Abstract
Cocaine abusers demonstrate faulty decision-making as manifested by their inability to discontinue self-destructive drug-seeking behaviors. The orbitofrontal cortex (OFC) plays an important role in decision-making. In this preliminary study we tested whether 25-day-abstinent cocaine abusers show alterations in normalized cerebral blood flow (rCBF) in the OFC using PET with 15O during the Iowa Gambling Task (a decision-making task). This task measures the ability to weigh short-term rewards against long-term losses. A control task matched the sensorimotor aspects of the task but did not require decision-making. Cocaine abusers (N = 13) showed greater activation during performance of the Iowa Gambling Task in the right OFC and less activation in the right dorsolateral prefrontal cortex (DLPFC) and left medial prefrontal cortex (MPFC) compared to a control group (N = 13). Better Iowa Gambling Task performance was associated with greater activation in the right OFC in both groups. Also, the amount of cocaine used (grams/week) prior to the 25 days of enforced abstinence was negatively correlated with activation in the left OFC. Greater activation in the OFC in cocaine abusers compared to a control group may reflect differences in the anticipation of reward while less activation in the DLPFC and MPFC may reflect differences in planning and working memory. These findings suggest that cocaine abusers show persistent functional abnormalities in prefrontal neural networks involved in decision-making and these effects are related to cocaine abuse. Compromised decision-making could contribute to the development of addiction and undermine attempts at abstinence.
Introduction
A survey in 2000 estimated that there were 1.2 million cocaine users in the United States 265,000 of whom were crack users (National Household Survey on Drug Abuse, 1999). The accumulated evidence suggests that repeated self-administration of cocaine despite its potential physical, psychological, and social consequences, is attributable to damage to specific neural networks involved in decision-making and performance monitoring (Volkow and Fowler, 2000; Bolla et al., unpublished observations). This model might explain why cocaine abusers continue to use cocaine despite their inability to relive the powerful pleasurable experiences of their first drug administration (Fischman et al., 1985). Taken together, these observations suggest that cocaine abusers might have difficulty making advantageous decisions. Therefore, it is important to identify and clarify the neural substrates that underlie decision-making. This may elucidate mechanisms contributing to continued high-risk behaviors in cocaine abusers. Better understanding of these mechanisms would then lead to improved prevention and intervention strategies. The orbitofrontal cortex (OFC) is a crucial component of a neural network that subserves positive reinforcement in primates (Rolls and Baylis, 1994). In humans, the OFC has reciprocal connections with many brain regions that mediate the rewarding effects of cocaine as well as decision-making and compulsive behaviors (Rolls, 2000; Volkow and Fowler, 2000). Damage to the OFC produces personality changes that include irresponsibility and persistence in self-destructive behaviors.
Evidence suggesting a change in the role of the OFC as a function of addiction comes from neuroimaging studies using positron emission tomography (PET). These studies have consistently revealed increased activation in the OFC, the anterior cingulate, the right insular region, and the amygdala in response to cocaine-related cues (Bonson et al., 2002; Childress et al., 1999; Wang et al. 1999; Grant et al., 1996). Other studies have also shown that cocaine abuse is related to a disruption in the striato-thalamo-orbitofrontal circuit (Volkow et al., 1993; Volkow and Fowler, 2000; Goldstein et al., 2001). Specifically, higher relative OFC metabolism was correlated with worse conflict monitoring (lower score on the Stroop) in nonaddicted individuals and with better conflict monitoring (higher score) in addicted individuals (Goldstein et al., 2001). These data suggest that addicted individuals are more dependent on the OFC in order to perform as well as controls (Goldstein et al., 2001). One limitation of this study was that the Stroop task was not administered during the PET - FDG scan acquisition. In addition to PET studies, investigations of brain morphology show smaller gray matter volumes in regions such as the ventromedial orbitofrontal, anterior cingulate, anteroventral insular, superior temporal cortices (Franklin et al., 2002), and the prefrontal cortex as a whole in cocaine abusers in comparison to controls (Lui et al., 1998).
In addition to the OFC, we reasoned that the dorsolateral prefrontal cortex (DLPFC) might also be dysfunctional in the cocaine abuser. The DLPFC plays a role in maintaining attentional demands of a task including planning, controlling performance (MacDonald et al., 2000), and learning and memory (de Zubicaray et al., 2001). For example, we found that cocaine abusers showed significantly less activation than controls in the right DLPFC during performance on the Stroop task compared to controls (Bolla et al., personal observations). Activation in the DLPFC showed a negative correlation with the amount of cocaine used prior to admission to the study. That is, the more cocaine used per week, the less the activation. Further understanding of functional alterations in the OFC and DLPFC in cocaine abusers may elucidate mechanisms of addiction and poor decision-making.
Neuropsychological testing has shown a variety of performance deficits in cocaine abusers including impairments in decision-making and judgment, when compared to controls (Strickland et al., 1993; Ardila et al., 1991; Bolla et al., 1999, 2000). The Iowa Gambling Task was developed to test decision-making in everyday life in patients with lesions of the ventromedial prefrontal cortex (VMPFC) (Bechara et al., 1994). Specifically, the task tests a person’s ability to choose between high gains with a risk for even higher losses, and low gains with a risk for smaller losses. Patients with lesions in the VMPFC consistently performed more poorly on this task than controls (Bechara et al., 2000a, 1999, 1997, 1994). Substance abusers also show performance deficits on this task (Bartzokis et al., 2000; Grant et al., 2000). Performance on the Iowa Gambling Task appears to be mediated by a neural network composed of the OFC, the amygdala, somatosensory/insular cortices, and the peripheral nervous system (Bechara et al., 1999, 1997; Ernst et al., 2002).
