Abstract
Chronic myeloid leukemia (CML) is a neoplasia characterized by proliferation of a myeloid cell lineage and chromosome translocation t(9;22) (q34;q11.2). As in the case of most cancers, the average telomere length in CML cells is shorter than that in normal blood cells. However, there are currently no data available concerning specific individual telomere length in CML. Here, we studied telomere length on each chromosome arm of CML cells. In situ hybridization with peptide nucleic acid probes was performed on CML cells in metaphase. The fluorescence intensity of each specific telomere was converted into kilobases according to the telomere restriction fragment results for each sample. We found differences in telomere length between short arm ends and long arm ends. We observed recurrent telomere length changes as well as telomere length maintenance and elongation in some individual telomeres. We propose a possible involvement of individual telomere length changes to some chromosomal abnormalities in CML. We suggest that individual telomere length maintenance is chromosome arm-specific associated with leukemia cells.
Introduction
Telomeres are the termini of eukaryotic chromosomes. Human telomeres are constituted of a tandem repeat of six base pairs (TTAGGG), which are wrapped in a protein complex [1,2]. Telomere sequences are lost in each round of cell replication because of the incomplete synthesis of the G-rich strand and the enzymatic processing of the C-rich strand [3,4]. The biogenesis of telomeres is regulated by a large number of proteins and an enzyme called telomerase. This enzyme is a ribonucleoprotein, which counteracts the loss of telomere sequences during cell division by adding telomeric repeats on the G-rich strand [5]. Telomerase is active in germ cells, adult stem cells, activated immune cells, and 90% of cancer cells [1,6]. However, it is absent or expressed at low levels in most adult differentiated cells and resting immune cells. McClintock [7] and Muller [8] initially pointed out the important role played by telomeres in chromosome stability by highlighting the chromosome end-to-end fusion process. Since these pioneering observations, the research on telomeres has been a focal point of aging and cancer studies. Telomere shortening and telomere maintenance by telomerase or by alternative lengthening of telomere (ALT) are some of the characteristic features of cancer cells [9,10].
Chronic myeloid leukemia (CML) is a myeloproliferative disease characterized by a proliferation of myeloid cell lineage and chromosome translocation t(9;22) (q34;q11.2) [11], the so-called Philadelphia (Ph) chromosome. This translocation generates a chimerical gene encoding the BCR-ABL oncogenic fusion protein that features constitutive high-tyrosine kinase activity [12]. Without effective therapy, CML irremediably progresses in three successive phases: chronic (CP), accelerated (AP), and blast crisis (BP). As is the case in all cancers, telomeres play a central role in the progression of CML. Telomere shortening has been reported in each of the three phases of CML, and this shortening is accentuated during progression of the disease [13,14]. Telomere length measurements in all the studies published to date have been based on global evaluations relying on techniques such as telomere restriction fragment (TRF) analysis and fluorescence in situ hybridization (FISH) using flow cytometry [13–15]. These two techniques provide a global picture of telomere lengths at the genomic and cellular levels by assessing their average length. The advent of telomere length measurement by quantitative FISH (Q-FISH) [16] has allowed a better understanding of telomere length at the chromosomal level. Besides the t(9;22)(q34;q11.2) translocation, other chromosomal aberrations have been reported during the progression of CML [17], and the critical shortening of telomeres may be related to these chromosomal changes. To the best of our knowledge, no extensive studies have been conducted on individual telomere length in CML as yet.
The assessment of individual telomere length profiles in CML will provide knowledge concerning specific individual telomere length changes associated with CML. On the one hand, the data will yield deeper insights regarding the relationship between individual telomere length changes and chromosomal abnormalities. Conversely, they will provide information on individual telomere length maintenance.
Materials and Methods
Samplings and Cell Cultures
Telomere length was measured in 21 CML patients at diagnosis. One patient was in the AP phase, whereas the others were in the CP phase. The average age of the patients was 45 years (range, 18–83 years; Table 1). Specimens of cryopreserved blood and bone marrow were obtained from the Quebec Leukemia Cell Bank (Centre de recherche, Hôpital Maisonneuve-Rosemont, Montreal, Quebec, Canada). Five to ten million cells of each sample were cultured in a 5-ml Marrow Max medium (Invitrogen, Ontario, Canada) containing 20% fetal bovine serum for 48 hours. Phytohemagglutinin-stimulated lymphocytes from 13 age-matched normal adults (4 males and 9 females) were used as controls. For each individual, 700 µl of blood was cultured following the standard cytogenetic techniques. After harvesting, the cells (normal lymphocytes or leukemia cells) were suspended in a methanol/acetic acid (3:1) fixative solution, and 10 µl of fixed nucleus suspension was spread onto cleaned slides in a modified environmental control unit Thermotron (CDS-5, Thermotron, Amsterdam, the Netherlands) [18], where temperature and relative humidity remained constant at 22°C and 55%, respectively. The local research ethic board of the Faculty of Medicine and Health Sciences of the University of Sherbrooke approved the protocol used in the study. Informed consent was obtained from each donor who provided samples of blood or bone marrow used in the study.
Table 1.
Clinical Data of CML Cases.
