Abstract
Objectives
The purpose of this study was to determine if changes in the position of the stimulating electrode in the cochlea could be used to elicit the electrically evoked auditory change complex (EACC) from Nucleus cochlear implant users.
Design
Nine postlingually deafened adults participated in this study. Each study participant had been using his or her Nucleus CI24 cochlear implant for at least 3 months prior to testing. The speech processor was bypassed and the output of the implanted receiver/stimulator was controlled directly. The stimulus was a 600 ms burst of a biphasic pulse train (1000 pps). In control conditions, the stimulating electrode was held constant and stimulation continued throughout the 600 ms recording interval. In experimental conditions, the EACC was elicited by introducing a change in the stimulating electrode 300 ms after the onset of the pulse train. The EACC was recorded using surface electrodes. Three recordings of 100 sweeps each were obtained for each stimulus condition. Bandpass filtering (1 – 100 Hz) was used to minimize contamination of the recordings by stimulus artifact. Averaged responses were then smoothed using a 40 ms wide boxcar filter and standard peak picking procedures were used to analyze these responses in the time domain.
Results
In each case, a clear onset response (P1-N1-P2) was recorded. In the experimental conditions, a second evoked potential, the EACC, was also recorded following the change in stimulating electrode. This second response had general morphologic characteristics that were very similar to those of the onset response. Increasing the separation between the two stimulating electrodes in the experimental conditions resulted in a general trend toward increased EACC amplitudes.
Conclusions
This report describes results of a set of experiments in which the speech processor of the cochlear implant was bypassed and the EACC was recorded in response to a change in stimulating electrode position. EACC amplitude was shown to increase as the separation between the two stimulating electrodes increased. While preliminary in nature, these results demonstrate the feasibility of recording the EACC in response to changes in stimulating electrode position from individual cochlear implant users.
Keywords: auditory evoked potential, cochlear implant, cortical evoked potential, electrical stimulation
INTRODUCTION
While a number of distinct, cortically generated, auditory evoked potentials have been described in the literature, the obligatory P1-N1-P2 complex is one of the most versatile and widely studied. The P1-N1-P2 complex is a transient auditory evoked potential that can be recorded from surface electrodes placed on the scalp in response to a wide range of stimuli. When recorded from normal hearing adult listeners in response to a brief acoustic stimulus, this obligatory cortical potential consists of a series of three peaks that are recorded within a latency range extending from 50 to 250 ms. The peaks are traditionally labeled individually as P1, N1 and P2. The distribution of these evoked potentials across the scalp has been well documented, as has the impact of changes in both stimulation and recording parameters (e.g Näätänen and Picton, 1987; Hyde, 1997). Additionally, although the P1-N1-P2 response complex does not reach its mature form until late adolescence or early adulthood, it can be recorded in young children using passive listening paradigms and the effects of development on this set of evoked potentials has been described (e.g. Ponton et al., 1996; Eggermont and Ponton, 2003; Wunderlich, et al., 2006).
The P1-N1-P2 response has a number of applications. It can be used to estimate hearing thresholds (e.g. Lightfoot and Kennedy, 2006; Hyde, 1997) and can be reliably recorded in individuals with varying degrees of hearing loss when sound is delivered through a hearing aid (Souza and Tremblay, 2006; Tremblay et al., 2006; Korczak et al., 2005). The P1-N1-P2 complex has been used to explore differences in how acoustic stimuli are processed within the auditory system by groups of normally hearing listeners as opposed to those with learning and/or central auditory processing disorders (Sabisch et al., 2006; Delb et al., 2003; Fuess et al., 2002; King et al., 2002; Maison et al., 1996; Tonnguist-Uhlen, 1996). Additionally, auditory training has been shown to influence both the amplitude and latency of the P1, N1 and P2 potentials (Hayes et al., 2003; Tremblay and Kraus, 2002; Tremblay et al., 2001; Tremblay, Kraus and McGee, 1998).
One distinct advantage that cortical potentials have over more peripherally generated responses, like the ABR, is the fact that cortical potentials can be elicited using a wider range of stimuli. It is possible, for example, to record the P1-N1-P2 complex in response to long duration tones, naturally or synthetically produced speech samples, or to a change in one or more parameters of an ongoing stimulus (e.g. Jerger and Jerger 1970; Yingling and Nethercut, 1983; Näätänen and Picton, 1987). Moreover, the amplitude and latency of the individual peaks that make up this cortically generated evoked potential have been shown to vary systematically depending on the specific acoustic characteristics of the speech sample (Obleser et al., 2003; Martin and Boothroyd, 2000; Sharma et al., 2000; Tremblay et al., 2004; Tremblay et al., 2003; Agung et al., 2006).
In 1998, Ostroff et al. recorded cortical potentials evoked by the repeated presentation of three different naturally produced speech tokens: /s/, /ei/ and /sei/ in normal hearing listeners. These investigators showed that the response evoked by the CV syllable /sei/ consisted of a series of two overlapping P1-N1-P2 responses generated by the fricative /s/ and vowel /ei/ respectively. The response recorded following presentation of the vowel /ei/ was significantly larger in amplitude when the vowel was presented in isolation, as opposed to when it was preceded by the consonant /s/ as in the CV syllable /sei/. Ostroff et al. (1998) referred to the response elicited by the vowel /ei/ when presented in the context of an ongoing syllable /sei/ as the “N1-P2 acoustic change complex (ACC)”. It should be noted that while the ACC and the P1-N1-P2 responses are similar in many ways, the ACC is more than a simple onset response. That is, short duration stimuli elicit a traditional P1-N1-P2 response but not an ACC. The ACC was only recorded when relatively long duration, time varying stimuli were used. Ostroff et al (1998) postulate that the ability of the auditory system to respond to acoustic changes in an ongoing stimulus, as demonstrated by a clearly evoked ACC, may be a prerequisite for higher levels of speech perception.
In a series of follow-up studies, Tremblay and colleagues (Tremblay et al., 2003; Tremblay et al., 2006) recorded cortical evoked auditory potentials using natural speech tokens /si/, and /∫i/. The morphology of the evoked potentials that were recorded in response to these two speech tokens varied systematically depending on the temporal and spectral properties of the speech signal. In addition, responses elicited by the vowel /i/ varied depending on whether the vowel was preceded by the fricative /s/ or /∫/. Like Ostroff et al. (1998), these authors interpreted their results as evidence that cortical auditory responses can reflect detection of change in the acoustic characteristics of a speech signal within a syllable of naturally produced speech.
Martin and Boothroyd (2000) took a different approach to measuring the ACC. Instead of using CV syllables where the transition from one phoneme to the next includes both spectral and temporal changes, they used long duration versions of the synthetic vowel /u/ to elicit cortical responses. Four hundred milliseconds after the onset of the vowel /u/, they introduced a change in either the RMS amplitude and/or frequency of the second formant of the vowel. At the onset of stimulation, the P1-N1-P2 complex is recorded. For many of the stimulus conditions, a second N1-P2 response, the ACC, was recorded following the “change” in the amplitude and/or frequency composition of the acoustic signal. Martin and Boothroyd (2000) noted that the ACC has a morphology that is very similar to the onset response, both in terms of latency and individual peak amplitude. The advantage of this stimulation paradigm is that it allows for separation of the two evoked potentials (the onset response and the change potential) in the time domain. Martin and Boothroyd (2000) describe the influence that systematic changes in formant frequencies and/or RMS intensity levels had on the change potential. ACC amplitudes were consistently larger when the stimulus change included both spectral as well as amplitude differences rather than when it included only changes in either the acoustic spectrum or the overall signal level.
More recently, Harris, Mills and Dubno (2007) report using the ACC to assess the impact of advancing age on intensity discrimination in normal hearing adults. Group averaged waveforms showed ACC responses for intensity increments as small as 2 dB. The authors do not report psychophysical measures of intensity discrimination for these study participants but note that the electrophysiological thresholds measured in this study were similar to those measured behaviorally in a previous study (He et al., 1998).
Results of these studies are important because they suggest that the ACC may function as an electrophysiologic measure of the neural processes that underlie detection of change in an ongoing acoustic signal. Martin and Boothroyd (2000), Ostroff et al. (1998), Tremblay et al. (2003) and Tremblay et al. (2006) all suggest that the ACC could potentially serve as a clinical tool for assessing speech perception capacity in very young children and mention that pediatric cochlear implant recipients represent one population where such measures might be particularly useful.
Electrically evoked, cortically generated evoked potentials were initially described in the literature in 1991 (Oviatt and Kileny, 1991; Kileny, 1991; Kaga et al., 1991). These early studies described methods for recording the N1-P2 and/or P300 response from cochlear implant users and compared these recordings to responses that are typically recorded using acoustic stimulation. Later studies focused on the relationship between the N1-P2 response and some measure of perception in cochlear implant users (e.g. Firszt et al., 2002a; Firszt et al., 2002b; Groenen et al., 1996; Beynon et al., 2002; Kraus, Micco and Koch, 1993). Ponton and colleagues (Ponton et al., 1996; Ponton et al., 1999; Ponton and Eggermont, 2001) showed that maturation of P1 was delayed in cochlear implant users relative to their age matched, normal hearing peers. Moreover, they suggest that the cortical potentials recorded from children who have experienced relatively long periods of deafness prior to cochlear implantation may never fully mature. Sharma et al. (2002a and 2002b) later expanded this work to show that the effects of development on the P1 response, as recorded from prelingually deaf, pediatric cochlear implant recipients, vary as a function of age at implant and Gordon et al. (2005) reported responses recorded from poor performers were atypical in terms of gross morphologic characteristics.
