Abstract
Objectives
Migrainous vertigo (episodic vertigo associated with migraine) is sometimes inherited as an autosomal dominant trait. However, neither disease genes nor loci that might be responsible have been reported. We sought to map the genetic locus for familial migrainous vertigo in a 4-generation family and to define the progression of disease in this family.
Methods
We studied 23 members in a family in whom migrainous vertigo was inherited as an autosomal dominant trait. Clinical information obtained included case histories and results of otolaryngological, neurologic, audiometric, and imaging evaluations. Genome-wide linkage analysis was performed with Affymetrix Genechip Human Mapping 10K microarrays. Genotyping of family members' DNA with microsatellite markers was used to further assess candidate loci identified from the whole-genome scan.
Results
Of 23 family members, 10 suffered from migrainous vertigo beginning after 35 years of age. Migraine headaches usually preceded the onset of vertigo by 15 to 20 years. Longitudinal audiometric studies over 12 years showed stable, high-frequency sensorineural hearing loss consistent with presbycusis. Low-frequency or fluctuating hearing loss was not observed. The results of vestibular testing and imaging studies were unremarkable. Genetic analysis defined a 12.0 MB interval on chromosome 5q35 between loci rs244895 and D5S2073 that contained the disease gene (logarithm of odds score, 4.21).
Conclusions
We report the first locus for familial migrainous vertigo, which mapped to 5q35.
Keywords: familial disease, gene, linkage study, migraine, vertigo
Introduction
The association between migraine and disorders of hearing and balance was recognized as early as ad 131, when Aretaeus of Cappadocia gave a particularly colorful description of the occurrence of hearing loss and vertigo during an attack of migraine.1 Otoneurologists commonly find that migraine is particularly frequent in patients with vertigo, and that patients with migraine have a high rate of vestibular symptoms. Auditory symptoms such as tinnitus, aural fullness, and hearing loss have also been described in patients with migraine and vertigo.1-8
The concept of migrainous vertigo (MV) has emerged recently, consisting of a complex of vestibular complaints in patients with migraine.2,9 Neuhauser et al2 proposed a set of clinical criteria to diagnose MV. Patients are considered to have definite MV if they have episodic vertigo, migraine according to International Headache Society (IHS) criteria,10 and at least 2 attacks of vertigo with 1 of the following: migrainous headache, photophobia, phonophobia, or visual or other auras. Probable MV is diagnosed if patients have episodic vertigo and at least 1 of the following: migraine according to IHS criteria, migrainous symptoms during vertigo, or migraine-specific precipitants of vertigo (eg, specific foods, sleep irregularities, hormonal changes). Vertigo is not considered to be an aura under this classification of MV.
In 1997, we described a large family, designated family A, with multiple members in whom migraine with aura as well as auditory and vestibular symptoms was inherited as an autosomal dominant trait.11-13 Members of this family have episodic vertigo and migraine with aura, and they meet the diagnostic criteria of Neuhauser et al2 for definite MV. In the present study, we describe the 12-year natural history of MV in affected members of family A and map the disease gene segregating in this family to chromosome 5q35.
Methods
Study Subjects
The Institutional Review Board of Brasília University Medical School approved this study. Individual family members were initially evaluated in the otolaryngology clinic at Brasília University Hospital. Informed consent was obtained from all subjects before enrollment in the study. A multidisciplinary team including an otolaryngologist, neurologist, and audiologist evaluated all family members. Each family member completed a questionnaire that included the following items: age of onset, duration, and frequency of headache; details of auras; age of onset, duration, and frequency of vertigo; details of auditory symptoms; a general review of systems; medical history; medications; and allergies. A diagnosis of migraine with aura was made from the IHS criteria.10 Vertigo was not considered as an aura for the diagnosis of migraine with aura.
