Abstract
Background
Several metabolic studies and a recent double-blinded placebo-controlled randomized trial have shown that higher vitamin C intake significantly reduces serum uric acid levels. Yet, the relation with the risk of gout is unknown.
Methods
We prospectively examined over a 20-year period (1986–2006) the relation between vitamin C intake and the risk of incident gout in 46,994 male participants with no history of gout at baseline. We used a supplementary questionnaire to ascertain the American College of Rheumatology criteria for gout. Vitamin C intake was assessed every four years through validated questionnaires.
Results
During the 20 years of follow-up, we documented 1,317 confirmed incident cases of gout. Compared with men with vitamin C intake < 250mg/day, the multivariate relative risk (RR) of gout was 0.83 (95% confidence interval [CI], 0.71 to 0.97) for total vitamin C intake 500–999 mg/day, 0.66 (0.52 to 0.86) for 1,000–1,499 mg/day, and 0.55 (0.38 to 0.80) for ≥ 1500 mg/day (P for trend < 0.001). The multivariate RR per 500mg increase in total daily vitamin C intake was 0.83 (95% CI, 0.77 to 0.90). Compared with men who did not use supplemental vitamin C, the multivariate RR of gout was 0.66 (95% CI, 0.49 to 0.88) for supplemental vitamin C intake 1,000–1,499 mg/day and 0.55 (0.36 to 0.86) for ≥ 1500 mg/day (P for trend < 0.001).
Conclusions
Higher vitamin C intake is independently associated with a lower risk of gout. Supplemental vitamin C intake may be beneficial in the prevention of gout.
Keywords: Gout, vitamin C, prospective, cohort
INTRODUCTION
Gout is the most common inflammatory arthritis in adult males.1 Epidemiologic studies suggest that the overall disease burden of gout is substantial and growing.2 Identifying the risk factors for gout that are modifiable with available measures is an important first step in prevention and management of this common and excruciatingly painful condition.3–5 In this context, identifying protective factors is equally important as hazardous factors for gout. Among those potentially useful protective factors against hyperuricemia and gout is vitamin C, an essential micronutrient for humans.
Previous studies have suggested that vitamin C supplementation lowers serum uric acid via a uricosuric effect,6–9 although these studies were small, of short duration, and used exceptionally high doses of vitamin C (one time ingestion of 3–12 gm for some days).10 The uricosuric effect of vitamin C may be due to competition for renal reabsorption via an anion-exchange transport system at the proximal tubules.7, 9 Recently, a randomized trial found that supplementation with 500 mg/day of vitamin C for two months reduced serum uric acid.10 Yet, the relation with the risk of gout (the clinical outcome of hyperuricemia) remains unknown. This effect, if confirmed, may have implications for the prevention and management of gout.
To examine these issues, we prospectively evaluated the relation between vitamin C intake and risk of incident gout in a cohort of 46,994 men with no history of gout.
METHODS
Study Population
The Health Professionals Follow-up Study is an ongoing longitudinal study of 51,529 male dentists, optometrists, osteopaths, pharmacists, podiatrists, and veterinarians who were predominantly white (91%) and aged 40 to 75 years in 1986. The participants returned a mailed questionnaire in 1986 concerning diet, medical history, and medications. Of the 49,776 men who provided complete information on vitamin C intake, 2,782 (5.6%) reported a history of gout on the baseline questionnaire. These prevalent cases at baseline were excluded from this analysis. The rate of follow-up for this cohort exceeded 90% during the study period.
Assessment of Vitamin C and Diet
To assess dietary intake including vitamin C intake, we used a semiquantitative food-frequency questionnaire that inquired about the average use of >130 foods and beverages during the previous year.3, 11, 12 In addition, respondents provided information on the use of supplemental vitamins, taken either alone or in multivitamin form. The baseline dietary questionnaire was completed in 1986 and was updated every four years. Nutrient intake (including dietary vitamin C intake) was computed from the reported frequency of consumption of each specified unit of food or beverage and from published data on the nutrient content of the specified portions.12 For supplemental vitamin C, respondents chose from the following categories: 0, 1 to 399, 400 to 700, 750 to 1250, and 1300 mg or more daily. The amount of vitamin C in multivitamin preparations was determined by the brand, type, and frequency of reported use. Food and nutrient intakes assessed by this dietary questionnaire have been validated previously against two 1-week diet records in this cohort.11, 13 Specifically, the Pearson correlation coefficient for energy adjusted total vitamin C intake between the dietary records and the questionnaire was 0.86.14 After adjustment for the week-to-week variation in vitamin C intake the correlation coefficient was 0.92. After excluding supplemental vitamin use the correlation coefficient for vitamin C was 0.77.