To date, two studies have been published in which the electrically evoked auditory change complex (EACC) was measured. Friesen and Tremblay (2006) used speech stimuli to record electrically evoked cortical potentials from a series of eight adults who used the Nucleus cochlear implant. The stimuli (/∫i/ and /si/), which were presented at one level in the sound field, were identical to those used in their earlier work (Tremblay et al., 2003) but in this case were processed though the speech processor of the listener’s cochlear implant. The electrically evoked responses that Friesen and Tremblay (2006) recorded were grossly similar to those they had recorded previously in response to acoustic stimulation (Tremblay et al., 2003). That is, for both the normal hearing and cochlear implant listeners, the response that was recorded using these two specific speech tokens consisted of a series of overlapping N1-P2 peaks. For the individuals who used a cochlear implant to communicate, the initial N1 peak, presumably evoked by detection of the initial consonant, was recorded approximately 40 ms earlier for /∫i/ stimulus than for /si/. The second negative peak, recorded following the vowel onset and reflecting detection of change in the speech signal, was on average 23 ms earlier for /∫i/ than for /si/. Repeated testing revealed that these electrically evoked responses were stable from test to retest and robust enough to be resolved in waveforms recorded from individual subjects. This study is important because it demonstrates clearly that longer duration speech or speech-like stimuli can be used to elicit the EACC from individual who use a cochlear implant to communicate. While there are clear advantages to using speech signals as stimuli, the overlap in the time domain between the onset response and the EACC can make analysis of the individual components of these responses difficult to characterize.
Recently, Martin (2007) reported results of a study in which the EACC was recorded from a single subject who used a Med-El cochlear implant to communicate. In this study, a series of long duration, synthetic vowels were presented in the sound field. Each stimulus was 786 ms in duration. The stimulus began with a format structure perceived by listeners as the vowel /u/. At the temporal midpoint in the signal, the F2 formant frequency was increased. A control stimulus that did not contain a change in F2 formant frequency was perceived by normal hearing listeners as a long duration /u/ while the stimulus with the largest F2 shift (1200 Hz) was perceived as /ui/. A neural response, the EACC, was elicited when the stimulation conditions included F2 formant frequency shifts that were large enough to be detected by the listener. Although this study includes data from only a single subject and much of the study dealt with identifying ways to manage the ongoing stimulus artifact recorded when this paradigm was used, the results suggest that it should be possible to record the EACC from a larger group of subjects using a stimulation paradigm similar to that originally described by Martin and Boothroyd (2000).
In both the Friesen and Tremblay (2006) and Martin (2007) studies, an acoustic stimulus was presented at a single level in the sound field and the study participants were tested using their own speech processor. While this approach may have ecological validity, interpretation of the results is complicated because the speech processor settings can vary widely from one cochlear implant user to the next. It is possible, for example, that a specific change in the frequency of the acoustic signal in the sound field may result in a change in place of stimulation in the cochlea that is greater for one subject than for another. Similarly, all cochlear implant speech processors are inherently nonlinear. An increase in the level of an acoustic signal presented in the sound field may or may not translate into a change in the amount of current provided by the cochlear implant. As a result, the magnitude of the EACC may be expected to vary significantly from patient to patient for the same set of stimuli. This concern applies not only to studies where the EACC is measured but to all studies where sound field stimulation is used and the acoustic stimulus is transmitted through the speech processor.
When measuring the EACC in response to frequency changes, results of Martin (2007) suggest that increasing the magnitude of the change in F2 formant frequency increases EACC amplitude. No EACC was measured when F2 frequency changes less than 75–150 Hz were used. EACC amplitudes increased as the magnitude of the change in F2 frequency change increased from 150 to 1200 Hz. Results of this study, however, are based on results of a single subject and no specific information is provided about how the output of the internal receiver stimulator changed for the range of frequency changes included in this study. Additionally, the subject used in this study was an adult diagnosed with auditory neuropathy/auditory dys-synchrony. As such, it is not clear how well results from this single subject can be expected to generalize to wider populations of cochlear implant users.
In this preliminary study, we expand on the work of Friesen and Tremblay (2006) and Martin (2007). Rather than presenting the stimulus in the sound field, we use Nucleus Implant Communicator (NIC) library of subroutines provided to us by Cochlear Corporation to control the output of the implanted receiver stimulator directly. This technique not only gives us better stimulus control, but limiting the stimulation at any point in time to a single electrode may have the added advantage of helping to limit contamination of the recorded responses by stimulus artifact. Our goals were (1) to explore the feasibility of recording the EACC in response to direct electrical stimulation in a larger group of cochlear implant users, and (2) to test the hypothesis that amplitude of the EACC will increase as the magnitude of the change in electrode position within the cochlea increases.
METHODS
Subjects
Nine postlingually deafened adults participated in this study. Each used the Nucleus CI24 cochlear implant system. Five were implanted unilaterally (M66, R13, R15, R80 and R82). Four were bilateral cochlear implant users (M54, M58, M52 and R47). Of the four bilateral cochlear implant users, data from both ears of subject M52 are included.
Each study participant received his/her cochlear implant at the University of Iowa Hospitals and Clinics. In each case, the surgery was uneventful and full electrode insertions were obtained. Each study participant had been using his/her cochlear implant for a minimum of 3 months prior to participating in this study. Table 1 lists general information about the study participants including gender, age at the time of testing, years of profound deafness prior to obtaining a cochlear implant, duration of cochlear implant use, etiology of deafness and the processing strategy used by the subject in every day life. Two subjects (M54R and M52R) had electrodes programmed out of their MAP because impedance testing revealed a short circuit. These electrodes were not used for the purposes of this study.
Table 1.
General demographic information about each of the nine study participants is listed including gender, age at implant, ear implanted, duration of profound deafness, duration of cochlear implant use at the time of testing, type of cochlear implant, preferred processing strategy and the number of electrodes disabled.
| Subject ID | Gender | Age (yrs) | Ear Implanted | Cochlear Implant type1 | Preferred Processing Strategy and Rate (Hz) | Electrodes disabled | Duration of profound HL prior to CI (yrs) |
Duration of CI use (yrs) | Etiology |
|---|---|---|---|---|---|---|---|---|---|
| M66 | M | 56 | R | CI24M | CIS 1800 | None | 2 | 7 | unknown |
| M54R | F | 58 | R | CI24M | ACE 1200 | 6, 11 (SC) | 7 | 7 | unknown |
| M58R | F | 65 | R | CI24M | ACE 720 | None | 1 | 6 | Meniere’s Disease |
| M52L M52R |
F | 66 | L R |
CI24M | ACE 720 | None 6, 22 (SC) |
0.25 | 6 | Virus/Fever |
| R13 | F | 68 | R | CI24R | CIS 1800 | None | 12 | 6 | Familial, progressive |
| R15 | M | 45 | R | CI24R | ACE 900 | None | 3 | 5 | Familial, progressive |
| R80 | M | 45 | L | CI24R | ACE 900 | None | 4 | 1 | Fever/ototoxicity |
| R82 | M | 81 | R | CI24R | ACE 500 | None | 02 | 1 | Unknown |
| R47R | F | 58 | R | CI24R | ACE 900 | None | 0.2 | 2.5 | Familial, progressive |
All of the study participants used a Nucleus device. CI24M refers to the straight electrode array. CI24R refers to the contoured electrode array.
This study participant had a severe hearing loss through 1000 Hz sloping to a profound loss at 4000 Hz at the time of surgery.
General procedures
Each evoked potential recording session lasted approximately three and a half hours. Participants were seated in a reclining chair and instructed to ignore the stimulus, minimize head movement and read or watch captioned TV. Each study participant was also asked not to sleep during the recording session and breaks were provided as necessary to insure they were able to comply with these instructions.
Recording parameters
Disposable, sterile Ag-AgCL surface recording electrodes were used to record the evoked responses. The EACC was recorded differentially between the vertex (Cz) and the mastoid contralateral to the cochlear implant. A second pair of electrodes positioned above and lateral to the eye contralateral to the implant were used to monitor eye-blink activity. A forehead ground was used for both recording channels.
Electrode impedance values ranged from 1000–3000 Ohms with no more than 2000 Ohms difference between any two electrodes. Artifact rejection was used to minimize contamination of the EEG recordings by muscle artifact. The artifact rejection level was set so that sweeps from both A/D channels were rejected if the channel used to record the eye-blink contained voltages larger than 100 µV.
Optically isolated, differential amplifiers (Dataq, BMA931) were used for recording. These amplifiers provided a gain of 10,000 and allowed for analog filtering between 1.0 Hz (high pass, 12 dB/octave) and 100 Hz (low pass, 12 dB/octave). Baseline correction was not required. The A/D system (National instruments DAQCard-6062E) had 12 bits of resolution and responses were recorded using a sampling rate of 100,000 Hz. LabVIEW software was used to control the averaging process and to display both the ongoing, raw EEG activity and the averaged responses. The recording time window was 600 ms. Pre- and post-stimulus baselines were not recorded.
Typically, three recordings of 100 sweeps were obtained for each stimulus condition. Control conditions, which did not include a change in the stimulating electrode, were interspersed randomly with the experimental conditions throughout the recording session.
Stimulation Parameters
NIC routines were used to control output of the internal receiver stimulator directly. This library of routines allows control of the output of the cochlear implant without a detailed knowledge of the system-level operating requirements of the device and allows for greater flexibility than is possible when the standard clinical interface is used. Custom software was developed that used these routines to control the output of the cochlear implant and to provide a trigger for the averaging software. In this experiment, the trigger pulse was always synchronized with the onset of the stimulating pulse train.
