Abstract
Synaptic activity recorded from low-density networks of cultured rat hippocampal neurons was monitored using microelectrode arrays (MEAs). Neuronal networks were patterned with poly-L-lysine (PLL) using microcontact printing (µCP). Polydimethysiloxane (PDMS) stamps were fabricated with relief structures resulting in patterns of 2 µm-wide lines for directing process growth and 20 µm-diameter circles for cell soma attachment. These circles were aligned to electrode sites. Different densities of neurons were plated in order to assess the minimal neuron density required for development of an active network. Spontaneous activity was observed at 10–14 days in networks using neuron densities as low as 200 cells/mm2. Immunocytochemistry demonstrated the distribution of dendrites along the lines and the location of foci of the presynaptic protein, synaptophysin, on neuron somas and dendrites. Scanning electron microscopy demonstrated that single fluorescent tracks contained multiple processes. Evoked responses of selected portions of the networks were produced by stimulation of specific electrode sites. In addition, the neuronal excitability of the network was increased by the bath application of high K+ (10–12 mM). Application of DNQX, an AMPA antagonist, blocked all spontaneous activity, suggesting that the activity is excitatory and mediated through glutamate receptors.
Keywords: synapse formation, microelectrode arrays, microcontact printing, neural networks
I. INTRODUCTION
Neuronal networks produced in cell cultures provide tremendous potential for investigation of synapse formation, development, and function (Gross et al., 1977; Gross et al., 1979; Pine et al., 1980; Grattarola et al., 1993; Gross et al., 1993; Potter et al., 2001; Corner et al., 2002; Marom et al., 2002; van Pelt et al., 2004; Gramowski et al., 2005). Planar microelectrode arrays (MEAs) provide a means for long-term, simultaneous recording of electrical activities from neural networks (Jimbo et al., 1992; James at al., 2004; Chang et al., 2001; Maher et al., 1999). Previous studies of neuronal networks on MEAs have used a large numbers of cells that are generally randomly organized on culture surfaces. Sophisticated analysis methods were then used to identify individual neurons electrophysiologically and describe the dynamics of the neural networks. Only when individual neurons are intracellularly recorded from, can the contributions of individual neuron be measured. (Makarov et al., 2005). To fully understand the function of a neuronal network and the neurons that compose it, we need to use electrophysiological and morphological methods to identify individual neurons and describe how they form connections to each other. This can be accomplished by patterning the network.
Several methods have been developed for patterning surfaces to control neuronal attachment and growth (Hammarback et al., 1985; Kleinfeld et al., 1988; Corey et al., 1991; Stenger et al., 1992; Matsuda et al., 1992; Lom et al., 1993; Hickman et al., 1994; Corey et al., 1997; Branch et al., 1998; Ravenscroft et al., 1998; Branch et al., 2000; James et al., 2000; Lauer et al., 2001). Using these techniques it is possible to direct neuron cell bodies to electrodes and thus provide the ability to study individual neurons within a neural network. Different semiconductor lithographic methods utilizing photoresist, laser or UV ablation, and microcontact printing have been used to pattern proteins and amino acids that influence the attachment and growth of cells. Microcontact printing provides a ready, precise, and reproducible means to control neuron attachment and growth (Corey et al., 1991; Chen et al., 1997; Branch et al., 1998; Branch et al., 2000; James et al., 2000; James et al., 2004; Chang et al., 2000; Chang et al., 2001).
The ideal networks for studies on synapse formation and function should provide (i) identification of each neuron in the network, (ii) monitoring of electrical activities from each neuron, and (iii) long-term network function in order to study development-, activity-, and pharmacology-dependent changes in synaptic/network function. To meet these goals, low-density cultures of neurons are necessary with a goal of individual neurons located at each MEA electrode site. Connections among neurons can then be directed along pre-defined pathways. The geometry of the pattern elements can control neuron cell body attachment to larger discs while narrow lines can direct process growth (Scholl et al., 2000). Aligned microcontact printing using such patterns can selectively control neuron cell bodies to attach on the electrode sites (James et al., 2000; Nam et al., 2004).
This paper describes methods to produce aligned protein patterns for controlling neuron attachment and growth on MEAs. Freshly dissociated rat hippocampal neurons were plated on these networks at different cell densities to determine the lowest cell densities needed to produce and maintain spontaneous synaptic activity. Network function was described by recording spontaneous and evoked potentials. Neuron organization was further analyzed using immunocytochemistry and scanning electron microscopy. These results present critical data for further development of MEAs and neuronal networks for understanding neuronal network function and development of cell-based biosensors.
II. MATERIALS AND METHODS
A. Microelectrode Array (MEA)
MEAs were fabricated using a semiconductor process as described previously (Jun et al., 2005). Briefly, thin titanium adhesion (30 nm) and gold (300 nm) layers were deposited on substrates (Pyrex #7740). An additional titanium layer (10 nm) was deposited on the gold layer to provide for adhesion to the insulation layer. The upper titanium layer, between the gold and insulation layers, prevents the breakdown of the insulation layer during repetitive culture cycles. The metal layers were patterned by wet etching with HF (1%) for titanium and HCl, HNO3 (3:1) solutions for gold. A triple stack insulating layer (SiO2/Si3N4/SiO2) was deposited by plasma enhanced chemical vapor with stress compensated thicknesses of each layer (Yoon et al., 2000). A photoresist (AZ1512, Clariant Corporation, Charlotte NC) mask was used for reactive ion etching of electrode and contact sites. The upper titanium layer was etched in the same cycle. Finally, a Teflon ring was attached to the MEAs with polydimethylsiloxane (PDMS, Sylgard 184; Dow Corning, Midland MI) to make a culture chamber. MEAs were designed to have 32 electrodes (4×8 array) with 200-µm site spacing. The exposed area of each electrode site was 100 µm2. The mean measured impedance was 1.91 MΩ in phosphate buffered solution at 1 kHz measured using a potentiostat (IM6e model, Zhaner Inc., Germany). In order to decrease electrode impedance, all sites were electroplated with platinum black. The electroplating solution contained 1% H2PtCl6, 1M NaCl for supplying conductive electrolytes, and 0.6 g/L lead acetate for uniform nucleation of platinum. Platinization was performed at −0.05 V vs. Ag/AgCl reference electrode for 90 sec. The impedance of platinized sites was 112±41 kΩ. After electroplating, MEAs were rinsed in deionized water several times. For recycling MEAs, household bleach was applied to remove cellular debris. Before beginning new cultures, MEAs were sonicated in acetone, ethyl alcohol, and deionized water (15 min for each step).