All subjects with a confirmed diagnosis of migraine were re-evaluated by an otoneurologist for the assignment of a clinical diagnosis of MV by means of the following diagnostic criteria proposed by Neuhauser et al2: 1) episodic vestibular symptoms of at least moderate severity (rotational vertigo, other illusory self or object motion, positional vertigo); 2) migraine according to the diagnostic criteria of the IHS10; 3) at least 1 of the following migrainous symptoms during at least 5 vertiginous attacks: migrainous headache, photophobia, phonophobia, visual or other auras; and 4) other causes of vertigo ruled out by appropriate investigations.
All affected individuals underwent standard pure tone audiometry with speech discrimination testing, as well as vestibular testing consisting of the bithermal caloric test and the sinusoidal vertical-axis rotation test.
Genetic Analysis
Genomic DNA was isolated from nucleated blood cells and/or saliva from 21 surviving family subjects and amplified by polymerase chain reaction.14,15 All available family members were first genotyped by use of microsatellite markers that assessed previously identified disease loci for migraine with aura (4q24; 11q24; 15q11-q13; 19p13)16-20 and familial benign recurrent vertigo (22q12).21
Initial genome-wide linkage scan was performed with the GeneChip Mapping 10K 2.0 Array (Affymetrix, Santa Clara, California). Preliminary multipoint analysis of whole-genome single nucleotide polymorphism (SNP) data was performed with a PERL script shell that optimized the Vitesse22 and Linkmap23,24 computer programs for SNP genotypes and assumed an autosomal dominant mode of inheritance, a disease allele frequency of 0.001, and disease penetrance of 85%. Because SNP genotypes, in which only 2 alleles are found among all family members, are frequently less informative than microsatellite alleles, in which multiple different alleles are found in family members, multipoint linkage analysis was used to calculate the maximum logarithm of odds (LOD) score in a region. The LOD scores were calculated by a 5-SNP sliding window approach, in which one LOD score was calculated, by multipoint linkage analysis, for each marker centered among its 4 closest neighbors, after nonpolymorphic SNPs were discarded. LOD scores are relatively insensitive to allele frequency in families in which there are relatively few missing (or deceased) family members. Therefore, for this preliminary analysis, the SNP allele frequencies were fixed at 0.5/0.5. This method provides an accurate, rapid assessment of LOD scores using SNP genotypes (S. R. DePalma and J. G. Seidman, unpublished data).
Genotyping of family members at microsatellite loci was used to further assess candidate loci identified from the whole-genome scan. Fluorescent-labeled polymerase chain reaction–amplified DNA fragments were size-fractionated on an Applied Biosystems 3730×l DNA Analyzer (Foster City, California). Two-point and multipoint LOD scores assessing linkage between microsatellite loci and MV were calculated with Vitesse.22 Allele frequencies for microsatellite markers on chromosome 5 were estimated from the genotypes of 25 healthy, unrelated Brazilian individuals used as controls, including 5 spouses from this family, with the program ILINK.25
Results
For this study, family A comprised 23 living and deceased family members descended from a single affected individual (Fig 1). Of these 23 subjects, 21 were over 35 years of age and were included in the genetic analysis. Ten of these 21 family members had definite MV. Of the 10 affected individuals, 6 were alive at the end of the 12-year follow-up of the study (1995 to 2006). Of the 6 living affected members, 3 were in the third generation and 3 in the fourth generation. All 6 subjects satisfied the criteria of Neuhauser et al2 for definite MV.
Fig 1.
