Abstract
Polyacrylamide and poly(ethylene glycol) diacrylate hydrogels were synthesized and characterized for use as drug-release and substrates for neuron cell culture. Protein release kinetics was determined by incorporating bovine serum albumin (BSA) into hydrogels during polymerization. To determine if hydrogel incorporation and release affects bioactivity, alkaline phosphatase was incorporated into hydrogels and released enzyme activity determined using the fluorescence-based ELF-97 assay. Hydrogels were then used to deliver brain derived neurotrophic factor (BDNF) from hydrogels polymerized over planar microelectrode arrays (MEA). Primary hippocampal neurons were cultured on both control and neurotrophin-containing hydrogel-coated MEAs. The effect of released BDNF on neurite length and process arborization was investigated using automated image analysis. Increased spontaneous activity as a response to released BDNF was recorded from the neurons cultured on top of hydrogel layers. These results demonstrate that proteins of biological interest can be incorporated into hydrogels to modulate development and function of cultured neural networks. These results also set the stage for development of hydrogel-coated neural prosthetic devices for local delivery of various biologically active molecules.
Keywords: poly(ethylene glycol) diacrylate, polyacrylamide, hydrogel, drug release, microelectrode arrays
Introduction
For more than two decades, microfabricated neural prosthetic devices have been used to stimulate and record from neurons following implantation into the central nervous system (CNS) [1, 2]. However, while neural prosthetic devices show great potential for the recording and stimulation of neural events within the CNS, reactive responses around the implanted devices ultimately reduces the quality of the interface over time by increasing the impedance of electrodes [3]. In order to circumvent these issues several strategies have been proposed – including the application of electric fields to reduce glial attachment to the electrodes and the release of drugs to prevent reactive gliosis [4-6]. Local release of biologically-active substances from hydrogels can also be used to control cell responses around inserted devices [7].
Hydrogels, a network of hydrophilic polymer chains, have been extensively used in various biomedical applications [8-11]. They have been synthesized with the ability to respond to temperature, pH, and the concentration of metabolites [12-15]. Common uses for hydrogels include soft contact lenses and wound healing materials. The hydrophilic and porous nature of hydrogels shows great promise as reservoirs for drug delivery systems [16]. The ability to tailor the release characteristics of hydrogels, e.g., by varying the length of the polymer or adjusting the cross-link density, enable their use as templates for controlled release of bioactive molecules both in vitro and in vivo.
Previously, we have demonstrated that hydrogels can be biologically safe and that functionalized hydrogel surfaces can be used as templates for protein deposition and as surfaces for the culture of neural cells [17]. We demonstrated that multiple proteins and enzymes could be patterned on the surface of photo-polymerized acrylamide-based hydrogels retaining biological and catalytic activity. LRM55 astroglioma and primary rat hippocampal neurons were successfully attached on patterned extracellular matrix (ECM) and neurons developed functionally active synapses on the hydrogel surfaces [18]. Thus these materials are good candidates for use as reservoirs for local release of materials to control neuron function.
Neurotrophins are proteins that play important roles in the survival and maintenance of neurons. By binding to specific receptors, secreted neurotrophins signal neurons to differentiate/grow, or prevent cell death. This class of proteins includes nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and neurotrophin-1, -3, and -4. Because of their crucial influence on neurons of the central nervous system, neurotrophins have been extensively studied especially in the fields of development, pharmacokinetics and pathophysiology [19-22]. Among various neurotrophins, BDNF is known to support the survival of existing neurons and strengthen the growth and differentiation of developing CNS neurons. Although the exact mechanisms are unclear, the relation between the level of BDNF and diseases such as depression, Alzheimer’s disease, epilepsy, and Huntington’s disease has been reported [23-27]. In this study, BDNF was used to investigate the effects of release from hydrogels on neuronal differentiation and increased neural activity.
We present results using a novel neural interface consisting of planar- microelectrode arrays (MEA) coated with neurotrophin-containing hydrogels. Protein release rates were measured and used to predetermine the amounts of BDNF needed to deliver targeted extracellular concentrations. Neurons cultured on hydrogel-MEAs demonstrated dose-dependent responses to released neurotrophin suggesting that these methods may provide for more stable conditions for studying neuron cell networks in vitro.
Materials and methods
Microelectrode arrays
MEAs were fabricated as described previously [28, 29]. Briefly, each MEA contained 32 electrodes (10×10 μm2; 4×8 arrays) with a 200-μm inter-electrode spacing (Figure 1). In order to fabricate a cell culture chamber over each MEA, a Teflon™ ring (diameter: 20mm, height: 7 mm) was attached to the MEAs using a biocompatible polydimethylsilaoxane elastomer (PDMS, Sylgard 184™; Dow Corning, Midland MI). In order to decrease the impedance of each electrode, prior to culture, electrodes were electroplated with platinum using the following solution: 1% H2PtCl6, 1M NaCl, 0.6 g/L lead acetate; electroplating conditions: constant voltage -0.05 V vs. an Ag/AgCl reference electrode for 90 sec. Following electroplating, MEAs were rinsed three times in deionized (DI) water to remove residual platinizing solution. Impedances were measured in phosphate buffered solution (PBS) in the frequency of 0.1 ~ 100 kHz using a potentiostat (IM6e model, Zhaner Inc., Germany). MEAs were recycled following each experiment. Recycling used treatments with household bleach to remove cellular debris, sequential sonications in acetone, ethyl alcohol, and DI water (15 min for each), and drying using a stream of nitrogen gas. Electrodes were re-platinized if electrode impedances were found to have increased following the cleaning process.
Figure 1.
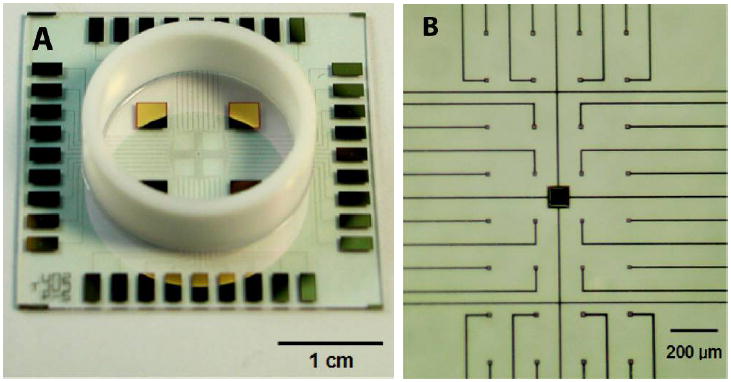
Photographs illustrating features of microelectrode array. (A) A lower magnification imagei llustrating the entire MEA including peripheral contacts, Teflon ring for developing culture space and larger reference electrodes. (B) A higher magnification image illustrating electrode distribution and conductor leads.