B. Microcontact printing
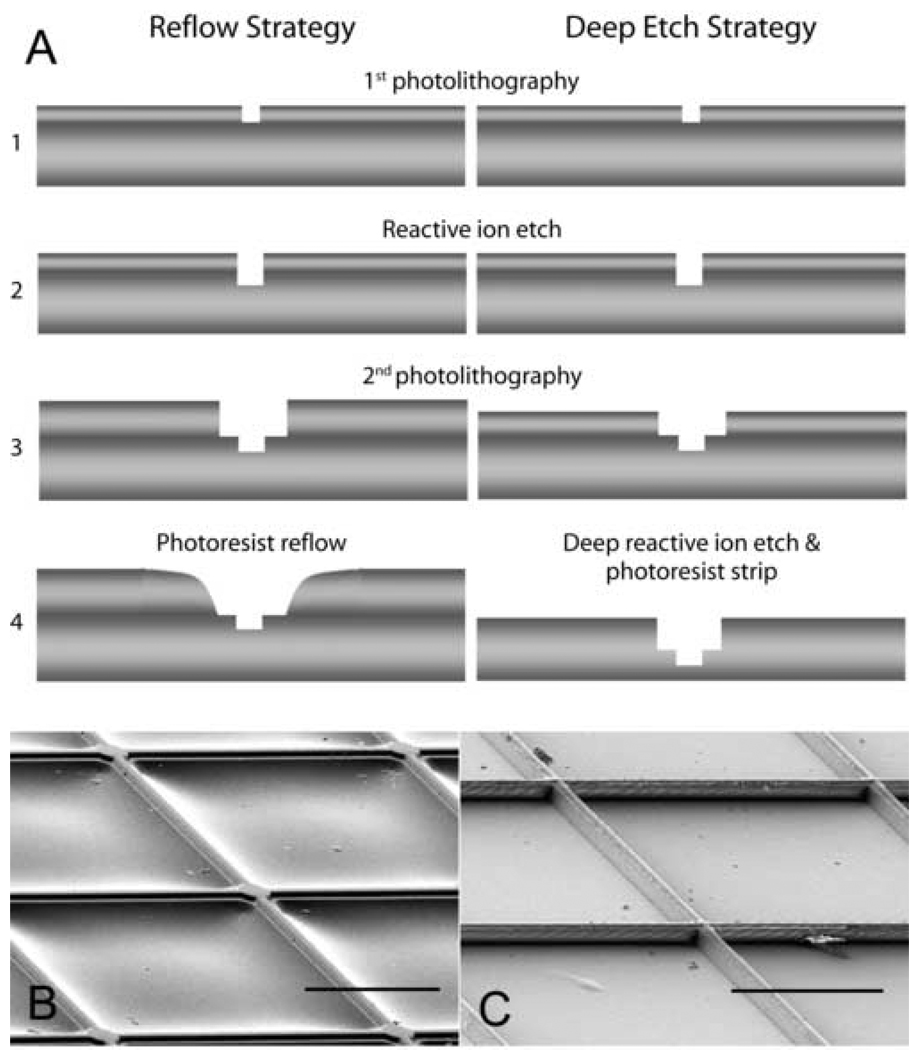
PDMS elastomer stamps were cast from masters designed to match the patterns of MEAs (Fig. 1). These contained orthogonally arrayed lines (2 µm-wide) and circles (20 µm-diameter) centered on line intersections. The lines were designed to provide connections between patterned electrode sites. These features were 2 µm high on the stamps. Because of the relatively large spacing of the 2 µm-wide connecting line stamp structures, 200 µm, it was anticipated that they might be distorted during stamping. Therefore, masters were produced using two different fabrication strategies to provide additional support (Fig. 1). In the first strategy, reflow of thick photo resist (AZ4620, Clariant Corporation, Charlotte NC) was used to make relieved structures (James et al., 2000). In the second strategy, deep silicon etching was used to make supporting structures. The same photolithographic masks were used for both methods.
Figure 1. Cartoon illustrating fabrication steps used to produce masters for microcontact printing and scanning electron micrographs of molded PDMS stamps.
(A) Two different strategies – reflow and deep-etch – were used to produce support for the 200-µm long lines connecting to the electrode sites. The pink layers represent photoresist. The gray layers represent the silicon wafers. Masters fabricated using resist reflow (B) produced structures containing a sloping base to support the raised surfaces that will be used to transfer proteins to the surfaces of the MEAs. Masters fabricated using the double deep-etch (C) strategy produced raised features on a stepped base. Scale bar = 100 µm.
Fluorescein isothiocyanate (FITC)-labeled poly-L-lysine (PLL) (1mg/mL in 0.1M borate buffer) was applied to the PDMS stamps for 1 hr. After blowing off excess PLL, PDMS stamps were mounted in a custom-made alignment tool to for x, y, z, and rotational alignment similar to the device previously used (James et al., 2000). After being aligned, the stamp were pressed to the surface of the MEA (pressure of 50 gram/cm2) and left in contact for approximately 1 hr. Before seeding neurons, all MEAs were sterilized by overnight incubation with 1% gentamycin (GIBCO, Carlsbad, CA) in PBS.
C. Cell Culturing
Primary hippocampal neurons were obtained as previously described (Dowell-Mesfin et al., 2004). Briefly, brains were isolated from embryonic day 18 rat pups (Sprague-Dawley rats; Taconic Farms, Germantown, NY). Hippocampi were dissected under a stereomicroscope and placed in ice-cold balanced saline solution (BSS) following dissection. Tissues were then incubated in 0.25% trypsin (Sigma, St Louis, MO) for 15 min at 37°C. After digestion, hippocampi were rinsed several times in BSS and triturated with a fire-polished Pasteur pipette. Neurons were seeded at densities of 100, 200, and 400 cells/mm2 on the PLL-patterned MEAs in minimal essential medium (MEM) supplemented with 10% horse serum and 0.1% pyruvic acid (GIBCO). Immediately after placing the cell suspensions in the MEA chambers, individual devices were shaken several times to ensure uniform cell distribution. After 4 hr, the plating medium was replaced with serum-free Neurobasal media (GIBCO) supplemented with B27 (GIBCO) and 0.5mM L-glutamine (Fluka, Biochemica, Milwaukee, WI) (Brewer et al., 1993). Cultures were maintained at 37°C in a 5% CO2, 95% air humidified atmosphere. Half of the media was replaced with fresh media twice a week. The Wadsworth Center Institutional Animal Care and Use Committee approved all animal procedures.
D. Electrophysiology
During electrophysiological experiments, growth medium was replaced with recording medium (HEPES-buffered Hanks’ saline (HBHS)). MEAs were mounted into a custom-made connector, which was placed on a resistively heated stage that maintained the media temperature at 36.5°C. An Ag/AgCl wire was immersed into the recording media in the culture chamber as the reference electrode for extracellular recording and stimulation. Signals from recording sites were amplified with a gain of 10,000 and filtered (0.3~5 kHz, 40 dB/decade) using a differential AC amplifier (Model 1700, A-M Systems, Inc., Sequim, WA). The signals were sampled at the frequency of 20 kHz and digitized by a data acquisition device (NI 6024E, National Instruments Corp., Austin TX). Recording was performed with a background noise less than 10 µVrms.
Using an isolated stimulator (ISO-Flex, AMPI, Israel), a 50 µs-width electrical current pulse was applied through a single electrode site for stimulation. The amplitude of the pulses was increased from 50 µA to 200 µA until evoked potentials were observed. Monophasic pulses were used for stimulation; both cathodic and anodic pulses were tested.
HBHS with high K+ was also used to stimulate neurons. The medium K+ concentration was increased from 4.6 to 12 mM by adding aliquots of a 1 M KCl solution. The osmolarity of the recording solution was adjusted to ~285 mM/kg using glucose. The osmolarity was measured using Vapor Pressure Osmometer 5520 (VAPRO™, Wescor, Logan UT).
The glutamate antagonist, 6,7-dinitroquinoxaline-2,3-dione (DNQX, Sigma) was used to characterize synaptic pharmacology. DNQX was used at 1µM and 2µM concentrations (Martin et al., 2003). After application and testing, DNQX was washed out of the MEA recording chamber and replaced with fresh recording media. All electrophysiological experiments were performed at least 2 weeks after initial cell plating.
E. Immunocytochemistry
Immunocytochemistry was employed to identify the locations of nuclei and the distribution of dendrites and synapses in the patterned neural networks. After electrophysiological experiments were completed, neurons were fixed with 4% paraformaldehyde (37°C for 15 min); permeablized with 1% Triton X-100 in HBHS for 15 min at room temperature; and incubated in 6% bovine serum albumin for 30 min at room temperature to block nonspecific binding. Samples were exposed to a mixture of rabbit polyclonal anti-MAP2 (Sigma, 1:500) and mouse monoclonal anti-synaptophysin (Sigma, 1:200) for 1 hr at 37°C. After washing, samples were additionally incubated with secondary antibodies, Texas Red goat anti-rabbit (Molecular Probes, Eugene OR, 1:200) and Alexa 488 goat anti-mouse (Molecular Probes, 1:200) for 1 hr at 37°C. Positive MAP-2 labeling identifies neuronal cell bodies and dendrites. Distribution of positive labeling for synaptophysin, a presynaptic protein, was used to identify potential synaptic sites (Fletcher et al., 1991). After immunocytochemistry, cell nuclei were labeled with DAPI in Milli-Q water (18.2 MΩcm) at room temperature for 30 min. The samples were washed and mounted under cover glass using mounting media (1:1 HBHS/glycerol containing n-propyl gallate). Images were collected with an Olympus BX41WI epifluorescence upright microscope using an Optronics Magnafire CCD camera (Olympus, Melville NY).
