Abstract
Previous in vitro studies showed that molecules in an extract of the mite Sarcoptes scabiei variety canis De Geer could modulate the secretion of cytokines from cultured normal human epidermal keratinocytes and dermal fibroblasts in the absence of proinflammatory cytokines in the cell culture media. The purpose of this study was to investigate whether scabies extract could also modulate cytokine and chemokine secretion from epidermal keratinocytes and dermal fibroblasts in the presence of proinflammatory cytokines that are likely present in the scabietic lesion in vivo. In particular, could the downmodulating properties of this ectoparasitic mite on skin cells be maintained in the presence of proinflammatory cytokines? We found that even in the presence of the proinflammatory cytokines interleukin (IL)-1α, IL-1β, and a mixture of tumor necrosis factor (TNF)α + IL-17, scabies extract still downregulated the levels of IL-8 secretion from keratinocytes and fibroblasts and of granulocyte/macrophage-colony stimulating factor (GM-CSF) secretion from fibroblasts that were induced by stimulation of the cells with proinflammatory cytokines alone. This study also showed that scabies molecules induced secretions of growth-related oncogene α (GROα), transforming growth factor α (TGFα), and cutaneous T-cell attracting chemokine (CTACK) from keratinocytes and IL-6 and granulocyte-colony stimulating factor (G-CSF) from fibroblasts. These findings, coupled with the previous findings that molecules in scabies extract could downregulate expression of intracellular adhesion molecule-1 (ICAM-1) and E-selectin by normal dermal microvascular endothelial cells and secretion of IL-1ra from keratinocytes, suggest that multiple factors from scabies mites play a role in the characteristic delayed inflammatory response to a primary infestation with S. scabiei. These are adaptations that favor invasion of the host by the parasite.
Keywords: scabies, keratinocytes, fibroblasts, cytokine, immunomodulation
Sarcoptes scabiei De Geer is an obligate ectoparasitic mite that lives in the stratum corneum of the skin of humans and other mammals. After an initial 4- to 8-wk delay on becoming infested with scabies for the first time, human patients present with various skin clinical symptoms including pruritus and local inflammation. Evidence is mounting suggesting that S. scabiei is the source of molecules that can downregulate the secretion of selected cytokines and chemokines and expression of adhesion molecules by resident and infiltrating cells of the epidermis and dermis, and this may contribute to the initial delay in inflammatory and immune responses to the scabies mite (Arlian et al. 1996b, 2003, 2004; Elder et al. 2006).
A previous in vitro study showed that molecules in a scabies extract prepared from whole mite bodies (variety canis) downregulated secretion of interleukin-1 receptor antagonist (IL-1ra) and IL-8 and stimulated secretion of IL-6 and vascular endothelial cell growth factor (VEGF) from cultured normal epidermal keratinocytes (Arlian et al. 2003). The same study showed that molecules in a scabies extract stimulated secretion of IL-6, IL-8, granulocyte colony stimulating factor (G-CSF), and VEGF from normal human dermal fibroblasts. Likewise, living burrowing mites have been shown to upregulate IL-1α and IL-1β secretion from human skin equivalents consisting of keratinocytes grown over a fibroblast-collagen matrix (Arlian et al. 1996b).
In addition to keratinocytes and fibroblasts, various other cell types that may control extravasation or that infiltrate scabietic lesions may respond to scabies mites and their products. Molecules in a scabies mite extract can inhibit the expression of intracellular adhesion molecule-1 (ICAM-1) and E-selectin by cultured normal human dermal microvascular endothelial cells (Elder et al. 2006) and stimulate T-regulatory (Treg) cells from normal human donors to produce the downregulating cytokine IL-10 (Arlian et al. 2006). Scabies extract (variety canis) has also been shown to induce secretion of IL-1β, IL-6, IL-8, and tumor necrosis factor-α (TNFα) from human peripheral blood mononuclear cells (mostly monocytes) in vitro and downregulate secretion of IL-6 and IL-8 that was induced by lipopolysaccharide (LPS)-stimulated dendritic cells derived from them (Arlian et al. 2004).
Taken together, a preponderance of in vitro data suggest that scabies mites in vivo may influence the initiation and course of inflammatory and immune reactions in the skin by modulation of cytokine and chemokine secretion and expression of adhesion molecules from resident skin cells such as fibroblasts, keratinocytes, and endothelial cells and infiltrating cells such as monocytes, dendritic cells derived from these, and lymphocytes.