Hydrogel synthesis
A polyacrylamide gel matrix consisting of 5% (w/v) acrylamide (19:1, 40% acrylamide:bis-acrylamide), in 30% (v/v) glycerol, 0.1% (v/v) Irgacure 2959 (Ciba Specialty Chemicals, Tarrytown, NY), and 100 mM Tris·Cl (pH 8.5) was prepared. A solution of 10% (wt/v) poly(ethylene glycol)-diacrylate (PEGDA) (Mw=3400; Nektar Therapeutics, Huntsville, AL) was prepared in HEPES-buffered Hanks’ saline (HBHS) containing 0.1% (v/v) Irgacure 2959. Bovine serum albumin (BSA), alkaline phosphatase, or BDNF were added to prepolymer solutions and polymerized in situ. Incorporated amounts of BDNF were determined based on the kinetics of BSA release experiments (see Section of BSA release from hydrogels at below).
Hydrogel polymerization
To ensure covalent attachment of hydrogels to surfaces, MEAs were pre-treated with Bind-Silane (3-(trimethoxyslyl)-propyl acrylate; Sigma, St. Louis, MO):acetic acid:dH2O (1:2:2) for 1 hour [17]. Hydrogels were synthesized by placing a 5 μL of pre-polymer solution onto a Bind-Silane treated MEA. To ensure hydrogel surfaces were planar, a second, separate set of glass coverslips (12 mm in diameter, Fischer Scientific International, Hampton, NH) were pre-treated with PlusOne Repel-Silane ES (dimethyldichlorosilane solution, 2% (w/v) in octamethylcyclo-tetrasiloxane; Amersharm Biosciences, Uppsala, Sweden) and placed on top of the hydrogel solution. Hydrogels were photopolymerized by exposure to UV light for 5 min on a 365 nm UV crosslinker (Spectrolinker™, Model XL-1000; Spectronics Corporation, Westbury, NY). Following polymerization the Repel-Silane treated coverslip was carefully removed from the hydrogel surface. The polymerization procedure is illustrated in Figure 2. In order to see how the electrochemical characteristics of MEAs were affected by hydrogel coating, MEA electrode impedances were re-measured using same protocol as described above.
Figure 2.
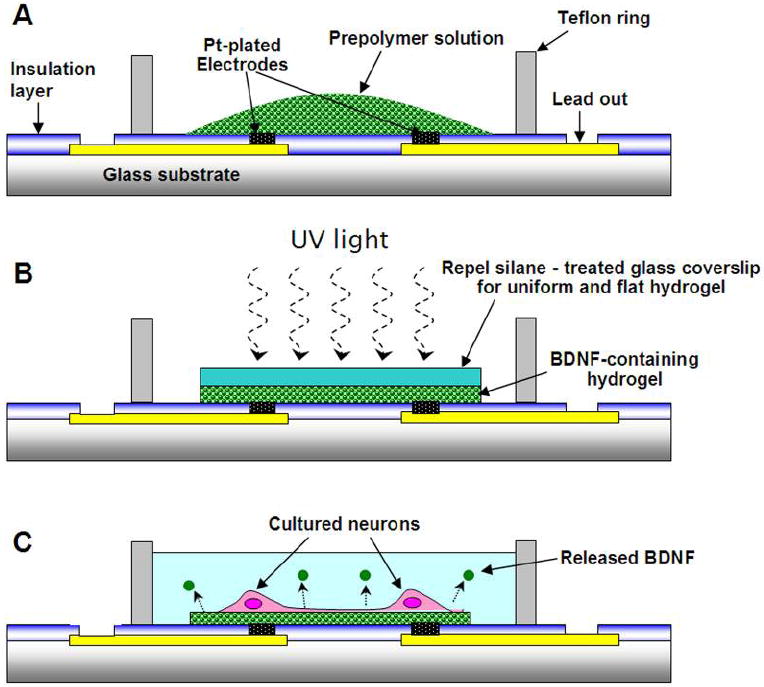
Hydrogel polymerization cross-section schematic representatives. (A) illustrates initial placement of pre-polymer hydrogel in chamber, (B) illustrates UV polymerization step including covering glass coverslip, (C) illustrates real application including cells growing on hydrogel surface and protein release.
BSA release from hydrogels
In order to characterize the release kinetics of proteins from both acrylamide- and PEGDA-based hydrogels, borosilicate glass coverslips (18mm in diameter, Fischer Scientific International, Hampton, NH) were used as backing templates for hydrogels polymerization rather than MEAs. BSA (1 mg/mL in PBS; Sigma, St. Louis, MO) was chosen as a test protein due to its ease of use, stability and cost. Hydrogels containing BSA (0.3 g/L) were polymerized were placed into 1 mL PBS, and incubated at 37°C for different times (1, 2, 4, 8, 24, 120, 288 hours). At the specified times, 100 μL of incubation medium was transferred to a well in a 96-well microplates and released BSA was quantified using the Bradford protein assay [30, 31] (Sigma, St. Louis, MO). The absorbance of each test sample (well) was measured at 515 nm using a spectrometer (PerkinElmer, Model: LS50B, Waltham, MA). The amount of released BSA was determined based on a BSA standard curve.
Enzymatic activity of Alkaline Phosphatase
To ensure the retention of biological activity of hydrogel-polymerized proteins, alkaline phosphatase was polymerized into hydrogels. The ELF-97-based assay (enzyme labeled fluorescence, 5’-chloro-2-phosphoryloxyphenyl)-6-chloro-4(3H)-quinazoline, Invitrogen) was used to determine the enzymatic activity of released alkaline phosphatase [32]. ELF-97 is virtually non-fluorescent in solution, but following hydrolysis generates a yellow-green-fluorescent alcohol precipitate (excitation: 350 nm, emission: 530 nm). Released alkaline phosphatase was collected following 7 days incubation at 37°C in PBS. Alkaline phosphatase enzyme activity for each release sample was determined using ELF-97 concentrations ranging from 0.01-1.0 mM and measuring changes in fluorescence intensity from 1-60 min. The calculated reaction rates were used for Michaelis-Menton kinetic analysis.