F. Scanning Electron Microscopy (SEM)
SEM images were taken in order to observe the patterned neural networks on MEA with high resolution. Fixed samples were rinsed several times in HBHS and dehydrated through graded ethanol solutions (30%, 50%, 60%, 75%, 95%, and 100% (v/v)) for 5 min each. After dehydration, ethyl alcohol was replaced with CO2 using a critical point dryer (Dowell-Mesfin et al., 2004). Dried samples were sputter-coated with gold and imaged using a LEO 1550 VP SEM at 7–8 keV (Carl Zeiss SMT Inc. Oberkochem, Germany).
III. RESULTS
A. Aligned Microcontact Printing
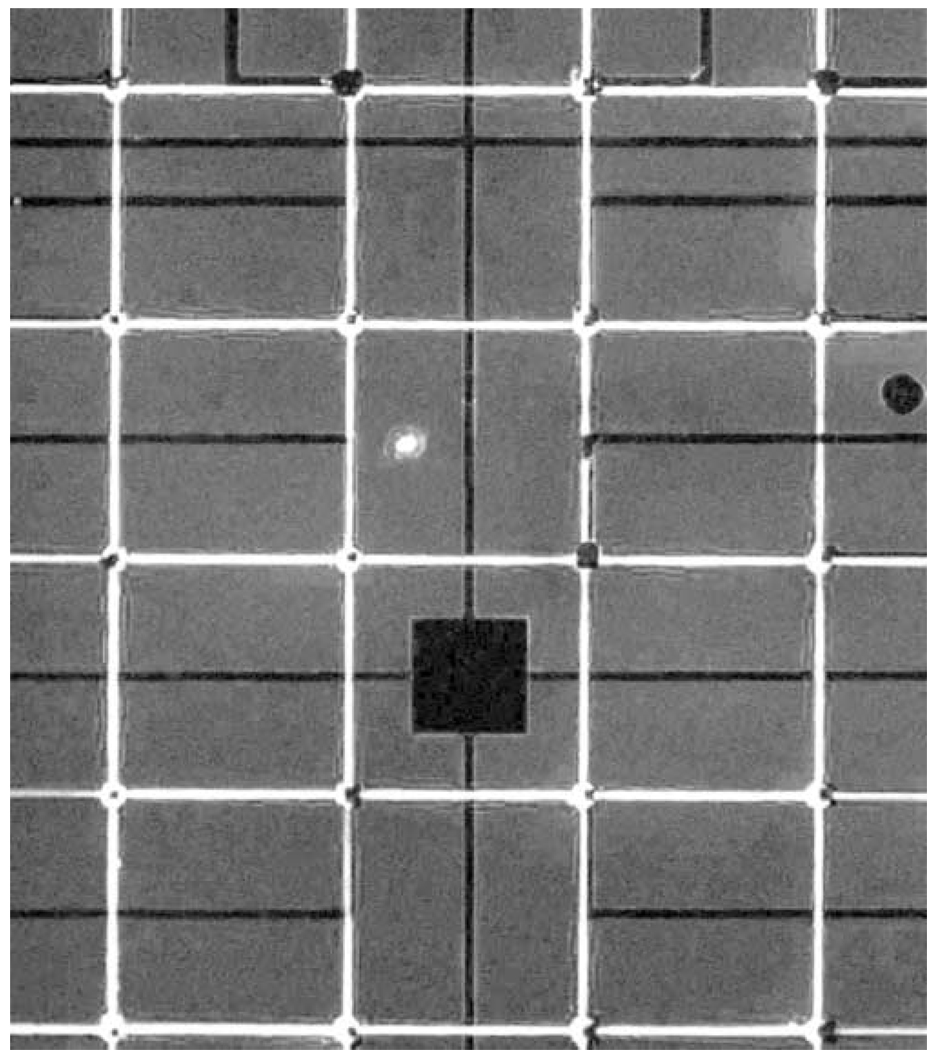
FITC-labeled PLL 2 µm-wide lines and 20 µm-diameter circles were transferred precisely and reproducibly onto MEAs with stamps made using both photo resist reflow and deep silicon etching (Fig. 2). Both strategies for supporting the line structures worked equally well; however, the reflowed supporting structures were sometimes torn while detaching cured PDMS stamps from the masters. Square non-fluorescent areas were observed in the center of disks due to the rough surface of electroplated Pt black electrodes. The lack of fluorescence signal may result from quenching of fluorescence signal due to the Pt black, or interference with the protein transfer by the roughened surfaces. Nevertheless, cell attachment to these sites did not seem to be affected.
Figure 2. Fluorescence micrograph illustrating aligned stamping of poly-L-lysine to a MEA.
FITC-labeled poly-L-lysine (white grid lines) was included in the stamping solution. Electrode sites are observed as dark squares inside the electrode sites. This maybe due to either quenching of the fluorescence signal due to the platinum black deposited on the electrode sites or lack of protein transfer to these regions because of the non-uniform surface produced by the platinum deposition. The darker profiles in the image represent the leads or the central square ground electrode.
B. Cell Patterning
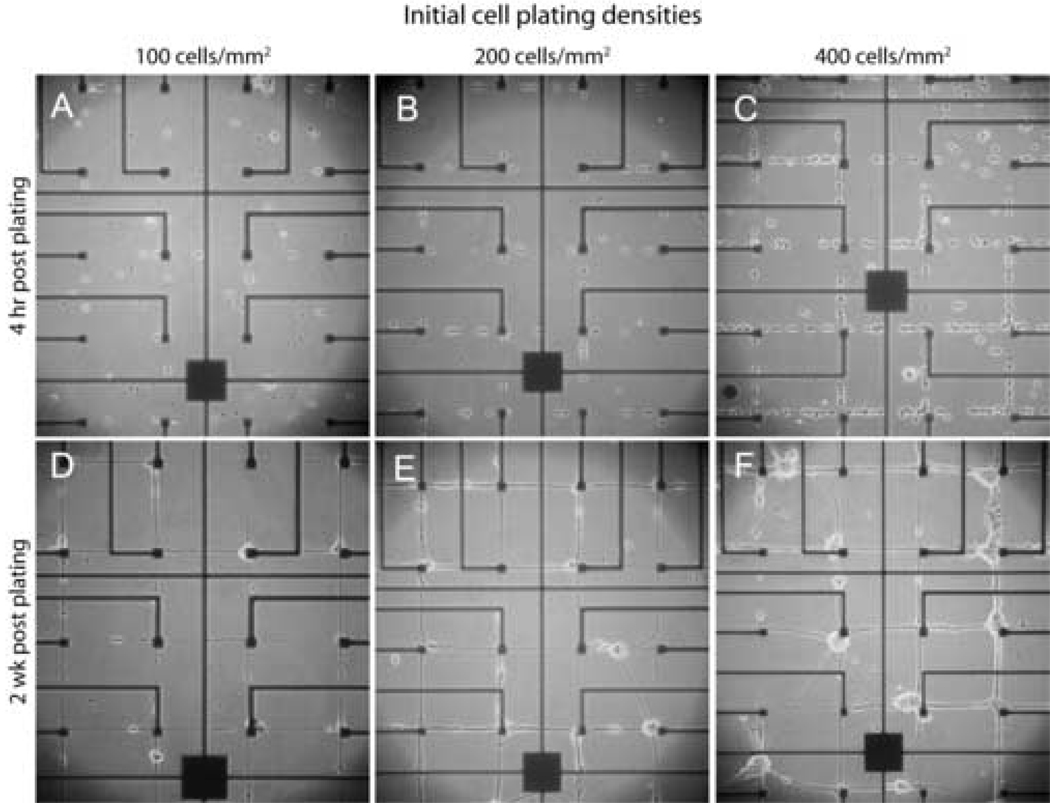
Immediately after seeding, neurons were observed on both 2 µm-wide connectors and 20 µm-diameter electrode sites. Within hours cell bodies migrated to the electrode sites located at the intersections of the orthogonally oriented connecting lines (Fig 3). When cells were plated at a density of 100 cells/mm2, almost all cell bodies were located on electrode sites and the 2 µm-wide connecting lines contained processes (Fig 3D). This outcome came closest to our goal of one cell body/electrode site (see Table 1). When cells were seeded at 200 cells/mm2 more variation was observed (Fig 3E). Multiple cell bodies were located on some electrode sites and a few cell bodies were observed along the connecting lines. When the cells were seeded at 400 cells/mm2 clusters of neurons were observed at electrode sites (Fig. 3F). Occasionally, bundles of processes detached from the patterned connecting lines resulting in diagonal connections between adjacent electrode sites. In order to confirm the reliability of our patterning methods for controlling cell attachment we compared the number of cells attached to electrode sites with those predicted from our plating densities (Table 1). These data indicate that ~25% of plated neurons attached when cells were plated at 100 cells/mm2. Increasing the plating density to 200 cells/mm2, resulted in a doubling of the number of cells observed on electrode sites. However, at the highest cell density and increased plating efficiency was observed. While the effective cell density is lower than the half of the plating cell density, these numbers reflect a short attachment period (4 hr) before the MEAs are rinsed and transferred to defined medium.