Assessment of Non-dietary Factors
At baseline, and every two years thereafter, the participants provided information on weight, regular use of medications (including diuretics), and medical conditions (including hypertension and chronic renal failure).5 Body-mass index was calculated by dividing the weight in kilograms by the square of the height in meters.
Ascertainment of Incident Cases of Gout
We ascertained incident cases of gout by the American College of Rheumatology survey gout criteria, as previously described.3 Briefly, on each biennial questionnaire, participants indicated whether they had received a physician diagnosis of gout. We mailed to those subjects with self-reported incident gout 1986 onwards a supplementary questionnaire to confirm the report and to ascertain the American College of Rheumatology survey gout criteria.3, 15 The primary end point in this study was an incident case of gout that met 6 or more of the 11 gout criteria.3, 15 To confirm the validity of the survey gout criteria in our cohort, we reviewed the relevant medical records from a sample of 50 of the men who had reported having gout.3 The concordance rate of confirming the report of gout between the gout survey criteria and the medical record review was 94% (47/50).3
Statistical Analysis
We computed person-time of follow-up for each participant from the return date of the 1986 questionnaire to the date of diagnosis of gout, death from any cause, or February 1, 2006, whichever came first. Men who died or had reported having gout on previous questionnaires were excluded from subsequent follow-up.
To represent long-term vitamin C (and other dietary) intake patterns of individuals, we used cumulative average intakes based on the information from 1986, 1990, 1994, 1998, and 2002 dietary questionnaires.3–5, 16, 17 For example, the incidence of gout from 1986 through 1990 was related to the vitamin C intake reported on the 1986 questionnaire, and incidence from 1990 through 1994 was related to the average intake reported on the 1986 and 1990 questionnaires. We repeated our analyses using baseline vitamin C (and other dietary) intake (1986) or updated vitamin C (and other dietary) intake every four years without cumulative averaging. When aggregating items to compute the composite dietary items (Table 1), we assumed that individual foods for which values were missing implied no intake.18, 19 Participants who failed to respond to a questionnaire during one follow-up cycle were not removed from the study; they were included in the next mailing of the questionnaire (they could skip answering a questionnaire but then answer the next).
Table 1.
Baseline Characteristics According to Total Vitamin C intake (1986)*
| Variable | Total Vitamin C Intake (mg/day) | All Participants |
||||
|---|---|---|---|---|---|---|
| <250 | 250–499 | 500–999 | 1,000–1,499 | ≥1500 | ||
| Participants (n) | 25430 | 9476 | 6349 | 3369 | 2370 | 46994 |
| Age (yr) | 54 | 55 | 56 | 55 | 54 | 55 (10) |
| Body mass index (kg/m2) | 25.0 | 24.8 | 24.6 | 24.6 | 24.5 | 24.8 (5) |
| Diuretic use (%) | 10 | 10 | 10 | 10 | 9 | 10 |
| History of hypertension (%) | 21 | 21 | 22 | 21 | 21 | 21 |
| History of renal failure (%) | 0.1 | 0.1 | 0.2 | 0.2 | 0.1 | 0.1 |
| Alcohol intake (g/d)* | 11 | 11 | 11 | 11 | 12 | 11 (15) |
| Total meat intake (servings/day)* | 1.4 | 1.4 | 1.3 | 1.2 | 1.2 | 1.4 (0.7) |
| Seafood intake (servings/day)* | 0.3 | 0.4 | 0.4 | 0.5 | 0.5 | 0.4 (0.3) |
| Dairy foods intake (servings/day)* | 1.9 | 2.1 | 2.0 | 1.8 | 1.8 | 1.9 (1.4) |
| Coffee intake (servings/day)* | 1.5 | 1.2 | 1.2 | 1.1 | 1.0 | 1.3 (1.6) |
| Fructose Intake (% of Energy)* | 4.6 | 5.7 | 5.6 | 5.4 | 5.7 | 5.1 (2.3) |
| Sweetened Soft Drink | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.3 (0.5) |
| Intake(servings/day)* | ||||||
| Dietary Vitamin C Intake (mg) | 131 | 215 | 202 | 182 | 196 | 164 (92) |
| Supplemental Vitamin C Intake (mg) | 14 | 127 | 493 | 1037 | 1599 | 255 (433) |
| Supplemental Vitamin C Use (%) | 24 | 74 | 97 | 99 | 100 | 53 |
Data are presented as mean (SD).