The basic stimulus used to evoke the P1-N1-P2 response was a 600-ms long train of biphasic current pulses. The individual pulses in the stimulus train were 25 µs/phase, had an inter-phase gap of 8 µs and were presented at a rate of 1000 pps. In all cases, monopolar stimulation between an active -cochlear electrode and the extra-cochlear ground electrode located in the temporalis muscle (MP1) was used. Cochlear implant listeners typically describe a stimulus such as this as a tone or buzz. The approximate pitch is dependent on the electrode that is stimulated. Stimulation of electrodes located near the base of the cochlea typically results in a higher pitch percept than stimulation of electrodes located near the apex.
For most subjects the stimulus train was presented once every 1.8 seconds. Subjects M54R, M66 and R13 showed evidence of adaptation when tested at this rate. For these three subjects, the stimulation rate was decreased to once every three seconds.
In control or “no change” conditions, the stimulating electrode was not changed during the entire 600-ms stimulus burst. In the experimental or “change” conditions, the stimulus began on one electrode at the 300 ms time point it was switched to a second electrode. A series of evoked potentials were recorded as the spatial separation of the two stimulating electrodes was varied.
Stimulation levels were set at or near the maximum acceptable comfort level for each subject and held constant throughout the entire stimulation period. Table 2 lists the behavioral thresholds and maximum comfortable levels used to estimate the loudest acceptable stimulation levels for the 1000 Hz pulse train. Also listed are the specific stimulation levels used for evoked potential testing. As is apparent in Table 2, the stimulation level was not changed when the stimulating electrode was changed. In this experiment, due primarily to time constraints, we did not specifically balance the two simulating electrodes in terms of loudness. Instead, study participants were selected only if their MAP C-levels and dynamic ranges varied little across electrodes. Additionally, when queried, the study participants described the change in stimulating electrode as a change in pitch rather than stimulation level. While we would argue that the effects of level should be minimal, it is possible that the change potentials recorded in this preliminary study reflect a neural response to change in both the place of stimulation and the perceived loudness of the stimulus.
Table 2.
Specific stimulation parameters used to record the individual responses shown in Figure 2 and 3 are listed. Also listed are the response latencies and peak to peak amplitudes for each subject. Electrode 1 refers to the electrode used for stimulation during the first 300 ms of the experimental trials. Electrode 2 is the stimulating electrode used in the second 300 ms of the experimental trials.
| Stimulation Parameters | Onset Response | EACC | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject ID |
Rate used for T&C levels |
Electrode 1 | Electrode 2 | EP Stimulus Level |
N1 latency (ms) |
P2 latency (ms) |
N1-P2 amplitude (µV) |
N1 latency (ms) |
P2 latency (ms) |
N1-P2 amplitude (µV) |
||||
| E1 | T-level | C- level |
E2 | T-level | C- level |
|||||||||
| M66 | 1000Hz | 10 | DNT | 222 | 15 | DNT | 222 | 222 | 95.0 | 195.0 | 12.00 | 92.0 | 188.0 | 6.37 |
| M54L | 1200Hz | 5 | 164 | 225 | 10 | 170 | 232 | 225 | 102.0 | 185.0 | 8.17 | 105.0 | 184.0 | 5.83 |
| M58R | 720Hz | 10 | 133 | 175 | 15 | 130 | 169 | 170 | 118.0 | 196.0 | 5.25 | 112.0 | 195.0 | 2.69 |
| M52L | 720Hz | 10 | 149 | 218 | 15 | 150 | 218 | 205 | 112.0 | 216.0 | 6.47 | 108.0 | 183.0 | 3.15 |
| M52R | 720Hz | 10 | 146 | 215 | 15 | 152 | 222 | 205 | 116.0 | 206.0 | 5.18 | 100.0 | 209.0 | 2.43 |
| R13 | 1000Hz | 14 | DNT | 185 | 19 | DNT | 189 | 185 | 125.0 | 259.0 | 5.45 | 114.0 | 266.0 | 4.31 |
| R15 | 900Hz | 17 | 155 | 207 | 21 | 157 | 206 | 205 | 120.0 | 184.0 | 3.69 | 108.0 | 173.0 | 2.75 |
| R80 | 900Hz | 15 | 134 | 204 | 20 | 135 | 201 | 210 | 134.0 | 224.0 | 6.85 | 119.0 | 191.0 | 2.39 |
| R82 | 1000Hz | 15 | DNT | 205 | 20 | DNT | 207 | 205 | 129.0 | 212.0 | 5.99 | 118.0 | 245.0 | 3.16 |
| R47R | 900Hz | 10 | 121 | 181 | 15 | 119 | 184 | 180 | 107.0 | 211.0 | 3.13 | 114.0 | 207.0 | 1.59 |
| Mean | 115.8 | 208.8 | 6.22 | 109.0 | 204.1 | 3.47 | ||||||||
| S.E. | 3.84 | 6.96 | 0.79 | 2.64 | 9.35 | 0.49 | ||||||||
Analysis Procedures
All of the recorded responses were analyzed off line using custom-designed MATLAB software. Recordings obtained using the same stimulation parameters were averaged together and then smoothed using a 40 ms wide boxcar filter prior to analysis. Typically, three replications of 100 sweeps were averaged for each condition before the individual peak amplitudes and latencies were determined. Peak-to-peak amplitude measures were measured between N1 and P2 for both the onset responses and the change potentials. N1 was defined as the minimum voltage recorded within a time window extending from 50 to 200 ms for the onset response and between 350 and 500 ms for the change potential. For both the onset and change potentials, P2 was defined as the maximum voltage recorded within a 120 ms window after the N1 peak. In cases where either the N1 or P2 peak was very broad, the midpoint of the peak was selected. All waveform analysis was done by experimenters familiar both with this experimental paradigm and with cortical potentials.
RESULTS
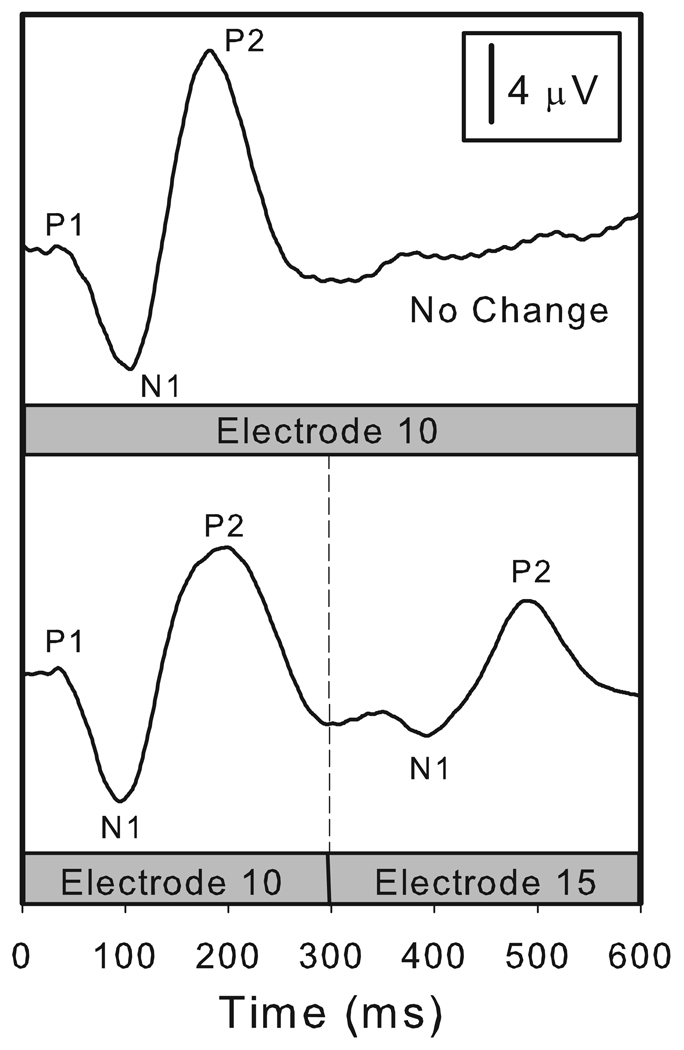
Figure 1 illustrates the stimulation paradigm used and shows a set of waveforms recorded from a single subject (M66). The stimulus used to record the response in the upper half of Figure 1 was a 600 ms burst of constant amplitude, biphasic current pulses presented at a rate of 1000 pps on electrode 10. This is a control or “no change” condition. The waveform shown in the upper half of Figure 1 contains only an onset response labeled P1, N1 and P2. The stimulus used to record the response in the lower half of Figure 1 was a 600 ms burst of constant amplitude, biphasic current pulses presented at a rate of 1000 pps. In this case, however, the stimulating electrode was changed from electrode 10 to electrode 15 after 300 ms of stimulation. This recording includes both an onset response and, in the second half of the recording interval, a change potential (the EACC). The EACC is similar to but smaller in amplitude than the onset response. The N1 and P2 peaks of the EACC are marked.
Figure 1.
Electrically evoked cortical potentials are shown for one subject (M66). The stimulus was a 600 ms burst of biphasic current pulses (1000 pps, 25 us/phase). In the control/no change condition (upper panel) stimulation was applied to electrode 10 for the full 600 ms recording interval. This recording contains only an onset response. In the lower panel, stimulation began on electrode 10 and at the 300 ms point was changed to electrode 15. This recording includes both an onset response and an EACC.
