, two hours of bilateral hindlimb ischemia of huPBL-SCID mice caused 50-90% mortality rate during reperfusion (data not shown). By decreasing injury severity, 100% survival was achieved using 1.5 hours of unilateral hindlimb ischemia followed by three hours of reperfusion. I/R injury of huPBL-SCID mice resulted in severe skeletal muscle damage, characterized by large area of rhabdomyolysis (Figure 3c). Control huPBL-SCID mice undergoing sham injury had no histologic evidence of skeletal muscle injury (Figure 3b). Histologic injury severity score was significantly greater in huPBL-SCID mice than in mice that received either saline (p ≤ 0.01) or normal human serum (p ≤ 0.01, Figure 4).
Figure 3.
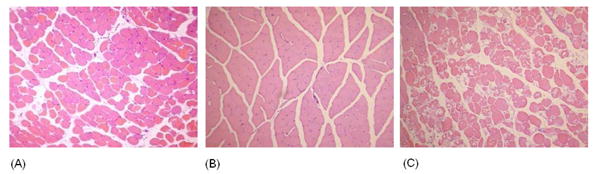
Engraftment with human PBL restores murine skeletal muscle I/R injury in antibody deficient mice. Transverse H&E cross sections of gastrocnemius muscle from (A) saline injected Rag2/γR -/, I/R treated, (B) huPBL-SCID mice, sham treated, and (C) huPBL-SCID mice, I/R treated are shown. 100× fields of areas of representative of greatest injury are shown.
Deposition of human IgM on skeletal muscle following IR injury
Cross sections of skeletal muscle from huPBL-SCID mice were examined for human IgM deposition by immunohistochemistry. Muscle from sham injured huPBL-SCID mice shows no hIgM staining on skeletal muscle (Figure 5). Strong hIgM staining can be seen within the small blood vessels in sham huPBL-SCID mice (arrows), showing both successful engraftment of human PBLs as well as serving as a positive control for hIgM staining. In contrast, strong labeling of muscle fibers can be seen in huPBL-SCID that have undergone I/R injury. Staining for hIgM is patchy, with labeling of some but not all fibers, similar to staining previously published for mouse IgM in murine skeletal muscle(25).
Figure 5.
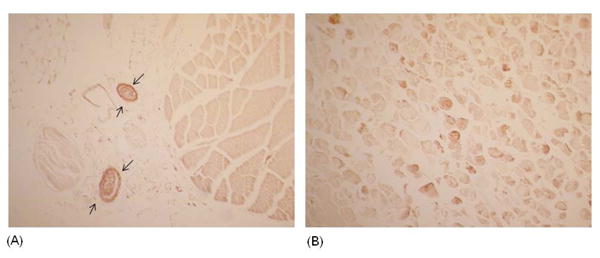
Deposition of human IgM on I/R treated murine skeletal muscle. Immunohistochemical staining for human IgM in skeletal muscle of huPBL-SCID that were (A) sham treated or (B) I/R treated. Arrows indicate blood vessels. 100× magnification.
Discussion
Multiple studies have demonstrated the critical role for natural IgM antibody in triggering the inflammatory tissue injury during ischemia-reperfusion in mice. In this study, we show that human serum can initiate murine skeletal muscle I/R injury. The activity of human serum was restricted to ischemia/reperfusion, as sham animals with human serum had no detectable muscle injury. This data is consistent with a previously proposed model of I/R injury in mice, where exposure of neoantigens on ischemic tissue leads to IgM binding and subsequent complement activation(17).
We also demonstrate increased severity of injury in PBL transplanted mice as compared to mice adoptively transferred with human serum. The increased severity in huPBL-SCID mice may be related to their higher circulating levels of total, and by inference, pathogenic hIgM. Assuming a circulating blood volume of 1.5 ml per mouse, the huPBL-SCID mice, with physiologic levels of human IgM in their serum, would have 2-3 times higher levels of total IgM as compared to the serum reconstituted mice. Variability of host levels of pathogenic IgM is unlikely to be a factor in these experiments, as PBL transplant had greater activity than serum transfer from the same donor. It is possible that transplanted lymphocytes, such as B, T or NK cells, could play a direct role in skeletal muscle injury. Arguing against this possibility is that fact that (1) no skeletal muscle injury is seen in the absence of ischemia and (2) no infiltrate of lymphocytes can be detected in skeletal muscle following I/R injury. Still, this study does not exclude a potential role of distant lymphocytes causing injury in an ischemia-dependent fashion.