We used Cox proportional hazards modeling (PROC PHREG) to estimate the relative risk (RR) for incident gout in all multivariate analyses (SAS Institute Inc, Cary, NC). We categorized total daily vitamin C intake in milligrams into five categories: less than 250, 250 to 499, 500 to 999, 1,000 to 1,499, 1500 or more.20, 21 Additionally, we categorized daily supplemental vitamin C intake in milligrams into five categories: none, 1 to 249, 250 to 499, 500 to 999, 1,000 to 1,499, 1500 or more and daily dietary vitamin C intake into five categories: less than 50, 50 to 99, 100 to 199, 200 to 299, 300 or more. Multivariate models were adjusted for age (continuous), total energy intake (continuous), alcohol (seven categories), body mass index (five categories), use of diuretics (thiazide or furosemide) (yes or no), history of hypertension (yes or no), history of chronic renal failure (yes or no), daily average intake of total meats (quintiles), seafood (quintiles), dairy foods (quintiles), fructose (quintiles), and coffee (regular and decaffeinated in four and three categories, respectively).3–5 Trends in gout risk across categories of vitamin C intake were assessed in Cox proportional hazards models by using the median values of intake for each category to minimize the influence of outliers. The RRs for the continuous measures for vitamin C intake indicate the increase in risk associated with an average increment of 500 mg per day. We conducted analyses stratified by body mass index (<25 kg/m2 vs ≥25 kg/m2), by alcohol use (≤5.5 g/day [median value] vs > 5.5 g/day), and dairy intake (≤1.6 servings/day [median value] vs >1.6 servings/day) to assess possible effect modification. We tested the significance of the interaction with a likelihood ratio test by comparing a model with the main effects of vitamin C intake and the stratifying variable and the interaction terms with a reduced model with only the main effects. For all RRs, we calculated 95% confidence intervals (CIs). All P values are two-sided.
RESULTS
Baseline Characteristics
During the 20-year follow-up, we documented 1,317 new cases of gout that met the American College of Rheumatology criteria.15 The characteristics of the cohort according to total daily vitamin C intake at baseline are shown in Table 1. With increasing vitamin C consumption, intake of total meat and coffee tended to decrease, but intake of total seafood intake increased slightly. Fructose intake was lower in the lowest category of vitamin C intake. Other variables were similar across the levels of vitamin C intake (Table 1).
Vitamin C Intake and Incident Gout
The incidence of gout decreased with increasing intake of total vitamin C intake (Table 2). Compared with men with vitamin C intake < 250mg/day, the multivariate relative risk (RR) of gout was 0.83 (95% confidence interval [CI], 0.71 to 0.97) for total vitamin C intake 500–999 mg/day, 0.66 (0.52 to 0.86) for 1,000–1,499 mg/day, 0.55 (0.38 to 0.80) for ≥ 1500 mg/day (P for trend < 0.001) (Table 2). Absolute risk reductions associated with total vitamin C intake categories (500–999, 1,000–1,499, and ≥ 1500 mg/day) were 27 cases, 51 cases, and 69 cases per 100,000 person years. The multivariate RR per 500mg increase in total daily vitamin C intake was 0.83 (95% CI, 0.77 to 0.90). When we repeated our analyses using updated vitamin C intake without cumulative averaging and baseline vitamin C intake, multivariate RRs of gout between extreme categories of total daily vitamin C intake were 0.64 (95% CI, 0.48 to 0.86; P for trend < 0.001) and 0.69 (95% CI, 0.51 to 0.92; P for trend < 0.001), respectively.
Table 2.