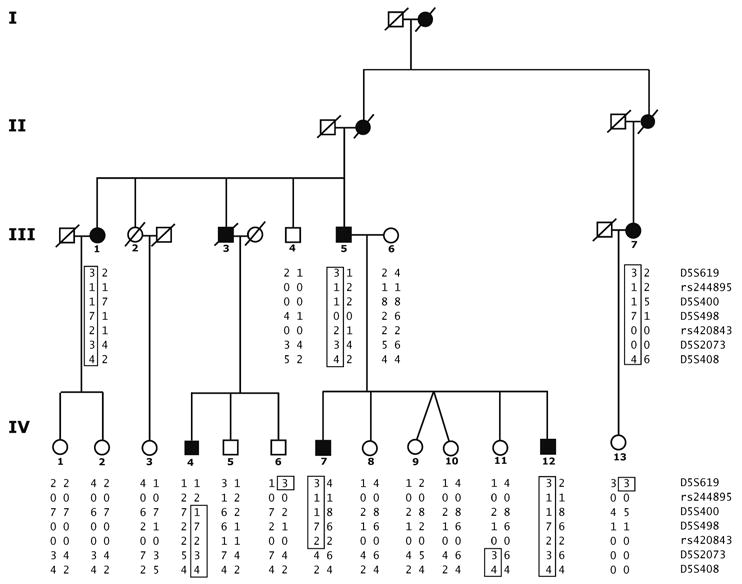
Family A pedigree indicates study subjects' genotypes, which defined disease haplotype and minimum interval containing disease gene. Affected and unaffected individuals are represented by black and open symbols, respectively. Four generations of family members were available for study (roman numerals). Disease haplotype (boxed) is indicated for affected individuals. Twelve individuals were genotyped with Affymetrix 10K array (III-1, III-5, III-6, IV-1, IV-2, IV-4, IV-6, IV-7, IV-8, IV-9, IV-10, IV-12).
All family members were evaluated for MV (Tables 1 and 2). All affected family members started to experience migraine with aura at a young age (mean, 12 years). Vestibular symptoms occurred much later, in the fourth or fifth decade (mean, 42.24 years). Audiometric evaluation typically showed high-frequency sensorineural hearing loss, which was consistent with a diagnosis of presbycusis (not shown). The hearing thresholds remained stable over the course of the study (12 years). Low-frequency or fluctuating sensorineural hearing loss was not observed. The predominant vestibular complaint was episodic rotational vertigo lasting for several hours, usually accompanied by bilateral tinnitus and phonophobia. The vertiginous episodes were frequently accompanied by migraine headaches. The interictal neurologic findings were unremarkable in all affected subjects. The results of caloric testing and rotation chair testing, performed in all affected members, were normal except in subject III-1, who displayed directional preponderance to the left. Cerebral imaging with magnetic resonance imaging or computed tomographic scanning yielded unremarkable findings in the 6 affected members.
TABLE 1.
Clinical Features of Migraine With Vertigo
| Patient | Current Age (y) |
Gender | Age at Onset (y) | Type of Vertigo |
Duration | Migraine During Vertigo |
Subjective Sense of Hearing Loss |
Tinnitus | Aural Fullness |
Phonophobia* | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Migraine | Vertigo | ||||||||||
| III-1 | 84 | F | 10 | 48 | RV, HMI | >1 d | Sometimes | No | 4/10 | No | No |
| III-4 | 74 | M | No | No | No | No | |||||
| III-5 | 72 | M | 12 | 30 | RV, PV | 1 h to 1 d | Always | No | 6/10 | No | No |
| III-6 | 66 | F | No | No | No | No | |||||
| III-7 | 81 | F | 10 | 40 | RV, HMI | 1 h to 1 d | Always | No | 7/10 | No | No |
| IV-1 | 50 | F | No | No | No | No | |||||
| IV-2 | 47 | F | No | No | No | No | |||||
| IV-3 | 44 | F | No | No | No | No | |||||
| IV-4 | 42 | M | 14 | 35 | RV | 5-60 min | Sometimes | No | 8/10 | No | No |
| IV-5 | 40 | M | No | No | No | No | |||||
| IV-6 | 38 | M | No | No | No | No | |||||
| IV-7 | 46 | M | 20 | 39 | RV, PV | 1 h to 1 d | Sometimes | Yes | 6/10 | Yes | Yes |
| IV-8 | 44 | F | No | No | No | No | |||||
| IV-9 | 42 | F | No | No | No | No | |||||
| IV-10 | 42 | F | No | No | No | No | |||||
| IV-11 | 41 | F | No | No | No | No | |||||
| IV-12 | 39 | M | 6 | 43 | RV, HMI | 1 h to 1 d | Sometimes | Yes | 7/10 | Yes | Yes |
| IV-13 | 36 | F | No | No | No | No | |||||
RV — rotational vertigo; HMI — head motion intolerance; PV — positional vertigo.