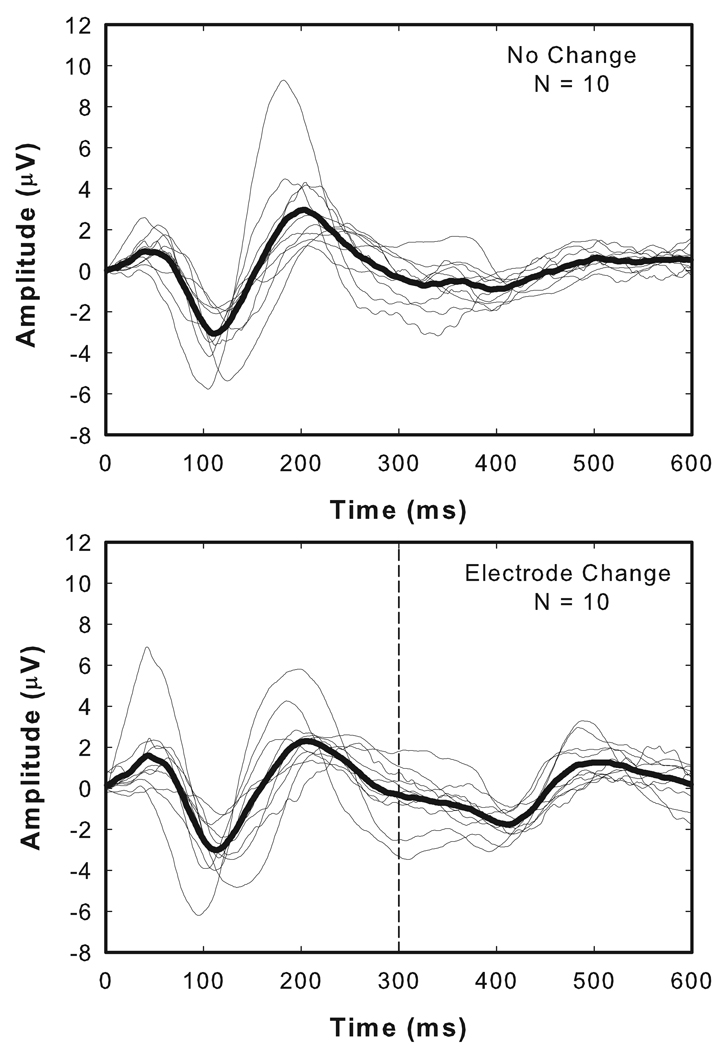
Figure 2 shows a collection of responses recorded from 10 ears of the 9 adults who participated in this study. Responses recorded from individual subjects are shown with thin lines. Superimposed on these individual responses is the grand mean average (thick lines). The responses shown in the upper panel of Figure 2 are control or “no change” conditions and were recorded in response to the repeated presentation of a 600 ms burst of biphasic current pulses (1000 pps, 25 µs/phase). No change in the stimulating electrode or in any of the other stimulation parameters occurred during this 600 ms stimulation/recording interval. The waveforms shown in the lower panel of Figure 2 were collected using a pulse train that started on one electrode and 300 ms later was switched to a second electrode located closer to the apex of the cochlea. For nine of the ten ears tested, the change was to an electrode located five electrodes away from the initial site of stimulation. For one subject, R15, stimulation began on electrode 17 and was changed to electrode 21. Details of the stimulation parameters are listed in Table 2.
Figure 2.
Thin lines represent waveforms recorded from each of the 9 adult study participants (10 ears). The thick line is the group mean average waveform. The upper panel is a control condition. The lower panel shows recordings from the same set of individuals. In this case, the stimulation level was held constant but 300 ms after stimulation began, the stimulating electrode was changed. In each case, the change was to an electrode located 4–5 electrodes apical to the initial stimulating electrode.
For each of the waveforms shown in Figure 2, an onset response is clearly visible with a negative peak (N1) recorded approximately 100 ms after the stimulus onset followed by a positive peak (P2) that occurs about 100 ms later. Onset responses are evident both in the individual waveforms and in the grand mean average waveforms. In the experimental conditions, EACC responses are also evident. In general, the EACC is smaller in terms of peak-to-peak amplitude than the onset response but shares most of the other morphologic characteristics of the onset response.
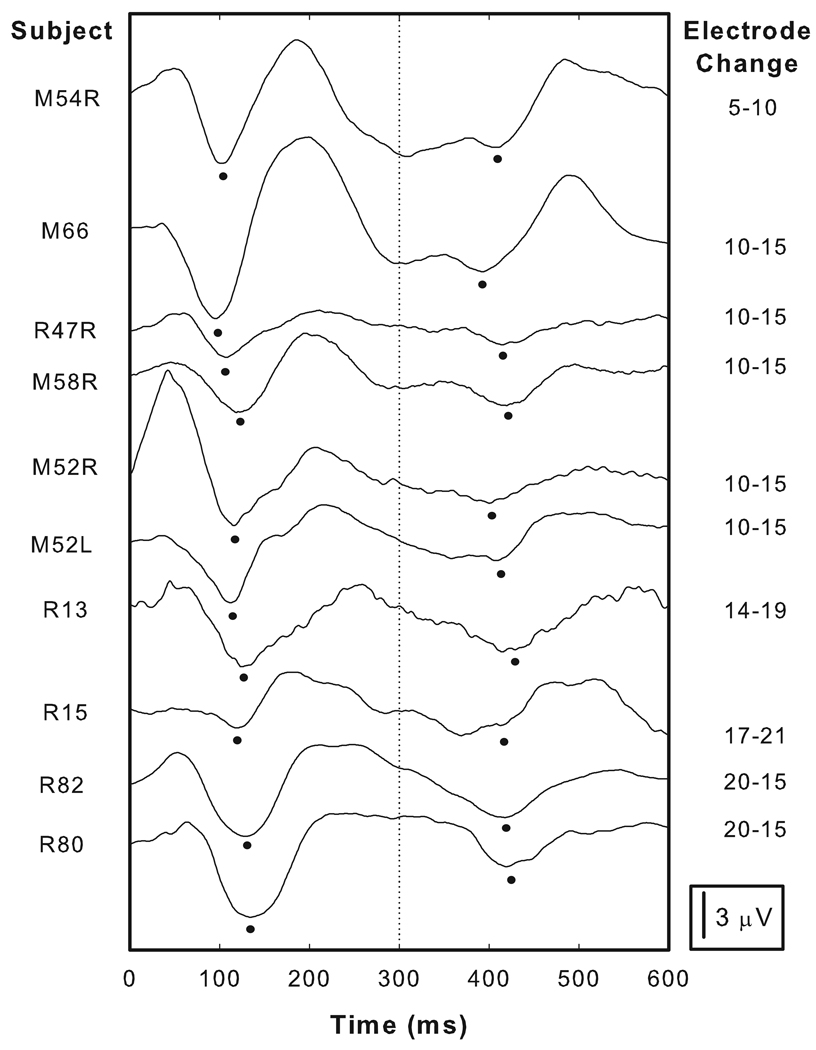
In order to allow cross subject variations to be more easily appreciated, the individual responses shown in the lower panel of Figure 2 have been offset from each other and re-plotted in Figure 3. Onset responses are clearly apparent in the first half of each waveform and the N1 peak is marked. The morphology of the onset response varied somewhat across subjects. For example, clear P1 peaks can be identified approximately 50 ms after the stimulus onset for many subjects (e.g. R82, R80 and M54L). For other subjects, P1 is not nearly as robust (e.g. M52L and R15). For all subjects the most identifiable component to the response was the N1 peak. N1 latencies ranged from 95 to 134 ms with a mean of 115.8 ms. P2 latencies exhibited more cross-subject variation and ranged in latency from 184 to 259 ms with a mean of 208.8 ms. N1-P2 amplitudes also varied across subjects ranging from 3.13 to 12 µV with a mean of 6.22 µV.
Figure 3.
The waveforms shown in the lower panel of Figure 2 have been have been shifted along the ordinate and re-plotted. The column on the right indicates the stimulating electrodes used to elicit these responses. For each subject, an onset response as well as an EACC is recorded and the dots indicate the N1 peaks picked for both the onset responses and for the EACC. More details about the stimulation parameters are listed in Table 2.
In many cases, the general morphologic characteristics of the EACC were similar to the onset response recorded for the same subject. For example, subjects with onset responses that included a clear P2 peak tended to have prominent P2 peaks in their EACC (e.g. M66 and M54L). Conversely, subjects with onset responses that did not have clearly identifiable P2 peaks, tended to have EACC waveforms with similar characteristics (e.g. R47R and R13). For most subjects, the P1 peak in the EACC was difficult to identify. Like the onset response, the most robust component of the EACC was N1. This potential is indicated by the small filled circle in Figure 3. When measured relative to the time the stimulating electrode was changed, EACC N1 latencies varied across subjects from 92 to 119 ms with a mean of 109 ms. P2 latencies ranged from 173 to 266 ms with a mean of 204.1 ms. N1-P2 amplitudes as measured in the individual EACC recordings ranged from 1.59 to 6.37 µV with a mean of 3.47 µV.
Two-tailed, paired t-tests were used to evaluate the differences in N1-P2 amplitude and latency between the onset potentials and the EACC. The EACC was significantly smaller in terms of peak-to-peak amplitude than the onset response (p <0.0002, df = 9), however, the latency differences observed between the onset and EACC responses were not significant at a p = 0.05 level.