Relative Risk of Incident Gout According to Total Vitamin C intake
| Total Vitamin C Intake (mg/day) | |||||||
|---|---|---|---|---|---|---|---|
| <250 | 250–499 | 500–999 | 1,000–1,499 | ≥1500 | P for trend | ||
| Cases | 693 | 326 | 202 | 67 | 29 | – | |
| Person-years | 436888 | 212384 | 153002 | 62415 | 32412 | – | |
| Age-adjusted RR (95% CI) | 1.0 | 1.00 | 0.85 | 0.68 | 0.55 | <0.001 | |
| (0.88, 1.15) | (0.73, 1.00) | (0.53, 0.88) | (0.38, 0.80) | ||||
| Multivariate RR (95% CI) | 1.0 | 0.97 | 0.83 | 0.66 | 0.55 | <0.001 | |
| (0.85, 1.12) | (0.71, 0.97) | (0.52, 0.86) | (0.38, 0.80) | ||||
RR denotes relative risk and CI confidence interval.
Age-adjusted models were adjusted for the total energy intake as well as age.
Multivariate models were adjusted for age, total energy intake, body mass index, diuretic use, history of hypertension, history of renal failure, and intake of alcohol, total meats, seafood, dairy foods, fructose, and coffee (regular and decaffeinated).
The incidence of gout also decreased with increasing intake of supplemental vitamin C intake (Table 3). Compared with men who did not use supplemental vitamin C, the multivariate RR of gout was 0.66 (0.49 to 0.88) for supplemental vitamin C intake 1,000–1,499 mg/day and 0.55 (0.36 to 0.86) for ≥ 1500 mg/day (P for trend < 0.001) (Table 3). The multivariate RR per 500 mg increase in supplemental daily vitamin C intake was 0.85 (95% CI, 0.77 to 0.93). The risk of gout did not significantly differ between the extreme categories of dietary vitamin C intake (multivariate RR between <50mg/day and > 300mg/day, 1.13; 95% CI, 0.69, 1.83), but the range of intake was substantially smaller than total or supplemental vitamin C intake.
Table 3.
Relative Risk of Incident Gout According to Supplemental Vitamin C intake
| Supplemental Vitamin C Intake (mg/day) | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 1–249 | 250–499 | 500–999 | 1,000–1,499 | ≥1500 | P for trend | |
| Cases | 374 | 583 | 129 | 157 | 53 | 21 | – |
| Person-years | 243731 | 361396 | 103744 | 111577 | 51476 | 24682 | – |
| Age-adjusted RR | 1.0 | 1.14 | 0.88 | 0.96 | 0.69 | 0.55 | <0.001 |
| (95% CI) | (0.99, 1.30) | (0.72, 1.08) | (0.79, 1.16) | (0.51, 0.92) | (0.36, 0.86) | ||
| Multivariate RR | 1.0 | 1.10 | 0.86 | 0.92 | 0.66 | 0.55 | <0.001 |
| (95% CI) | (0.96, 1.26) | (0.70, 1.06) | (0.76, 1.11) | (0.49, 0.88) | (0.36, 0.86) | ||
RR denotes relative risk and CI confidence interval.
Age-adjusted models were adjusted for the total energy intake as well as age.
Multivariate models were adjusted for age, total energy intake, body mass index, diuretic use, history of hypertension, history of renal failure, and intake of alcohol, total meats, seafood, dairy foods, fructose, coffee (regular and decaffeinated), and dietary vitamin C.
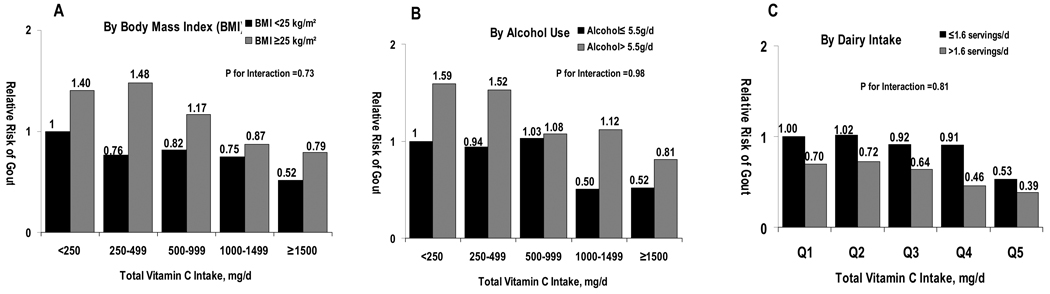
We conducted stratified analyses to evaluate whether the association between vitamin C intake and the risk of gout varied according to body mass index, alcohol use (≤ 5.5 g/day [median value] vs > 5.5 g/day), and dairy intake (≤ 1.6 servings/day [median value] vs > 1.6 servings/day). Relative risks from these stratified analyses consistently suggested inverse associations similar to those from the main analyses, and no significant interaction was found with these variables (all P values for interaction >0.73; Figure 1).