The primary aim of this study was to determine if 25-day-abstinent cocaine abusers demonstrated differences in brain activation (normalized rCBF) compared to non-drug users during performance on the Iowa Gambling Task compared to a control task. We hypothesized that the cocaine abusers would show abnormalities in relative rCBF primarily in the OFC and DLPFC while performing the task compared to a group of control participants. Based on our previous PET work and neuropsychological data, we also hypothesized that grams per week of cocaine used prior to the 25 days of enforced abstinence that preceded the study would be negatively correlated with activation in the OFC and DLPFC of cocaine abusers during performance of the Iowa Gambling Task (Bolla et al., 1999, 2000). The characterization of persistent neurobehavioral deficits during the initial phase of abstinence from drug use is important because such deficits could impair the individual’s ability to discontinue self-destructive addictive behavior.
Methods
Participants
The institutional review boards of the National Institute on Drug Abuse - Intramural Research Program (NIDA-IRP), the Johns Hopkins Medical Institutions Joint Committee on Clinical Investigation, and the Johns Hopkins Bayview Medical Institutional Review Boards approved this protocol. All participants gave written informed consent and were compensated for their time. Control subjects and cocaine abusers were recruited using newspaper advertisements. Participant selection was based on drug use history obtained using a structured interview, Drug Use Survey Questionnaire (DUSQ) (Smith, 1991), Addiction Severity Index (ASI) (McLellan et al., 1980), and the Psychiatric Diagnostic Interview Schedule (DIS) (Robins et al., 1981). Subjects received a full medical screening which included a complete physical, neurological examination, urine toxicology, and a pregnancy test for women. All participants were right-handed, and English was their native language.
Control group
Potential participants were included in the control group if they reported consuming 10 alcoholic drinks/week or less, and using marijuana less than 8 days/month. Individuals were excluded if they reported current use of any illicit drug other than marijuana.
Cocaine group
The cocaine group consisted of individuals who reported that cocaine was their drug of choice, used cocaine by any route for at least 2 years, administered cocaine at least four times/month, had two urine toxicology screens, at least 48 h apart, that were positive for cocaine metabolites, and reported alcohol consumption of 10 or fewer alcoholic drinks/week. The positive toxicology screens confirmed cocaine use during the prior 24 to 72 h to ensure that all participants were abstinent for a uniform period. Participants were excluded if they met the Diagnostic and Statistical Manual of Mental Disorders - IV (DSM - IV) criteria gleaned from the DIS for current or past dependence on any other psychoactive substance other than cocaine, including alcohol, or if their urine toxicology screen was positive for substances other than cocaine and its metabolites.
Exclusion criteria for all participants
Volunteers were excluded for a past or current Axis I disorder other than nicotine dependence by DSM-IV criteria using the DIS (e.g., anxiety disorder, posttraumatic stress disorder, and major depressive disorder). Volunteers were also excluded for a past or current history of neurological illness (e.g., head trauma resulting in loss of consciousness, seizure disorder, stroke), an abnormal neurological examination, pregnancy or left-handedness. No volunteer was included if there was current or past use of psychoactive medication.
Data collection
At the initial visit to the Clinical Inpatient Research Unit (CIRU) at NIDA-IRP all participants had a full medical screening. They were then admitted to the CIRU for approximately 25 days. Random drug screens were performed during the inpatient stay to ensure abstinence. No treatment or medications for drug abuse were given over the 25-day stay.
Design
Subjects participated in one PET session (on Day 25 of the residential stay for the cocaine abusers and Day 3 of the residential stay for control participants) during which they received six injections of H2 15O water (10 mCi each). Each injection was followed by a 1-min acquisition scan. Three cognitive conditions were studied: rest—R (eyes fixated on a target); active task—A (Iowa Gambling Task); and control task—C (sensorimotor control task). Two scans were acquired for each of the three conditions (rest, Iowa Gambling Task, control task). The order of these tasks was counter-balanced between participants. Tasks began 1 min prior to injection of the tracer and continued until the end of the task, when the participant had selected 100 cards (approximately 5 min). Participants were permitted to smoke cigarettes and drink caffeinated beverages as they wished, but were instructed to abstain from both for 3 h prior to the study.