| Information at Time of Sampling | No. of Metaphases Analyzed (Q-FISH) | ||||||
| Samples | Age | Sex | Tissues | Blasts | Karyotype | Clinical Phases | |
| 02-H055 | 45 | M | Blood | 5%/Blood | 46,XY,t(9;22)(q34;q11.2)23 | CP | 10 |
| 02-H058 | 26 | F | Bone marrow | 2%/BM | 46,XX,t(9;22)(q34;q11.2)21 | CP | 11 |
| 02-H077 | 45 | M | Blood | 3%/Blood | 46,XY,t(9;22)(q34;q11.2)20 | CP | 15 |
| 03-H034 | 45 | M | Bone marrow | 1%/BM | 46,XY,t(9;22)(q34;q11.2)19/46,idem,?der(7)del(7)(q11.2q21)del(7)(q36)2 | CP | 10 |
| 03-H044 | 64 | F | Blood | 5%/Blood | 46,XX,t(9;22)(q34;q11.2)21 | CP | 15 |
| 03-H056 | 18 | M | Blood | 3%/Blood | 46,XY,t(9;22)(q34;q11.2)21 | CP | 13 |
| 03-H061 | 44 | F | Bone marrow | 10%/BM | 46,XX,t(9;22)(q34;q11.2)23 | AP | 15 |
| 03-H077 | 35 | F | Bone marrow | 5%/BM | 46,XX,t(9;22)(q34;q11.2)29 | CP | 9 |
| 03-H079 | 50 | F | Bone marrow | 2%/BM | 46,XX,t(9;22)(q34;q11.2)20 | CP | 11 |
| 04-H013 | 52 | M | Bone marrow | 5%/BM | 46,XY,t(9;22)(q34;q11.2)22 | CP | 5 |
| 04-H015 | 38 | F | Bone marrow | 3%/BM | 46,XX,t(9;22)(q34;q11.2)21 | CP | 11 |
| 04-H035 | 61 | F | Bone marrow | 2%/BM | 46,XX,t(9;22)(q34;q11.2)20 | CP | 8 |
| 04-H057 | 44 | M | Blood | 3%/Blood | 46,XY,t(9;22)(q34;q11.2)20 | CP | 9 |
| 04-H131 | 33 | F | Bone marrow | 4%/BM | 46,XX,t(9;22;13)(q34;q11.2;p13)20 | CP | 8 |
| 05-H020 | 57 | F | Bone marrow | 3%/BM | 46,XX,t(9;22)(q34;q11.2)20 | CP | 9 |
| 05-H043 | 45 | F | Bone marrow | 2%/BM | 46,XX,t(9;22)(q34;q11.2)20 | CP | 10 |
| 05-H049 | 83 | F | Bone marrow | 2%/BM | 46,XX,t(9;22)(q34;q11.2)18 | CP | 9 |
| 05-H166 | 40 | M | Bone marrow | 2%/BM | 46,XY,t(9;22)(q34;q11.2)20 | CP | 10 |
| 06-H047 | 39 | F | Bone marrow | 5%/BM | 46,XX,t(9;22)(q34;q11)22 | CP | 7 |
| 06-H069 | 53 | M | Bone marrow | 2%/BM | 46,XY,t(9;22)(q34.1;q11.2)20 | CP | 8 |
| 06-H097 | 32 | F | Blood | 7%/Blood | 46,XX,t(9;22)(q34;q11.2)20 | CP | 9 |
Samplings were done at diagnosis and before the initiation of treatment. The translocation t(9;22) was the most frequent cytogenetic abnormality. More than 80% of the patients were at the CP, and 13 of 21 patients were females.
AP indicates accelerated phase; CP, chronic phase.
Peptide Nucleic Acid FISH
The FISH technique was performed using peptide nucleic acid (PNA) telomere probe FITC-(CCCTAA)3 (Applied Biosystems, Foster City, CA). Hybridizations were carried out following the protocol provided by the manufacturer, with modifications based on our own and others' previous protocols [19,20]. Briefly, slides were left for 24 hours at 37°C after spreading and then fixed in a 3.7% formaldehyde solution, followed by a proteinase K treatment. Next, the slide preparations were denatured at 80°C for 3 minutes under a 24-mm x 30-mm coverslip in the presence of 10 µl of PNA telomere probe. Hybridization was then carried out in the dark for 30 minutes at room temperature. A posthybridization wash was done using 0.4x SSC containing 0.3% NP-40 at 65°C for 2 minutes and 2x SSC/0.1% NP-40 for 2 minutes. The slides were counterstained using 125 ng/ml 4,6-diamidino-2-phenylindole (DAPI) mixed with 1 mg/ml p-phenylenediamine (Sigma Aldrich, St Louis, MO). The slides were kept in the dark for 2 days at room temperature to enhance banding patterns on chromosomes.
Imaging and Fluorescence Measurement
The slides were examined under an Olympus BX61 microscope (Olympus America Inc., Center Valley, PA) equipped with appropriate filters. For each sample, a number of metaphases were digitally captured using a Compulog IMAC-CCD S30 videocamera module (MetaSystems Group Inc., Waltham, MA). Standard DAPI and fluorescein isothiocyanate filters were used to display chromosome and telomere-specific signals, respectively. Approximately 5 to 15 metaphases were karyotyped (Table 1) based on the quality of DAPI-banding pattern. To determine a minimum number of metaphases to be analyzed, a widely used standard [21] was followed. According to this standard, whereas at least 50% of cells carrying the same chromosome anomaly (very long or short telomeres at the same chromosome ends in this study), the minimum number of five cells needs to be analyzed on a 95% confidence limit (power) and under a 99% analytical sensitivity of a specific technique such as FISH [19]. Telomere measurements were done using an in situ imaging system software (ISIS 2; MetaSystems). Fluorescein isothiocyanate fluorescence intensity was measured on both the short-arm (p-end) and the long-arm (q-end) of each 1 of the 46 chromosomes per cell. Therefore, each metaphase presented 92 telomere length measurements. The length of a specific individual telomere for a given specimen was the average of these individual telomere lengths in different metaphases.
TRF Technique
DNA was extracted from six normal individuals and from all leukemia donors using the standard method of phenol-chloroform. The TRF technique was performed using the TeloTAGGG Telomere Length Assay kit (Roche Applied Science, Laval, Quebec, Canada). Briefly, 1.5 µg of DNA was digested using HinfI and RasI restriction enzymes. The digested DNA was electrophoretically resolved on 0.7% Agarose gel, transferred to a nylon membrane, and blotted with digoxigenin-labeled (CCCTAA)3 DNA probe. The membrane was exposed to a phosphorimager screen and detected on the PhosphorImager Storm 860 (Molecular Dynamics, Sunnyvale, CA) and then on an x-ray film. Telomere size was measured using the Image J freeware (National Institutes of Health, Bethesda, MD).