Figure 1. Multivariate Relative Risk of Incident Gout According to Total Vitamin C intake and Body Mass Index (BMI), Alcohol Use, and Dairy Intake.
The reference groups for comparisons in were the men who had total daily vitamin C intake <250 mg and BMI <25 kg/m2 for panel A, men who had total daily vitamin C intake <250 mg and alcohol intake ≤5.5g/day for panel B, and men who had total daily vitamin C intake <250 mg and total daily dairy intake ≤1.6 serving for panel C. The relative risks were adjusted for the same covariates included in the multivariate models of Table 2.
DISCUSSION
Our objective was to evaluate prospectively the suspected protective effect of vitamin C intake against the risk of gout in a large cohort of men. Using the American College of Rheumatology criteria for gout,15 we found that the risk of gout decreased with increasing vitamin C intake, up to a 45% lower risk at the top vitamin C intake category of 1500 mg or more. These associations were independent of dietary and other risk factors for gout such as body mass index, age, hypertension, diuretic use, alcohol, and chronic renal failure. The decreasing risk persisted across subgroups stratified by body mass index, alcohol use, and dairy intake. The current study provides the first prospective evidence about the inverse association between vitamin C intake and the risk of gout.
The suspicion for a potential protective effect of vitamin C intake against gout originally stemmed from metabolic experiments that examined the impact of short-term loading of high-dose vitamin C on the serum uric acid levels. For example, ingestion of a single dose of 4 g vitamin C doubled the fractional excretion of uric acid and a daily ingestion of 8 g of vitamin C for 3 to 7 days reduced serum uric acid by 2.0 to 3.1 mg/dL as a result of uricosuria.9 Recently, a double-blinded placebo-controlled randomized trial (n=184) showed that supplementation with vitamin C as low as 500 mg daily for two months reduced serum uric acid by 0.5mg/dl, compared to no change in the placebo group.10 Furthermore, a retrospective Taiwanese case-control study (91 gout cases and 91 controls) reported an inverse association between vitamin C intake and the presence of gout (unadjusted odds ratios between the extreme tertiles, 0.31; 95% CI, 0.15 to 0.35), although no multivariate adjustment for the link was reported.22
The uricosuric effect of vitamin C is likely due to a competition for renal reabsorption of uric acid via an anion-exchange transport system at the proximal tubule.7, 9 Recent advances in molecular mechanisms of renal urate transport suggest that the uricosuric effect may be through cis-inhibition of URAT1 (urate transporter 1, the key target of typical uricosurics),23 Na+-dependent anion cotransporter (e.g. SLC5A8/A12), or both in the proximal tubules.2 Furthermore, the recent randomized trial showed vitamin C supplements (500 mg/day) significantly increased glomerular filtration rate, providing another potential mechanism for the uricosuric effect of vitamin C intake.10 It remains speculative whether the antioxidant action of vitamin C may have a protective effect against gouty inflammation, as was suggested for reducing the risk of inflammatory polyarthritis according to a recent prospective study.24
While our data suggest that total vitamin C intake of 500 mg/day or more is associated with a reduced risk, the potential benefit of lower intake is not clear. According to a recent analysis of nine prospective studies, compared with subjects who did not take supplemental vitamin C, those who took > 700 mg supplemental vitamin C/day had a 25% lower risk of coronary heart disease (95% CI, 7%, 40%; P for trend < 0.001).25 The same study found that supplemental vitamin E intake was not significantly related to reduced CHD risk.25 This potential cardiovascular benefit of vitamin C may be particularly relevant among gout patients given their increased risk of cardiovascular morbidity and mortality.26, 27 Given the general safety profile associated with Vitamin C intake, particularly, within the generally consumed ranges as in our study (e.g. tolerable upper intake level of vitamin C < 2000 mg in adults according to the Food and Nutrition Board, Institute of Medicine),28 vitamin C may provide a useful option in the prevention of gout.