Abnormal sensitivity to sound.
TABLE 2.
Evolution of Migraine Headaches and Vertigo Over 12-Year Span in Affected Family Members
| Patient | Migraine | Vertigo | ||||||
|---|---|---|---|---|---|---|---|---|
| In 1995 | In 2006 | In 1995 | In 2006 | |||||
| Episodes | Intensity* | Episodes | Intensity* | Episodes | Intensity† | Episodes | Intensity† | |
| III-1 | 100 | 9 | 25 | 6 | 100 | Moderate | 50 | Severe |
| III-5 | 50 | 9 | 25 | 9 | 50 | Severe | 100 | Severe |
| III-7 | 100 | 8 | 50 | 7 | 10 | Mild | 40 | Moderate |
| IV-4 | 25 | 9 | 10 | 6 | 10 | Moderate | 25 | Moderate |
| IV-7 | 10 | 9 | 10 | 8 | 10 | Severe | 25 | Severe |
| IV-12 | 10 | 9 | 10 | 8 | 2 | Moderate | 10 | Moderate |
Intensity of migraine was measured with visual analog scale that ranged from 1 to 10.
Moderate — does not prohibit daily activities; severe — prohibits daily activities.
Two members (patients IV-7 and IV-12) also experienced aural fullness, a sense of hearing loss, and phonophobia during the episodic vertigo. Although the initial clinical presentation11-13 in both cases was suggestive of Meniere's disease, we were unable to document a significant low-frequency or fluctuating sensorineural hearing loss by audiometric testing during 12 years of follow-up in either individual.
The penetrance of the disease gene segregating in this family was estimated to be greater than 85% from family history. That is, assuming that disease occurred after 35 years of age, 20 individuals who were the offspring of the original affected individual were sufficiently old to express disease. Of these 20 individuals, 9 were affected and 11 were unaffected (Fig 1 and Table 1).
DNA from 6 affected and 15 nonaffected members was available for genetic analyses. DNA from 12 family members was genotyped with the Affymetrix GeneChip Human Mapping 10K array. LOD scores were calculated with optimized versions of Vitesse and LINKMAP (see Methods). The Affymetrix 10K SNP array genome scan excluded 40% of the autosomal genome at an LOD of −2.0 or lower. Two loci, on chromosomes 5 and 11, were observed in which the LOD score was greater than 2.5 (Fig 2A). Each locus was greater than 20 cM. The locus on chromosome 5q35 was located near the q terminal end of the chromosome (Fig 2B).
Fig 2.
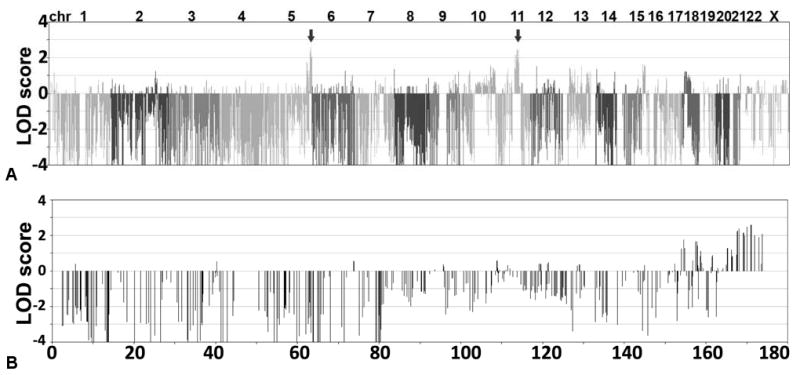
Genome-wide scan to identify loci linked to migrainous vertigo locus. A) Logarithm of odds (LOD) scores at 10,000 loci determined by genotyping 12 family A members (see Fig 1) with 10K Affymetrix single nucleotide polymorphism (SNP) array. LOD scores were calculated at each locus by use of sliding 6-SNP window. Two loci, on chromosomes 5 and 11, achieved LOD score greater than 2.5 (arrows). B) LOD scores obtained on chromosome 5; locus on 5q was validated by microsatellite analyses.