The expression of primary proinflammatory cytokines such as IL-1α, IL-1β, and TNFα in the skin is essential to initiating and sustaining the inflammatory/immune response to exogenous stimuli in the skin. Although IL-1α and IL-1β can be produced by activated keratinocytes and fibroblasts, IL-1α, IL-1β, and TNFα are also produced by activated monocytes and macrophages. These cytokines can act on several different cell types including keratinocytes, fibroblasts, and endothelial cells and amplify their cytokine and chemokine responses. In addition, the newly described cytokine IL-17 may play a role in mediating cutaneous inflammation through possible synergy with TNFα as has been reported for skin and lung fibroblasts (Katz et al. 2001, Letuve et al. 2006).
In the previous studies in which fibroblasts and keratinocytes were stimulated with scabies extract, these cells were not co-stimulated with proinflammatory cytokines. In vivo, proinflammatory cytokines are likely present in the vicinity of the burrowing mite, and these may influence the response of keratinocytes and fibroblasts to mite products and the course of inflammation. Thus, the purpose of this research was to investigate whether scabies extract could modulate cytokine and chemokine secretion from keratinocytes and fibroblasts in the presence of some other proinflammatory cytokines that are likely present in the scabietic lesion.
Materials and Methods
Reagents
A whole body homogenate extract of S. scabiei variety canis mites of all life stages was prepared in glass-distilled water as previously described (Morgan and Arlian 1994). The extract was filtered through a 0.22-μm filter into sterile vials and its protein concentration was determined by the method of Bradford (1976) using bovine serum albumin as the standard.
Recombinant human IL-4, IL-13, IL-17, and TNFα were purchased from R&D Systems (Minneapolis, MN), and IL-1α and IL-1β were obtained from eBioscience (San Diego, CA). All ELISA kits (DuoSet Quantikine kits) were purchased from R&D Systems and were used according to the manufacturer's directions.
Human Cells
Cryopreserved keratinocytes and fibroblasts obtained from adult donors along with all media, supplemental growth factors, and cell culture reagents were purchased from Lonza Walkersville (Walkersville, MD). Cells were grown in their recommended growth media at 37°C in 5–6% CO2. Cells were maintained and passaged according to the supplier's directions. For testing, cells were plated in 24- or 48-well Costar tissue culture plates (Corning, NY) and grown to ≈80% confluence. At the beginning of each experiment, the medium was removed from the cells and was replaced with fresh growth media containing the stimulants (scabies extract ± proinflammatory cytokine) of interest. Cells exposed to medium in the absence of stimuli served as controls. Cell-free culture supernatants were collected at 8, 24, or 48 h and were stored at −80°C for subsequent cytokine analysis.
All experiments were performed at least twice with each experiment including at least three replicate wells for each test stimulant. Data presented here are from one representative experiment and are reported as mean ± SEM.
Results
Preliminary Studies
We are particularly interested in the downregulation that scabies mites seem to be able to impose on the host inflammatory and immune reactions. To determine downregulatory effects, it is necessary to first stimulate upregulated function of cells that do not constitutively express a cytokine and then challenge them with scabies products in the absence and presence of proinflammatory mediators. Proinflammatory cytokines chosen for study were IL-1α, IL-1β, IL-17, and tumor necrosis factor-α (TNFα). Therefore, dose–response experiments were conducted to first determine the effect of proinflammatory cytokines on keratinocytes and fibroblasts to determine the appropriate dose of these cytokines to use in experiments in which upregulated cells were co-stimulated (dual stimulation) with S. scabiei extract together with the proinflammatory cytokine.
We determined that IL-1α and IL-1β each at 1.0 ng/ml induced optimal cytokine secretion from keratinocytes and fibroblasts (datanot shown). Co-stimulation with these two proinflammatory cytokines at this dose did not result in enhanced secretion over the effect induced by each individual cytokine alone.
IL-17 was chosen for study because the Th-17 subset of T-cells (formerly included in the Th-1 phenotype of T-cells) secrete the proinflammatory cytokine IL-17. Fibroblasts and many other cells types including venus endothelial cells have receptors for IL-17 and cells respond to this cytokine by expressing other proinflammatory cytokines including IL-1β, IL-6, IL-8, and TNFα (Fossiez et al. 1996, Jovanovic et al. 1998, Linden 2006, Schmidt-Weber et al. 2007). IL-17 has also been reported to synergize the stimulatory effects of TNFα (Katz et al. 2001) so we tested IL-17 and TNFα alone and in combination.