Several strengths and potential limitations of our study deserve comment. Our study was substantially larger than previous studies concerning gout1, 29–34 and dietary data including vitamin C information were prospectively collected and validated. Potential biased recall of diet was avoided in this study because the intake data were collected before the diagnosis of gout. Because dietary consumption was self-reported by questionnaire, some misclassification of exposure is inevitable. However, the food frequency questionnaire has been extensively validated in a sub-sample of this cohort, and any remaining misclassification would have likely biased the results toward the null. The use of repeated dietary assessments in the analyses not only accounts for changes in dietary consumption over time but also decreases measurement error.11, 13 However, our study was observational; thus, we cannot rule out the possibility that unmeasured factors might contribute to the observed associations. As in other epidemiologic studies of gout,1, 29–32 our primary definition of gout did not require observation of urate crystals in joint fluid examination. While presence of a tophus or urate crystal in joint fluid would be diagnostic of gout,15 the sensitivity of these findings is too low especially in a population study such as ours because arthrocentesis is performed infrequently. Thus, its application would likely miss the vast majority of genuine gout cases. The reliable information provided by health professionals in our cohort, the obvious nature of clinical presentation of gout, and the ready access to medical care for these men would have helped ensure a high level of sensitivity in our detection of gout. In our study, fulfillment of six of the 11 American College of Rheumatology survey criteria15 showed a high-degree of concordance with medical record review and the incidence rate of gout fulfilling the criteria in our cohort closely agreed with that estimated among male physicians in the Johns Hopkins Precursor Study1 (1.5 vs. 1.7 per 1000 person-years, respectively).
The restriction to health professionals in our cohort is both a strength and a limitation. The cohort of well-educated men minimizes potential for confounding associated with socioeconomic status and we were able to obtain high quality data with minimal loss to follow-up. Although the absolute rates of gout and distribution of vitamin C intake may not be representative of a random sample of US men, the biological effects of vitamin C on gout should be similar. Of note, other dietary and life style risk factors of gout observed in this cohort3, 4, 35, 36 have all been found significant in the studies based on the NHANES III.37–40 Our findings are most directly generalizable to men 40 years old and older (the most gout-prevalent population29) with no history of gout. Given the potential influence of female hormones on the risk of gout in women41 and an increased role of dietary impact on uric acid levels among patients with existing gout,42 prospective studies of these populations would be valuable.
In conclusion, these prospective data indicate that vitamin C intake is strongly associated with a lower risk of gout. Increasing vitamin C intake may be beneficial in the prevention of gout.
ACKNOWLEDGMENTS
This work was supported in part by grants from the National Institute of Health (DK58573, HL35464, and CA55075) and TAP Pharmaceuticals. The funding sources had no role in the design, conduct, or reporting of the study or in the decision to submit the manuscript for publication.
REFERENCES
- 1.Roubenoff R, Klag MJ, Mead LA, Liang KY, Seidler AJ, Hochberg MC. Incidence and risk factors for gout in white men. JAMA. 1991;266:3004–3007. [PubMed] [Google Scholar]
- 2.Choi HK, Mount DB, Reginato AM. Pathogenesis of gout. Ann Intern Med. 2005;143:499–516. doi: 10.7326/0003-4819-143-7-200510040-00009. [DOI] [PubMed] [Google Scholar]