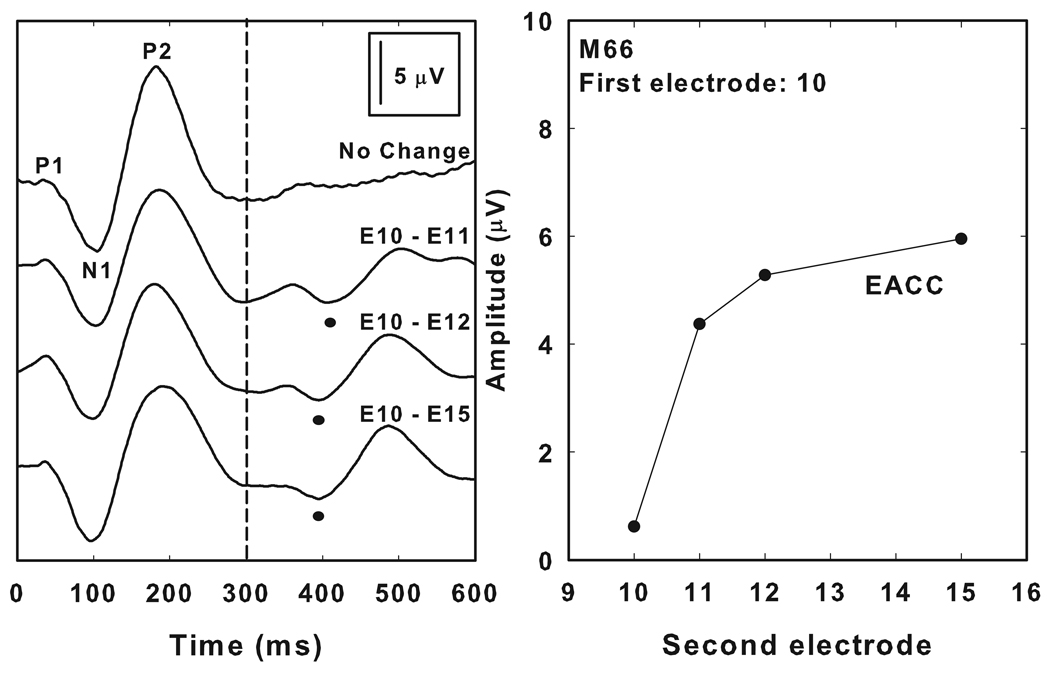
Figure 4 shows the effect that varying the separation between the two stimulating electrodes had on EACC amplitudes. The waveforms in the left panel of Figure 4 were recorded from a single, postlingually deafened adult cochlear implant recipient (M66) using a 600 ms burst of biphasic current pulses (1000 pps, 25 µs/phase). Stimulation began on electrode 10. The waveform shown at the top of the figure is a control condition and did not contain a change either in the stimulating electrode or in any of the other stimulation parameters for the entire 600 ms recording interval. The other waveforms shown in Figure 4 were obtained using a stimulus that included a change in the site of stimulation from electrode 10 to electrodes 11, 12 and 15 respectively 300 ms after the onset of stimulation. All four waveforms show an onset response. In conditions that included a stimulus change, electrically evoked auditory change potentials are also recorded. These responses have morphologic characteristics that are similar to the onset response and the negative peak of the change complex is observed approximately 100 ms after the change was introduced. The amplitude of the change potential is smallest and the latency slightly prolonged when the distance between the two stimulating electrodes was small (e.g. when the stimulating electrode changed from electrode10 to electrode 11). The amplitude increased as the distance between the two stimulating electrodes was increased. The panel on the right in Figure 4 graphically illustrates this effect. This subject could clearly detect the change in stimulating electrode even when that change was only between two adjacent electrodes (i.e. electrode 10 to electrode 11) and perceived this change as a change in pitch.
Figure 4.
The left panel shows a series of onset and EACC responses recorded from an individual study participant (M66). In each case, stimulation began on electrode 10. The upper waveform is a control/no change condition. As indicated on the figure, the stimulating electrode was changed 300 ms after stimulation was begun to a second electrode located apical to electrode 10. The right panel shows amplitude of these EACC responses as measured between N1 and P2. In this graph, response amplitude is plotted as a function of the second electrode in the stimulus pair. In the control/no change condition, the second electrode stimulated was electrode 10. The separation between the two stimulating electrodes increased as the second electrode was changed from electrode 11 to electrode 15.
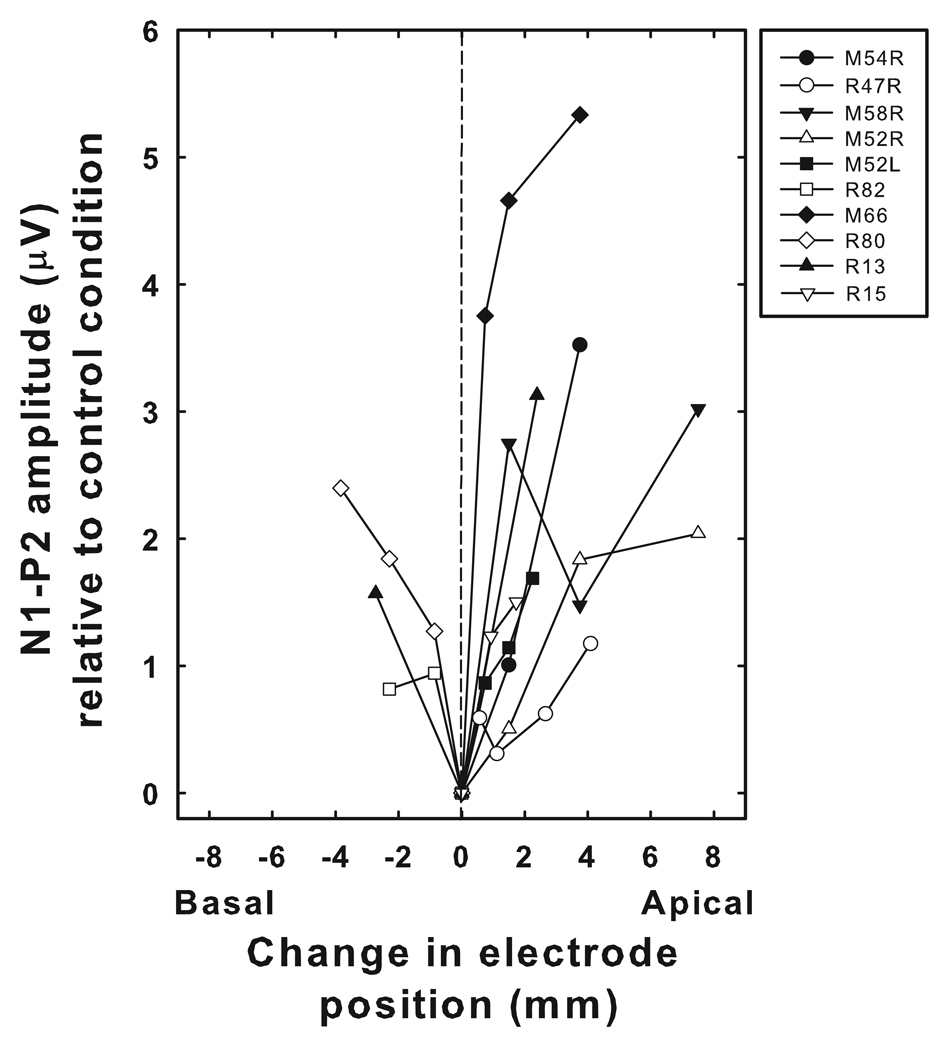
Figure 5 shows data collected from each of the 9 study participants. This figure shows how changes in stimulating electrode position affected EACC amplitudes. The abscissa shows the relative change in electrode position used to evoke the EACC. The intra-cochlear array of the Nucleus cochlear implant is 17 mm long. As indicated in Table 2, some study participants used the CI24M cochlear implant. Other study participants used the Nucleus CI24R cochlear implant. The CI24M device has straight electrode array in which the 22 intra-cochlear electrodes are equally spaced at 0.75 mm intervals. The CI24R device has a pre-coiled electrode array in which the inter-electrode distance varies approximately exponentially as electrode number increases from 1 to 22. Inter-electrode distances are wider at the base of the cochlea and decrease for more apical electrode locations. In order to account for these differences in electrode position across the array and across subjects, the change in electrode position used to evoke the EACC has been converted to millimeters. Changes in the position of the stimulating electrode in an apical direction are indicated with positive numbers while changes in stimulating electrode position toward the base are indicated with negative numbers. Because of the exploratory nature of the study at this point, and the fact that we were choosing electrodes to stimulate that had roughly similar C-levels and behavioral dynamic ranges, the specific electrodes tested for each subject varies. The maximum peak-to-peak voltage difference that occurred during a time window extending from 375 ms to 575 ms was calculated. For conditions that included an electrode change, this voltage difference is the peak-to-peak amplitude of the EACC. For control conditions, where there was no change in the stimulating electrode, this voltage difference is a measure of the amount of physiologic noise and uncancelled stimulus artifact in the recording. The ordinate in Figure 5 shows the difference in microvolts between the peak-to-peak amplitude measured in the experimental conditions and the peak-to-peak amplitude measured in the control condition. As a result of this subtraction, conditions that did not include a change in the stimulating electrode (control conditions) have a corrected EACC amplitude of 0 µV in Figure 5.
Figure 5.
Change in amplitude of the EACC as a function of the distance between the two stimulating electrodes used to elicit the EACC is shown. The ordinate shows the difference between the EACC N1-P2 amplitudes measured in stimulation conditions that included a change in the stimulating electrode and the maximum peak-to-peak amplitudes recorded over the same time window in the control or “no change” condition. Changes in the stimulating electrode in a basal direction (e.g. from electrode 15 to electrode 10) are indicated by negative numbers. Changes in the stimulating electrode in the apical direction (e.g. from electrode 15 to electrode 20) are indicated by positive numbers.