Neuron cell culture
Due to the hydrophilic surface of acrylate-based hydrogels, surfaces were not amenable to neural cell adhesion. Thus, prior to cell seeding, poly-L-lysine (PLL; Sigma, St Louis, MO) was transferred onto hydrogel surfaces using microcontact printing. PDMS stamps with plain surfaces were inked with PLL solution (1mg/mL in borate buffer) for 30 min, then placed onto the hydrogel surface for 5 min after excess PLL solution was removed using a brief blast of air. Primary hippocampal neurons were prepared as previously described [33]. Briefly, brains were isolated from embryonic day 18 rat pups (Sprague-Dawley rats; Taconic Farms, Germantown, NY). Hippocampi were dissected under a stereomicroscope and placed in ice-cold balanced saline solution (BSS) following dissection. Tissues were then incubated in 0.25 % trypsin (Sigma, St Louis, MO) for 15 min at 37 °C. After digestion, hippocampi were rinsed three times in BSS for 10 min each, before being triturated with a fire-polished Pasteur pipette. Neurons were seeded at densities of 200 cells/cm2 on hydrogel-coated MEAs and PLL-treated MEAs for control experiments in minimal essential medium (MEM) supplemented with 10 % horse serum and 0.1 % pyruvic acid (Invitrogen). Immediately after placing the cell suspensions in the MEA chambers, individual devices were gently agitated several times to ensure uniform cell distribution. After 4 hr incubation, the MEM plating medium was replaced with serum-free Neurobasal media (Invitrogen) supplemented with B27 (Stem Cell Technologies, Vancouver, Canada) and 2.0 mM GlutaMAX (Invitrogen) [34]. Cultures were maintained at 37 °C in a 5 % CO2, 95 % air humidified atmosphere. Wadsworth Center Institutional Animal Care and Use Committee approved all animal procedures.
Electrophysiological recordings
Electrical measurements were made from cultured neural networks on hydrogel-coated MEAs at 10 days in vitro (DIV). BDNF was polymerized into hydrogels to produce 100 ng/mL of BDNF at 3 days in vitro. Spontaneous activity was measured using on-board electrodes. During electrophysiological experiments, the medium was replaced with recording medium (HEPES-buffered Hanks’ saline (HBHS)). The media temperature was maintained at 36.5 °C by placement on a resistive heated stage. An Ag/AgCl wire was immersed into the recording media in the culture chamber as the reference electrode for extracellular recording. Signals from recording electrodes were amplified with a gain of 10,000 and filtered (0.3~5 kHz, 40 dB/decade) using a differential AC amplifier (Model 1700, A-M Systems, Inc., Sequim, WA). The signals were sampled at 20 kHz and digitized by a data acquisition device (NI 6024E, National Instruments Corp., Austin, TX). Recordings were performed with background noise less than 15 μVrms. For all recording experiments, we recorded the spontaneous activity from neurons located directly on the electrodes.
Image analysis
After 10 days in culture, cells were processed for immunocytochemistry using established protocols [33]. Briefly, hydrogel-coated MEAs coverslips were rinsed in Ca2+ Mg2+-free HBHS pre-warmed to 37 °C for 2 min. Cells were fixed in 4 % paraformaldehyde for 20 min at 37 °C. Cells were then incubated in HBHS for 5 min and blocked in 6% BSA for 30 min at room temperature. MEAs were incubated in an anti-βIII tubulin primary antibody (chicken polyclonal, 1:500; Chemicon) for 1 hour at 37 °C. Samples were then rinsed three times in HBHS and incubated with a secondary antibody (Alexa 647 goat anti-chicken; Invitrogen, 1:500) for 1 hour at 37 °C. Cell nuclei were stained with DAPI (25 μg/mL). Samples were finally rinsed three times in HBHS. Glass coverslips were mounted onto MEAs with a mounting medium consisting of 50:50 HBHS and glycerol saturated with n-propyl gallate. Fluorescently labeled cells were imaged using an inverted Nikon TE2000 epifluorescence upright microscope with an Optronics Magnafire CCD camera (Olympus, Melville NY), and the Image-Pro AMS processing software (Media Cybernetics, Inc. Silver Spring MD).
The resulting images were analyzed using automated process tracing and soma segmentation algorithms [35]. For refined analysis of neurite bifurcation (branch) points, a recently developed algorithm was used [36]. Unlike interactive tracing algorithms such as NeuronJ [37], these algorithms allow automated tracing. Furthermore, they provide accurate and objective measurements of soma and branch points. The output files of these algorithms are a comprehensive dataset including process traces, soma segmentations, coordinates of branch points, and measurements of branch angles. These files were inspected and edited for minor tracing errors using Neurolucida (MBF Biosciences, Williston, VT). From these validated data, measurements of Number of processes per cell, number of primary processes, total length of processes, and number of branching points were extracted. Statistical analysis was performed using analysis of variance (ANOVA) procedure. The analysis was performed with three different groups of surfaces (control, polyacrylamide, PEGDA) and different BDNF concentrations (0, 50, 100 ng/mL). When significant differences were indicated by ANOVA, data sets were further analyzed using Tukey’s post hoc test.
Results
Hydrogel properties
Following UV polymerization, the nonhydrated hydrogel thickness was 44 μm calculated using the formula, volume = πr2h (where h is the thickness, volume = 5 μL , and r = 0.6 cm). According to the 3-D confocal measurements using fluorescence detection of biotin-fluorescein-incubated gels, the hydrogel swelled approximately 30 % following hydration in buffer solution. The hydrophilicity of hydrogel surfaces was characterized by contact angle goniometry. The contact angles were 56° ± 2° and 54° ± 3° for polyacrylamide and PEGDA, respectively. These contact angles were not significantly different from that of the silanized glass surface (59° ± 2°) [17].
BSA release
BSA release experiments were performed to determine the release kinetics of hydrogel entrapped biomolecules (Figure 3). BSA release kinetics was affected by the hydrogel chemistry. Release curves for BSA were fitted by nonlinear regression to the following logarithmic equation with two coefficients:
where y is the percentage of released BSA, t is incubation time in hours.