TNFα at 5.0 ng/ml induced secretion of IL-6, IL-8, G-CSF, and granulocyte/macrophage colony stimulating factor (GM-CSF) from fibroblasts and of IL-8 from keratinocytes (Tables 1, 2). IL-17 at 10 and 100 ng/ml by itself stimulated secretion of IL-8 by keratinocytes but little or no other cytokine secretion from keratinocytes or fibroblasts was elicited by IL-17 at any of the concentrations tested. TNFα at 5.0 ng/ml and IL-17 at 10.0 ng/ml combined synergistically to induce secretion of IL-6 from keratinocytes and of IL-6, G-CSF and GM-CSF from fibroblasts. For both cell types, TNFα and IL-17 together induced an additive effect on the secretion of IL-8 compared with stimulation with TNFα or IL-17 alone (data not shown). Hence, the subsequent experiments used TNFα + IL-17 at 5.0 and 10.0 ng/ml, respectively.
Table 1. Summary of the modulatory effects of various stimuli on normal human epidermal keratinocytes.
| Stimulant | Cytokine secreted | ||||||
|---|---|---|---|---|---|---|---|
| IL-8 | IL-6 | GROα | TGFα | CTACK | TARC | TSLP | |
| None | bld | bld | + | + | + | bld | bld |
| IL-1α | ? | bld | ↑ | ND | ↑ | bld | bld |
| IL-1β | ? | bld | ↑ | ND | ↑ | bld | bld |
| IL-17 | ? | bld | ND | ↑ | ND | ND | ND |
| TNFα | ? | bld | ND | ↑ | ↑ | Tr | Tr |
| TNFα + IL-17 | ? | ↑ | ↑ | ↑ | ND | ND | ND |
| SS | bld | Tr | ↑ | ↑ | ↑ | Tr | Tr |
| SS + IL-1α | ↓ | bld | = | ND | ND | bld | bld |
| SS + IL-1β | ↓ | bld | = | ND | ND | bld | bld |
| SS + TNFα + IL-17 | ↓ | ↑ | = | ↑ | ↑ | ND | ND |
Cytokine levels were measured at 24 h for all except CTACK, TARC, and TSLP that were determined at 48 h.
SS, Sarcoptes scabiei extract; bld, below the limit of detection; +, constitutively produced; ND, not determined; Tr, trace amounts produced; ↑, amount increased compared with constitutive level or level with single stimulant; ↓, amount decreased compared with stimulant without SS; =, level equal to that of stimulant in the absence of SS.
Table 2. Summary of the modulatory effects of various stimuli on normal human dermal fibroblasts.
| Stimulant | Cytokine secreted | |||||
|---|---|---|---|---|---|---|
| IL-8 | GM-CSF | IL-6 | G-CSF | TARC | TSLP | |
| None | bld | bld | bld | bld | bld | bld |
| IL-1α | ? | ↑ | ↑ | ↑ | ND | ND |
| IL-1β | ? | ↑ | ↑ | ↑ | ND | ND |
| IL-17 | bld | bld | bld | bld | bld | bld |
| TNFα | ? | ↑ | ↑ | ↑ | bld | bld |
| TNFα + IL-17 | ? | ↑ | ↑ | ↑ | bld | bld |
| SS | ? | bld | ↑ | ↑ | bld | bld |
| SS + IL-1α | ↓ @ 8 h = @ 24 h | ↓ | = | = | ND | ND |
| SS + IL-1β | ↓ @ 8 h = @ 24 h | ↓ | = | = | ND | ND |
| SS + TNFα + IL-17 | ↓ @ 8 h = @ 24 h | ↓ | = | = | ND | ND |
Cytokine levels were measured at 24 h for all except as noted and for TARC and TSLP that were determined at 48 h.
SS, Sarcoptes scabiei extract; bld, below the limit of detection; +, constitutively produced; ND, not determined; ↑, amount increased compared with constitutive level or level with single stimulant; ↓, amount decreased compared with stimulant without SS; =, level equal to that of stimulant in the absence of SS.
Normal Human Epidermal Keratinocytes
Sarcoptes scabiei Extract Downregulates the Secretion of IL-8 (CXCL8) in the Presence of the Proinflammatory Cytokines IL-1α, IL-1β, and TNFα + IL-17
Keratinocytes constitutively secreted very little IL-8 and stimulation with scabies extract had little effect on these levels (Fig. 1; Table 1). Stimulation of keratinocytes with either IL-1α, IL-1β, or TNFα + IL-17 dramatically increased secretion of IL-8 above constitutive levels. In a dose-dependent fashion, co-stimulation with scabies extract and the mixture of IL-1α, IL-1β, or TNFα + IL-17 decreased IL-8 secretion to levels below those induced by the proinflammatory cytokines in the absence of S. scabiei extract.