Figure 3. Phase micrographs of live neurons growing as networks on patterned poly-L-lysine MEAs.
Cells were plated at initial seeding densities of 100, 200, and 400 cells/mm2. Images are presented illustrating cultures at 4 hrs and 2 wks after plating.
Table 1. Predicted and observed densities of neurons on electrodes.
Predicted values represent all of the cells that would be found in a cell seeding area of 1600 × 800 µm2. This area represents the region containing the 32 electrode sites and a 100µm-wide border. The predicted values were calculated from the total number of neurons in the region (1.28 mm2 seeding density) divided by 32 electrodes each tiling (100µm × 100µm). We have made the assumption that cells in each tiling area might travel to move from its touch-down site to an electrode pad in the 4 hours between plating and changing media.
| 100 cells/mm2 | 200 cells/mm2 | 400 cells/mm2 | |
|---|---|---|---|
| Predicted | 4.0 | 8.0 | 16.0 |
| Observed | 0.9 ± 1.3 | 1.9 ± 3.7 | 5.3 ± 6.1 |
C. Electrophysiology
Few spontaneous signals were recorded from MEAs with 100 cells/mm2 plating densities, even when examined for as long as one month after plating. When MEAs were plated at 200 cells/mm2 spontaneous activity was regularly observed. Frequently, signals were observed to propagate through the networks. Burst of action potentials were observed when neurons were plated at 400 cells/mm2. For all samples, signals were recorded for 2 min and the firing rate of the spontaneous activity was shown for each density of neural networks (Table 2). As the density of cells increases, the rapid increase of activity was observed. On average, the numbers of active electrodes were 1.3 ± 0.11 (N = 5), 1.31 ± 1.38 (N = 5), and 8.0 ± 13.36 (N = 5) in the cultures plated at 100, 200, and 400 cells/mm2, respectively. Even at the density of 400 cells/mm2, the number of active electrodes was less than half of total number of recording electrodes. The results were summarized in Table 2. For all subsequent electrophysiological experiments, neurons were seeded with a density of 200 cells/mm2. At this density, we most closely approached our goals of having a single neuron/electrode site and culture conditions promoted spontaneous development of network activity.
Table 2. Number of active electrodes and firing rate of spontaneous activity.
The number of active electrodes is the mean value of the number of electrodes from which neural activities were recorded among total 32 electrodes of an MEA. Firing rates were calculated from spontaneous activities recorded from the active electrodes.
| 100 cells/mm2 | 200 cells/mm2 | 400 cells/mm2 | |
|---|---|---|---|
| Number of active electrodes |
1.3 ± 1.5 | 6.3 ± 1.5 | 14.3 ± 5.1 |
| Firing rate (Hz) | 0.1 ± 0.1 | 1.3 ± 1.4 | 8.0 ± 13.4 |
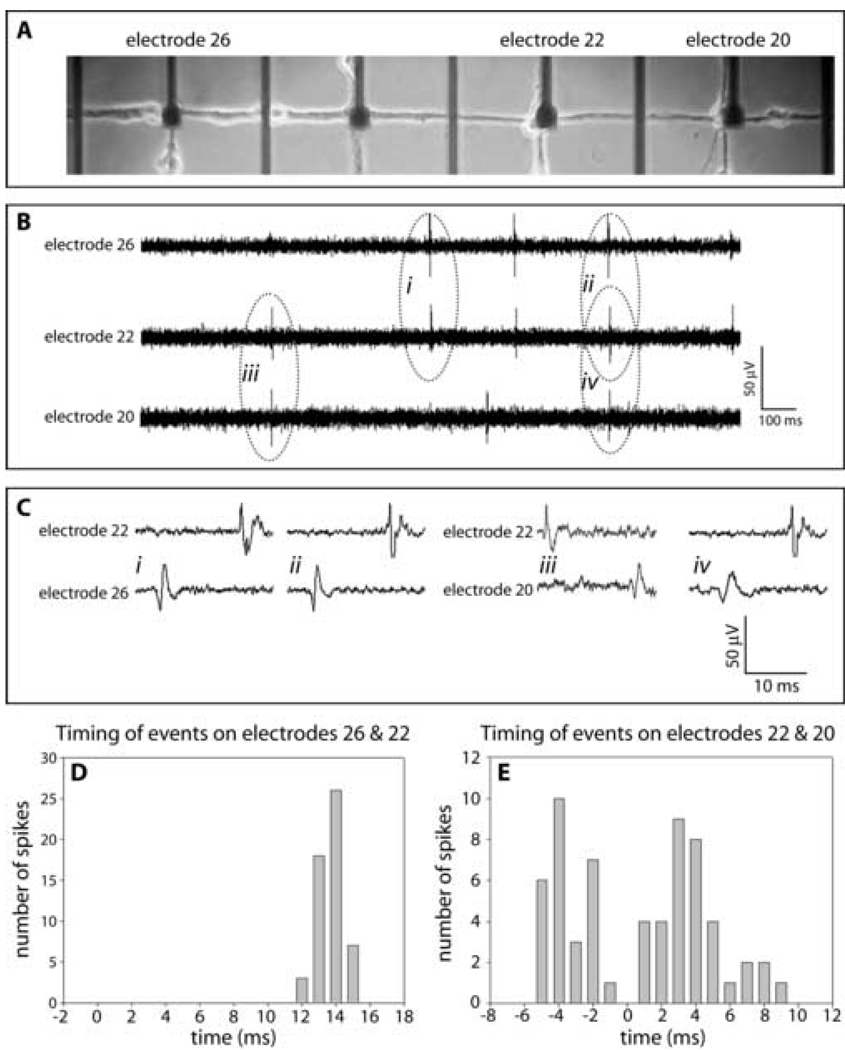
Synchronized action potentials were frequently detected between adjacent electrodes (Fig. 4B). In this example, 76% (54 spikes of 71 spikes for 2 min) of the action potentials recorded on electrode 26 were followed by action potentials on electrode 22. The time interval between these recorded events was very consistent (Fig. 4C & D). The mean value of the time delay was 14 ms; the calculated conduction velocity was 2.8 cm/s. Complex, extensive, unmyelinated axons of the Schaffer collateral network have been shown to have conduction velocities ranging from 20 to 35 cm/s (Anderson et al., 1978). These reported conduction velocities were consistent for axon diameter of 0.1–0.3 µm (Ishizuka et al., 1990). The slow conduction velocity reported here indicates that these events were not the result of direct action potential propagation from electrode 26 to electrode 22, but rather, propagation through polysynaptic pathways. The synchronized signals between electrodes 22 and 20, demonstrated different characteristics. Only 56 % (62 spikes of 111 spikes for 2 min) of the action potentials recorded at electrode 22 were correlated with potentials recorded at electrode 20. The time delays of these recorded potentials showed greater variability (Fig. 4E). In addition, the latencies between recorded potentials from these electrodes indicated that signals were propagating in both directions. This could occur by axons from different neurons traversing in opposite directions on a single connector or by other routes resulting in signals being recorded at this electrode sites.
Figure 4. Representative data illustrating propagation of signals between two electrode sites recorded at DIV20.
(A) Phase microscopic image of living culture. (B) Signal recordings on electrodes 26, 22, and 20 demonstrating temporal correlations. (C) Signal recordings indicated in B with more temporal resolution. (D & E) Histograms plotting numbers of observations vs. latencies between events measured at the indicated electrodes.