Figure 3.
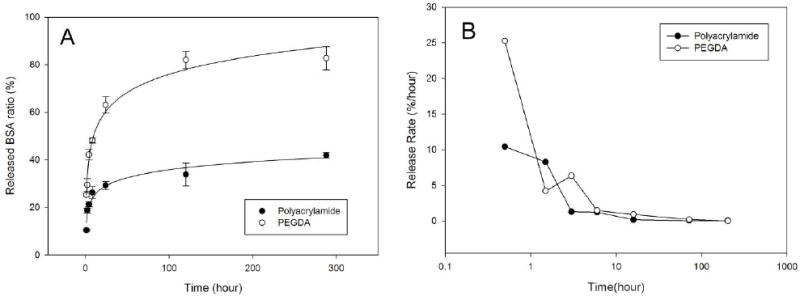
BSA release from polyacrylamide and PEGDA hydrogels (A) time course of BSA release. (B) semi-log plot of BSA release rate. Release rate was calculated as (the total amount of released BSA)/(loaded BSA)
This analysis demonstrated that BSA release rate (b) was approximately two times greater for PEGDA than polyacrylamide hydrogels (11.0 vs. 4.8 %/hr). In addition, the recovery of protein (released BSA/loaded BSA) was greater for PEGDA (83% vs. 42%). Thus, during 12 day incubations almost twice as much BSA was released from PEGDA hydrogels. Since both hydrogels were polymerized in the presence of the same amount of BSA, this difference may be attributed to the differences in the amount of protein bound to the hydrogels during polymerization.
Using the parameters calculated from the BSA measurements (TABLE 1), BDNF loading conditions were determined to deliver effective BDNF concentrations of 0, 50, 100 ng/mL at 3 days in vitro. This time point was chosen since it has been shown that at this time BDNF has an important influence on primary neurons, including dendritic branching and total process length. Since neuron plating media was replaced with neuron-specific growth media at 4 hrs, calculations were made to correct for the amount of BDNF released within the initial 4 hours and removed at the media change. For example, in order to achieve a concentration of 50 ng/mL of BDNF at 3 days in vitro, 145.9 ng of BDNF was loaded into acrylamide hydrogels, while 69.1 ng was loaded into PEGDA hydrogels.
Table 1.
Parameters for hydrogel-released BSA fitting.
| a | b | R2 | |
|---|---|---|---|
| Polyacrylamide | 13.7 | 4.8 | 0.9810 |
| PEGDA3400 | 25.2 | 11.0 | 0.9527 |
Enzymatic functionality
In order to confirm the biological activity of hydrogel-released biomolecules, enzyme kinetic analysis was performed on released alkaline phosphatase. Enzyme samples were collected after a 7-day release period, a time that would have permitted maximal BSA release (see Figure 3). The rate of ELF-97 metabolism (υ) was determined at different substrate concentrations (Figure 4b) and Michaelis-Menton analysis performed (TABLE 2). This analysis demonstrated enzyme-substrate affinity is retained in protein released from both hydrogels (KM=0.12, 0.10, 0.11 for control, acrylamide and PEGDA samples, respectively); however, total enzymatic activity was reduced in the different hydrogel samples (VMAX = 1.24 × 10-3, 0.10 × 10-3, 0.27 × 10-3, for control, acrylamide and PEGDA samples, respectively). These reductions are similar to those observed for BSA release confirming that more protein is released from acrylamide than PEGDA hydrogels.
Figure 4.
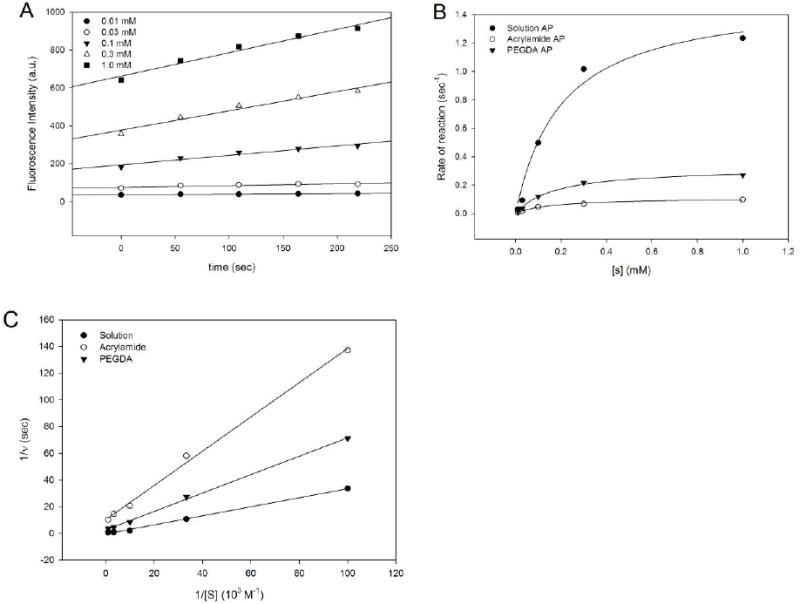
Kinetic analyses of released alkaline phosphatase enzyme activity. (A) Representative graph illustrating ELF-97 product produced using different substance concentrations. These data were obtained using freshly prepared alkaline phosphatase solution. (B) Plots the calculated enzymatic rates from solution, polyacrylamide-released, and PEGDA-released alkaline phosphatase as a function of substrate (ELF-97) concentration. (C) Michaelis-Menton plots of rates (1/v) for the date presented in B. Lines were drawn for linear regression analysis (A and C) and hyperbola nonlinear regression for (B)
Table 2.