Determination of Physical Length of Individual Telomeres
We converted individual telomere signal intensities obtained from Q-FISH to physical length in kilobases using TRF results from CML and normal cells, as described in previous reports [20,22], with some modifications. Briefly, the average fluorescence intensity of metaphases of a given case was equated to the average length in kilobases of TRF measurements from the same case. Next, the equivalent of a fluorescence unit of each individual telomere was converted into kilobases. The normal individual telomere lengths from peripheral blood cells were adjusted to reflect those of hematopoietic stem cells, according to Sakoff et al. [23].
Statistical Analysis
Descriptive statistics, including means, SDs, and confidence intervals, were generated by the “proc means” and the “proc univariate” procedures of the Statistical Analysis System (SAS) software, version 9.1.3 (SAS Institute, Inc, Cary, NC). Analysis of variance was performed using four fix effects: group, with 2 levels (normal and leukemia); chromosome, with 24 levels (autosomes and sex chromosomes); chromosome arm, with 2 levels (p-end and q-end); and sexes, with 2 levels (female and male). Subjects (individual cases) served as a random effect. The dependent variable was the physical telomere length in kilobases. We also used the PDIFF function for the pairwise comparisons of the least square means, which provides a defensible estimate of interesting effects. Tukey and Dunnet adjustments were used to keep the experiment-wise rate of type I error to 5%. The individual telomere length for each CML sample was compared with its counterpart in normal samples used as reference. Individual telomere lengths for CML samples were defined as elongated or shortened when their lengths were significantly longer or shorter (P < .05) than their counterparts in the normal reference. Otherwise, they were defined as maintained when no statistical difference (P > .05) was observed between CML cases and normal reference cases. We used orthogonal contrasts to compare each individual telomere length of a given case to its average telomere length. The validity of these models was confirmed by a normal distribution of all residuals. The statistical significance was established for all comparisons for P < .05 at a 95% confidence interval. The descriptive graphics were produced with the Statistical Package for Social Sciences (SPSS) software, version 17.0 (SPSS, Inc, Chicago, IL).
Results
Longer Mean Telomere Lengths on p-Ends Rather Than on q-Ends in CML
First, we found that telomeres on both p- and q-ends in the leukemia group were significantly shorter than their counterparts in the normal group (P < .001 for both arms; Table 2). Second, we observed that in the CML group, the mean individual telomere length for females (7.12 ± 0.11 kb) was significantly longer (P < .001) than that for males (5.85 ± 0.121 kb), whereas the normal adult group did not display such a difference (P = .43; Figure W1). Interestingly, we further found that, in the leukemia group, the mean telomere length for the p-ends (6.91 ± 0.12 kb) was statistically longer (P < .001) than the mean for the q-ends (6.33 ± 0.10 kb), whereas the mean telomere lengths for the two chromosome ends in the normal group did not differ (P = .41; Table 2). In summary, we observed significant differences in telomere length between p-ends and q-ends as well as between females and males in the CML group.
Table 2.
Representation of Average Telomere Length in CML and Normal Groups.
| CML Group | SD | SE | Normal Group | SD | SE | P | |
| p | 6.91 | 2.83 | 0.12 | 8.46 | 1.48 | 0.136 | <.001 |
| q | 6.33 | 2.37 | 0.10 | 8.53 | 1.00 | 0.092 | <.001 |
| P | <.001 | .41 |
Average telomere length of all telomeres, on p-arms or q-arms of CML cells, was statistically shorter and widely distributed than their corresponding entities in normal cells (P < .001). The values are expressed in kilobases. The statistical method used is described in the Materials and Methods section. p indicates chromosome short arms; q, chromosome long arms.
Nonrandom Individual Telomere Length Changes in CML
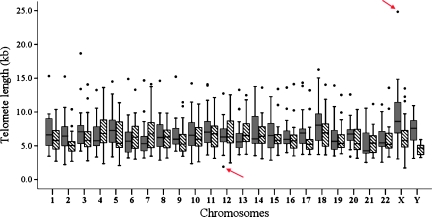
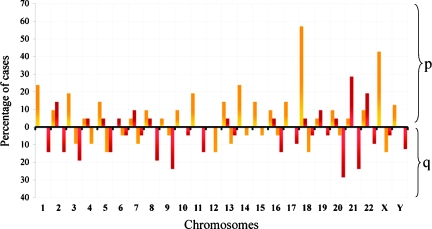
We observed that the mean individual telomere lengths for each specific chromosome ends from all cases ranged from 1.70 to 24.83 kb (Figure 1) in the CML group. Because assessing the individual telomere lengths of all the CML cases collectively could hide important information regarding the telomere length profile of each case, we determined the specific individual telomere length profile for each single case. To establish the specific individual telomere profile, we determined the longest and the shortest telomeres in relative length for each case by comparing the mean of each specific individual telomere length to the mean of all telomeres in the sample using orthogonal contrasts. When the mean length for a given individual telomere was significantly greater or less than the telomere length for that case (P < .05), it was considered to be among the longest or the shortest telomeres, respectively. It was found that in the CML samples, the p-ends harbored the longest telomeres, whereas the shortest telomeres were mainly found on the q-ends. In CML cases, the telomeres on 18p and Xp were among the longest in 57.14% and 42.85% of cases, respectively, whereas the telomeres on 21p and 21q were among the shortest in 28.57% and 23.80% of cases, respectively (Figure 2). In normal adult samples, the telomeres on 5p, 3p, and 4p were among the longest individual telomeres in 57.14%, 42.4%, and 38.46% of cases, respectively (Figure W2A). In contrast, the telomeres on 19p, 17p, and 20q were among the shortest in 46.15%, 38.46%, and 38.46% of cases, respectively (Figure W2B). In conclusion, specific telomere length changes of the longest individual telomeres on 18p and Xp, and the shortest individual telomeres on 21p and 21q seemed to be a characteristic of CML in the CP phase.
Figure 1.
Box plot of individual telomere lengths (kb) in the CML group. Black boxes correspond to chromosome short arms; shaded boxes, chromosome long arms. The individual telomere length was shown by the mean of the specific telomere lengths in 10 metaphases. The dots represent the extreme value of an individual telomere length in each case. The red arrows indicate the minimum and maximum individual telomere lengths on Xp (24.83 kb) and 12p (1.70 kb), respectively.