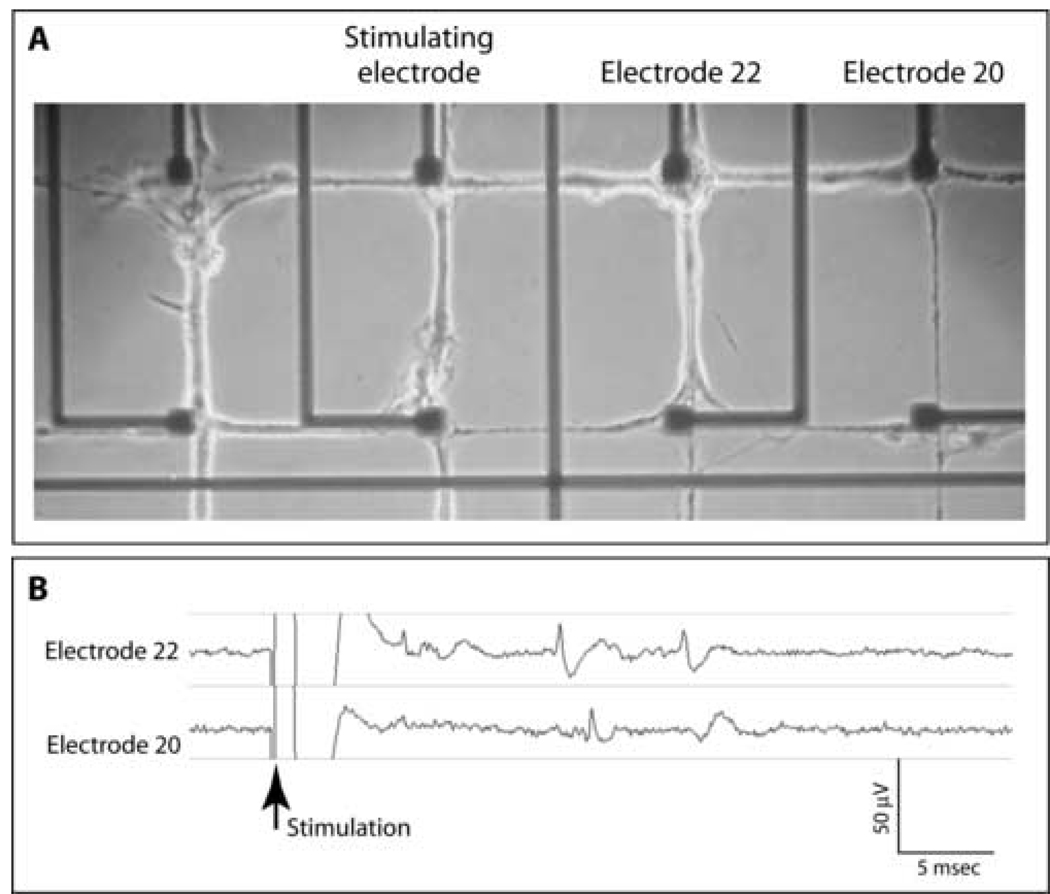
When electrodes were used for stimulation (Fig 5A), cathodic electrical current pulses (amplitude = 200 µA; pulse width = 50 µs) routinely produced evoked potentials (Fig 5B). No responses were produced using anodic stimulation. Typically, action potentials with similar characteristics were recorded at different electrodes indicating that multiple neuronal processes were activated on a single electrode.
Figure 5. Representative data illustrating stimulation through an electrode site.
(A) Phase micrograph of living culture demonstrating the relative positions of the stimulating and recording electrodes. (B) Examples of evoked signals produced using a 200 µA, 50 µs current pulse.
D. Pharmacology
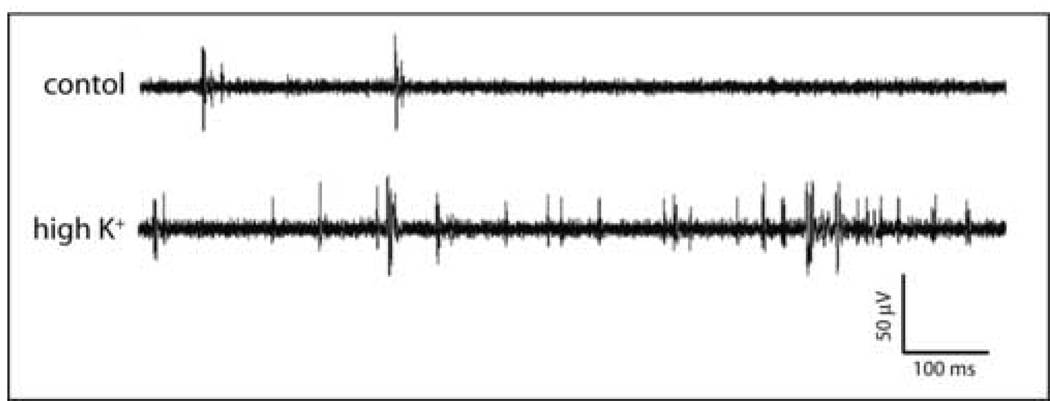
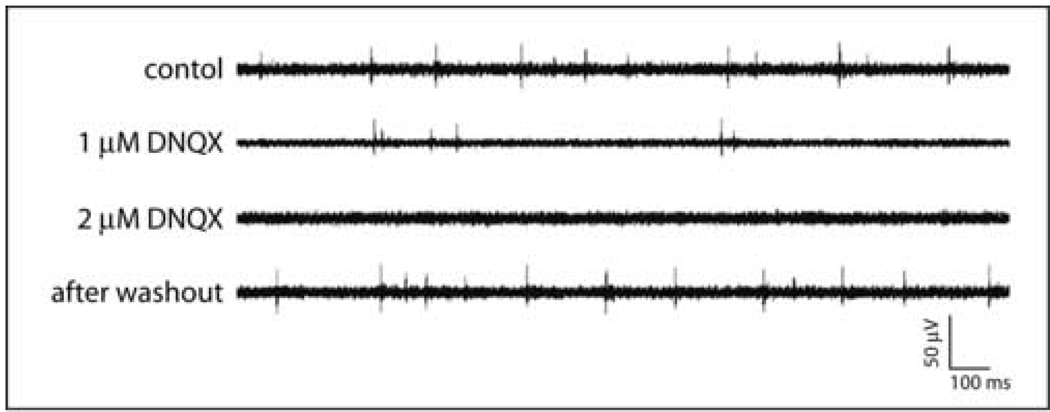
Chemical activation with high-K+ HBHS dramatically increased activity throughout the networks (Fig. 6). The AMPA receptor antagonist DNQX inhibited spontaneous activity in a dose-dependent fashion. Approximately half of the spontaneous activity was inhibited by 1 µM DNQX, while the application of 2 µM DNQX inhibited nearly all activity (Fig. 7). These effects were reversible with spontaneous activities returning to initial rates after washout (Fig. 7). The firing rates were 9.43±1.18 Hz, 4.77±2.24 Hz, 0.07±0.51 Hz, and 10.13±2.31 Hz for the initial state, 1 µM, 2 µM DNQX application, and washout state, respectively (N=5). These results demonstrate that excitatory glutamatergic synapses were responsible for spontaneous network activity. All pharmacological studies were performed using cultures at least 21 days after plating.
Figure 6. Representative data illustrating spontaneous activities and high potassium evoked activities.
The spontaneous activity was recorded immediately before changing the media potassium concentrations. This data was recorded using a patterned neuronal network at DIV 21.
Figure 7. Spontaneous activity is inhibited by DNQX.
(A) Representative recordings demonstrating dose-dependent inhibition of neuronal activity and reversal after washout. (B) Quantification of DNQX inhibition of spontaneous activity.