To determine whether the disease gene segregated with loci on chromosomes 5 or 11, we performed further genotyping using 8 microsatellite markers (Fig 1). These microsatellites were significantly more informative than were individual or even combined SNPs from the Affymetrix GeneChip 10K. This genotype information was used for 2-point LOD score calculations. In addition, a few additional family members (III-4, IV-1, IV-2, IV-3, IV-6, and IV-13) were included in the analysis. These analyses excluded a region on chromosome 11 as a potential location for the disease locus (LOD score less than −2.0; data not shown). Two-point LOD score analyses with microsatellite markers demonstrated a maximum LOD score of +3.34 at 0% for marker D5S498 at 173.7 MB on chromosome 5 (Table 3).
TABLE 3.
Linkage Between Loci on Chromosome 5q35 and Migrainous Vertigo in Family A
| Markers | Position | Recombination Fraction (θ) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.01 | 0.02 | 0.05 | 0.10 | 0.15 | 0.20 | ||
| D5S619 | 166814145 | −1.882* | 0.001 | 0.287 | 0.636 | 0.829 | 0.865 | 0.820 |
| D5S400 | 168375587 | 3.666 | 3.599 | 3.531 | 3.326 | 2.974 | 2.610 | 2.234 |
| D5S1456 | 168965187 | 2.099 | 2.069 | 2.038 | 1.940 | 1.759 | 1.563 | 1.353 |
| D5S498 | 173746386 | 3.560 | 3.495 | 3.430 | 3.232 | 2.891 | 2.537 | 2.168 |
| D5S408 | 179920997 | −1.386 | 1.101 | 1.346 | 1.577 | 1.591 | 1.469 | 1.284 |
LOD scores were calculated over a region spanning the interval between loci D5S619 and D5S408 by means of multipoint linkage analysis. A disease haplotype was constructed from 8 loci from this chromosomal region (Fig 1). All 6 affected individuals available for genetic analysis in this family carried the disease haplotype. Multipoint linkage calculation, using penetrance of 0.85 and allele frequencies determined from unaffected family members, identified peak LOD scores between D5S400 and D5S498 (Fig 3A). Similar results were obtained with a range of penetrances and allele frequencies (data not shown). In summary, multilocus linkage analysis provided conclusive evidence that the MV locus maps in the D5S619 to D5S408 region, with a maximum LOD score of 4.21.
Fig 3.
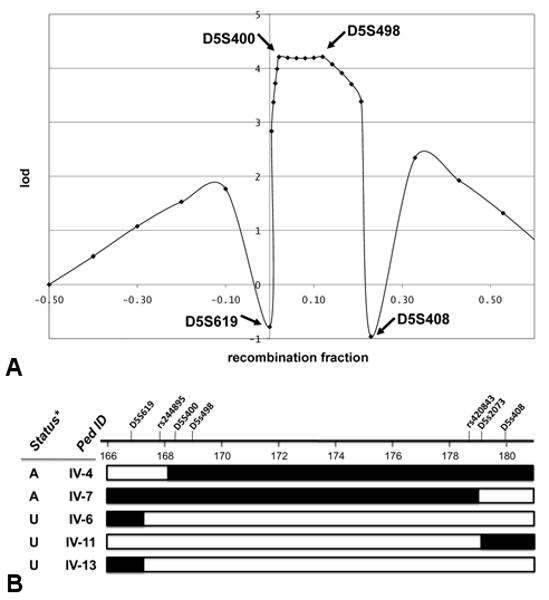
Migrainous vertigo disease locus maps to chromosome 5q (chr5:167897063179920771 bases). A) Multipoint linkage analyses in family A using microsatellite loci D5S619, D5S400, D5S498, and D5S408. B) Interval of 12.0 MB on chromosome 5 was defined by crossover events reflected in 2 affected and 3 unaffected individuals. SNP locations and microsatellite locations were based on human genome build hg18. Status* — disease status: affected (A) or unaffected (U).