Figure 2.
Percentage of cases showing relative individual telomere lengths among the longest (yellow bars) and the shortest (red bars) in the CML group. In each sample, a specific individual telomere length was compared with the mean of all telomeres. When an individual telomere was shorter or longer than the mean telomere of a case (P < .05), it was considered among the shortest or longest telomere of that case. Chromosome numbers are shown on the x-axis. Frequently observed longest telomeres are at 18p and Xp, which were seen in 54.14% and 42.85% of cases, respectively, whereas the shortest, at 20q, 21p, and 21q, were seen in 28.57%, 28.57%, and 23.80% of cases, respectively.
Individual Telomere Shortening Rate in CML
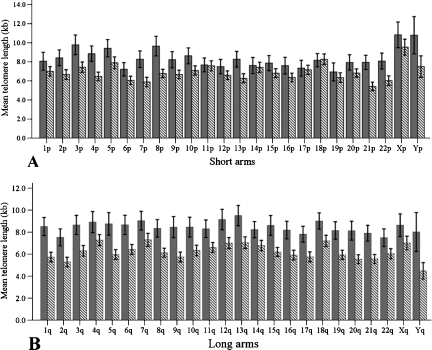
To evaluate the possible evolution of specific individual telomere lengths during leukemogenesis (initiation of CML until diagnosis), we assessed individual telomere length shortening rates in the CML group by measuring the relative difference [(A − B / A) x 100] in length between each specific individual telomere (A) in the normal group and its corresponding individual telomere (B) in the CML group. We found that individual telomeres on the q-ends had a higher shortening rate (26.38%) than those on the p-ends (17.97%; P < .001). Moreover, individual telomere losses were more uniform on the q-ends (0.31%) than on the p-ends (0.88%) as variances (P = .007). Next, the study of individual telomere shortening rates showed that telomeres on Yp, Yq, 1q, 5q, 9q, 8p, and 21p had the highest telomere attrition rates, and their lengths were at least 30% shorter than their counterparts in the normal group (Figure 3, A and B). In summary, the individual telomere lengths on both ends of the Y chromosome presented the highest telomere shortening rate (p-end = 34.02%, q-end = 44.52%) in the CML group.
Figure 3.
Means of individual telomere lengths on chromosome arms in normal and CML groups. The gray columns represent the mean of individual telomere lengths in normal adults, whereas the hatched columns correspond to the mean of individual telomere lengths in CML cases. (A) Means of individual telomere lengths on the chromosome short arms. (B) Means of individual telomere lengths on the chromosome long arms. Error bars, 95% confidence interval.
Maintenance of Some Individual Telomere Lengths in CML
We investigated whether there were differences for specific individual telomeres between the leukemia and the normal groups by comparing the mean of each individual telomere length in the leukemia group with its counterpart in the normal group. Using the PDIFF function for pairwise comparisons of the least square means, we found that telomeres on 11p, 14p, 17p, 18p, 19p, and Xp were maintained in the CML group because data showed no statistical difference (P > .05) in mean telomere lengths between the CML and the normal groups for these particular telomeres (Figure 3A). In contrast, all individual telomeres on the q-ends in the normal group were statistically longer than the corresponding individual telomeres in the leukemia group (P < .05; Figure 3B).
Presence of Some Long Telomeres in CML in the CP Phase
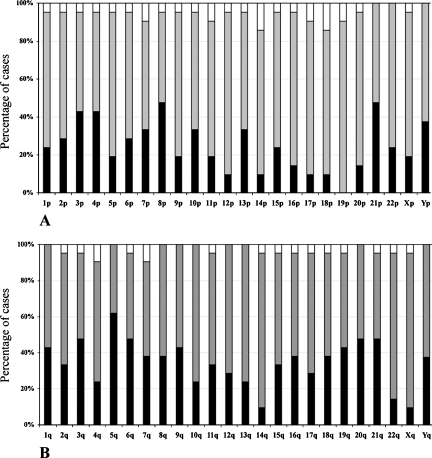
To evaluate the elongation of each specific individual telomere in CML cases, we compared the mean length for each specific individual telomere in each CML case to its counterpart mean in the normal group. We used the PDIFF function and Dunnet adjustment for multiple comparisons. This approach helped to eliminate the group effect and to show the elongation or shortening of a specific individual telomere in each sample in reference to the normal. We noticed telomere elongation events on some chromosome arms, such as 14p and 18p, in 14.3% of CML cases (Figure 4A). Interestingly, in all CML cases, telomere sequences on 19p were maintained or elongated because no shortening was observed for this chromosome end. In addition, individual telomeres on 5p, 9p, 11p, 12p, 14p, 16p, 17p, 18p, 20p, Xp, 14q, and Xq were elongated or maintained in 80% of the CML cases (Figure 4, A and B). Furthermore, we found three CML cases that presented very long individual telomeres on Xp (24.83 kb), 3p (18.66 kb), and 18p (16.23 kb), respectively (Figure 5, A–D). These results were confirmed by the TRF technique, which showed specific bands distinct from the telomere smear (Figure 6). We concluded that some CML cells harbor specific individual long telomeres (16–25 kb) despite average telomere shortening of the remaining telomeres.
Figure 4.
Percentages of short, maintained, and long individual telomeres in CML samples. The average of each specific individual telomere length in each CML case was compared with its corresponding individual telomere length in normal control group. Short or long individual telomeres in each leukemia sample were defined when their respective lengths were statistically (P < .05) shorter (black columns) or longer (white columns) than the corresponding individual telomere length in normal control. If there was no statistical difference, they were considered as maintained (gray columns). (A) Individual telomere on the chromosome short arm. The individual telomere on 19p was not shortened in any CML sample. (B) Individual telomere on the chromosome long arms. The 5q was shortened in almost two-thirds of CML cases.
Figure 5.