This study is also limited by the fact that we cannot definitely conclude that the skeletal muscle I/R injury we observe is caused solely by human IgM, as we did not fractionate the serum to exclude pathogenic activity of IgG or other factors. In the mouse, skeletal muscle I/R activity is confined to IgM(3). Moreover, purified human IgM restores I/R injury in mouse intestine(22). We also show that I/R injury correlates with deposition of human IgM on ischemic muscle fibers. Together, it seems likely that it is the IgM fraction of antibody that causes skeletal muscle I/R injury in our model, but further studies will be required to prove this conclusively.
The patchy pattern of human IgM deposition that we observe is strikingly similar to the pattern of mouse IgM deposition in wild type skeletal muscle, which has been shown to correlate with fast and slow twitch muscle fibers(25). Conservation of this staining pattern suggests that host pathways underlying ischemia mediated exposure of self antigens is unaltered in the huPBL-SCID mice. Interestingly, in the intestinal model, human IgM deposition on injured intestine could not be visualized. This finding may also be related to low level (50 μg per animal) of human IgM in the intestinal model compared to the huPBL-SCID model (physiologic IgM concentrations).
Together, our study suggests that the proposed mechanism of murine I/R injury may be conserved in man. In mice, NHMCII has been identified as a target self antigen exposed during ischemia. Whether pathogenic human IgM also recognizes NHMCII or a NHMCII like antigen is unknown. NHMCII itself, including the putative binding epitope of CM22, is highly conserved between mouse and man(17). Whether a single (or family) of CM22 analogues exist in the human natural IgM repertoire is an important unanswered question for the development of IgM targeted reperfusion therapies. The efficacy of NHMCII blockers, such as peptides that mimic the binding site of CM22 or non-complement activating anti-myosin antibodies, in ameliorating reperfusion injury in the huPBL-SCID mouse will be an important test to determine the specificity of pathogenic human natural antibodies.
Our finding that human PBL engraftment restores I/R injury suggests that circulating B cells may harbor pathogenic antibodies in humans. Alternatively, human PBL contains B1 cells or their progenitors, which could repopulate the peritoneal B cell compartment when transferred into Rag2/γR -/- mice. Future characterization of the peripheral and peritoneal human B lymphocyte population in huPBL-SCID mice may help distinguish these alternatives. Regardless, our results suggest that human PBL, rather than relatively inaccessible peritoneal B cells, may be a source for further cloning and identification of pathogenic human IgM. The huPBL-SCID mouse may also be an useful model to test potential therapies for ischemia-reperfusion injury.
Acknowledgments
Research supported by NIH grants P50GM052585 (F.D.M., M.C.C.) and F32GM084639 (E.G.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cotran RS, Kumar V, Collins T, Robbins SL. Robbins pathologic basis of disease. 6th. Philadelphia: Saunders; 1999. [Google Scholar]
- 2.Chan RK, Ibrahim SI, Verna N, Carroll M, Moore FD, Jr, Hechtman HB. Ischaemia-reperfusion is an event triggered by immune complexes and complement. Br J Surg. 2003;90(12):1470–8. doi: 10.1002/bjs.4408. [DOI] [PubMed] [Google Scholar]
- 3.Weiser MR, Williams JP, Moore FD, Jr, Kobzik L, Ma M, Hechtman HB, et al. Reperfusion injury of ischemic skeletal muscle is mediated by natural antibody and complement. J Exp Med. 1996;183(5):2343–8. doi: 10.1084/jem.183.5.2343. PMCID: 2192547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weisman HF, Bartow T, Leppo MK, Marsh HC, Jr, Carson GR, Concino MF, et al. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science. 1990;249(4965):146–51. doi: 10.1126/science.2371562. [DOI] [PubMed] [Google Scholar]
- 5.Lindsay TF, Hill J, Ortiz F, Rudolph A, Valeri CR, Hechtman HB, et al. Blockade of complement activation prevents local and pulmonary albumin leak after lower torso ischemia-reperfusion. Ann Surg. 1992;216(6):677–83. doi: 10.1097/00000658-199212000-00010. PMCID: 1242715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill J, Lindsay TF, Ortiz F, Yeh CG, Hechtman HB, Moore FD., Jr Soluble complement receptor type 1 ameliorates the local and remote organ injury after intestinal ischemia-reperfusion in the rat. J Immunol. 1992;149(5):1723–8. [PubMed] [Google Scholar]