Haplotype analysis revealed 2 recombination events in affected individuals and 3 events in unaffected individuals that defined the maximum interval containing the disease locus (Fig 1 and Fig 3B). The disease gene lies between loci rs244895 and D5S2073, a 12.0 MB interval encoding at least 150 known genes taken from the hg18 human reference sequence (NCBI Build 36.1).26
Discussion
A 12-year follow-up study of family A defined the progression of MV and demonstrated linkage of the disease gene to chromosome 5q35. The affected family members had all of the features associated with patients with migraine (with or without vertigo).27-30 The first description of this family, in 1997, described affected family members as having migraine and Meniere's disease,11 because 2 members (patients IV-7 and IV-12) also experienced aural fullness and a subjective sense of hearing loss during the episodic vertigo. Although the initial clinical presentation in these cases was suggestive of Meniere's disease, we were unable to document a clear sensorineural hearing loss (a hallmark of Meniere's disease31) by audiometric testing over 12 years of follow-up in these 2 individuals. Furthermore, the other affected members did not have any auditory symptoms. Our subsequent clinical evaluations showed that the natural history of the syndrome was compatible with the new concept of MV.
The pathophysiology and site of lesion for the audiovestibular symptoms (vertigo, tinnitus, phonophobia, etc) in affected members is unknown. On theoretical grounds, both peripheral and central lesions can explain the audiovestibular complaints. The audiometric and vestibular studies did not show any clear evidence of peripheral or central lesions (except presbycusis); however, these studies were done in intervals between attacks, and therefore, transient abnormalities may have been missed.
A genome-wide screen for loci linked to MV segregating in this large family and subsequent fine structure mapping demonstrated that the disease gene is located between loci rs244895 and D5S2073. The likelihood that co-inheritance of the chromosome 5 loci with disease would have occurred by chance is less than 1 in 15,800 (LOD score = 4.20 at θ = 0). In this family, the minimal 12.0 MB disease interval is defined by 2 recombination events that were detected in affected individuals IV-4 and IV-7 and 3 events in unaffected individuals (IV-6, IV-11, and IV-13; Fig 3B). Because MV is a very rare condition, the cases in the affected family members all derive from the inheritance of a shared disease gene from a common ancestor. Unfortunately, a minimal disease interval of 12.0 MB is large, and identifying the disease-causing mutation will require the development of new DNA sequencing technologies. However, if other families are identified that map to overlapping genomic regions, these families will allow further reduction of the disease interval.
This is the first reported locus for MV. Based on the pathophysiology of inner ear dysfunction and migraine, we chose putative candidate genes in this region, including KCNMB1 (Kv channel interacting protein 1 isoform 1); KCNIP1 (potassium large conductance calcium activated); ATP6V0E (ATPase, H+ transporting, lysosomal, V0 subunit E); SLC34A1 (solute carrier family 34 sodium phosphate); GABRP (gamma-amino butyric acid-GABA A receptor); DRD1 (dopamine receptor D1); and HRH2 (histamine receptor H2) for initial sequencing studies. No mutations were detected. Eventual identification of the MV disease gene in this family should provide a better understanding of the pathophysiology of this disorder.
Acknowledgments
We thank the family for their cooperation and enthusiastic support.