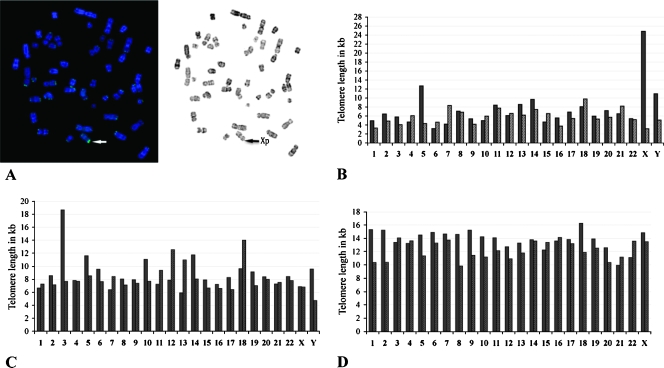
Individual telomere lengths in CML cases. Some CML cases showing individual long telomeres. Black bars represent the p-ends, whereas gray bars represent the q-ends. (A) Representative metaphase of a CML case (02H077) showing very bright fluorescence on chromosome X. (B) Same case (02H077) representing the value of individual telomere in kilobases. The telomeres on Xp measured 24.83 kb, and the average telomere of the case was 6.60 kb. (C) Case 06H069 showing elongation of telomere on 3p (18.66 kb). Its average telomere length was 8.43 kb. (D) Case 06H097 displayed homogenous with long telomere lengths. Its average telomere length was 12.75 kb, and the longest telomere was on 18p (16.27 kb).
Figure 6.
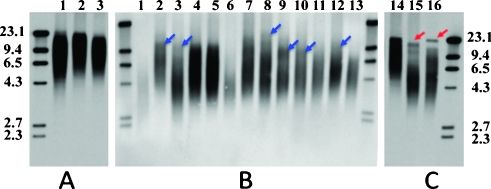
TRF results from three normal individuals (A) and 16 CML cases (B and C). General shortened telomeres can be seen in all samples except for one case (lane 14, case 06H097). Some CML show uncommon telomere fragments, which appear as fuzzy bands (blue arrows in panel B) and represent long telomeres. Two cases, 06H069 (lane 15) and 02H077 (lane 16), the long telomere fragments seem as typical sharp bands (red arrows in panel C) distinct from those short telomere fragments that are distributed as smears in the gel. The molecular markers (kb) are labeled at left and right sides of the panels. Their individual telomere lengths are also shown in Figure 5.
Discussion
Progressive telomere shortening is well described in hematological malignancies, especially at the time of diagnosis and disease relapse [24–27]. However, little is known regarding telomere length changes associated with specific chromosome ends in tumor cells. Owing to the uneven distribution of telomere length on individual chromosome arms, studies on specific telomere changes could have impact on chromosome-specific factors in relation with chromosome stability in tumor cells. Here, we report a study of individual telomere lengths in CML patients at diagnosis before treatment. To our knowledge, this is the first extensive study to determine specific individual telomere lengths in CML.
General Comparison of Telomere Lengths between CML and Normal Cells
We showed that the average telomere length for CML patients was shorter than for normal controls, in agreement with other reports [13,15]. This finding was valid for telomere lengths on both chromosome ends because the mean telomere lengths on p- and q-ends in CML were statistically shorter than their counterparts in normal samples. Interestingly, we found that the average telomere length on p-ends was significantly longer than on q-ends in CML samples. This phenomenon can be attributed to disparate telomere erosion rates on p- and q-ends in CML, which telomeres on q-ends had higher and more uniform erosion rates than those on p-ends. In contrast, telomere lengths in normal individuals did not show the difference between p- and q-ends. Similar to previous reports from other groups, the study on individual telomere length in vivo and in vitro during normal aging did not show differences between p- and q-ends in telomere lengths and in telomere shortening dynamic or telomere erosion rate [22,28]. In addition, we found that the telomeres of female patients were significantly longer than those of male patients. These observations pointed to the differences between CML and normal cells with regard to telomere shortening on chromosome arms. These differences lead to the heterogeneity of individual telomere length profiles in CML cases and may confer a proliferative advantage for the malignant cells.
Shortest and Longest Individual Telomeres
Tumor cells pass through many more replicative cycles than normal cells. Consequently, telomere shortening becomes a common finding for many types of tumors. However, little is known whether the shortest and the longest telomeres are associated with specific chromosomes in malignant disorders. Such a finding could imply chromosome-specific factors involved in telomere length regulation. Our approach allowed us to determine the shortest and longest telomeres associated with specific chromosome ends. We found that telomeres on 19p, 17p, and 20q had higher percentages of being among the shortest in normal samples, in agreement with the findings of other groups [16,22]. However, telomeres on 20q, 21p, 21q, and 9q in the CML group had the highest proportion of being among the shortest telomeres. Similarly to the shortest telomeres, we noticed a swift in the profile of the longest telomeres in normal and CML samples. The telomeres on 18p, Xp, 1p, and 14p were more represented among the longest telomeres in CML samples, whereas those on 5p, 3p, 4q, and 1p had the highest frequencies of being the longest telomeres in normal samples. In summary, telomeres on 5p, 14p, 17p, 19p, Xp, 21p, 4q, and 9q were more prone to change their profile during leukemogenesis.
It is worth noting that, although individual telomere lengths shorten in normal cells during aging and are different in different tissues, they maintain the same profile under these two conditions [22,28,29]. However, individual telomere length profiles in cancer cells are different from those of normal cells even if they derive from the same stem cells. Zheng et al. [30] have shown a different profile of 9p telomere length in lymphocytes from normal women and breast cancer patients, and they have suggested that this finding may be a risk factor in breast cancer. The maintenance of telomere length as well as the rate of shortening may have an influence on the profile change of individual telomere in CML. In clinical settings, this change in profile could be used as a marker to monitor the progression of the disease and the success of the treatment. It would be of great interest to investigate the profile changes of the individual telomeres as the disease progresses through the AP and BP and to understand the biological meaning of these changes. Moreover, the findings could foster research on individual telomere length in other malignancies that lack reliable biomarkers.