Iowa Gambling Task (active task)
Since the primary aim was to study OFC functioning in the context of decision-making in abstinent cocaine users, we chose the Iowa Gambling Task as our activation task. Lesion studies show that the OFC plays an important role in the ability to perform well on this task (Bechara et al., 2000b). The Iowa Gambling Task measures the participant’s ability to choose between high gains with a risk of extremely high losses, and low gains with a risk of smaller losses. Participants were instructed to win as much money as possible by picking one card at a time from each of the 4 decks (A, B, C, and D) in any order until the computer instructed them to stop (after the selection of the 100th card). While performing the task, participants were informed of the amount of money they had left after each card was selected. Participants selected on average about 20 cards per minute. For further detail on this task see Ernst et al., (2002). The measure of performance used for all subsequent analyses was the net global outcome score (net score). This was calculated by subtracting the total number of cards selected from the disadvantage decks (A+B) from the total number of cards selected from the advantage decks (C+D) in trial 1 and trial 2 and then deriving a mean for both trials. The PET acquisition scan started 1 min after the beginning of the task and lasted for approximately 1 min during the scan to ensure that the participant was cognitively engaged in the task. The participant continued to play until the computer informed them to stop after selecting 100 cards. Participants were instructed that for each “game dollar” they won, they would receive one cent and could therefore make up to $20.00 in “real money” per session.
Control task
The control task was designed to be analogous to the Iowa Gambling Task with respect to sensorimotor demands and exposure to gains and losses. Unlike the active task, the gains and losses associated with the control task were equal between decks and participants were instructed to select cards sequentially in the fixed order of A-B-C-D-A-B-C-D, -etc. Consequently, card selection did not require decision-making. Although the sensory and motor aspects of the control task were identical to those in the active task, rewards and penalties were contingent upon behavior in the active task but noncontingent in the control task.
Scans
Scans were acquired with a Siemens ECAT EXACT HR +, in 63 planes with a 15.5-cm field of view in 3D mode. Images were reconstructed using a Hann filter with 0.5 cutoff frequency. The average transverse resolutions (full-width half-maximum (FWHM)) of the scanner at 1 and 10 cm from the center of the field of view, measured in 3D mode and determined using Fluorine-18 line source, and a ramp filter (with a 0.5 cutoff frequency) were 4.66 and 5.45 mm, respectively. Axial resolutions of the scanner (FWHM), measured using a point source of F 18 and the same reconstruction algorithm, were 4.21 mm and 5.0 mm at 0 and 10 cm from the center, respectively. In case of application of a Hann filter with a 0.5 cutoff frequency, used for brain images reconstruction, the average transverse resolutions were 6.52 and 7.16 mm, respectively. For the same reconstruction algorithm, the average axial resolution at 0 cm from the center was 3.72 mm and at 10 cm, 5.64 mm.
Image processing and statistical analyses
PET images were realigned, spatially normalized into the Montreal Neurological Institute (MNI) coordinate system, and smoothed with a 12 × 12 × 12 mm Gaussian kernel using Statistical Parametric Mapping Software (SPM 99; Welcome Department of Cognitive Neurology). A two-stage procedure was implemented for statistical analyses. In the first stage, PET images from each participant were used to create an adjusted mean image, representing the relative change in rCBF between the active and the control tasks (all scans from the active task minus the control task). Thus, the adjusted mean image represents the change in normalized rCBF between the active task and the control task. This change in rCBF was taken to reflect the process of decision-making by subtracting the motor, auditory, and visual components involved in the task (control task) from the higher cognitive functions of decision-making (active task). In the computation of these adjusted images, within-session variations in global signal were adjusted using proportional scaling. Importantly, there were no significant differences in the correlation between global signal and the conditions of interest (Aguirre et al., 1998; Desjardins et al., 2001; Andersson et al., 1997, 2001; Worsley et al., 1996). The second stage of the procedure involved entering the combined adjusted image from each participant into a random-effects two-sample t test (24 degrees of freedom). Based on the literature and our previously published data using the identical task, we hypothesized group differences bilaterally in the OFC and DLPFC, predominately on the right side (Ernst et al., 2002).
Correlation analyses were performed to examine the relationship between behavioral performance and relative rCBF and to test the hypothesis that the amount of weekly drug use would be related to the amount of activation in the OFC and DLPFC during performance on the Iowa Gambling Task.
Results
Demographics and drug use (Table 1)
Table 1.
Demographic characteristics of control group and cocaine abusers
| Characteristic | Control group (n = 13) | Cocaine group (n = 13) |
|---|---|---|
| Age, years | 30.0 ± 6.3 (22-42) | 36.1 ± 4.8* (28-43) |
| Education, years | 13.2 ± 2.9 (8-19) | 13.1 ± 2.7 (12-18) |
| Mother’s years of education | 12.8 ± 2.6 (9-17) | 12.0 ± 3.1 (8-20) |
| Shipley IQ | 98.1 ± 10.3 (83-118) | 95.8 ± 11.9 (77-113) |
| Hollingshead SES | 4.3 ± 1.2 | 4.2 ± 0.8 |
| Sex, M/F | 10/3 | 10/3 |
| Race, A.A./C. | 6/6 1 Hispanic | 9/3 1 Hispanic |
| Cocaine use | ||
| Days/week | 0 | 5.2 ± 1.7 (3-7) |
| Grams/week | 0 | 3.2 ± 2.7 (0.9-10.5) |
| Longest use/years | 0 | 7.3 (2-15) |
| Route of administration | 0 | 100% |
| Smoke | ||
| Alcohol use | ||
| Drinks/week | 0.7 ± 1.7 (0-6) | 3.2 ± 3.7 (0-10) |
| Cigarette smokers | 7/13 | 11/13 |
Note. Numbers in parentheses are ranges.