Characterization of released alkaline phosphatase. Kinetics of substrate metabolism.
| control | polyacrylamide | PEGDA | |
|---|---|---|---|
| Vmax (M s-1) | 1.24 × 10-3 | 0.10 × 10-3 | 0.27 × 10-3 |
| KM (mM) | 0.12 | 0.10 | 0.11 |
| kcat (s-1) | 347.2 | 28.0 | 75.6 |
| kcat/KM (M-1s-1) | 2.89 × 106 | 0.28 × 106 | 0.69 × 106 |
Effects of BDNF-treatment on neurons
Hydrogel systems were loaded to deliver three different BDNF concentrations (0, 50, 100 ng/mL). For control experiments, the same concentrations of BDNF were supplemented to the neurons cultured on MEAs without hydrogels. BDNF delivery increased both neuron process lengths and process branching (Figure 5). Automated process tracing was used to quantify concentration-dependent changes in total process length, number of processes, number of branching points, and number of primary processes (Figure 6). The results of ANOVA indicated that there was no significant difference between substrates (control, polyacrylamide, and PEGDA). Dose-dependent trends were observed in the number of processes/cell, total process length and the number of branch points/cell. Statistically significant increases were observed between 0 ng/mL and 100 ng/mL deliveries for controls and PEGDA-delivery conditions for number of processes/cell and total process length (Figure 6A and C). Significant increases were observed with polyacrylamide-delivery systems for total process length and number of branching points (Figure 6C and D). A clear trend was observed for PEGDA delivery, but this did not reach statistical significance (Figure 6D). BDNF did not change the number of primary processes per cell (Figure 6B). These data clearly demonstrate that biologically active BDNF can be released from hydrogels.
Figure 5.
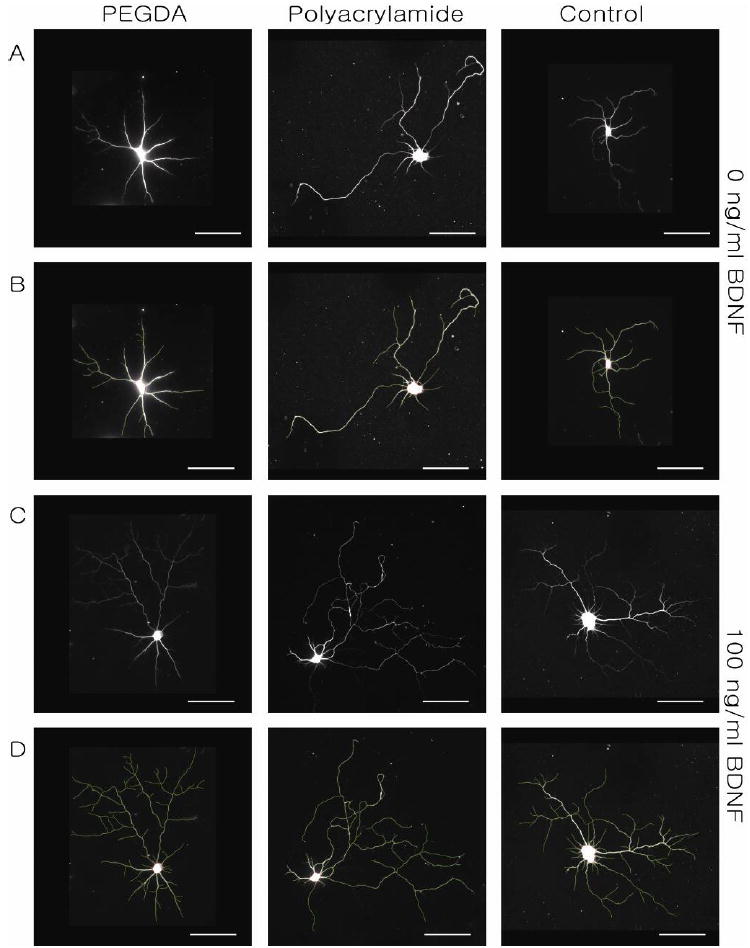
Dose-dependent responses of cultured hippocampal neurons to BDNF. (A) β-III tubulin stained neurons cultured on PEGDA, polyacrylamide, and glass coverslip in order. (B) Neurite tracing of images in Panel A. (C) β-III tubulin staining of BDNF-treated neurons cultured on PEGDA, polyacrylamide, and glass coverslip and (D) traced images of images in Panel C. Neurons were imaged at DIV 10. Scale bars are 50 μm.
Figure 6.
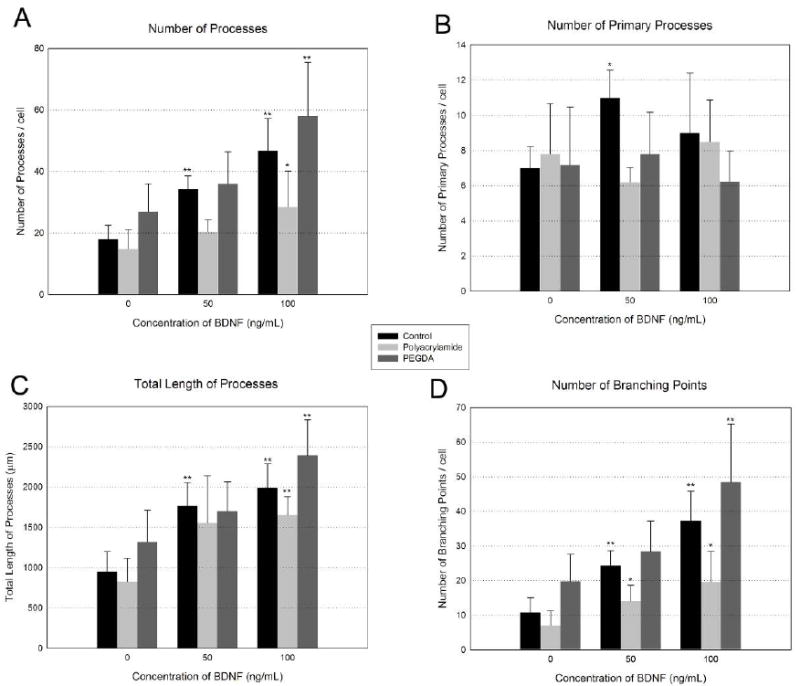
The effects of released-BDNF on cultured neurons. (A) Number of processes per neuron, (B) number of primary processes per neuron (C) Total length of processes, (D) number of branching points per neuron. Values are given as means ± standard deviation. There was no significant difference between substrates (control, polyacrylamide, and PEGDA). The asterisk indicates measures which are significantly different than the corresponding values from 0 ng/mL BDNF. (ANOVA followed by Tukey’s post hoc test, *: P<0.05, **:P<0.01, n=5 for each condition)
Electrochemical impedance spectroscopy
Prior to the study of the possible impact of released BDNF on neuronal electrical activity, impedance measurements were first made to determine how hydrogel coating would affect electrode performance. Measurements were made over a range of frequencies (Figure 7A). This data demonstrated that hydrogel coatings generally produced increased impedance values at all frequencies, though this effect was more pronounced for polyacrylamide than PEGDA. When measurements were compared at 1 kHz, where the spectrum of neural signal mostly exists, the average impedance magnitudes were 188 kΩ for polyacrylamide-coated MEAs and 149 kΩ for PEGDA-coated MEAs. When compared with electrode impedance measurements made without hydrogel coating MEA, these values represent 53 % and 21 %, increases, respectively.