Individual Telomere Shortening in CML
The terms of the “telomere shortening” and the “short telomere” should be differentiated because the former is a dynamic process (telomere sequence losses), whereas the latter is correlated with telomere length profile. Our study on individual telomere shortening in CML samples showed that telomeres on Yp, Yq, and 5q shortened more rapidly than the others. Similarly, Rashid-Kolvear et al. [31] have observed in breast cancer cells that telomeres on 17q shortened more rapidly than the average telomere. It is tempting to suggest that the differential shortening of individual telomeres may be a property inherent to cancer cells.
The continuous and rapid losses of telomere sequences on both arms of Y chromosome and on the long arm of chromosome 5 may account for their losses in CML in late phases. These observations have been reported as ones of the recurrent chromosomal abnormalities observable during the progression of CML [32,33]. Besides the possible involvement of short individual telomeres in chromosomal abnormalities, they may also account for an estimation of the rate of cell proliferation. Recently, Keler et al. have estimated, based on the 1-kb difference of average telomere length between normal and CML at the CP, that the malignant cells may undergo approximately 10 times more cell divisions than normal cells [34]. However, this suggestion may be an underestimation. For instance, we found that Yp and Yq had the highest telomere sequence losses of 3.70 and 3.60 kb, respectively. We can thus estimate that the number of cell divisions of CML cells may be 30 times more than those of normal cells'. This accelerated telomere shortening on some individual telomeres could also be explained by an increase in enzymatic processing of specific telomeres due to DNA damage, which has been reported on short telomeres [35], or the disruption of telomere sheltering. Other studies will be needed to elucidate mechanisms involved in the accelerated shortening of some individual telomeres in CML and to establish their occurrence in other malignancies.
Individual Telomere Maintenance or Lengthening
Long telomeres could have very different effects for the maintenance of chromosome stability in comparison with short telomeres. It could be therefore suggested that long telomeres located at some specific chromosome ends may play a role to protect some “key” chromosomes during cell proliferation in tumor progression. In the present study, we found that some specific telomeres on 11p, 14p, 17p, 18p, 19p, and Xp were maintained in the CML group, and this maintenance was consistent in individual CML cases. Despite their average telomere shortening, more than 80% of these cases showed telomere length maintenance or lengthening on these chromosomes arms. In this connection, Krejci et al. [36] have reported a single individual telomere lengthening on 11q and estimated its length of more than 20 kb, whereas the average telomere length was short in one case of acute lymphoblastic B-cell leukemia. Moreover, the presence of individual telomere lengthening has been observed in some cancer cell lines, especially in those using ALTmechanism to maintain their telomeres [37]. Similar to telomere shortening, individual telomere length maintenance or lengthening may be involved in some chromosomal abnormalities seen in CML. For instance, trisomy 19 is a recurrent chromosomal abnormality in CML, appearing during progression of the disease [38]. Although in our normal samples, the individual telomere on 19p was among the shortest telomeres, it was maintained or elongated in all of our CML cases. One explanation for this maintenance may be the need for cells to keep chromosome 19 intact to fulfill their neoplastic fate and even to reinforce their malignancy commitment by adding one additional copy of chromosome 19. One key element contributing to the occurrence of trisomy 19 in CML late phases may be the maintenance of telomere length on chromosome 19p.
The lengthening of different individual telomeres in CML at the CP could correlate with different mechanisms on the maintenance of chromosome stability. First, the presence of some important genes on some chromosome arms may trigger some mechanisms preserving the integrity of these chromosomes arms by protecting telomeres' lengths. For instance, 17p harbors some important genes, such as p53, which has been implicated in the pathogenesis of more than 50% of cancer cases and 10% to 12% of leukemia cases [39], especially in the progression of CML from the CP to the AP [40–42]. Thus, maintenance of the telomere length may protect the chromosomes bearing the important genes in CML cells. Second, the possible preferential action of telomere length maintenance mechanisms on short telomeres could ensure cell survival [43,44]. As a result, telomerase and other telomere maintenance mechanisms may preferentially act on these telomeres to maintain their length. Finally, the cis-acting regulation of an individual telomere length may involve its chromosome arm. Telomere recombination has been implicated in telomere maintenance [44,45], and there is a large body of evidence suggesting a relationship between telomere recombination and epigenetic changes in subtelomeric DNA and chromatin [46,47]. Thus, we can speculate that each individual chromosome arm may have specific epigenetic features in its subtelomere region, enabling it to have control over individual telomere length regulation in CML at the CP.
In addition to normal or low telomerase activity in more than 90% of CML samples at the CP [15], the differential telomere shortening, the maintenance and lengthening of individual telomere, as well as the presence of short and long telomeres as presented in this study are suggestive of the presence of ALT mechanism to maintain telomere length in CML cells at the CP. Further work is obviously required to decipher the involvement of ALT in the maintenance of individual telomere length during the different phases of CML.
Conclusions
This study sheds light on the involvement of individual telomere changes in length in CML at diagnosis. We have defined the individual telomere length profiles and shown nonrandom individual telomere length changes. We have suggested the possible involvement of telomere length changes in CML chromosome abnormalities. Besides a defined role of telomerase in these changes, we have suggested the presence of an ALTmechanism that would maintain telomere length in some individual telomeres in CML at the CP.
Supplementary Material
Acknowledgments
The authors thank Gilles Dupuis and Régen Drouin for reviewing the manuscript and making important comments and suggestions. The authors also thank Rina Kampeas for thoroughly and carefully editing the manuscript.
Footnotes
The study reported here was supported by Fonds de la Recherche en Santé du Québec, the Fondation des étoiles/Foundation of Stars, the Faculty of Medicine and Health Sciences of the Université de Sherbrooke, and the Centre de Recherche Clinique Étienne LeBel.
This article refers to supplementary materials, which are designated by Figures W1 and W2 and are available online at www.neoplasia.com.