P < 0.05.
The control group (n = 13) was matched to the cocaine group (n = 13) on Shipley IQ score and sex. No group differences were found in years of education, Shipley IQ, maternal education, or Hollingshead Index of socioeconomic status. The cocaine group was older (36 years versus 30 years). Also, there were more cigarette smokers in the cocaine group than the control group (11/13 versus 7/13). Smoking was the main route of cocaine administration for all the cocaine users (84% smoked exclusively). Overall average amount and frequency of cocaine use (grams/week) were estimated. Grams/week were estimated from participants’ self-reports of how much money was spent each week ($100/g for 50% purity which the Drug Enforcement Agency reports for the Baltimore area).
Brain activation during the Iowa Gambling Task
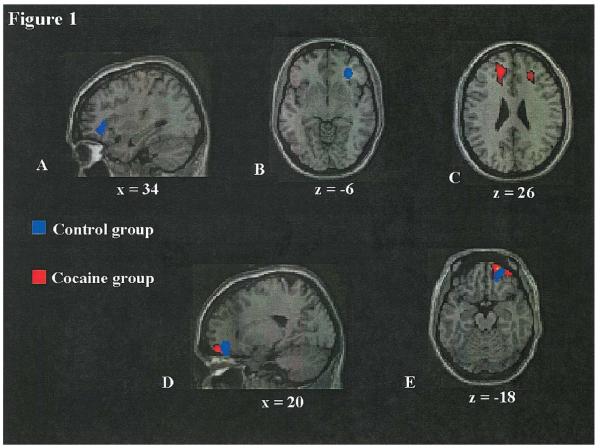
A priori, we expected differences to be observed between cocaine abusers and nondrug users in the OFC and the DLPFC. These findings confirmed our hypothesis; specifically, cocaine abusers showed significantly more activation than controls in the right OFC and significantly less activation than controls in the right DLPFC and left MPFC (Table 2 and Figs. 1A,B, and C)
Table 2.
Group differences in signal intensity during performance on the gambling task
| Cocaine group > Control group | Side | Talairach coordinates |
BA | Voxel T | Cluster size k | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| A priori region | |||||||
| Orbitofrontal cortex | R | 34 | 35 | −8 | 47 | 3.72* | 46 |
| Other regions | |||||||
| Putamen | L | −24 | −6 | −6 | 4.23* | 67 | |
| Postcentral gyrus | L | −55 | −15 | 43 | 1, 3, 4 | 3.44* | 44 |
| Cocaine group > Control group | Side | Talairach coordinates |
BA | Voxel T | Cluster size k | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| A priori regions | |||||||
| Medial prefrontal cortex | L | −16 | 38 | 20 | 9 | 3.84* | 46 |
| Dorsolateral prefrontal cortex | R | 34 | 23 | 36 | 9 | 3.84* | 109 |
| Other regions | |||||||
| Superior parietal lobe | R | 24 | −69 | 50 | 7 | 4.42* | 155 |
| Medial frontal gyrus | L | −12 | 16 | 45 | 6, 32 | 3.84* | 36 |
| Middle temporal gyrus | L | −53 | −18 | −6 | 21 | 3.70* | 64 |
| Cerebellum | R | 10 | −37 | −32 | 3.49* | 26 | |
Note. Peak height threshold P < 0.005, extent threshold 25, uncorrected for multiple comparisons, with 24 degree of freedom.
P < 0.001, uncorrected.
Fig. 1.
(A and B) Sagittal and axial views. Cocaine abusers showed more activation than controls in the right OFC (peak located at 34, 35, −8) [BA 47]; (P < 0.001, k = 46 voxels, uncorrected) during performance on the Iowa Gambling Task (active task minus control task). (C) Cocaine abusers showed less activation than controls in the right DLPFC (peak located at 34, 23, 36) [BA 9]; and left MPFC (peak located at −16, 38, 20) [BA 9]; (P < 0.001; right DLPFC, k = 109 voxels and left MPFC, k = 46 voxels, uncorrected) during performance on the Iowa Gambling Task (active task minus control task); (D and E) Sagittal and axial views. Significant correlation between better performance on the Iowa Gambling Task and greater activation in the right medial OFC in the cocaine abusers (blue) (peak located at 20, 38, −17, 223 voxels), [BA 11] and control group (red) (peak located at 20, 42, −19, 94 voxels), [BA 11]; P < 0.001).
Post hoc exploratory analyses of the entire brain also revealed group differences during the Iowa Gambling Task in regions other than the a priori regions of interest (Table 2). Cocaine abusers showed greater activation in the left putamen and the left postcentral gyrus than the control group. On the other hand, cocaine abusers showed less activation than controls in the right superior parietal lobule, the left medial frontal gyrus, left middle temporal gyrus, and the right cerebellum. It is important to note that these latter analyses were exploratory in nature.