E. Immunocytochemistry
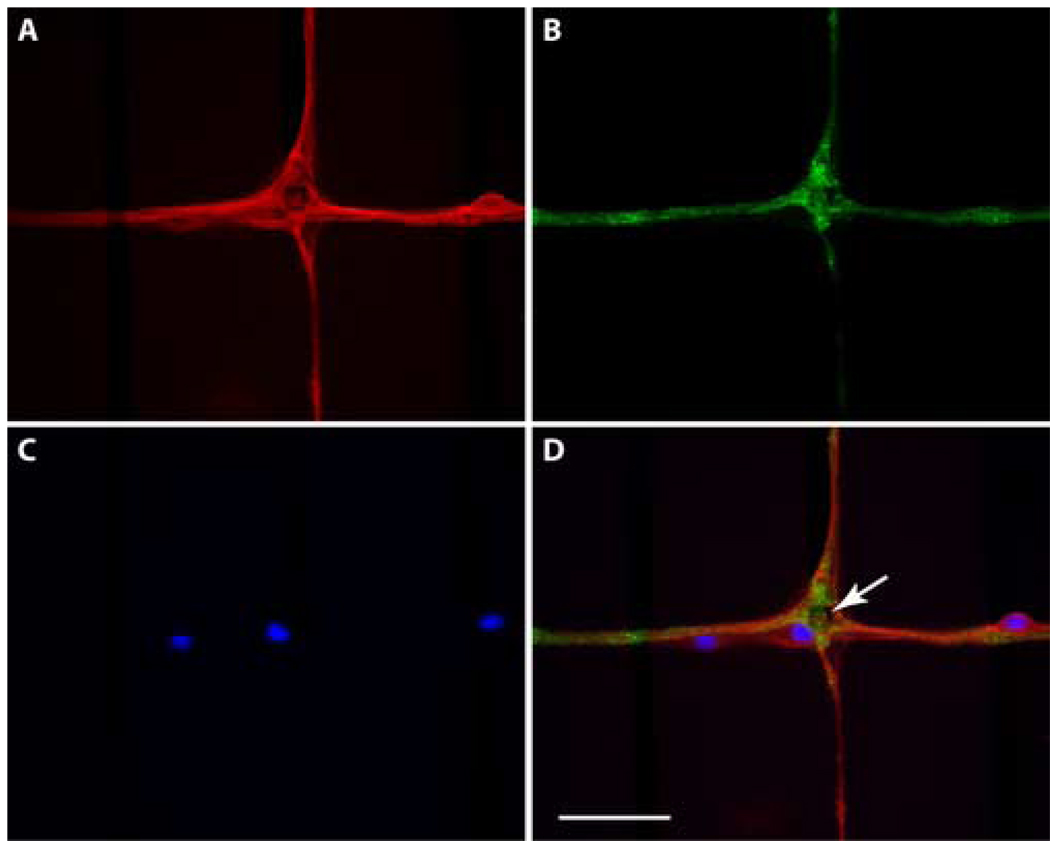
The dendrites and synapses in the patterned network were identified using immunocytochemistry. Nuclei were labeled using DAPI. Nuclear staining and MAP2 immunocytochemistry confirmed cell somas can be isolated to electrode sites (Fig. 8). At these longer times in cell culture, cells are also observed not only near electrode sites, but also out along the patterned lines. While these cells may have landed at these sites, it is more likely that they migrated out from electrode sites. Sometimes cells are observed the synaptophysin labeling was observed as puncta near cell bodies, along dendrites, and in thicker bundles of processes. These results demonstrate that presynaptic sites were distributed throughout the neural networks.
Figure 8. Fluorescence micrographs of neurons growing on a pattern MEA.
(A) Labeling of dendrites and cell bodies using MAP-2 primary antibodies. (B) Distribution of the presynaptic protein synaptophysin. (C) Labeling of cell nuclei using DAPI. (D) Merged images demonstrating that there are three neurons in this field. One cell body is located on the electrode site and two others are observed along the lines connecting electrode sites. The white arrow indicates the electrode site. Scale bar = 50 µm.
F. SEM imaging
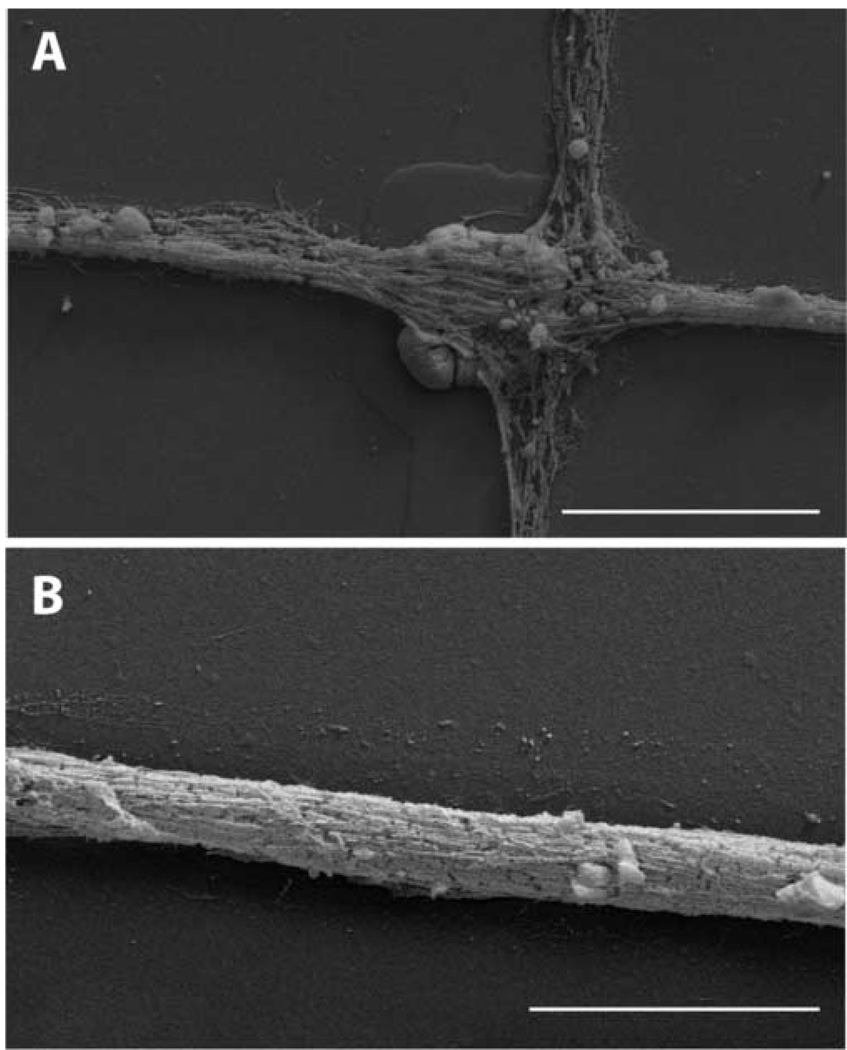
SEM imaging permitted higher resolution observations of the neural networks. Neuron somas were well attached to electrode sites despite the deposition of Pt black on the electrodes (Fig 9A). Many neuronal processes were observed to cross the electrode sites (Fig 9A). These processes formed very compact fascicles of processes on the connector lines (Fig 9B). These appeared to be tightly adhered to the patterned lines. Together with the immunocytochemical analysis these data indicate that these networks are highly complex with many neuron-to-neuron connections.
Figure 9. SEM images of neurons in patterned neuronal networks on an MEA.
(A) An image collected at an electrode site. At least one nucleus may be discerned near the lower left corner of the electrode site. Many neuron processes course over the electrode site and along the connecting lines. (B) An image collected along a connecting line. This image demonstrates the tight packing of processes along these lines. These images were made from samples that been prepared for immunocytochemistry. Scale bars = 20 µm
IV. DISCUSSION
Microcontact printing of patterns, containing circles for neuron soma attachment and narrow lines for neuron process growth, directed development of neural networks of hippocampal neurons. Aligned printing of these patterns to MEAs resulted in regular placement of neurons on electrodes. The number of neurons associated with each electrode was dependent on initial neuron plating density. Spontaneous electrical activity was observed within two weeks of plating when cell densities of 200 cells/mm2 or higher were used.