References
- 1.Lee HW, Blasco MA, Gottlieb GJ, Horner JW, II, Greider CW, DePinho RA. Essential role of mouse telomerase in highly proliferative organs. Nature. 1998;392:569–574. doi: 10.1038/33345. [DOI] [PubMed] [Google Scholar]
- 2.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 3.Sfeir AJ, Chai W, Shay JW, Wright WE. Telomere-end processing the terminal nucleotides of human chromosomes. Mol Cell. 2005;18:131–138. doi: 10.1016/j.molcel.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 4.Lingner J, Cooper JP, Cech TR. Telomerase and DNA end replication: no longer a lagging strand problem? Science. 1995;269:1533–1534. doi: 10.1126/science.7545310. [DOI] [PubMed] [Google Scholar]
- 5.Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 6.Lansdorp PM. Telomeres, stem cells, and hematology. Blood. 2008;111:1759–1766. doi: 10.1182/blood-2007-09-084913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McClintock B. The behavior in successive nuclear divisions of a chromosome broken at meiosis. Proc Natl Acad Sci USA. 1939;25:405–416. doi: 10.1073/pnas.25.8.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller HJ. The re-making of chromosomes. Collecting Net, Woods Hole. 1938;13:181–198. [Google Scholar]
- 9.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 10.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 11.Rowley JD. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243:290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- 12.Quintas-Cardama A, Cortes J. Molecular biology of bcr-abl1-positive chronic myeloid leukemia. Blood. 2009;113:1619–1630. doi: 10.1182/blood-2008-03-144790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boultwood J, Fidler C, Shepherd P, Watkins F, Snowball J, Haynes S, Kusec R, Gaiger A, Littlewood TJ, Peniket AJ, et al. Telomere length shortening is associated with disease evolution in chronic myelogenous leukemia. Am J Hematol. 1999;61:5–9. doi: 10.1002/(sici)1096-8652(199905)61:1<5::aid-ajh2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Brummendorf TH, Holyoake TL, Rufer N, Barnett MJ, Schulzer M, Eaves CJ, Eaves AC, Lansdorp PM. Prognostic implications of differences in telomere length between normal and malignant cells from patients with chronic myeloid leukemia measured by flow cytometry. Blood. 2000;95:1883–1890. [PubMed] [Google Scholar]
- 15.Ohyashiki K, Ohyashiki JH, Iwama H, Hayashi S, Shay JW, Toyama K. Telomerase activity and cytogenetic changes in chronic myeloid leukemia with disease progression. Leukemia. 1997;11:190–194. doi: 10.1038/sj.leu.2400560. [DOI] [PubMed] [Google Scholar]
- 16.Martens UM, Zijlmans JM, Poon SS, Dragowska W,, Yui J, Chavez EA, Ward RK, Lansdorp PM. Short telomeres on human chromosome 17p. Nat Genet. 1998;18:76–80. doi: 10.1038/ng0198-018. [DOI] [PubMed] [Google Scholar]
- 17.Radich JP. The biology of CML blast crisis. Hematology Am Soc Hematol Educ Program. 2007;2007:384–391. doi: 10.1182/asheducation-2007.1.384. [DOI] [PubMed] [Google Scholar]
- 18.Spurbeck JL, Zinsmeister AR, Meyer KJ, Jalal SM. Dynamics of chromosome spreading. Am J Med Genet. 1996;61:387–393. doi: 10.1002/(SICI)1096-8628(19960202)61:4<387::AID-AJMG15>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 19.Yan J, Chen BZ, Bouchard EF, Drouin R. The labeling efficiency of human telomeres is increased by double-strand PRINS. Chromosoma. 2004;113:204–209. doi: 10.1007/s00412-004-0310-8. [DOI] [PubMed] [Google Scholar]
- 20.Lansdorp PM, Verwoerd NP, van de Rijke FM, Dragowska V, Little MT, Dirks RW, Raap AK, Tanke HJ. Heterogeneity in telomere length of human chromosomes. Hum Mol Genet. 1996;5:685–691. doi: 10.1093/hmg/5.5.685. [DOI] [PubMed] [Google Scholar]
- 21.Dewald G, Stallard R, Al Saadi A, Arnold S, Bader PI, Blough R, Chen K, Elejalde BR, Harris CJ, Higgins RR, et al. A multicenter investigation with interphase fluorescence in situ hybridization using X- and Y-chromosome probes. Am J Med Genet. 1998;76:318–326. [PubMed] [Google Scholar]
- 22.Perner S, Brüderlein S, Hasel C, Waibel I, Holdenried A, Ciloglu N, Chopurian H, Nielsen KV, Plesch A, Högel J, et al. Quantifying telomere lengths of human individual chromosome arms by centromere-calibrated fluorescence in situ hybridization and digital imaging. Am J Pathol. 2003;163:1751–1756. doi: 10.1016/S0002-9440(10)63534-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakoff JA, De Waal E, Garg MB, Denham J, Scorgie FE, Enno A, Lincz LF, Ackland SP. Telomere length in haemopoietic stem cells can be determined from that of mononuclear blood cells or whole blood. Leuk Lymphoma. 2002;43:2017–2020. doi: 10.1080/1042819021000015970. [DOI] [PubMed] [Google Scholar]
- 24.Yamada O, Kawauchi K, Akiyama M, Ozaki K, Motoji T, Adachi T, Aikawa E. Leukemic cells with increased telomerase activity exhibit resistance to imatinib. Leuk Lymphoma. 2008;49:1168–1177. doi: 10.1080/10428190802043861. [DOI] [PubMed] [Google Scholar]
- 25.Ohyashiki K, Ohyashiki JH, Fujimura T, Kawakubo K, Shimamoto T, Saito M, Nakazawa S, Toyama K. Telomere shortening in leukemic cells is related to their genetic alterations but not replicative capability. Cancer Genet Cytogenet. 1994;78:64–67. doi: 10.1016/0165-4608(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 26.Terasaki Y, Okumura H, Ohtake S, Nakao S. Accelerated telomere length shortening in granulocytes: a diagnostic marker for myeloproliferative diseases. Exp Hematol. 2002;30:1399–1404. doi: 10.1016/s0301-472x(02)00969-4. [DOI] [PubMed] [Google Scholar]