Iowa Gambling Task performance
A Mann-Whitney U test was used to test for group differences between controls and cocaine abusers in net outcome score because the net scores were not normally distributed within groups (controls, mean net score, 14.3 (23.9); cocaine abusers, mean net score 6.17 (25.7)). The cocaine group performed more poorly than the control group, although this difference was not statistically significant. We also examined group differences in performance between the first and the second trials of the Iowa Gambling Task using a Wilcoxon signed-rank test. This comparison could be considered a measure of learning. The controls showed a slightly greater change from trial 1 to trial 2 (trial 1, mean = 8.2 (18.9), and trial 2, mean = 20.5 (38.2) than the cocaine group (trial 1, mean = 3.8 (23.3), and trial 2, mean = 8.3 (32.1); however, these differences were not significant.
Iowa Gambling Task performance and rCBF
Using a fixed-effects analysis of the 13 healthy controls we isolated a cluster of peak activation in the right OFC (x = 18, y = 36, x = 22 [BA 11]; t (38) = 4.25). This epicenter of the cluster of peak activation in the OFC correlated significantly with performance scores in both groups separately (Table 3 and Figs. 1D and E). For each group, the better the performance, the greater the activation in the right medial OFC. No correlations were found between performance and activation in either the right or the left DLPFC.
Table 3.
Correlation of signal intensity with performance on the Gambling Task and amount of cocaine used (grams/week)
| Performance and signal intensity | Side | Talairach coordinates |
BA | Cluster size k | Voxel T | Correlation coefficienta | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Control group | ||||||||
| Orbitofrontal cortex | R | 20 | 42 | −19 | 11 | 94 | 3.88* | 0.71 |
| Cocaine group | ||||||||
| Orbitofrontal cortex | R | 20 | 38 | −17 | 11 | 223 | 4.66* | 0.75 |
| Cocaine (grams/week) and signal intensity |
Side | Talairach coordinates |
BA | Cluster size k | Voxel Tb | Correlation coefficient | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Orbitofrontal cortex | L | −22 | 40 | −18 | 11 | 36 | 4.27* | −0.82 |
Peak height threshold P < 0.005, extent threshold 25, uncorrected, with 11 degrees of freedom
Peak height threshold P < 0.005, extent threshold 25, uncorrected, with 9 degrees of freedom.
P < 0.001, uncorrected.
Cocaine used (grams/week) and rCBF
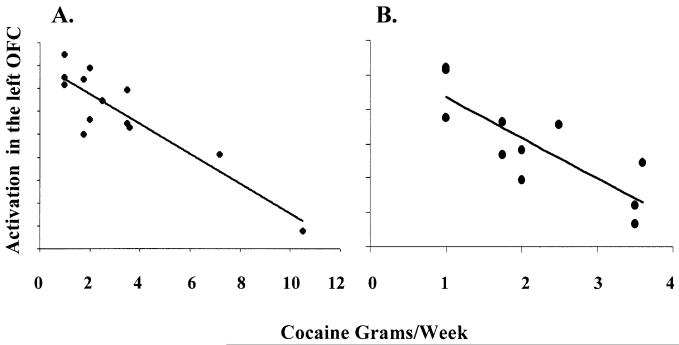
We hypothesized a priori that the amount of cocaine used would be negatively associated with activation in the OFC and DLPFC. Correlation analyses between activation in the OFC and grams per week of cocaine used per week revealed three significant clusters. The first cluster was located in the right OFC and the second and third clusters were located in two separate areas in the left OFC (P < 0.005, k = 25 voxels, uncorrected). The greater the amount of cocaine used (grams per week) the lower the activation in the OFC (Fig. 2A). We were concerned that our two cocaine abusers with the highest cocaine use (7.2 and 10.5 g) were responsible for these findings since they could be considered “extreme cases” with respect to the amount of cocaine used as well as magnitude of activation. We therefore, reran the correlation analyses without these 2 “extreme cases” and found a significant correlation between grams of cocaine used and less activation in the left OFC (Table 3 and Fig. 2B). No correlation was found between grams per week of cocaine used and activation in the DLPFC with the extreme cases included or excluded.
Fig. 2.
(A) Regression between activation in the 13 cocaine abusers at the epicenter of the left OFC (peak located at −26, 20, −18) and the number of grams of cocaine used per week prior to the 25 days of enforced abstinence (r =−0.89, P < 0.001, k = 59 voxels, uncorrected). (B) Regression between activation in the 11 cocaine abusers at the epicenter of the left OFC (peak located at −22, 40, −18) and the number of grams of cocaine used per week prior to the 25 days of enforced abstinence (r =−0.82, P < 0.005, k = 36 voxels, uncorrected). The two “extreme cases” with respect to the number of grams of cocaine used per week and magnitude of activation intensity were omitted from this analysis. Only 10 data points are readily apparent because two cases with 1 g of cocaine use overlap.
Since we did not perform absolute quantification of cerebral blood flow, we examined whole brain normalized CBF differences between the groups for the mean of the two resting scans and the mean of the two scans obtained during the control task. No significant normalized CBF differences were found between groups even at a threshold of P < 0.05—uncorrected, for either the resting or the control condition. We also addressed the possibility that group differences in vascular reactivity may be responsible for the observed effects in the OFC, DLFPC, and MPFC by examining changes in normalized rCBF in the motor cortex where we expected normal levels of activation in both groups (Callicott et al., 1998). We found no group differences in motor cortex (−26, −44, 65) (Kiehl and Liddle, 2001). This suggests that the cocaine abusers activate this brain region in a fashion similar to control participants while at the same time fail to activate the right DLPFC and left MPFC, and over-activate the right OFC. This effect supports the notion that the observed changes in these specific brain regions are not due to global group differences in vascular reactivity. No between-group differences for the resting or control conditions suggest that cocaine abusers did not have a different baseline level of activation than controls. Rather, they failed to activate to the same degree as controls during the active task in the right DLPFC and left MPFC and showed greater activation in the right OFC.