EACC amplitudes generally increase as the distance between the two electrodes is increased, either in an apical or basal direction. There is variability across subjects. Some subjects show very rapid increases in amplitude as the distance between the two stimulating electrodes increased (e.g. M66). In other subjects, the rate of change is much less dramatic (e.g. R82) or even nonmonotonic (e.g. M66 or M58R).
DISCUSSION
Electrically evoked auditory change potentials have been described previously (Friesen and Tremblay, 2006; Martin, 2007). Friesen and Tremblay (2006) used natural speech tokens as stimuli to record EACCs from eight Nucleus cochlear implant users. Martin (2007) used a set of synthesized vowels that contained variations in the F2 formant frequency to elicit the EACC from a single MedEl cochlear implant user who had been diagnosed with auditory neuropathy/dys-synchrony. In both studies, the stimuli were presented in the sound field and the cochlear implant listeners were tested while using their own speech processors. One limitation of this approach is that it complicates, to some extent, how the results can be interpreted. For example, it is possible (if not probable) that the output of the cochlear implant could have varied across subjects in terms of which electrodes were stimulated, at what level and at what time.
In this study, one of our primary objectives was to develop and test a procedure for recording the EACC in response to direct electrical stimulation in a group of Nucleus cochlear implant users. In order to gain more precise stimulus control, we elected to bypass the speech processor and to use NIC routines to control the output of the implanted receiver/stimulator directly. The focus of this preliminary report was to determine the extent to which changes in place of stimulation within the cochlea impact the EACC.
Generally, our results compare well with results reported by Martin and Boothroyd (1999, 2000) and later Martin (2007). For example, both the electrically and acoustically evoked change responses are smaller in amplitude than the onset response but share many of the same basic morphologic characteristics (e.g. see Figure 1, Figure 2 and Figure 3). Additionally, in our work, the amplitude of the EACC was shown to increase as the distance between the two stimulating electrodes, and presumably the perceptual salience of the stimulus change, increased (see Figure 5). This is similar to results reported previously by Martin and Boothroyd (2000) and Martin (2007) who showed that the amplitude of the change complex increased as the magnitude of the change in either formant frequency and/or stimulus level increased.
While they do not report correlations to perception, Martin and Boothroyd (2000) found the ACC to be sensitive to changes in RMS amplitude that were as small as 1–2 dB. Martin (2007) did report results of a psychophysical test designed to determine how accurately their study participant could detect changes in F2 frequency for the stimuli used in their study. The stimulus set tested included F2 changes that ranged from 0 Hz to 1200 Hz. Stimuli that could not be reliably distinguished on the behavioral task did not elicit an EACC. These results are promising because they suggest that the EACC might serve as an electrophysiologic correlate to performance. However, this conclusion is based on results obtained from a single subject who had been diagnosed with auditory neuropathy/dys-synchrony and as such may not be generalizable to the wider implant population. Additionally, it is not clear from Martin (2007) why some of the changes in F2 frequency did not elicit an EACC. For example, it is possible that the changes in the acoustic signal resulted in a change in the electrical stimulus produced by the cochlear implant that was too small to be perceived by the listener and as such no EACC was evoked. It is also possible that the acoustic frequency change was too small to create a change in the electrical output of the device. This would not be an issue if the device output were controlled directly as was done in the current study.
In this study, we expand on previously published results to include a wider range of listeners using stimulus conditions where the primary change used to evoke the response was a change in place of stimulation within the cochlea. One limitation of this investigation is that we cannot rule out the possibility that the stimuli we used to evoke the EACC may have contained concurrent changes in loudness as well as pitch. In order to minimize effects of level, we specifically chose electrodes to use that had similar C-level and dynamic ranges and we presented the stimuli at or near C-level for each study participant (See Table 2). All study participants, when queried, described the stimulus change as a change in pitch. However, in this preliminary work we did not explicitly balance loudness across the electrodes. Additional research is currently underway to assess sensitivity of the EACC to changes in electrode position for stimuli that are balanced in loudness and to assess the effects of changes in level for stimuli presented on a single electrode.
Another issue that may have had an impact our results, as well as those of others, is the extent to which the responses we record are contaminated by stimulus artifact. Stimulus artifact is considerably more problematic for evoked potential studies involving cochlear implant listeners than it is for studies that use listeners with normal hearing or hearing impaired listeners who use conventional amplification devices. Many different approaches have been used to help minimize contamination of the evoked potentials by stimulus artifact. Typical approaches include the use of contralateral recording montages and short duration stimuli. Other techniques that have been used successfully include alternating the polarity of the stimulus in the average, widening the band-pass filter settings, limiting the gain on the recording amplifiers and/or use of some method of artifact subtraction. When long duration stimuli are used, contamination of the recorded responses by stimulus artifact becomes even more problematic. In fact, Martin and Boothroyd (1999) commented that although the AAC may have potential application for assessing hearing in pediatric cochlear implant recipients, doing so may not be easy given that recording these responses requires the use of long duration electrical stimuli. Gilley et al. (2006) describes a technique for minimizing cochlear implant artifact in cortical auditory evoked potential recordings that involves systematically moving the reference electrode used to make the differential recordings in order to minimize stimulus artifact. The main goal of the Martin (2007) paper is to determine if it is possible to separate the EACC from stimulus artifact. A range of different artifact suppression techniques was compared. While the study concluded it was possible to separate neural response from ongoing stimulus artifact, the recordings published in that work clearly illustrate the scope of the challenge.
In the current study we dealt with stimulus artifact by choosing recording electrode sites that are remote from the internal receiver/stimulator. Additionally, we use relatively low gain to avoid saturation of the recording amplifier. Unlike previous investigators, however, we also elected to bypass the speech processor of the cochlear implant and control the output of the implanted receiver/stimulator directly. Doing so insured that we were able to stimulate a single electrode at a relatively high pulse rate. The EACC contains primarily low frequency energy (< 30 Hz). The electrical pulse train used to elicit the EACC creates artifact in the recorded waveform that is much higher in frequency that the neural response. This relatively wide separation in the frequency domain between the neural response and the electrical stimulus artifact allows for the use of simple filtering techniques to minimize stimulus artifact contamination of the EACC. We are not the first investigators to adopt this approach to separation of the neural response and the stimulus artifact. Friesen and Tremblay (2006), also recorded electrically evoked change potentials from cochlear implant recipients that were relatively free from stimulus artifact contamination. Like the current study, these investigators dealt with stimulus artifact contamination by choosing an electrode montage that minimized the amount of artifact in the recordings and by filtering the responses between 0.15 and 100 Hz. In the current study, the process of minimizing stimulus artifact was made easier because the output of the implanted receiver/stimulator was controlled directly allowing us to create a stimulus that was less complex both temporally and spectrally than the stimulus used by Friesen and Tremblay.
Examination of the individual EACC waveforms shown in Figure 2 and Figure 3 does reveal some residual low amplitude noise in our recordings. This noise is likely the result of a combination of residual stimulus artifact from the electrical pulse train, physiologic noise and/or contamination of the responses by artifact associated with the line voltage. Additionally, M52R has a response with a particularly large amplitude, vertex positive peak that does not look physiologic in nature at approximately the time one might expect to record P1. For this subject, use of an alternative recording electrode montage, may have resulted in recordings that were more similar to the responses reported for our other study participants. In general, however, we would argue that based on the results reported in this study, it is possible to record the EACC even when long duration electrical pulse trains are used for stimulation.
Another fairly major difference between this study and those reported previously is that other investigators have used speech stimuli to elicit the change complex (Friesen and Tremblay, 2006; Tremblay et al., 2006). When CV syllables are used as stimuli, a series of overlapping cortical potentials are recorded. The longer latency peaks in their series of cortically generated responses, regardless of whether they are elicited in response to acoustic or electrical stimulation, most likely reflect detection of change in some aspect of the stimulus and, as such, those peaks have been classified as change potentials. Tremblay et al. (2003) have suggested that the acoustic change complex may be an ideal tool for assessing the ability of a subject with impaired speech understanding to detect changes in an ongoing acoustic signal.
Recently, Friesen and Tremblay (2006) and Martin (2007) have shown that it is possible to record this response using speech stimuli in cochlear implant recipients. There are clearly benefits to recording cortical potentials such as the EACC using speech stimuli (natural or synthetic). However, by controlling the output of the implanted components of the cochlear implant directly rather than using an acoustic signal that is presented in the sound field and transmitted through the speech processor we were able to attain greater stimulus control than has been possible in previous studies. As such, we were able to explore the impact that systematically varying the separation between two stimulating electrodes had on the EACC (see Figure 4 and Figure 5). It should also be possible to adapt the stimulation paradigm described in this report to allow exploration of the relative salience of many different aspects of an electrical stimulus. Studies aimed at assessing the extent to which changes in stimulus level and/or other temporal properties of an electrical stimulus are effective in evoking the EACC are currently underway in our laboratory.
One potential application for the EACC is as a tool for assessing speech perception or discrimination capabilities of pediatric cochlear implant recipients. Studies have shown that cortical evoked potentials are related, at least grossly, to the speech perception abilities of adult cochlear implant users (Kelly et al., 2005; Makhdoum et al., 1998). Specifically, Makhdoum et al. (1998) found statistically significant correlations between both P2 latency and N1-P2 amplitude measures and speech perception. Kelly et al. (2005) replicated the earlier findings of Makhdoum et al. (1998) and also showed that P2 latency was negatively correlated the duration of deafness prior to implantation for postlingually deafened, adult cochlear implant users. More recently, Friesen et al. (2006) and Martin (2007) recorded ACC waveforms from cochlear users. Results of these two studies, although somewhat limited, suggest that there may be a correlation between the EACC and behavioral measures of how sound is perceived by cochlear implant users. Friesen et al. (2006) found differences in the grand mean EACC waveforms elicited from a small set of patients who are good performers relative to patients who are poor performers. Martin (2007) showed good correspondence between behavioral measures of frequency discrimination ability and the magnitude of frequency changes needed to elicit a measurable EACC in a single cochlear implant user. If future research continues to show reasonable correlations between EACC measures and the ability of the listener to perceive change in an ongoing stimulus, it may be reasonable to expect that this potential could be used as an objective estimate of auditory capacity in pediatric implant recipients. Clearly, more data are needed to determine how feasible it will be to measures this response in the youngest group of implant recipients (e.g. children under 2 years of age).