- 7.Zhang M, Takahashi K, Alicot EM, Vorup-Jensen T, Kessler B, Thiel S, et al. Activation of the lectin pathway by natural IgM in a model of ischemia/reperfusion injury. J Immunol. 2006;177(7):4727–34. doi: 10.4049/jimmunol.177.7.4727. [DOI] [PubMed] [Google Scholar]
- 8.Williams JP, Pechet TT, Weiser MR, Reid R, Kobzik L, Moore FD, Jr, et al. Intestinal reperfusion injury is mediated by IgM and complement. J Appl Physiol. 1999;86(3):938–42. doi: 10.1152/jappl.1999.86.3.938. [DOI] [PubMed] [Google Scholar]
- 9.Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annu Rev Immunol. 2002;20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]
- 10.Ochsenbein AF, Fehr T, Lutz C, Suter M, Brombacher F, Hengartner H, et al. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 1999;286(5447):2156–9. doi: 10.1126/science.286.5447.2156. [DOI] [PubMed] [Google Scholar]
- 11.Ansel KM, Harris RB, Cyster JG. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity. 2002;16(1):67–76. doi: 10.1016/s1074-7613(01)00257-6. [DOI] [PubMed] [Google Scholar]
- 12.Carroll MC, Holers VM. Innate autoimmunity. Adv Immunol. 2005;86:137–57. doi: 10.1016/S0065-2776(04)86004-8. [DOI] [PubMed] [Google Scholar]
- 13.Chan RK, Ding G, Verna N, Ibrahim S, Oakes S, Austen WG, Jr, et al. IgM binding to injured tissue precedes complement activation during skeletal muscle ischemia-reperfusion. J Surg Res. 2004;122(1):29–35. doi: 10.1016/j.jss.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Zhang M, Austen WG, Jr, Chiu I, Alicot EM, Hung R, Ma M, et al. Identification of a specific self-reactive IgM antibody that initiates intestinal ischemia/reperfusion injury. Proc Natl Acad Sci U S A. 2004;101(11):3886–91. doi: 10.1073/pnas.0400347101. PMCID: 374339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suber F, Carroll MC, Moore FD., Jr Innate response to self-antigen significantly exacerbates burn wound depth. Proc Natl Acad Sci U S A. 2007;104(10):3973–7. doi: 10.1073/pnas.0609026104. PMCID: 1820693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austen WG, Jr, Zhang M, Chan R, Friend D, Hechtman HB, Carroll MC, et al. Murine hindlimb reperfusion injury can be initiated by a self-reactive monoclonal IgM. Surgery. 2004;136(2):401–6. doi: 10.1016/j.surg.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Zhang M, Alicot EM, Chiu I, Li J, Verna N, Vorup-Jensen T, et al. Identification of the target self-antigens in reperfusion injury. J Exp Med. 2006;203(1):141–52. doi: 10.1084/jem.20050390. PMCID: 2118091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang M, Carroll MC. Natural antibody mediated innate autoimmune response. Mol Immunol. 2007;44(13):103–10. doi: 10.1016/j.molimm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 19.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7(2):118–30. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 20.King M, Pearson T, Shultz LD, Leif J, Bottino R, Trucco M, et al. A new Hu-PBL model for the study of human islet alloreactivity based on NOD-scid mice bearing a targeted mutation in the IL-2 receptor gamma chain gene. Clin Immunol. 2008;126(3):303–14. doi: 10.1016/j.clim.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 21.van Rijn RS, Simonetti ER, Hagenbeek A, Hogenes MC, de Weger RA, Canninga-van Dijk MR, et al. A new xenograft model for graft-versus-host disease by intravenous transfer of human peripheral blood mononuclear cells in RAG2-/- gammac-/- double-mutant mice. Blood. 2003;102(7):2522–31. doi: 10.1182/blood-2002-10-3241. [DOI] [PubMed] [Google Scholar]
- 22.Zhang M, Alicot EM, Carroll MC. Human natural IgM can induce ischemia/reperfusion injury in a murine intestinal model. Mol Immunol. 2008;45(15):4036–9. doi: 10.1016/j.molimm.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304(5667):104–7. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 24.Chan RK, Verna N, Afnan J, Zhang M, Ibrahim S, Carroll MC, et al. Attenuation of skeletal muscle reperfusion injury with intravenous 12 amino acid peptides that bind to pathogenic IgM. Surgery. 2006;139(2):236–43. doi: 10.1016/j.surg.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 25.Chan RK, Austen WG, Jr, Ibrahim S, Ding GY, Verna N, Hechtman HB, et al. Reperfusion injury to skeletal muscle affects primarily type II muscle fibers. J Surg Res. 2004;122(1):54–60. doi: 10.1016/j.jss.2004.05.003. [DOI] [PubMed] [Google Scholar]