Discussion
Consistent with our hypothesis, we found functional abnormalities in brain activity in cocaine abusers. Specifically, abstinent cocaine abusers show greater activation during the Iowa Gambling Task in the right OFC and less activation in the right DLPFC when compared to controls. Less activation in the left MPFC was also observed in the cocaine group. In addition, the amount of cocaine used (grams/week) prior to the 25 days of abstinence was negatively correlated with activation in the left OFC. These results suggest that cocaine abusers have persistent functional abnormalities in prefrontal neural networks involved in decision-making and that these effects are related to cocaine use. These findings are relevant since compromised decision-making would likely contribute to the maintenance of addiction and undermine attempts at abstinence.
There are a variety of plausible explanations for the greater activation of the right OFC of cocaine abusers relative to the control group during this decision-making task. One explanation is supported by the strong correlation between better performance and higher activation in the right medial OFC for both cocaine abusers and controls. This suggests that the right OFC in both groups is performing a role in decision-making; however, the functioning of the right OFC of cocaine abusers may not be as efficient as in that of controls. Therefore, to compensate for an inefficient right OFC, the cocaine abusers may overactivate the right OFC in an attempt to meet the demands of the task. Further support for the plausibility of our finding of greater right OFC activation in the cocaine group comes from reports that right unilateral lesions may affect decision-making preferentially, while left unilateral lesions seem to have little affect on decision-making (Tranel et al., 2000, 2002).
Hypersensitivity to reward may offer another explanation for the greater activation in the right OFC in the cocaine abusers. In cocaine abusers, the potential for gain and reward may trigger much stronger representations of reward-related thoughts in the OFC. In the present study both cocaine abusers and controls showed increase activation in the right medial OFC with better performance. This finding is consistent with the idea that responses to reward are a result of medial OFC activation (O’Doherty et al., 2001). Thus, it is possible that the hyperactivity of the OFC in cocaine abusers during the Iowa Gambling Task reflects an abnormally intense focus and thinking about the winning/rewarding emotional aspects of the task. This thought process would exaggerate the value of the high reward and suppresses the negative value of the high loss. Overall, this process would most likely result in poor decision-making. On a more practical level, a cocaine abuser would be more likely to focus on the positive reinforcement of cocaine, while ignoring its destructive consequences.
Others have also reported hyperactivity in the OFC in substance abusers in the literature (Goldstein et al., 2001). However, unlike previous findings, our data do not support a reversal of roles of the OFC (i.e., higher activation and better performance in substance abusers and higher activation and worse performance in controls) (Goldstein et al., 2001). This may be because we used a different task and because the task was performed simultaneously with the measurements of brain function.
Significantly less activation in the right DLPFC in cocaine abusers than controls during performance of the Iowa Gambling Task replicates our previous findings of less activation in the right DLPFC during performance of the Stroop task. The present data also support our previous finding of a dose-related relationship between number of grams/week of cocaine used and performance on a neurobe-havioral match-to-sample task (Bolla et al., 1999) that is sensitive to lesions of the DLPFC in primates (Mishkin, 1964). The DLPFC is believed to be involved in planning and working memory that are skills required to perform the Iowa Gambling Task adequately. Therefore, based on the present finding and previous findings showing less activation in the DLPFC in cocaine abusers than controls while performing the Stroop (Bolla et al., unpublished observations), the right DLPFC appears to be an important component of the prefrontal neural network thought to be affected by cocaine abuse. The cocaine group also showed less activation in the left medial prefrontal cortex (MPFC) that is a region also involved in planning and performance on the Iowa Gambling Task. While this was not an a priori region of interest (Manes et al., 2002) showed that patients with discreet lesions to the MPFC were selectively impaired on the Iowa Gambling Task when compared to two other decision-making tasks. Patients with damage to the MPFC also showed difficulties with planning. Therefore, our current finding is consistent with previous reports.
A possible mechanism for these group differences in OFC, DLPFC, and MPFC function may be related to abnormal blood flow that is found in the cerebral vasculature of chronic cocaine abusers (Herning et al., 1999). These changes in flow resistance might have led to parenchymal damage and associated abnormal responses in the brain regions supplied by the anterior and middle cerebral vasculature. For example, increased activation in the right OFC could be secondary to denervation supersensitivity, which is an exaggerated sensitivity of neurons to a neurotransmitter following the destruction of presynaptic neurons (Yarbrough and Phillis, 1975). Thus, cellular damage associated with the abnormal relative blood flow observed in cocaine abusers might have led to decreased synaptic functions that are compensated during resting periods but show increases during stressful periods, like during task performance (Zigmond and Hastings, 1998). The group differences in activation in the OFC, DLPFC, and MPFC might also be related to cigarette use or age since the cocaine users contained more smokers and were older. However, we found no differences in activation in the OFC, DLPFC, and MPFC between smokers and nonsmokers and no correlation was found between age and activation in these same brain regions.