- 27.Engelhardt M, Ozkaynak MF, Drullinsky P, Sandoval C, Tugal O, Jayabose S, Moore MA. Telomerase activity and telomere length in pediatric patients with malignancies undergoing chemotherapy. Leukemia. 1998;12:13–24. doi: 10.1038/sj.leu.2400889. [DOI] [PubMed] [Google Scholar]
- 28.Martens UM, Chavez EA, Poon SS, Schmoor C, Lansdorp PM. Accumulation of short telomeres in human fibroblasts prior to replicative senescence. Exp Cell Res. 2000;256:291–299. doi: 10.1006/excr.2000.4823. [DOI] [PubMed] [Google Scholar]
- 29.Graakjaer J, Bischoff C, Korsholm L, Holstebroe S, Vach W, Bohr VA, Christensen K, Kolvraa S. The pattern of chromosome-specific variations in telomere length in humans is determined by inherited, telomere-near factors and is maintained throughout life. Mech Ageing Dev. 2003;124:629–640. doi: 10.1016/s0047-6374(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 30.Zheng YL, Loffredo CA, Shields PG, Selim S. Chromosome 9 arm-specific telomere length and breast cancer risk. Carcinogenesis. 2009;30:1380–1386. doi: 10.1093/carcin/bgp151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rashid-Kolvear F, Pintilie M, Done SJ. Telomere length on chromosome 17q shortens more than global telomere length in the development of breast cancer. Neoplasia. 2007;9:265–270. doi: 10.1593/neo.07106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitelman F. The cytogenetic scenario of chronic myeloid leukemia. Leuk Lymphoma. 1993;11(Suppl 1):11–15. doi: 10.3109/10428199309047856. [DOI] [PubMed] [Google Scholar]
- 33.Mitelman F, Levan G, Nilsson PG, Brandt L. Non-random karyotypic evolution in chronic myeloid leukemia. Int J Cancer. 1976;18:24–30. doi: 10.1002/ijc.2910180105. [DOI] [PubMed] [Google Scholar]
- 34.Keller G, Brassat U, Braig M, Heim D, Wege H, Brummendorf TH. Telomeres and telomerase in chronic myeloid leukaemia: impact for pathogenesis, disease progression and targeted therapy. Hematol Oncol. 2009;27:123–129. doi: 10.1002/hon.901. [DOI] [PubMed] [Google Scholar]
- 35.Zou Y, Sfeir A, Gryaznov SM, Shay JW, Wright WE. Does a sentinel or a subset of short telomeres determine replicative senescence? Mol Biol Cell. 2004;15:3709–3718. doi: 10.1091/mbc.E04-03-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krejci K, Stentoft J, Koch J. Molecular cytogenetics investigation of the telomeres in a case of Philadelphia positive B-ALL with a single telomere expansion. Neoplasia. 1999;1:492–497. doi: 10.1038/sj.neo.7900065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perrem K, Colgin LM, Neumann AA, Yeager TR, Reddel RR. Coexistence of alternative lengthening of telomeres and telomerase in hTERT-transfected GM847 cells. Mol Cell Biol. 2001;21:3862–3875. doi: 10.1128/MCB.21.12.3862-3875.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johansson B, Fioretos T, Mitelman F. Cytogenetic and molecular genetic evolution of chronic myeloid leukemia. Acta Haematol. 2002;107:76–94. doi: 10.1159/000046636. [DOI] [PubMed] [Google Scholar]
- 39.Murray-Zmijewski F, Slee EA, Lu X. A complex barcode underlies the heterogeneous response of p53 to stress. Nat Rev Mol Cell Biol. 2008;9:702–712. doi: 10.1038/nrm2451. [DOI] [PubMed] [Google Scholar]
- 40.Ashur-Fabian O, Adamsky K, Trakhtenbrot L, Cohen Y, Raanani P, Hardan I, Nagler A, Rechavi G, Amariglio N. Apaf1 in chronic myelogenous leukemia (CML) progression: reduced Apaf1 expression is correlated with a H179R p53 mutation during clinical blast crisis. Cell Cycle. 2007;6:589–594. doi: 10.4161/cc.6.5.3900. [DOI] [PubMed] [Google Scholar]
- 41.Ahuja H, Bar-Eli M, Advani SH, Benchimol S, Cline MJ. Alterations in the p53 gene and the clonal evolution of the blast crisis of chronic myelocytic leukemia. Proc Natl Acad Sci USA. 1989;86:6783–6787. doi: 10.1073/pnas.86.17.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feinstein E, Cimino G, Gale RP, Alimena G, Berthier R, Kishi K, Goldman J, Zaccaria A, Berrebi A, Canaani E. P53 in chronic myelogenous leukemia in acute phase. Proc Natl Acad Sci USA. 1991;88:6293–6297. doi: 10.1073/pnas.88.14.6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hemann MT, Strong MA, Hao LY, Greider CW. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001;107:67–77. doi: 10.1016/s0092-8674(01)00504-9. [DOI] [PubMed] [Google Scholar]
- 44.Morrish TA, Greider CW. Short telomeres initiate telomere recombination in primary and tumor cells. PLoS Genet. 2009;5:e1000357. doi: 10.1371/journal.pgen.1000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Londono-Vallejo JA, Der-Sarkissian H, Cazes L, Bacchetti S, Reddel RR. Alternative lengthening of telomeres is characterized by high rates of telomeric exchange. Cancer Res. 2004;64:2324–2327. doi: 10.1158/0008-5472.can-03-4035. [DOI] [PubMed] [Google Scholar]
- 46.Gonzalo S, Jaco I, Fraga MF, Chen T, Li E, Esteller M, Blasco MA. DNA methyltransferases control telomere length and telomere recombination in mammalian cells. Nat Cell Biol. 2006;8:416–424. doi: 10.1038/ncb1386. [DOI] [PubMed] [Google Scholar]
- 47.Vera E, Canela A, Fraga MF, Esteller M, Blasco MA. Epigenetic regulation of telomeres in human cancer. Oncogene. 2008;27:6817–6833. doi: 10.1038/onc.2008.289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.