Finally, results of this study have demonstrated variations across subjects (see Figure 3 and Figure 5). It is of interest to evaluate how that sensitivity may be related to other electrophysiologic measures of sensitivity to changes in place of stimulation within the cochlea. Previous work in our laboratory has evaluated channel interaction at the level of the auditory nerve (Abbas et al, 2004; Hughes and Abbas, 2006). Such measures have also shown variations across subjects but have not shown strong correlations to the ability of subjects to discriminate differences between these electrodes behaviorally. The extent to which differences in CNS processing may play a role is clearly an issue in making such comparisons. Future work is needed comparing results of cortical responses obtained using this paradigm with peripheral measures of channel interaction and spread of excitation within the cochlea as well as with psychophysical measures of discrimination and with electrophysiologic measures of cortical processing obtained using synthetic as well as natural speech stimuli.
This study might best be described as a feasibility study. We have described a technique that we think has the potential to be useful in future studies that focus on how changes in an acoustic stimulus as perceived by a cochlear implant user are processed within the central auditory system. In this study, the focus has been on describing the effect that changes in place of stimulation within the cochlea had on the EACC response. Clearly, the ability of a listener to detect changes in place of stimulation within the cochlea is one factor that may be important for understanding speech. However, the paradigm itself can easily be adapted to allow us to assess sensitivity to a range of other changes in an ongoing stimulus including variations in stimulation level and/or a change in the temporal properties of the signal.
In future work, it may be possible to use the EACC to detect perception of differences between more spectrally complex signals and it may also be possible to adapt this stimulation and recording paradigm to assess sensitivity to changes in interaural time or intensity cues for bilateral cochlear implant users. While we view these results as preliminary in nature, our goal for future studies is to identify a set of experimental conditions that can be shown to correlate with perception in adults and then to expand that work by modifying these procedures for pediatric applications.
CONCLUSIONS
This report describes preliminary results obtained using a technique, modeled after one initially used by Martin and Boothroyd (2000), for recording the EACC in Nucleus cochlear implant users. While this is not the first time that electrically evoked auditory change potentials have been reported in the literature, this study does represent the first time that this has been accomplished using direct stimulation of the implanted electronics. The responses that we have recorded were elicited in response to a change in the place of stimulation within the cochlea. Results reveal that the EACC can be recorded using this technique for individual cochlear implant recipients. Moreover, considerable variability across subjects was revealed. Generally, EACC amplitudes increased as the distance between the two stimulating electrodes was increased.
ACKNOWLEDGEMENTS
This work was supported by grants from the NIH/NIDCD (DC00242), the NIH/NCRR (RR00059) and the Iowa Lions Sight and Hearing Foundation. The authors would like to thank three anonymous reviewers for their very helpful comments on an earlier version of this manuscript. We are also indebted to Wenjun Wang and Sean Sweeney for help with programming.
Support: This work was supported in part by grants from NIH/NIDCD (P50 DC00242), NIH/NCRR (RR00059) and the Iowa Lions Sight and Hearing Foundation.
Footnotes
Electrically evoked, auditory potentials were measured from Nucleus cochlear implant users. The speech processor was bypassed and the implanted electronics were controlled directly. A 600 ms train of biphasic current pulses was presented. In experimental conditions, the stimulus train included a change in the stimulating electrode position. The amplitude of the responses that were elicited by this change in electrode location tended to increase as the distance between the two stimulating electrodes increased. These results suggest that it may be feasible to use electrophysiologic techniques to assess sensitivity to change in various aspects of an ongoing electrical stimulus.
REFERENCES
- Abbas PJ, Hughes ML, Brown CJ, et al. Channel interaction in cochlear implant users evaluated using electrically evoked compound action potentials. Audiology & Neurotology. 2004;9:203–213. doi: 10.1159/000078390. [DOI] [PubMed] [Google Scholar]
- Agung K, Purdy SC, McMahon CM, et al. The use of cortical auditory evoked potentials to evaluate neural encoding of speech sounds in adults. Journal of the American Academy of Audiology. 2006;17:559–572. doi: 10.3766/jaaa.17.8.3. [DOI] [PubMed] [Google Scholar]
- Beynon AJ, Snick A, van den Brock P. Evaluation of cochlear implant benefit with auditory cortical evoked potentials. International Journal of Audiology. 2002;41:429–435. doi: 10.3109/14992020209090420. [DOI] [PubMed] [Google Scholar]
- Delb W, Strauss DJ, Hohenberg G, et al. The binaural interaction component (BIC) in children with central auditory processing disorders (CAPD) International Journal of Audiology. 2003;42:401–412. doi: 10.3109/14992020309080049. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Ponton CW. Auditory-evoked potential studies of cortical maturation in normal hearing and implanted children: correlations with change in structure and speech perception. Acta Oto-Laryngologica. 2003;123:249–252. doi: 10.1080/0036554021000028098. [DOI] [PubMed] [Google Scholar]
- Firszt JB, Chambers RD, Kraus N, Reader RM. Neurophysiology of cochlear implant users I: effects of stimulus current level and electrode site on the electrical ABR, MLR and N1-P2 response. Ear and Hearing. 2002a;23:502–515. doi: 10.1097/00003446-200212000-00002. [DOI] [PubMed] [Google Scholar]
- Firszt JB, Chambers RD, Kraus N. Neurophysiology of cochlear implant users II: comparison among speech perception, dynamic range, and physiological measures. Ear and Hearing. 2002b;23:516–531. doi: 10.1097/00003446-200212000-00003. [DOI] [PubMed] [Google Scholar]
- Friesen LM, Tremblay KL. Acoustic change complexes recorded in adult cochlear implant listeners. Ear and Hearing. 2006;27:678–685. doi: 10.1097/01.aud.0000240620.63453.c3. [DOI] [PubMed] [Google Scholar]
- Fuess VL, Bento RF, da Silveira JA. Delay in maturation of the auditory pathway and its relationship to language acquisition disorders. ENT: Ear, Nose & Throat Journal. 2002;81:706–710. [PubMed] [Google Scholar]
- Gilley PM, Sharma A, Dorman M, Finley C, Panch A, Martin K. Minimization of cochlear implant stimulus artifact in cortical auditory evoked potentials. Clinical Neurophysiology. 2006;117:1772–1782. doi: 10.1016/j.clinph.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Gordon KA, Tanaka S, Papsin BC. Atypical cortical responses underlie poor speech perception in children using cochlear implants. NeuroReport. 2005;16:2041–2045. doi: 10.1097/00001756-200512190-00015. [DOI] [PubMed] [Google Scholar]
- Groenen PAP, Makhdoum M, van den Brink JL, Stollman M, Snik A, van den Broek P. The relation between electric auditory brain stem and cognitive responses and speech perception in cochlear implant users. Acta Otolaryngoogica. 1996;116:785–790. doi: 10.3109/00016489609137926. [DOI] [PubMed] [Google Scholar]
- Harris KC, Mills JH, Dubno JH. Electrophysiologic correlates of intensity discrimination in cortical evoked potentials of younger and older adults. Hearing Research. 2007;228:58–68. doi: 10.1016/j.heares.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes EA, Warrier CM, Nicol TG, et al. Neural plasticity following auditory training in children with learning problems. Clinical Neurophysiology. 2003;114:673–684. doi: 10.1016/s1388-2457(02)00414-5. [DOI] [PubMed] [Google Scholar]
- He N, Dubno JR, Mills JH. Frequency and intensity discrimination measured in a maximum-likelihood procedure from young and aged normal-hearing subjects. Journal of the Acoustical Society of America. 1998;103:553–565. doi: 10.1121/1.421127. [DOI] [PubMed] [Google Scholar]
- Hughes ML, Abbas PJ. The relation between electrophysiologic channel interaction and electrode pitch ranking in cochlear implant recipients. Journal of the Acoustical Society of America. 2006;119:1527–1537. doi: 10.1121/1.2163273. [DOI] [PubMed] [Google Scholar]
- Hyde M. The N1 response and its applications. Audiology & Neuro-Otology. 1997;2:281–307. doi: 10.1159/000259253. [DOI] [PubMed] [Google Scholar]
- Jerger J, Jerger S. Evoked response to intensity and frequency change. Archives of Otolaryngology. 1970;91:433–436. doi: 10.1001/archotol.1970.00770040627007. [DOI] [PubMed] [Google Scholar]
- Kaga K, Kodera K, Hirota E, Tsuzuka T. P300 response to tones and speech sounds after cochlear implant: A case report. Laryngoscope. 1991;101:905–907. doi: 10.1288/00005537-199108000-00017. [DOI] [PubMed] [Google Scholar]
- Kelly AS, Purdy SC, Thorne PR. Electrophysiological and speech perception measures of auditory processing in experienced adult cochlear implant users. Clinical Neurophysiology. 2005;116:1235–1246. doi: 10.1016/j.clinph.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Kileny PR. Use of electrophysiologic measures in the management of children with cochlear implants: brainstem, middle latency and cognitive (P300) responses. American Journal of Otology. 1991;12:37–42. [PubMed] [Google Scholar]