Fig. 1.
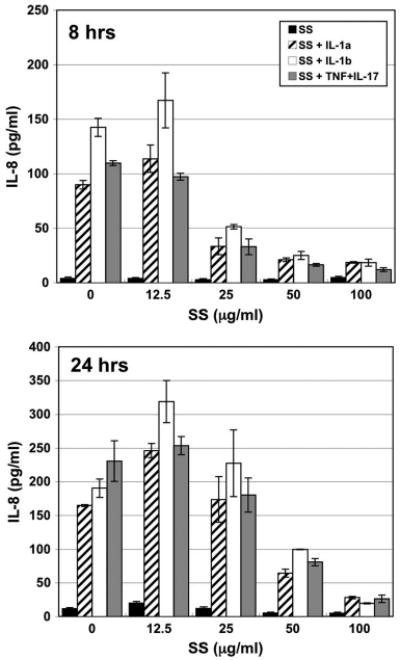
IL-8 (CXCL8) secretion by normal human epidermal keratinocytes in response to various stimuli. Cells were incubated for 8 or 24 h with varying doses of S. scabiei (SS) extract alone or in combination with IL-1α (1.0 ng/ml), IL-1β (1.0 ng/ml), or TNFα + IL-17 (5.0 + 10.0 ng/ml, respectively).
Sarcoptes scabiei Extract Upregulates Secretion of IL-6, Growth-Related Oncogene α (GROα/CXCL1), and Transforming Growth Factor α (TGFα) But Did Not Modulate Proinflammatory Stimuli-Induced Secretion of These Cytokines
IL-6
Cultured keratinocytes constitutively secreted very little IL-6 (Table 1). Stimulation of keratinocytes with varying doses of scabies extract did induce some secretion of IL-6 in a dose-dependent fashion. Stimulation of keratinocytes with TNFα + IL-17 together increased IL-6 secretion. Addition of scabies extract at varying doses along with TNFα + IL-17 resulted in additive secretion of IL-6. Neither IL-1α nor IL-1β elicited IL-6 secretion above baseline levels.
GROα (CXCL1)
Keratinocytes constitutively secreted GROα and stimulation of these cells with scabies extract increased secretion of GROα above constitutive levels in a dose- and time-dependent fashion (Table 1). Stimulation with IL-1α, IL-1β, or TNFα + IL-17–induced secretion of large amounts of GROα. Keratinocytes co-stimulated with scabies extract along with IL-1α, IL-1β, or TNFα + IL-17 did not appreciably modulate the stimulant-induced GROα secretions.
TGFα
Keratinocytes constitutively produced TGFα and stimulation of these cells with TNFα + IL-17 slightly increased TGFα secretion (Table 1). Stimulation with varying doses of scabies extract yielded a dose-dependent secretion of TGFα. Co-stimulation with scabies extract along with TNFα + IL-17 resulted in a mite extract dose-dependent increase in TGFα above the TNFα + IL-17–induced levels.
Cutaneous T-cell Attracting Chemokine (CTACK/CCL27) Secretion Requires Prolonged Stimulation With Scabies Extract
CLA (cutaneous lymphocyte associated antigen) is the surface ligand of a subset of memory T cells. These T cells bind to E-selectin that is expressed on microvascular endothelial cells during local inflammation and whose expression on dermal endothelial cells is suppressed in the presence of scabies extract (Elder et al. 2006). CLA+ memory T cells preferentially home to the skin. CTACK is an attractant for CLA+ memory T cells that is produced in the skin by keratinocytes (Morales et al. 1999, Homey et al. 2002) and TNFα and IL-1β have been reported to induce CTACK production (Homey et al. 2002).
No CTACK secretion by keratinocytes was detected before 48 h. Stimulation with varying doses of scabies extract increased CTACK production from keratinocytes above constitutive levels by nearly three-fold at all doses tested (Fig. 2; Table 1). Although addition of IL-1α, IL-1β, IL-4, or IL-13 at 10.0 ng/ml doubled the amount of CTACK secreted, stimulation with TNFα at 10.0 ng/ml elicited a five-fold increase in CTACK production. Stimulation with scabies extract at 12.5,25, and 50 μg/ml along with TNFα (10 ng/ml) increased CTACK production above TNFα-induced level (Fig. 2).