Supported by grants from the National Institutes of Health (J. G. Seidman and C. E. Seidman, and U24DC008559 to Merchant), by the National Council for Scientific and Technological Development-CNPq, Brazil (Bahmad), and by Lakshmi Mittal and Axel Eliasen.
References
- 1.Kayan A, Hood JD. Neuro-otological manifestations of migraine. Brain. 1984;107:1123–42. doi: 10.1093/brain/107.4.1123. [DOI] [PubMed] [Google Scholar]
- 2.Neuhauser H, Leopold M, von Brevern M, Arnold G, Lempert T. The interrelations of migraine, vertigo, and migrainous vertigo. Neurology. 2001;56:436–41. doi: 10.1212/wnl.56.4.436. [DOI] [PubMed] [Google Scholar]
- 3.Baloh RW. Neurotology of migraine. Headache. 1997;37:615–21. doi: 10.1046/j.1526-4610.1997.3710615.x. [DOI] [PubMed] [Google Scholar]
- 4.Eggers SD. Migraine-related vertigo: diagnosis and treatment. Curr Pain Headache Rep. 2007;11:217–26. doi: 10.1007/s11916-007-0193-5. [DOI] [PubMed] [Google Scholar]
- 5.Maione A. Migraine-related vertigo: diagnostic criteria and prophylactic treatment. Laryngoscope. 2006;116:1782–6. doi: 10.1097/01.mlg.0000231302.77922.c5. [DOI] [PubMed] [Google Scholar]
- 6.Bikhazi P, Jackson C, Ruckenstein MJ. Efficacy of anti-migrainous therapy in the treatment of migraine-associated dizziness. Am J Otol. 1997;18:350–4. [PubMed] [Google Scholar]
- 7.Dieterich M, Brandt T. Episodic vertigo related to migraine (90 cases): vestibular migraine? J Neurol. 1999;246:883–92. doi: 10.1007/s004150050478. [DOI] [PubMed] [Google Scholar]
- 8.Cass SP, Furman JM, Ankerstjerne K, Balaban C, Yetiser S, Aydogan B. Migraine-related vestibulopathy. Ann Otol Rhinol Laryngol. 1997;106:182–9. doi: 10.1177/000348949710600302. [DOI] [PubMed] [Google Scholar]
- 9.Marcus DA, Kapelewski C, Rudy TE, Jacob RG, Furman JM. Diagnosis of migrainous vertigo: validity of a structured interview. Med Sci Monit. 2004;10:CR197–CR201. [PubMed] [Google Scholar]
- 10.Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Headache Classification Committee of the International Headache Society. Cephalalgia. 1988;8(suppl 7):1–96. [PubMed] [Google Scholar]
- 11.Oliveira CA, Bezerra RL, Araújo MF, Almeida VF, Messias CI. Meniere's syndrome and migraine: incidence in one family. Ann Otol Rhinol Laryngol. 1997;106:823–9. doi: 10.1177/000348949710601004. [DOI] [PubMed] [Google Scholar]
- 12.Oliveira CA, Ferrari I, Messias CI. Occurrence of familial Meniere's syndrome and migraine in Brasília. Ann Otol Rhinol Laryngol. 2002;111:229–36. doi: 10.1177/000348940211100307. [DOI] [PubMed] [Google Scholar]
- 13.Bezerra RL. Meniere's syndrome and migraine: familial incidence and genetic transmission [Dissertation] Brasília, Brazil: Brasília University; 2002. [Google Scholar]
- 14.Haines JL, Korf BR, Morton CC, Seidman CE, Seidman JG, Smith DR, editors. Current protocols in human genetics. Newark, NJ: John Wiley and Sons; 2008. [Google Scholar]
- 15.Song L, DePalma SR, Kharlap M, et al. Novel locus for an inherited cardiomyopathy maps to chromosome 7. Circulation. 2006;113:2186–92. doi: 10.1161/CIRCULATIONAHA.106.615658. [DOI] [PubMed] [Google Scholar]