Figure 7.
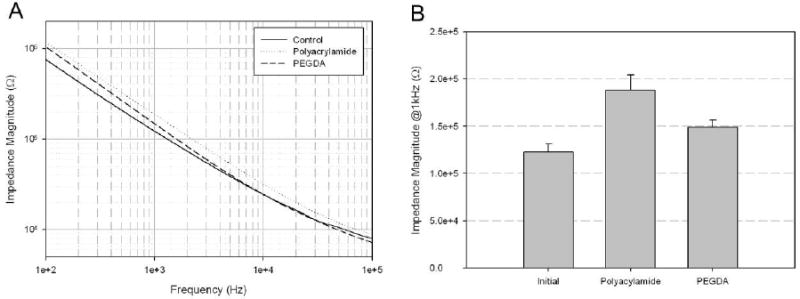
Impedance characteristics of hydrogel-coated microelectrode arrays. (A) Electrochemical impedance spectroscopy, (B) Comparison of impedance magnitude at 1 kHz after hydrogel coating (n=16)
Neuron culture and recording
Neurons were cultured on polyacrylamide and PEGDA hydrogels loaded to release 0, 50, and 100 ng/mL BDNF. At DIV 10, spontaneous neuronal activity was only observed in cultures with 100 ng/mL BDNF delivery (Figure 8). The frequencies of the spontaneous activity are measured from 100ng/mL BDNF-treated neurons cultured on different substrates (Figure 8D). The most robust responses (e.g. increased numbers and spike amplitudes) were observed on PEGDA hydrogels when compared to polyacrylamide hydrogels. In contrast, spontaneous activity was detected from neurons cultured on MEAs when BDNF was applied directly to the culture medium at both 50 and 100 ng/mL. Signals recorded from cultures on hydrogel-coated MEAs had lower amplitudes compared to signals recorded on uncoated MEAs (Figure 8). The lower amplitudes can be attributed to the increased impedances due to the hydrogel coatings (Figure 8). Activity was detected only from electrodes where neuronal cell bodies were located on or immediately nearby the electrode. (Figure 9; The underlying electrode can be visualized in the DAPI image (Figure 9B)). The electrode pattern is out of focus due to the thickness of hydrogel coating.
Figure 8.
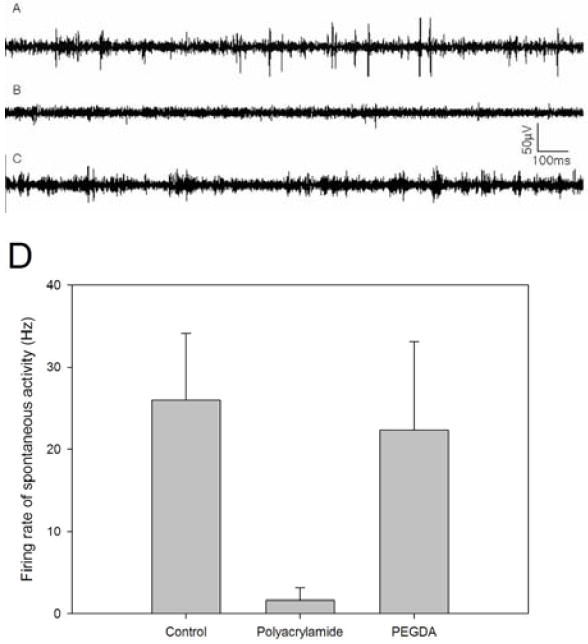
Representative activity recorded at DIV 10 from neural networks cultured (A) on a bare MEA with bath application of 100 ng/mL BDNF, (B) on polyacrylamide hydrogel with 100 ng/mL released-BDNF and (C) on PEGDA hydrogel with 100 ng/mL released-BDNF. (D) Firing frequency plots from 100ng/mL BDNF treated neurons cultured on different substrates (n=3 for each condition).
Figure 9.
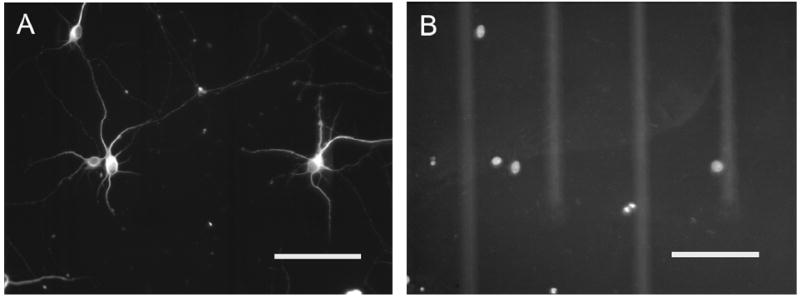
Immunocytochemistry of cultured neurons on hydrogel-coated (PEGDA) microelectrode arrays at DIV 10. (A) β-III tubulin staining for neuronal cell bodies and processes, (B) DAPI staining for nuclei. Brightness has been adjusted to provide clear representation of electrodes and conductor leads. Scale bars are 100 μm.
Discussion
These results demonstrate that biologically active neurotrophins (BDNF) can be loaded into hydrogel coatings on MEAs and released with sufficient delivery control to affect neuron process growth and spontaneous electrical activity. Conditions for neurotrophin release were developed by measuring release of BSA and alkaline phosphatase. Release of these proteins provided kinetic measures of protein release from hydrogel coatings and demonstrated that incorporation of BDNF in hydrogels during polymerization did not destroy enzyme activity. While the buffer composition of the Polyacrylamide and PEGDA-based hydrogels were different, no significant difference was observed in the biological activity of released biomolecules. Initial tests were conducted to determine whether the ionic composition resulted in a significant effect on the biological activity of released biomolecules. As seen in Figure 4C, no significant differences were seen for alkaline phosphatase activity between either hydrogel, when compared to solution-based alkaline phosphatase. Based on this data, we did not expect significant differences in the resulting bioactivity for BDNF following incorporation into each of the hydrogels (polyacrylamide versus PEGDA).