- 3.Choi HK, Atkinson K, Karlson EW, Willett WC, Curhan G. Purine-Rich Foods, Dairy and Protein Intake, and the Risk of Gout in Men. New Eng J Med. 2004;350:1093–1103. doi: 10.1056/NEJMoa035700. [DOI] [PubMed] [Google Scholar]
- 4.Choi HK, Atkinson K, Karlson EW, Willett WC, Curhan G. Alcohol Intake and Risk of Incident Gout in Men - A Prospective Study. Lancet. 2004;363:1277–1281. doi: 10.1016/S0140-6736(04)16000-5. [DOI] [PubMed] [Google Scholar]
- 5.Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, Weight Change, Hypertension, Diuretic Use, and Risk of Gout in Men - The Health Professionals Follow-Up Study. Arch Intern Med. 2005;165:742–748. doi: 10.1001/archinte.165.7.742. [DOI] [PubMed] [Google Scholar]
- 6.Mitch WE, Johnson MW, Kirshenbaum JM, Lopez RE. Effect of large oral doses of ascorbic acid on uric acid excretion by normal subjects. Clin Pharmacol Ther. 1981;29:318–321. doi: 10.1038/clpt.1981.42. [DOI] [PubMed] [Google Scholar]
- 7.Berger L, Gerson CD, Yu TF. The effect of ascorbic acid on uric acid excretion with a commentary on the renal handling of ascorbic acid. Am J Med. 1977;62:71–76. doi: 10.1016/0002-9343(77)90351-5. [DOI] [PubMed] [Google Scholar]
- 8.Sutton JL, Basu TK, Dickerson JW. Effect of large doses of ascorbic acid in man on some nitrogenous components of urine. Hum Nutr Appl Nutr. 1983;37:136–140. [PubMed] [Google Scholar]
- 9.Stein HB, Hasan A, Fox IH. Ascorbic acid-induced uricosuria. A consequency of megavitamin therapy. Ann Intern Med. 1976;84:385–388. doi: 10.7326/0003-4819-84-4-385. [DOI] [PubMed] [Google Scholar]
- 10.Huang HY, Appel LJ, Choi MJ, et al. The effects of vitamin C supplementation on serum concentrations of uric acid: results of a randomized controlled trial. Arthritis Rheum. 2005;52:1843–1847. doi: 10.1002/art.21105. [DOI] [PubMed] [Google Scholar]
- 11.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 12.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 13.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93:790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 14.Giovannucci E, Colditz G, Stampfer MJ, et al. The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol. 1991;133:810–817. doi: 10.1093/oxfordjournals.aje.a115960. [DOI] [PubMed] [Google Scholar]
- 15.Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yu TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20:895–900. doi: 10.1002/art.1780200320. [DOI] [PubMed] [Google Scholar]
- 16.Hu FB, Stampfer MJ, Manson JE, et al. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997;337:1491–1499. doi: 10.1056/NEJM199711203372102. [DOI] [PubMed] [Google Scholar]
- 17.Hu FB, Stampfer MJ, Manson JE, et al. Dietary protein and risk of ischemic heart disease in women. Am J Clin Nutr. 1999;70:221–227. doi: 10.1093/ajcn.70.2.221. [DOI] [PubMed] [Google Scholar]
- 18.Caan B, Hiatt RA, Owen AM. Mailed dietary surveys: response rates, error rates, and the effect of omitted food items on nutrient values. Epidemiology. 1991;2:430–436. [PubMed] [Google Scholar]
- 19.Joshipura KJ, Hu FB, Manson JE, et al. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med. 2001;134:1106–1114. doi: 10.7326/0003-4819-134-12-200106190-00010. [DOI] [PubMed] [Google Scholar]
- 20.Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A prospective study of the intake of vitamins C and B6, and the risk of kidney stones in men. J Urol. 1996;155:1847–1851. [PubMed] [Google Scholar]
- 21.Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Intake of vitamins B6 and C and the risk of kidney stones in women. J Am Soc Nephrol. 1999;10:840–845. doi: 10.1681/ASN.V104840. [DOI] [PubMed] [Google Scholar]
- 22.Lyu LC, Hsu CY, Yeh CY, Lee MS, Huang SH, Chen CL. A case-control study of the association of diet and obesity with gout in Taiwan. Am J Clin Nutr. 2003;78:690–701. doi: 10.1093/ajcn/78.4.690. [DOI] [PubMed] [Google Scholar]
- 23.Enomoto A, Kimura H, Chairoungdua A, et al. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002;417:447–452. doi: 10.1038/nature742. [DOI] [PubMed] [Google Scholar]