- 16.Wessman M, Kallela M, Kaunisto MA, et al. A susceptibility locus for migraine with aura, on chromosome 4q24. Am J Hum Genet. 2002;70:652–62. doi: 10.1086/339078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cader ZM, Noble-Topham S, Dyment DA, et al. Significant linkage to migraine with aura on chromosome 11q24. Hum Mol Genet. 2003;12:2511–7. doi: 10.1093/hmg/ddg252. [DOI] [PubMed] [Google Scholar]
- 18.Russo L, Mariotti P, Sangiorgi E, et al. A new susceptibility locus for migraine with aura in the 15q11-q13 genomic region containing three GABA-A receptor genes. Am J Hum Genet. 2005;76:327–33. doi: 10.1086/427521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaunisto MA, Tikka PJ, Kallela M, et al. Chromosome 19p13 loci in Finnish migraine with aura families. Am J Med Genet B Neuropsychiatr Genet. 2005;132B:85–9. doi: 10.1002/ajmg.b.30082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Brevern M, Ta N, Shankar A, et al. Migrainous vertigo: mutation analysis of the candidate genes CACNA1A, ATP1A2, SCN1A, and CACNB4. Headache. 2006;46:1136–41. doi: 10.1111/j.1526-4610.2006.00504.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee H, Jen JC, Wang H, et al. A genome-wide linkage scan of familial benign recurrent vertigo: linkage to 22q12 with evidence of heterogeneity. Hum Mol Genet. 2006;15:251–8. doi: 10.1093/hmg/ddi441. [DOI] [PubMed] [Google Scholar]
- 22.O'Connell JR, Weeks DE. The VITESSE algorithm for rapid exact multilocus linkage analysis via genotype set-recoding and fuzzy inheritance. Nat Genet. 1995;11:402–8. doi: 10.1038/ng1295-402. [DOI] [PubMed] [Google Scholar]
- 23.Lathrop GM, Lalouel JM. Easy calculations of lod scores and genetic risks on small computers. Am J Hum Genet. 1984;36:460–5. [PMC free article] [PubMed] [Google Scholar]
- 24.Cottingham RW, Jr, Idury RM, Schäffer AA. Faster sequential genetic linkage computations. Am J Hum Genet. 1993;53:252–63. [PMC free article] [PubMed] [Google Scholar]
- 25.Kong A, Gudbjartsson DF, Sainz J, et al. A high-resolution recombination map of the human genome. Nat Genet. 2002;31:241–7. doi: 10.1038/ng917. [DOI] [PubMed] [Google Scholar]
- 26.Karolchik D, Kuhn RM, Baertsch R, et al. The UCSC Genome Browser Database: 2008 update. Nucleic Acids Res. 2008;36:D773–D779. doi: 10.1093/nar/gkm966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuritzky A, Toglia UJ, Thomas D. Vestibular function in migraine. Headache. 1981;21:110–2. doi: 10.1111/j.1526-4610.1981.hed2103110.x. [DOI] [PubMed] [Google Scholar]
- 28.Olsson JE. Neurotologic findings in basilar migraine. Laryngoscope. 1991;101:1–41. doi: 10.1002/lary.1991.101.s52.1. [DOI] [PubMed] [Google Scholar]
- 29.Fedorova ML. Vestibular syndrome in migraine. Klin Med (Mosk) 1970;48:70–6. in Russian. [PubMed] [Google Scholar]
- 30.Parker W. Migraine and the vestibular system in adults. Am J Otol. 1991;12:25–34. [PubMed] [Google Scholar]
- 31.Committee on Hearing and Equilibrium guidelines for the diagnosis and evaluation of therapy in Menière's disease; American Academy of Otolaryngology–Head and Neck Foundation, Inc. Otolaryngol Head Neck Surg. 1995;113:181–5. doi: 10.1016/S0194-5998(95)70102-8. [DOI] [PubMed] [Google Scholar]