Protein release was faster and more complete from PEGDA than polyacrylamide hydrogels. PEGDA hydrogels also produced smaller increases in electrode-media impedance measurements. These results suggest that PEGDA hydrogels may have a more open structure, thus allowing for more rapid and complete protein release and producing a smaller change in measure electrode impedance. The slower release kinetics and less efficient release from polyacrylamide hydrogrels may result from the extent of crosslinking in the polymer resulting in more trapped protein. The consistent impedance increases measured from polyacrylamide-coated MEAs are also consistent with tighter crosslinking and therefore more protein entrapment. Alternatively, protein release from polyacrylamide may be decreased because protein becomes bound into the acrylamide polymer during cross linking.
One of the main advantages of incorporating neurotrophins in a hydrogel solution prior to polymerization is the ability to load precise amounts of BDNF into gels. This method requires only micrograms of neurotrophin and thus provides a great advantage over diffusion-based, bulk loading systems that can require milligram quantities of protein. The short UV exposure times required for PEGDA hydrogel polymerization had no deleterious effects on protein function as demonstrated by the alkaline phosphatase results, e.g. no polymerization-related change in enzyme- substrate affinity.
While it was clear that the hydrogel layer produced an increase in electrode impedance measures (Figure 7A), it was still possible to detect action potentials using hydrogel-coated microelectrode arrays (Figure 8). (The thickness of these hydrogels is expected to be ~44 μm [17].) Electrochemical impedance spectroscopy demonstrated that impedance changes were less than two-fold. Due to the porous structure of the hydrogel, we suggest that there are sufficient pathways for electrical current to flow. This result is consistent with the previous reports that demonstrated neural recordings through polymer coated microelectrodes increased impedance by a factor of 2 – 3 [38]. As frequency increased, the impedance magnitude of hydrogel-coated MEAs approached that of bare MEAs, which indicates that the capacitance of the electrode did not significantly increase. The frequency-dependent change in impedance was more pronounced for PEGDA than polyacrylamide.
Despite the small hydrogel-associated impedance changes, the amplitude of neural signal was attenuated approximately by half with PEGDA hydrogel coatings (Figure 8). This is most likely due to the distance between neurons and electrodes. At the increased distance between neurons growing on hydrogels and electrodes, a portion of the ionic currents from neurons will not flow directly towards an underlying electrode. Obviously, in this study, the thickness of the hydrogel determined the minimum distance between neurons and electrodes; however, there is a possibility that actually distance between electrodes and neurons can be smaller than the thickness of hydrogels because of the bumped structure of Pt-plated electrodes. Also, the distance can be minimized by reducing the initial amount of hydrogel to be polymerized or by directing neuron attachment to electrode sites using soft lithographic methods. Alternatively, selective polymerization can be used to make voids in the hydrogel layer immediately above electrodes and thus allow neurons make direct contacts with the electrodes.
Beyond the use for neurotrophin release there are several additional applications for the hydrogel coating on MEAs. For instance, other CNS cells can be encapsulated within hydrogel matrices. For example, neurons can be co-cultured with astrocytes confined to, entrapped in, the hydrogels for supplying nutrients and growth factors for neural survival, growth, and long-term maintenance. Hydrogels can also be used to provide a necessary environment for patterning proteins to control neuron attachment and growth [17, 18]. Hydrogels can also be synthesized to respond to pH, electrical fields, temperature, light, and organic compounds. Incorporating these properties may make it possible to change hydrogel function or structure during an experiment.
Acknowledgments
This work was supported by the International Collaboration Program, NBS-ERC (Nano Bioelectronics and Systems Engineering Research Center)/KOSEF (Korea Science and Engineering Foundation), by the Nanobiotechnology Center (NBTC), an STC Program of the National Science Foundation under Agreement No. ECS-9876771, the National Institutes of Health under Agreement No. R01-NS044287 (W.S.) and by the National Institute of Biomedical Imaging And Bioengineering under Agreement No. R21EB007782 (M.R.H.). The authors acknowledge use of the Wadsworth Center Advanced Light Microscopy & Image Analysis Core Facility. We would also like to thank Shirley Madewell and Adriana Verschoor for critical review of the manuscript.