Fig. 2.
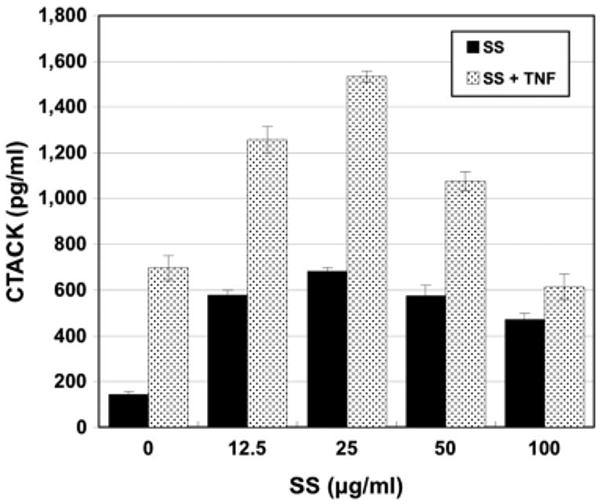
CTACK (CCL27) secretion by normal human epidermal keratinocytes in response to various stimuli. Cells were incubated for 48 h with varying doses of S. scabiei (SS) extract alone or in combination with TNFα (10.0 ng/ml).
Scabies Extract Has No Effect on the Secretion of Thymus- and Activation-Regulated Chemokine (TARC/CCL17) and Thymic Stromal Lymphopoietin (TSLP)
TARC is a cytokine produced by dendritric cells, keratinocytes, fibroblasts, and bronchial epithelial cells and is a specific chemoattractant for Th-2 type T-cells to sites of inflammation (Yu et al. 2002). Fibroblasts require dual stimulation with IL-4 and TNFα to produce TARC (Yu et al. 2002). TSLP is a keratinocyte-produced cytokine that promotes dendritic cell-mediated allergic inflammation (Bogiatzi et al. 2007, Liu 2007, Soumelis et al. 2002).
Keratinocytes did not constitutively secrete TARC or TSLP and no more than trace amounts of these compounds were produced when cells were stimulated with scabies extract, with TNFα alone, or in combination with IL-1α, IL-1β, IL-4, or IL-13 (Table 1).
Normal Human Dermal Fibroblasts
Scabies Extract Downregulates Induced Secretion of IL-8 (CXCL8) and GM-CSF
IL-8 (CXCL8)
Unstimulated fibroblasts did not secrete measurable amounts of IL-8 (Fig. 3; Table 2). At 8 h, fibroblasts stimulated with scabies extract increased IL-8 secretion; however, IL-8 secretion decreased as scabies extract concentrations increased (Fig. 3). By 24 h, IL-8 secretion was induced by scabies extract in a dose-dependent manner. Stimulation of fibroblasts with IL-1α, IL-1β, or TNFα + IL-17 elicited secretion of tremendous amounts of IL-8. At 8 h, the stimulant-induced IL-8 secretion was decreased by co-incubation with increasing doses of scabies extract but by 24 h the scabies extract had no effect on the level of IL-8 secretion from fibroblasts induced by these proinflammatory mediators (Fig. 3).
Fig. 3.
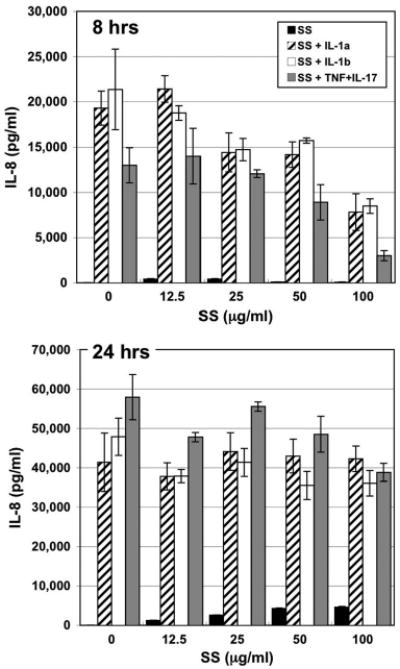
IL-8 (CXCL8) secretion by normal human dermal fibroblasts in response to various stimuli. Cells were incubated for 8 or 24 h with varying doses of S. scabiei (SS) extract alone or in combination with IL-1α (1.0 ng/ml), IL-1β (1.0 ng/ml), or TNFα + IL-17 (5.0 + 10.0 ng/ml, respectively).