- 24.Pattison DJ, Silman AJ, Goodson NJ, et al. Vitamin C and the risk of developing inflammatory polyarthritis: prospective nested case-control study. Ann Rheum Dis. 2004;63:843–847. doi: 10.1136/ard.2003.016097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knekt P, Ritz J, Pereira MA, et al. Antioxidant vitamins and coronary heart disease risk: a pooled analysis of 9 cohorts. Am J Clin Nutr. 2004;80:1508–1520. doi: 10.1093/ajcn/80.6.1508. [DOI] [PubMed] [Google Scholar]
- 26.Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation. 2007;116:894–900. doi: 10.1161/CIRCULATIONAHA.107.703389. [DOI] [PubMed] [Google Scholar]
- 27.Krishnan E, Svendsen K, Neaton JD, Grandits G, Kuller LH. Long-term cardiovascular mortality among middle-aged men with gout. Arch Intern Med. 2008;168:1104–1110. doi: 10.1001/archinte.168.10.1104. [DOI] [PubMed] [Google Scholar]
- 28.Hathcock JN, Azzi A, Blumberg J, et al. Vitamins E and C are safe across a broad range of intakes. Am J Clin Nutr. 2005;81:736–745. doi: 10.1093/ajcn/81.4.736. [DOI] [PubMed] [Google Scholar]
- 29.Campion EW, Glynn RJ, DeLabry LO. Asymptomatic hyperuricemia. Risks and consequences in the Normative Aging Study. Am J Med. 1987;82:421–426. doi: 10.1016/0002-9343(87)90441-4. [DOI] [PubMed] [Google Scholar]
- 30.Shadick NA, Kim R, Weiss S, Liang MH, Sparrow D, Hu H. Effect of low level lead exposure on hyperuricemia and gout among middle aged and elderly men: the Normative Aging Study. J Rheumatol. 2000;27:1708–1712. [PubMed] [Google Scholar]
- 31.Abbott RD, Brand FN, Kannel WB, Castelli WP. Gout and coronary heart disease: the Framingham Study. J Clin Epidemiol. 1988;41:237–242. doi: 10.1016/0895-4356(88)90127-8. [DOI] [PubMed] [Google Scholar]
- 32.Hochberg MC, Thomas J, Thomas DJ, Mead L, Levine DM, Klag MJ. Racial differences in the incidence of gout. The role of hypertension. Arthritis Rheum. 1995;38:628–632. doi: 10.1002/art.1780380508. [DOI] [PubMed] [Google Scholar]
- 33.Sharpe CR. A case-control study of alcohol consumption and drinking behaviour in patients with acute gout. Can Med Assoc J. 1984;131:563–567. [PMC free article] [PubMed] [Google Scholar]
- 34.Gibson T, Rodgers AV, Simmonds HA, Court-Brown F, Todd E, Meilton V. A controlled study of diet in patients with gout. Ann Rheum Dis. 1983;42:123–127. doi: 10.1136/ard.42.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi HK, Willett W, Curhan G. Coffee consumption and risk of incident gout in men: A prospective study. Arthritis Rheum. 2007;56:2049–2055. doi: 10.1002/art.22712. [DOI] [PubMed] [Google Scholar]
- 36.Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. BMJ. 2008;336:309–312. doi: 10.1136/bmj.39449.819271.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi HK, Curhan G. Beer, Liquor, Wine, and Serum Uric Acid Level - The Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2004:1023–1029. doi: 10.1002/art.20821. [DOI] [PubMed] [Google Scholar]
- 38.Choi HK, Curhan G. Coffee, tea, and caffeine consumption and serum uric acid level: The third national health and nutrition examination survey. Arthritis Rheum. 2007;57:816–821. doi: 10.1002/art.22762. [DOI] [PubMed] [Google Scholar]
- 39.Choi HK, Liu S, Curhan G. Intake of Purine-Rich Foods, Protein, Dairy Products, and Serum Uric Acid Level - The Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2005;52:283–289. doi: 10.1002/art.20761. [DOI] [PubMed] [Google Scholar]
- 40.Choi JW, Ford ES, Gao X, Choi HK. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2008;59:109–116. doi: 10.1002/art.23245. [DOI] [PubMed] [Google Scholar]
- 41.Nicholls A, Snaith ML, Scott JT. Effect of oestrogen therapy on plasma and urinary levels of uric acid. Br Med J. 1973;1:449–451. doi: 10.1136/bmj.1.5851.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gibson T, Hannan SF, Hatfield PJ, et al. The effect of acid loading on renal excretion of uric acid and ammonium in gout. Adv Exp Med Biol. 1977;76B:46–56. doi: 10.1007/978-1-4684-3285-5_6. [DOI] [PubMed] [Google Scholar]