Action potentials were recorded from approximately one third of the electrode sites on each MEA even though neurons were observed on nearly all the electrode sites. This is consistent with previous studies (Chang et al., 2001). This inefficiency for functional recording sites may be an obstacle for optimal MEA use. One possible contributing factor for this observation is low neuron density. We observed no spontaneous activity when cells were plated at 100 cells/mm2. There are several possible explanations for this observation. Low synaptic input and/or insufficient trophic interactions may be major contributors. At lower densities neurons may not receive sufficient synaptic input to develop into functional networks. Neurons in vitro may have far fewer synapses when compared to neurons in vivo. There are several experimental strategies that might overcome this problem. One is to provide electrical stimulation during network development (Nam et al., 2003). This might provide greater neurotransmitter release and promote more synapse formation (Grubb et al., 2004). Another strategy would be to include an NMDA (N-methyl-D-aspartate) receptor antagonist in the culture media during network development. This procedure has been shown to increase the total number of synapses and spontaneous neuronal activity (Corner et al., 2002; Martinoia et al., 2005). Trophic signals could also be increased by using more cells in the neuronal network, by applying specific trophic factors, or providing feeder cells. The first is not consistent with efforts to develop neural networks with limited elements, but does increase spontaneous electrical activity in network (unpublished observation). Application of brain-derived neurotrophic factor has also been reported to increase spontaneous activity in neuronal cultures (Legrand et al., 2005). This approach is direct, but also requires knowledge of the necessary trophic factors for optimal effects. Close association of feeder layers of neurons and/or astrocytes might provide the necessary trophic factors without increasing neuron numbers on the MEAs and without specific knowledge of the precise factors required. Feeder layers of astrocytes could be used, since these cells are critical for neuron development and function in the brain. Such feeder cultures could be included using opposing cultures (Banker and Goslin, 1997) or by including cells constrained to regions outside the recording regions of the MEAs. We are exploring these possibilities.
Our pharmacological experiments demonstrated that excitatory glutamate receptors, AMPA receptors, are critical to network activity in more mature cultures, e.g. after 21 days. More complete pharmacological studies will indicate the importance of other types of glutamate receptors and the possible role of other neurotransmitters. For instance, the synaptic signals occurring between hippocampal neurons during early developmental stages are mediated by GABAergic synapses before the maturation of glutamatergic transmission (Ma et al., 1998). Consistent with this we have observed suppression of spontaneous activity by bicuculline methiodide, a GABAA receptor antagonist, at 7 days in culture (unpublished observation).
Our morphological analysis of these neuronal networks demonstrated that neuronal somas are usually located on electrode sites providing close association with electrode sites. Pre-synaptic proteins were observed near neuronal somas, along MAP-2 positive processes and in regions of larger bundles of neuronal processes. SEM imaging (Fig 9) clearly demonstrates that the neuronal organization of these cultures is more complex than the cytochemistry suggests. Tightly compacted bundles of neuronal processes were observed along lines and coursing over electrode sites. Thus the signals observed at a particular electrode site may be initiated by neurons at different locations.
Additional evidence of the complexity of these networks can be derived from the electrophysiological recordings. The conduction velocities recorded from specific electrode sites was 0.03 m/sec (see Fig. 4), which is significantly slower than reported conduction velocities of 0.2–0.35 m/s, expected of single unmyelinated axons (Andersen et al., 1978). Thus many of connections we are observing may be part of more circuitous multi-synaptic pathways. Simultaneous recording from the entire network and tracing of individual neurons will be needed to understand the contributions of each neuron to the network activity.
These data demonstrate that low-density neuronal networks can be used to advance our knowledge of neuron networks on MEAs, and demonstrate that these methods will enable more precise investigations of network function. MEA-based systems show great promise for the study of nervous system functions, including memory, learning, and development of biosensors for pharmacology and other applications.
ACKNOWLEDGMENTS
This work was supported by the International Collaboration Program, NBS-ERC (Nano Bioelectronics and Systems Engineering Research Center)/KOSEF (Korea Science and Engineering Foundation) and NIH, R01NS-044287, NSF, ECS-9876771. Authors also appreciate help from T.H. Lee and J.K. Lee for assistance in the fabrication of MEAs and stamp masters.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andersen P, Sifvenius H, Sungberg SH, Sveen O, Wigstrom H. Functional characteristics of unmyelinated fibers in the hippocampal cortex. Brain Res. 1978;144:11–18. doi: 10.1016/0006-8993(78)90431-6. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR, Everge EK, Price PJ. Optimized survival of Hippocampal neurons in B27-supplemented neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- Banker G, Goslin K. Culturing Neurons. Cambridge: MIT Press; 1998. [Google Scholar]
- Branch DW, Corey JM, Weyhenmeyer JA, Brewer GJ, Wheeler BC. Microstamp patterns of biomolecules for high-resolution neuronal networks. Med Biol Eng Comput. 1998;36:135–141. doi: 10.1007/BF02522871. [DOI] [PubMed] [Google Scholar]
- Branch DW, Wheeler BC, Brewer GJ, Leckband DE. Long-term maintenance of patterns of hippocampal pyramidal cells on substrates of polyethelene glycol and microstamped polylysine. IEEE Trans Biomed Eng. 2000;47:290–300. doi: 10.1109/10.827289. [DOI] [PubMed] [Google Scholar]
- Chang JC, Brewer GJ, Wheeler BC. Modulation of neural network activity by patterning. Biosen & Bioelec. 2001;16:527–533. doi: 10.1016/s0956-5663(01)00166-x. [DOI] [PubMed] [Google Scholar]
- Chang JC, Brewer GJ, Wheeler BC. Microelectrode array recordings of patterned hippocampal neurons for four weeks. Biomed Microdev. 2000;2:245–253. [Google Scholar]
- Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- Corey JM, Wheeler BC, Brewer GJ. Compliance of hipppocampal neurons to patterned substrate networks. J Neurosci Res. 1991;30:300–307. doi: 10.1002/jnr.490300204. [DOI] [PubMed] [Google Scholar]
- Corey JM, Brunette AL, Chen MS, Weyhenmeyer JA, Brewer GJ, Wheeler BC. Differentiated B104 neuroblastoma cells are a high-resolution assay for micropatterned substrates. J Neurosci Methods. 1997;75:91–97. doi: 10.1016/s0165-0270(97)00062-9. [DOI] [PubMed] [Google Scholar]
- Corner MA, van Pelt J, Wolters PS, Baker RE, Nuytinck RH. Physiological effects of sustained blockade of excitatory synaptic transmission on spontaneously active developing neuronal networks-an inquiry into the reciprocal linkage between intrinsic biorhythms and neuroplasticity in early ontogeny. Neuroscience and Biobehavioral Reviews. 2002;26:127–185. doi: 10.1016/s0149-7634(01)00062-8. [DOI] [PubMed] [Google Scholar]
- Dowell-Mesfin NM, Abdul-Karim MA, Turner AMP, Schanz S, Craighead HG, Roysam B, Turner JN, Shain W. Topographically modified surfaces affect orientation and grown of Hippocampal neurons. J Neural Eng. 2004;1:78–90. doi: 10.1088/1741-2560/1/2/003. [DOI] [PubMed] [Google Scholar]