GM-CSF
Fibroblasts did not constitutively secrete GM-CSF nor did addition of scabies extract elicit secretion of this cytokine (Fig. 4; Table 2). Stimulation of fibroblasts with IL-1α, IL-1β, or TNFα + IL-17–induced substantial GM-CSF secretion. Co-stimulation of fibroblasts with scabies extract and any of the three proinflammatory stimuli induced a dose-dependent downregulation of GM-CSF secretion below the proinflammatory-induced levels at both 8 and 24 h.
Fig. 4.
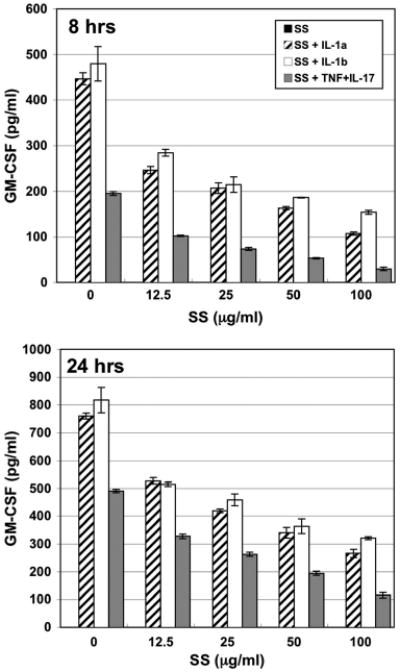
GM-CSF secretion by normal human dermal fibroblasts in response to various stimuli. Cells were incubated for 8 or 24 h with varying doses of S. scabiei (SS) extract alone or in combination with IL-1α (1.0 ng/ml), IL-1β (1.0 ng/ml), or TNFα + IL-17 (5.0 + 10.0 ng/ml, respectively).
Sarcoptes scabiei Extract Upregulates Secretion of IL-6 and G-CSF by Dermal Fibroblasts
IL-6
Cultured fibroblasts did not constitutively produce IL-6 (Table 2). Stimulation of fibroblasts with varying doses of scabies extract increased IL-6 secretion in both a dose-dependent and time-dependent fashion. Stimulation of fibroblasts with IL-1α, IL-1β, or TNFα + IL-17 also induced increased secretion and these levels of IL-6 secretion were unchanged by co-stimulation with scabies extract compared with proinflammatory-induced expression.
G-CSF
Fibroblasts did not constitutively secrete G-CSF (Table 2). Stimulation with varying doses of scabies extract induced G-CSF secretion in a dose- and time-dependent fashion. Stimulation with either IL-1α, IL-1β, or TNFα + IL-17 also increased G-CSF secretion. Co-stimulation with scabies extract and proinflammatory cytokines did not affect the G-CSF levels induced by the proinflammatory stimuli alone.
Scabies does not induce the secretion of TARC (CCL27) and TSLP
Fibroblasts did not constitutively secrete TARC or TSLP nor were they expressed when cells were stimulated with scabies extract or with TNFα alone or in combination with IL-4, IL-13, or IL-17 (Table 2).
Discussion
A previous study found that molecules in scabies extract downregulated the constitutive and LPS-induced secretion of IL-8 from normal cultured keratinocytes (Arlian et al. 2003). In that particular study, no proinflammatory cytokines were added to the culture media along with the scabies extract. In vivo, it is likely that proinflammatory cytokines such as IL-1α, IL-1β, TNFα, and IL-17 are released by other cells in the vicinity of a scabies mite in the epidermis. Therefore, in this study we co-stimulated keratinocytes with scabies extract and IL-1α, IL-1β, and a TNFα + IL-17 mixture. We observed that keratinocytes constitutively secreted very little IL-8 but IL-8 secretion was induced when the cells were stimulated with IL-1α, IL-1β, and TNFα + IL-17. Importantly, this finding was that scabies extract, even in the presence of the proinflammatory cytokines IL-1α, IL-1β, and a TNFα + IL-17 mixture, dose-dependently downregulated the level of IL-8 secretion that was induced by the proinflammatory cytokines alone. IL-8 is a potent chemotatic factor for neutrophils. Neutrophils are a major component of the cell infiltrate into a scabietic lesion once extravasation begins (Arlian et al. 1994, 1996a).