- King C, Warrier LM, Hayes E, et al. Deficits in auditory brainstem pathway encoding of speech sounds in children with learning problems. Neuroscience Letters. 2002;319:111–115. doi: 10.1016/s0304-3940(01)02556-3. [DOI] [PubMed] [Google Scholar]
- Korczak PA, Kurtzberg D, Stapells DR. Effects of sensorineural hearing loss and personal hearing aids on cortical event related potential and behavioral measures of speech-sound processing. Ear and Hearing. 2005;26:165–185. doi: 10.1097/00003446-200504000-00005. [DOI] [PubMed] [Google Scholar]
- Kraus N, Micco AG, Koch DB, et al. The mismatch negativity cortical evoked potential elicited by speech in cochlear implant users. Hearing Research. 1993;65:118–124. doi: 10.1016/0378-5955(93)90206-g. [DOI] [PubMed] [Google Scholar]
- Lightfoot G, Kennedy V. Cortical electric response audiometry hearing threshold estimation: accuracy, speed, and the effects of stimulus presentation features. Ear and Hearing. 2006;27:443–456. doi: 10.1097/01.aud.0000233902.53432.48. [DOI] [PubMed] [Google Scholar]
- Maison S, Duclaux R, Ferber-Viart C, et al. Clinical interest of brainstem auditory evoked potentials in 72 children with inadequate language development. International Journal of Neuroscience. 1996;88:261–272. doi: 10.3109/00207459609000619. [DOI] [PubMed] [Google Scholar]
- Makhdoum MJ, Groenen PA, Snik AF, et al. Intra- and inter-individual correlations between auditory evoked potentials and speech perception in cochlear implant users. Scandinavian Audiology. 1998;27:13–20. doi: 10.1080/010503998419650. [DOI] [PubMed] [Google Scholar]
- Martin BA. Can the acoustic change complex be recorded in an individual with a cochlear implant? Separating neural responses from cochlear implant artifact. Journal of the American Academy of Audiology. 2007;18:126–140. doi: 10.3766/jaaa.18.2.5. [DOI] [PubMed] [Google Scholar]
- Martin BA, Boothroyd A. Cortical auditory event-related potentials in response to periodic and aperiodic stimuli with the same spectral envelope. Ear and Hearing. 1999;20:33–44. doi: 10.1097/00003446-199902000-00004. [DOI] [PubMed] [Google Scholar]
- Martin BA, Boothroyd A. Cortical, auditory evoked potentials in response to changes of spectrum and amplitude. Journal of the Acoustical Society of America. 2000;107:2155–2161. doi: 10.1121/1.428556. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Picton T. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Obleser J, Elbert T, Lahiri A, et al. Cortical representation of vowels reflects acoustic dissimilarity determined by formant frequencies. Brain Research: Cognitive Brain Research. 2003;15:207–213. doi: 10.1016/s0926-6410(02)00193-3. [DOI] [PubMed] [Google Scholar]
- Ostroff JM, Martin BA, Boothroyd A. Cortical evoked response to acoustic change within a syllable. Ear and Hearing. 1998;19:290–297. doi: 10.1097/00003446-199808000-00004. [DOI] [PubMed] [Google Scholar]
- Oviatt DL, Kileny PR. Auditory event-related potentials elicited from cochlear implant recipients and hearing subjects. American Journal of Audiology. 1991;1:48–55. doi: 10.1044/1059-0889.0101.48. [DOI] [PubMed] [Google Scholar]
- Ponton CW, Eggermont JJ. Of kittens and kids: altered cortical maturation following profound deafness and cochlear implant users. Audiology & Neurotology. 2001;6:363–380. doi: 10.1159/000046846. [DOI] [PubMed] [Google Scholar]
- Ponton CW, Moore JK, Eggermont JJ. Prolonged deafness limits auditory system developmental plasticity: evidence from an evoked potentials study in children with cochlear implants. Scandinavian Audiology (suppl.) 1999;51:13–22. [PubMed] [Google Scholar]
- Ponton CW, Don M, Eggermont JJ, et al. Maturation of human cortical auditory function: differences between normal hearing children and children with cochlear implants. Ear and Hearing. 1996;17:430–437. doi: 10.1097/00003446-199610000-00009. [DOI] [PubMed] [Google Scholar]
- Sabisch B, Hahne A, Glass E, et al. Auditory language comprehension in children with developmental dyslexia: evidence from event-related brain potentials. Journal of Cognitive Neuroscience. 2006;18:1676–1695. doi: 10.1162/jocn.2006.18.10.1676. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman MF, Spahr AJ. A sensitive period for the development of the central auditory system in children with cochlear implants: Implications for age of implant. Ear and Hearing. 2002a;23:532–539. doi: 10.1097/00003446-200212000-00004. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman MF, Spahr AJ. Rapid development of cortical auditory evoked potentials after early cochlear implantation. Neuroreport. 2002b;13:1365–1368. doi: 10.1097/00001756-200207190-00030. [DOI] [PubMed] [Google Scholar]
- Sharma A, Marsh CM, Dorman MF. Relationship between N1 evoked potential morphology and the perception of voicing. Journal of the Acoustical Society of America. 2000;108:3030–3035. doi: 10.1121/1.1320474. [DOI] [PubMed] [Google Scholar]
- Souza PE, Tremblay KL. New perspectives on assessing amplification effects. Trends in Amplification. 2006;10:119–143. doi: 10.1177/1084713806292648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnguist-Uhlen J. Topography of auditory evoked long latency potentials in children with severe language impairment, the P2 and N2 component. Ear and Hearing. 1996;17:314–326. doi: 10.1097/00003446-199608000-00003. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Billings CJ, Friesen LM, et al. Neural representation of amplified speech sounds. Ear and Hearing. 2006;27:93–103. doi: 10.1097/01.aud.0000202288.21315.bd. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Billings C, Rohila N. Speech evoked cortical potentials: effects of age and stimulus presentation rate. Journal of the American Academy of Audiology. 2004;15:226–237. doi: 10.3766/jaaa.15.3.5. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Friesen L, Martin BA, et al. Test-retest reliability of cortical evoked potentials using naturally produced speech sounds. Ear and Hearing. 2003;24:225–232. doi: 10.1097/01.AUD.0000069229.84883.03. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Kraus N. Auditory training induces asymmetrical changes in cortical neural activity. Journal of Speech, Language, & Hearing Research. 2002;45:564–572. doi: 10.1044/1092-4388(2002/045). [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Kraus N, McGee T, et al. Central auditory plasticity: changes in the N1-P2 complex after speech sound training. Ear and Hearing. 2001;22:79–90. doi: 10.1097/00003446-200104000-00001. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Kraus N, McGee T. The time course of auditory perceptual learning: neurophysiological changes during speech sound training. Neuroreport. 1998;9:3557–3360. doi: 10.1097/00001756-199811160-00003. [DOI] [PubMed] [Google Scholar]
- Wunderlich JL, Cone-Wesson BK, Shepherd R. Maturation of the cortical auditory evoked potential in infants and young children. Hearing Research. 2006;212:185–202. doi: 10.1016/j.heares.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Yingling CD, Nethercut GE. Evoked response to frequency shifted tones: tonotopic and contextual determinants. International Journal of Neuroscience. 1983;22:107–118. doi: 10.3109/00207459308987389. [DOI] [PubMed] [Google Scholar]
RELATED PUBLICATION
- Friesen LM, Tremblay KL. Acoustic change complexes recorded in adult cochlear implant listeners. Ear and Hearing. 2006;27:678–685. doi: 10.1097/01.aud.0000240620.63453.c3. [DOI] [PubMed] [Google Scholar]
- Gilley PM, Sharma A, Dorman M, Finley C, Panch A, Martin K. Minimization of cochlear implant stimulus artifact in cortical auditory evoked potentials. Clinical Neurophysiology. 2006;117:1772–1782. doi: 10.1016/j.clinph.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Martin BA. Can the acoustic change complex be recorded in an individual with a cochlear implant? Separating neural responses from cochlear implant artifact. Journal of the American Academy of Audiology. 2007;18:126–140. doi: 10.3766/jaaa.18.2.5. [DOI] [PubMed] [Google Scholar]
- Martin BA, Boothroyd A. Cortical auditory event-related potentials in response to periodic and aperiodic stimuli with the same spectral envelope. Ear and Hearing. 1999;20:33–44. doi: 10.1097/00003446-199902000-00004. [DOI] [PubMed] [Google Scholar]
- Martin BA, Boothroyd A. Cortical, auditory evoked potentials in response to changes of spectrum and amplitude. Journal of the Acoustical Society of America. 2000;107:2155–2161. doi: 10.1121/1.428556. [DOI] [PubMed] [Google Scholar]
- Ostroff JM, Martin BA, Boothroyd A. Cortical evoked response to acoustic change within a syllable. Ear and Hearing. 1998;19:290–297. doi: 10.1097/00003446-199808000-00004. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Billings CJ, Friesen LM, et al. Neural representation of amplified speech sounds. Ear and Hearing. 2006;27:93–103. doi: 10.1097/01.aud.0000202288.21315.bd. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Billings C, Rohila N. Speech evoked cortical potentials: effects of age and stimulus presentation rate. Journal of the American Academy of Audiology. 2004;15:226–237. doi: 10.3766/jaaa.15.3.5. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Friesen L, Martin BA, et al. Test-retest reliability of cortical evoked potentials using naturally produced speech sounds. Ear and Hearing. 2003;24:225–232. doi: 10.1097/01.AUD.0000069229.84883.03. [DOI] [PubMed] [Google Scholar]