- Fletcher TL, Cameron P, Camilli PD, Banker G. The distribution of synapsin I and synaptophysin in hippocampal neurons developing in culture. J Neurosci. 1991;11(6):1617–1626. doi: 10.1523/JNEUROSCI.11-06-01617.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grattarola M, Martionoia S. Modeling the neuron-microtransducer junction:from extracellular to patch recording. IEEE Trans Biomed Eng. 1993;40(1):35–41. doi: 10.1109/10.204769. [DOI] [PubMed] [Google Scholar]
- Gross GW, Rieske E, Kreutzberg GW, Meyer A. A new fixed-array multielectrode system designed for long-term monitoring of extracellular single unit neuronal activity in vitro. Neurosci Lett. 1977;6:101–105. doi: 10.1016/0304-3940(77)90003-9. [DOI] [PubMed] [Google Scholar]
- Gross GW. Simultaneous single unit recording in vitro with a photoetched laser deinsulated gold multimicroelectrode surface. IEEE Trans Biomed Eng. 1979;26(5):273–279. doi: 10.1109/tbme.1979.326402. [DOI] [PubMed] [Google Scholar]
- Gross GW, Rhoades BK, Reust DL, Schwalm FU. Stimulation of monolayer networks in culture through thin-film indium-tin oxide recording electrodes. J Neurosci Methods. 1993;50(2):131–143. doi: 10.1016/0165-0270(93)90001-8. [DOI] [PubMed] [Google Scholar]
- Gramowski A, Juugelt K, Weiss DG, Gross GW. Substance identification by quantitative characterization of oscillatory activity in murine spinal cord networks on microelectrode arrays. European J Neurosci. 2005;19:2815–2825. doi: 10.1111/j.0953-816X.2004.03373.x. [DOI] [PubMed] [Google Scholar]
- Grubb MS, Thompson ID. The influence of early experience on the development of sensory system. Curr Opinion Neurobiol. 2004;14:503–512. doi: 10.1016/j.conb.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Hammarback JA, Palm SL, Furcht LT, Letourneau PC. Guidance of neurite outgrowth by pathways of substratum-adsorbed laminin. J Neurosci Res. 1985;13:213–220. doi: 10.1002/jnr.490130115. [DOI] [PubMed] [Google Scholar]
- Hickman JJ, Bhatia SK, Quong JN, Shoen P, Stenger DA, Pike CJ, Cotman CW. Rational pattern design for in-vitro cellular networks using surface photochemistry. J Vac Sci Tech A. 1994;12:607–616. [Google Scholar]
- Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rats. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- James C, Spence A, Dowell-Mesfin N, Hussain R, Smith K, Craighead H, Isaacson M, Shain W, Turner J. Extracellular recording from patterned neuronal networks using planar microelectrode arrays. IEEE Trans Biomed Eng. 2004;51:1640–1648. doi: 10.1109/TBME.2004.827252. [DOI] [PubMed] [Google Scholar]
- James C, Davis R, Meiyer M, Turner A, Turner S, Wither G, Kam L, Banker G, Craighead H, Isaacson M, Turner J, Shain W. Aligned microcontact printing of micrometer-scale poly-L-lysine structures for controlled growth of cultured neurons on planar microelectrode arrays. IEEE Trans Biomed Eng. 2000;47:17–21. doi: 10.1109/10.817614. [DOI] [PubMed] [Google Scholar]
- Jimbo Y, Kawana A. Electrical stimulation and recording from cultured neurons using a planar electrode array. Bioelectrochem Bioenerg. 1992;29:193–204. [Google Scholar]
- Jun SB, Hynd M, Dowell-Mesfin N, Smith KL, Turner J, Shain W, Kim SJ. Analysis of low-density neuronal networks on microcontact printed microelectrode arrays. The 7th International Conference on Cellular Engineering (ICCE); Seoul, Korea. 2005. [Google Scholar]
- Kleinfeld D, Kahler KH, Hockberger PE. Controlled outgrowth of dissociated neurons on patterned substrates. J Neurosci. 1988;8:4098–4120. doi: 10.1523/JNEUROSCI.08-11-04098.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer L, Klein C, Offenhaeusser A. Spot compliant neuronal networks by structure optimized microcontact printing. Biomaterials. 2001;22:1925–1932. doi: 10.1016/s0142-9612(00)00379-3. [DOI] [PubMed] [Google Scholar]
- Legrand JC, Darbon P, Streit J. Effect of brain-derived neurotrophic factor(BDNF) on activity mediated by NMDA receptors in rat spinal cord cultures. Neurosci Lett. 2005;390:145–149. doi: 10.1016/j.neulet.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Lom B, Healy KE, Hockberger PE. A versatile technique for patterning biomolecules on glass coverslips. J Neurosci Methods. 1993;50:385–397. doi: 10.1016/0165-0270(93)90044-r. [DOI] [PubMed] [Google Scholar]
- Ma W, Liu QY, Jung D, Manos P, Pancrazio JJ, Schaffner AE, Barker JL, Stenger DA. Central neuronal synapse formation on micropatterned surfaces. Dev Brain Res. 1998;111:231–243. doi: 10.1016/s0165-3806(98)00142-4. [DOI] [PubMed] [Google Scholar]
- Maher MP, Pine J, Wright J, Tai YC. The neurochip: a multielectrode device for stimulating and recording from cultured neurons. J Neurosci Methods. 1999;87:45–56. doi: 10.1016/s0165-0270(98)00156-3. [DOI] [PubMed] [Google Scholar]
- Makarov VA, Panetsos F, Feo OD. A method for determining neural connectivity and inferring the underlying network dynamics using extracellular spike recordings. J Neurosci Methods. 2005;144:265–279. doi: 10.1016/j.jneumeth.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Marom S, Shahaf G. Development, learning and memory in large random networks of cortical neurons: lessons beyond anatomy. Quarterly Reviews of Biophysics. 2002;35(1):63–87. doi: 10.1017/s0033583501003742. [DOI] [PubMed] [Google Scholar]
- Martin A, Recasens M, Guiramand J. DNQX-induced toxicity in cultured rat Hippocampal neurons: an apparent AMPA receptor-independent effect? Neurochem International. 2003;42:251–260. doi: 10.1016/s0197-0186(02)00089-x. [DOI] [PubMed] [Google Scholar]
- Martinoia S, Bonzano L, Chiappalone M, Tedesco M. Electrophysiological activity modulation by chemical stimulation in networks of cortical neurons coupled to microelectrode arrays: A biosensor for neuropharmacological applications. Sensors and Actuators B. 2005;108:589–596. [Google Scholar]
- Matsuda T, Sugawara T, Inoue K. Two-dimensional cell manipulation technology an artificial neural circuit based on surface microphotoprocessing. ASAIO J. 1992:243–247. doi: 10.1097/00002480-199207000-00029. [DOI] [PubMed] [Google Scholar]
- Nam Y, Khatami D, Wheeler BC, Brewer GJ. Electrical stimulation of patterned neuronal networks in vitro. Proceedings of the 25th Annual International Conference of the IEEE EMBS; Cancun, Mexico. 2003. [Google Scholar]
- Nam Y, Chang J, Khatami D, Brewer GJ, Wheeler BC. Patterning to enhance activity of cultured neuronal networks. IEE Proc Nanobiotechnol. 2004;151:109–115. doi: 10.1049/ip-nbt:20040706. [DOI] [PubMed] [Google Scholar]
- Pine J. Recording action potentials from cultured neurons with extracellular microcircuit electrodes. J Neurosci Methods. 1980;2(1):19–31. doi: 10.1016/0165-0270(80)90042-4. [DOI] [PubMed] [Google Scholar]
- Potter SM, DeMarse TB. A new approach to neural cell culture for long-term studies. J Neurosci Methods. 2001;110(12):17–24. doi: 10.1016/s0165-0270(01)00412-5. [DOI] [PubMed] [Google Scholar]
- Ravenscroft MS, Bateman KE, Shaffer KM, Schessler HM, Jung DR, Schneider TW, Montgomery CB, Custer TL, Schaffner AE, Liu QY, Li YX, Barker JL, Jickman JJ. Developmental neurobiology implications from fabrication and analysis of Hippocampal neuronal networks on patterned silane-modified surfaces. J Am Chem Soc. 1998;120:12169–12177. [Google Scholar]
- Scholl M, Sprössler C, Denyer M, Krause M, Nakajima K, Maelicke A, Knoll W, Offenhäusser A. Ordered networks of rat hippocampal neurons attached to silicon oxide surfaces. J Neurosci Methods. 2000;104:65–75. doi: 10.1016/s0165-0270(00)00325-3. [DOI] [PubMed] [Google Scholar]
- Stenger DA, Georger JH, Jr, Dulcey CS, Hickman JJ, Rudolph AS, Nielsen TB, McCort SM, Calvert JM. Coplanar molecular assemblies of amino- and perfluorinated alkylsilanes: characterization and geometric definition of mammalian cell adhesion and growth. J Am Chem Soc. 1992;114:8435–8442. [Google Scholar]
- van Pelt J, Wolters PS, Corner MA, Rutten WL, Ramakers GJ. Long-term characterization of firing dynamics of spontaneous bursts in cultured neural networks. IEEE Trans Biomed Eng. 2004;51(11):2051–2062. doi: 10.1109/TBME.2004.827936. [DOI] [PubMed] [Google Scholar]
- Yoon TH, Hwang EJ, Shin DY, Park SI, Oh SJ, Jung SC, Shin HC, Kim SJ. A micromachined silicon depth probe for multi-channel neural recording. IEEE Trans Biomed Eng. 2000;47:1082–1087. doi: 10.1109/10.855936. [DOI] [PubMed] [Google Scholar]