The temporal effect of scabies extract on fibroblast IL-8 production over time was also interesting. At 24 h, scabies induced a dose-dependent increase in IL-8 production as we had seen in our earlier studies (Arlian et al. 2003). The addition of the proinflammatory cytokines induced secretion of large amounts of IL-8 that was downregulated by scabies extract at the shorter time but not at the longer time. This is consistent with our observations in TNFα-stimulated dermal endothelial cells where scabies extract (200 μg/ml) inhibited IL-8 production by 85% at 6 h but by lesser amounts as time progressed (52% at 12 h; Elder et al. 2006). In fact, this observation of scabies down-modulation of endothelial cell cytokine secretion at shorter times is one of the reasons that we conducted the current experiments at shorter times.
We hypothesize that the apparent inconsistency between the ability of scabies extract to induce IL-8 secretion from fibroblasts while also suppressing proinflammatory-induced IL-8 production is caused by the presence of at least two distinct sets of bioactive molecules in the scabies extract. One (the “IL-8 suppressor”) interferes with proinflammatory-induced IL-8 production, perhaps by binding to the receptor through which these stimulatory molecules act. The second stimulates IL-8 secretion by a mechanism independent of the one used by the other stimuli, and by 24 h, the amount of IL-8 produced in response to this molecule overcomes the inhibition.
Another important finding was that S. scabiei extract could dose-dependently downregulate the secretion of GM-CSF from fibroblasts that was induced by stimulation with proinflammatory cytokines. The functions of GM-CSF include stimulating the differentiation of granulocytes and monocyte progenitors and the growth of endothelial cells. It is not clear what the effect might be in a scabietic lesion but perhaps monocytes would not differentiate into macrophages in the lesion or even infiltrate from the blood stream into the lesion. Temporal histological studies have shown that there are few macrophages in scabietic lesions compared with neutrophils and lymphocytes (Arlian et al. 1994, 1996a).
Although scabies mites are the source of molecules that can downregulate selected aspects of fibroblast, keratinocyte, and endothelial cell function, these mites also stimulate inflammatory events by these same cells and others. This study showed that scabies molecules induce secretions of GROα, TGFα, and CTACK from keratinocytes and IL-6 and G-CSF from fibroblasts. In most cases, scabies extract acted in an additive fashion to increase the effect of the proinflammatory cytokines IL-1α, IL-1β, and TNFα + IL-17 on these cells. The interesting question is how do these upregulated and downregulated effects influence the overall host response temporally after the mite infestation is initiated. Clearly, the downregulation events induced by the mites are dominant early in an infestation even in the presence of proinflammatory cytokines and lipid-mediators. The depressed response shifts to an inflammatory response as an infestation progress. In our in vitro system, we stimulate cells with scabies mite whole body extracts that contain all soluble mite molecules. One possibility is that in vivo the effector molecules, and their quantities are released at different rates and/or at different times. The down-modulating molecules are likely released by live mites so they can establish a population in the host protected from the host's damaging response. Live mites produce downregulating molecules but as the mite population increases and mites die and disintegrate in the skin, they may be the source of molecules that induce the inflammatory response that eventually overrides the initial downregulation.
Overall, these results suggest that molecules in scabies extract can both down- and upmodulate cytokine and chemokine expression from keratinocytes and fibroblasts even in the presence of potent proinflammatory mediators. These findings that the secretion of IL-8 from keratinocytes and fibroblasts and of GM-CSF from fibroblasts can be downregulated coupled with our previous finding that scabies could downregulate expression of IL-8, ICAM-1, and E-selectin by dermal microvascular endothelial cells (Elder et al. 2006) and IL-1ra from keratinocytes (Arlian et al. 2003) suggest that scabies mites are the source of multiple factors that may play a role in the delayed inflammatory response observed in an early primary infestation with scabies. The interesting host–parasite relationship that has evolved favors the parasite's early successful exploitation of the host and the survival of the parasite.
Acknowledgments
This study was funded by a grant to L.G.A. from the U.S. National Institutes of Health, National Institute of Allergy and Infectious Disease (AI-017252).