Despite significant group differences in brain activation, only a tendency was observed with the cocaine group performing more poorly than the control group on the Iowa Gambling Task. This nonsignificant group difference in task performance does not agree with the results of others (Grant et al., 2000). This discrepancy between studies can be attributed to differences in group characteristics (i.e., cocaine versus polysubstance abusers, sample size, length of abstinence, and the mode of test administration— computer versus examiner administration). Failure to detect large performance differences is most likely related to low statistical power to find such effects, given our sample size. Neuro-imaging analyses are sensitive enough to detect changes in a sample of this size; however, behavioral performance measures usually require a larger sample size. Therefore the performance measures (i.e., net score and learning) may only show significant group differences with a larger sample size. Alternatively, abstinence from cocaine for 25 days may have reduced high-risk responses on the Iowa Gambling Task because of recovery of performance (Mann et al., 1999; Bartzokis et al., 2000). On the other hand, the absence of large performance differences between groups emphasizes that the group differences in activation do not reflect differences in attention or effort while performing the Iowa Gambling Task. Nevertheless, it is important to pursue this line of investigation since poorer decision-making and reduced learning capacity could render a cocaine abuser a poor candidate for treatments currently available during early abstinence.
After the imaging data were reanalyzed excluding the two cocaine abusers considered to be “extreme cases” (out-liers) in the amount of cocaine they administered, a strong negative correlation was found between grams of cocaine used prior to the study and activation in the left OFC. While we cannot posit causality from this study, this finding of a dose-response relationship supports the hypothesis that chronic heavy use of cocaine is at least partially responsible for persistent prefrontal lobe dysfunction. This finding is consistent with our past work showing dose-related neurobehavioral effects in a separate group of cocaine abusers (Bolla et al., 1999, 2000). This relationship between greater cocaine use and less activation in the left OFC was not reflected as a significant group effect in performance on the Iowa Gambling Task possibly because this task appears to rely more heavily on the integrity of the right OFC than the left OFC. This apparent lateralized effect may also explain why there appears to be a dissociation between greater activation in the right OFC in cocaine abusers than controls, but less activation in the left OFC as the amount of cocaine used increases.
It appears that individuals with right OFC dysfunction, which include cocaine abusers, may not make associations between present emotions and past negative experiences, despite knowing the adverse consequences (Bechara, 2000b). Therefore, understanding the roles of the OFC and DLPFC in relation to substance abuse could facilitate the development of more appropriately targeted prevention and treatment programs for those who suffer from addiction. Nevertheless, these data should be interpreted with caution as they are preliminary and will need to be replicated. Future studies using fMRI and event-related designs would make it possible to determine more detailed mechanisms of decision-making and the roles of the OFC and DLPFC in this complex cognitive process.
Acknowledgments
This work was supported by NIH Grants DA 11426 (KB) and the JHBMC-GCRC (MO1 RR02719) and the DHHS/NIH/NIDA Intramural Research Program. We thank all the nurses and staff at NIDA-IRP, the Brain Imaging Center, and the Bayview GCRC who contributed to this project. We especially thank Debra Hill, B.A., for computer and database support and Steve Grant, Ph.D., for his intellectual contributions to this work.
References
- Aguirre GK, Zarahn E, D’Esposito M. The variability of human, BOLD hemodynamic responses. NeuroImage. 1998;8:360–369. doi: 10.1006/nimg.1998.0369. [DOI] [PubMed] [Google Scholar]
- Andersson JL. How to estimate global activity independent of changes in local activity. NeuroImage. 1997;6:237–244. doi: 10.1006/nimg.1997.0302. [DOI] [PubMed] [Google Scholar]
- Andersson JL, Ashburner J, Friston KJ. A global estimator unbiased by local changes. NeuroImage. 2001;13:1193–1206. doi: 10.1006/nimg.2001.0763. [DOI] [PubMed] [Google Scholar]
- Ardila A, Rosselli M, Strumwasser S. Neuropsychological deficits in chronic cocaine abusers. Int. J. Neurosci. 1991;57:73–79. doi: 10.3109/00207459109150348. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Beckson M, Rapoport R, Grant S, Wiseman EJ, London ED. Abstinence from cocaine reduces high-risk responses on a Iowa Gambling task. Neuropsychopharmacology. 2000;22:102–103. doi: 10.1016/S0893-133X(99)00077-9. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb. Cortex. 2000a;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J. Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;28:1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000b;123:2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Funderburk FR, Cadet JL. Differential effects of cocaine and cocaine + alcohol on neurocognitive performance. Neurology. 2000;54:2285–2292. doi: 10.1212/wnl.54.12.2285. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Rothman RB, Cadet JL. Dose-related neurobehavioral effects of chronic cocaine use. J. Neuropsychiatry Clin. Neurosci. 1999;11:361–369. doi: 10.1176/jnp.11.3.361. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Grant S, Contoreggi C, Links J, Metalfe J, Weyl LH, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Ramsey NF, Tallent K, Bertolino A, Knable MB, Coppola R, Goldberg T, van Gelderen P, Mattay VS, Frank JA, Moonen CT, Weinberger DR. Functional magnetic resonance imaging brain mapping in psychiatry: methodological issues illustrated in a study of working memory in schizophrenia. Neuropsychopharmacology. 1998;18(3):186–196. doi: 10.1016/S0893-133X(97)00096-1. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am. J. Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zubicaray G, McMahon K, Wilson SJ, Muthiah S. Brain activity during encoding, retention, and retrieval of stimulus representations. Learning Memory. 2001;8:243–251. doi: 10.1101/lm.40301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins AE, Kiehl KA, Liddle PF. Removal of confounding effects of global signal in functional magnetic resonance imaging analyses. NeuroImage. 2001;13:751–758. doi: 10.1006/nimg.2000.0719. [DOI] [PubMed] [Google Scholar]
- Ernst M, Bolla K, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, London ED. Decision-making in a risk-taking task: A PET study. Neuropsychopharmacology. 2002;26:682–691. doi: 10.1016/S0893-133X(01)00414-6. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Schuster CR, Javaid J, Hatano Y, Davis J. Acute tolerance development to cardiovascular and subjective effects of cocaine. J. Pharmacol. Exp. Ther. 1985;235:677–682. [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol. Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, Wang G, Fowler JS, Rajaram S. Addiction changes the orbitofrontal gyrus function: involvement in response inhibition. Cogn. Neurosci.Neuropsychol. 2001;12:2595–2599. doi: 10.1097/00001756-200108080-00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision-making. Neuropsychologia. 2000;38:1180–1187. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc. Natl. Acad. Sci. USA. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herning RI, King DE, Better WE, Cadet JL. Neurovascular deficits in cocaine abusers. Neuropsychopharmacology. 1999;21:110–118. doi: 10.1016/S0893-133X(98)00141-9. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle RF. An event-related functional magnetic resonance imaging study of an auditory oddball task in schizophrenia. Schizoph. Res. 2001;30(48):159–171. doi: 10.1016/s0920-9964(00)00117-1. [DOI] [PubMed] [Google Scholar]
- Lui X, Matochik JA, Cadet JL, London ED. Smaller volume of prefrontal lobe in polysubstance abusers: A magnetic resonance imaging study. Neuropsychopharmacology. 1998;18:243–252. doi: 10.1016/S0893-133X(97)00143-7. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, Robbins T. Decision-making processes following damage to the prefrontal cortex. Brain. 2002;125:624–639. doi: 10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- Mann K, Gunther A, Stetter F, Ackerman K. Rapid recovery from cognitive deficits in abstinent alcoholics: a controlled test-retest study. Alcohol Alcoholism. 1999;34:567–574. doi: 10.1093/alcalc/34.4.567. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O’Brien CP. An improved evaluation instrument for substance abuse patients: the Addiction Severity Index. J. Nervous Mental Disord. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- Mishkin M. Perseveration of central sets after frontal lesions in monkeys. In: Warren JM, Akert K, editors. The Frontal Granular Cortex and Behavior. McGraw-Hill; New York: 1964. pp. 219–241. [Google Scholar]
- U.S. Department of Health and Human Services. Substance Abuse and Mental Health Services Administration Office of Applied Studies . National Household Survey on Drug Abuse. U.S. Gov. Printing Office; Washington, DC: 1999. [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornack J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule: its history, characteristics, and validity. Arch. Gen. Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cereb. Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Baylis LL. Gustatory, olfactory, and visual convergence within the primate orbitofrontal cortex. J. Neurosci. 1994;14:5437–5452. doi: 10.1523/JNEUROSCI.14-09-05437.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS. Addictive drug survey manual. NIDA Addiction Research Center; Baltimore: 1991. [Google Scholar]
- Strickland TL, Mena I, Villanueva-Meyer J, Miller BL, Cummings J, Mehringer CM, Satz P, Myers H. Cerebral perfusion and neuropsychological consequences of chronic cocaine use. J. Neuropsychiatry Clin. Neurosci. 1993;5:419–427. doi: 10.1176/jnp.5.4.419. [DOI] [PubMed] [Google Scholar]
- Tranel D, Bechara A, Damasio, Damasio AR. Decision-making in patients with unilateral ventromedial prefrontal cortex lesions [abstract] Soc. Neurosci. Abst. 2000;26:S49. [Google Scholar]
- Tranel D, Bechara A, Denberg NL. Asymmetric functional roles of right and left ventromedial prefrontal cortices in social conduct, decision-making, and emotional processing. Cortex. 2002;38:589–612. doi: 10.1016/s0010-9452(08)70024-8. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb. Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Wang G, Volkow ND, Fowler JS, Cervany P, Hitzemann R, Pappas N, Wong CT, Felder C. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci. 1999;64:775–784. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Yarbrough GG, Phillis JW. Supersensitivity of central neurons— a brief review of an emerging concept. Can. J. Neurol. Sci. 1975;2:147–152. doi: 10.1017/s0317167100020187. [DOI] [PubMed] [Google Scholar]
- Zigmad MJ, Hastings TG. Neurochemical responses to lesions of dopaminergic neurons: implications for compensations and neuropathology. Adv. Pharmaco. 1998;42:788–792. doi: 10.1016/s1054-3589(08)60865-0. [DOI] [PubMed] [Google Scholar]