References Cited
- Arlian LG, Rapp CM, Vyszenski-Moher DL, Morgan MS. Sarcoptes scabiei: histopathological changes associated with acquisition and expression of host immunity to scabies. Exp Parasitol. 1994;78:51–63. doi: 10.1006/expr.1994.1005. [DOI] [PubMed] [Google Scholar]
- Arlian LG, Morgan MS, Rapp CM, Vyszenski-Moher DL. The development of protective immunity in canine scabies. Vet Parasitol. 1996a;62:133–142. doi: 10.1016/0304-4017(95)00854-3. [DOI] [PubMed] [Google Scholar]
- Arlian LG, Vyszenski-Moher DL, Rapp CM, Hull BE. Production of IL-1 alpha and IL-1 beta by human skin equivalents parasitized by Sarcoptes scabiei. J Parasitol. 1996b;82:719–723. [PubMed] [Google Scholar]
- Arlian LG, Morgan MS, Neal JS. Modulation of cytokine expression in human keratinocytes and fibroblasts by extracts of scabies mites. Am J Trop Med Hyg. 2003;69:652–656. [PubMed] [Google Scholar]
- Arlian LG, Morgan MS, Neal JS. Extracts of scabies mites (Sarcoptidae: Sarcoptes scabiei) modulate cytokine expression by human peripheral blood mononuclear cells and dendritic cells. J Med Entomol. 2004;41:69–73. doi: 10.1603/0022-2585-41.1.69. [DOI] [PubMed] [Google Scholar]
- Arlian LG, Morgan MS, Paul CC. Evidence that scabies mites (Acari: Sarcoptidae) influence production of interleukin-10 and the function of T-regulatory cells (Tr1) in humans. J Med Entomol. 2006;43:283–287. doi: 10.1603/0022-2585(2006)043[0283:etsmas]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Bogiatzi SI, Fernandez I, Bichet JC, Marloie-Provost MA, Volpe E, Sastre X, Soumelis V. Cutting edge: proinflammatory and Th2 cytokines synergize to induce thymic stromal lymphopoietin production by human skin keratinocytes. J Immunol. 2007;178:3373–3377. doi: 10.4049/jimmunol.178.6.3373. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Elder BL, Arlian LG, Morgan MS. Sarcoptes scabiei (Acari: Sarcoptidae) mite extract modulates expression of cytokines and adhesion molecules by human dermal microvascular endothelial cells. J Med Entomol. 2006;43:910–915. doi: 10.1603/0022-2585(2006)43[910:ssasme]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homey B, Alenius H, Muller A, Soto H, Bowman EP, Yuan W, McEvoy L, Lauerma AI, Assmann T, Bunemann E, et al. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med. 2002;8:157–165. doi: 10.1038/nm0202-157. [DOI] [PubMed] [Google Scholar]
- Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F, Pelletier JP. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol. 1998;160:3513–3521. [PubMed] [Google Scholar]
- Katz Y, Nadiv O, Beer Y. Interleukin-17 enhances tumor necrosis factor alpha-induced synthesis of interleukins 1,6, and 8 in skin and synovial fibroblasts: a possible role as a “fine-tuning cytokine” in inflammation processes. Arthrit Rheum. 2001;44:2176–2184. doi: 10.1002/1529-0131(200109)44:9<2176::aid-art371>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Letuve S, Lajoie-Kadoch S, Audusseau S, Rothenberg ME, Fiset PO, Ludwig MS, Hamid Q. IL-17E upregulates the expression of proinflammatory cytokines in lung fibroblasts. J Allergy Clin Immunol. 2006;117:590–596. doi: 10.1016/j.jaci.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Linden A. Interleukin-17 and airway remodelling. Pulm Pharmacol Ther. 2006;19:47–50. doi: 10.1016/j.pupt.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Liu YJ. Thymic stromal lymphopoietin and OX40 ligand pathway in the initiation of dendritic cell-mediated allergic inflammation. J Allergy Clin Immunol. 2007;120:238–244. doi: 10.1016/j.jaci.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Morales J, Homey B, Vicari AP, Hudak S, Oldham E, Hedrick J, Orozco R, Copeland NG, Jenkins NA, McEvoy LM, Zlotnik A. CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells. Proc Natl Acad Sci USA. 1999;96:14470–14475. doi: 10.1073/pnas.96.25.14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MS, Arlian LG. Serum antibody profiles of Sarcoptes scabiei infested or immunized rabbits. Folia Parasitol (Praha) 1994;41:223–227. [PubMed] [Google Scholar]
- Schmidt-Weber CB, Akdis M, Akdis CA. TH17 cells in the big picture of immunology. J Allergy Clin Immunol. 2007;120:247–254. doi: 10.1016/j.jaci.2007.06.039. [DOI] [PubMed] [Google Scholar]
- Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- Yu B, Koga T, Urabe K, Moroi Y, Maeda S, Yanagihara Y, Furue M. Differential regulation of thymus- and activation-regulated chemokine induced by IL-4, IL-13, TNF-alpha and IFN-gamma in human keratinocyte and fibroblast. J Dermatol Sci. 2002;30:29–36. doi: 10.1016/s0923-1811(02)00046-4. [DOI] [PubMed] [Google Scholar]