References
- 1.Schwartz AB, Cui XT, Weber DJ, Moran DW. Brain-Controlled Interfaces: Movement Restoration with Neural Prosthetics. Neuron. 2006;52:205–220. doi: 10.1016/j.neuron.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 2.Buzsaki G. Large-scale recording of neuronal ensembles. Nat Neurosci. 2004;7:446–451. doi: 10.1038/nn1233. [DOI] [PubMed] [Google Scholar]
- 3.Shain W, Spataro L, Dilgen J, Haverstick K, Retterer S, Isaacson M, Saltzman M, Turner JN. Controlling cellular reactive responses around neural prosthetic devices using peripheral and local intervention strategies. Neural Systems and Rehabilitation Engineering, IEEE Transactions on [see also IEEE Trans on Rehabilitation Engineering] 2003;11:186–188. doi: 10.1109/TNSRE.2003.814800. [DOI] [PubMed] [Google Scholar]
- 4.Retterer ST, Smith KL, Bjornsson CS, Neeves KB, Spence AJH, Turner JN, Shain W, Isaacson MS. Model neural prostheses with integrated microfluidics: a potential intervention strategy for controlling reactive cell and tissue responses. Biomedical Engineering, IEEE Transactions on. 2004;51:2063–2073. doi: 10.1109/TBME.2004.834288. [DOI] [PubMed] [Google Scholar]
- 5.Otto KJ, Johnson MD, Kipke DR. Voltage pulses change neural interface properties and improve unit recordings with chronically implanted microelectrodes. Biomedical Engineering, IEEE Transactions on. 2006;53:333–340. doi: 10.1109/TBME.2005.862530. [DOI] [PubMed] [Google Scholar]
- 6.Johnson MD, Otto KJ, Kipke DR. Repeated voltage biasing improves unit recordings by reducing resistive tissue impedances. Neural Systems and Rehabilitation Engineering, IEEE Transactions on [see also IEEE Trans on Rehabilitation Engineering] 2005;13:160–165. doi: 10.1109/TNSRE.2005.847373. [DOI] [PubMed] [Google Scholar]
- 7.Gunasekaran S, Ko S, Xiao L. Use of whey proteins for encapsulation and controlled delivery applications. Journal of Food Engineering. 2007;83:31–40. [Google Scholar]
- 8.Zubtsov DA, Savvateeva EN, Rubina AY, Pan’kov SV, Konovalova EV, Moiseeva OV, Chechetkin VR, Zasedatelev AS. Comparison of surface and hydrogel-based protein microchips. Analytical Biochemistry. 2007;368:205–213. doi: 10.1016/j.ab.2007.04.040. [DOI] [PubMed] [Google Scholar]
- 9.Kiyozumi T, Kanatani Y, Ishihara M, Saitoh D, Shimizu J, Yura H, Suzuki S, Okada Y, Kikuchi M. The effect of chitosan hydrogel containing DMEM/F12 medium on full-thickness skin defects after deep dermal burn. Burns. 2007;33:642–648. doi: 10.1016/j.burns.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Cacou C, Tinkler J. In response to ‘A late complication following the insertion of hydrogel breast implants’. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2007;60:967. doi: 10.1016/j.bjps.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 11.Zhang C, Xing D, Li Y. Micropumps, microvalves, and micromixers within PCR microfluidic chips: Advances and trends. Biotechnology Advances. 2007;25:483–514. doi: 10.1016/j.biotechadv.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Park HY, Choi CR, Kim JH, Kim WS. Effect of pH on drug release from polysaccharide tablets. Drug Delivery. 1998;5:13–18. doi: 10.3109/10717549809052022. [DOI] [PubMed] [Google Scholar]
- 13.Deyao K, Tao P, Goosen MFA, Min JM, He YY. pH-sensitivity of hydrogels based on complex-forming chitosan-polyether interpenetrating polymer network. Journal of Applied Polymer Science. 1993;48:343–354. [Google Scholar]
- 14.Strachotova B, Strachota A, Uchman M, Slouf M, Brus J, Plestil J, Matejka L. Super porous organic-inorganic poly(N-isopropylacrylamide)-based hydrogel with a very fast temperature response. Polymer. 2007;48:1471–1482. [Google Scholar]
- 15.Xu X-D, Zhang X-Z, Wang B, Cheng S-X, Zhuo R-X, Wang Z-C. Fabrication of a novel temperature sensitive poly(N-isopropyl-3-butenamide) hydrogel. Colloids and Surfaces B: Biointerfaces. 2007;59:158–163. doi: 10.1016/j.colsurfb.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Dillon G, Yu X, Sridharan A, Ranieri J, Bellamkonda R. The influence of physical structure and charge on neurite extention in a 3D hydrogel scaffold. J Biomater Sci Polym Ed. 1998;9:1049–1069. doi: 10.1163/156856298x00325. [DOI] [PubMed] [Google Scholar]
- 17.Hynd M, Frampton J, Burnham M, Martin D, Dowell-Mesfin N, Turner J, Shain W. Functionalized hydrogel surfaces for the patterning of multiple biomolecules. Biomaterials. 2007;81:347–54. doi: 10.1002/jbm.a.31002. [DOI] [PubMed] [Google Scholar]
- 18.Hynd M, Frampton J, Dowell-Mesfin N, Turner J, Shain W. Directed cell growth on protein-functionalized hydrogel surfaces. J Neurosci Methods. 2007;162:255–63. doi: 10.1016/j.jneumeth.2007.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan DR, Miller FD. Developing with BDNF: A Moving Experience. Neuron. 2007;55:1–2. doi: 10.1016/j.neuron.2007.06.025. [DOI] [PubMed] [Google Scholar]
- 20.Chao MV. NEUROTROPHINS AND THEIR RECEPTORS: A CONVERGENCE POINT FOR MANY SIGNALLING PATHWAYS. Nature Reviews Neuroscience. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 21.Shooter EM. Early days of the nerve growth factor proteins. Annual Review of Neuroscience. 2001;24:601–629. doi: 10.1146/annurev.neuro.24.1.601. [DOI] [PubMed] [Google Scholar]
- 22.Kalb R. The protean actions of neurotrophins and their receptors on the life and death of neurons. Trends in Neurosciences. 2005;28:5–11. doi: 10.1016/j.tins.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Post RM. Role of BDNF in bipolar and unipolar disorder: Clinical and theoretical implications. Journal of Psychiatric Research. 2007;41:979–990. doi: 10.1016/j.jpsychires.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Lee B-H, Kim H, Park S-H, Kim Y-K. Decreased plasma BDNF level in depressive patients. Journal of Affective Disorders. 2007;101:239–244. doi: 10.1016/j.jad.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Koennings S, Sapin A, Blunk T, Menei P, Goepferich A. Towards controlled release of BDNF-Manufacturing strategies for protein-loaded lipid implants and biocompatibility evaluation in the brain. Journal of Controlled Release. 2007;119:163–172. doi: 10.1016/j.jconrel.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Tsai S-J. TrkB partial agonists: Potential treatment strategy for major depression. Medical Hypotheses. 2007;68:674–676. doi: 10.1016/j.mehy.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 27.Kozisek ME, Middlemas D, Bylund DB. Brain-derived neurotrophic factor and its receptor tropomyosin-related kinase B in the mechanism of action of antidepressant therapies. Pharmacology & Therapeutics. 2008;117:30–51. doi: 10.1016/j.pharmthera.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Jun SB, Hynd MR, Dowell-Mesfin N, Smith KL, Turner JN, Shain W, Kim SJ. Low-density neuronal networks cultured using patterned poly-L-lysine on microelectrode arrays. Journal of Neuroscience Methods. 2007;160:317–326. doi: 10.1016/j.jneumeth.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoon TH, Hwang EJ, Shin DY, Park SI, Oh SJ, Jung SC, Shin HC, Kim SJ. A micromachined silicon depth probe for multichannel neural recording. IEEE Transactions On BioMedical Engineering. 2000;47:1082–1087. doi: 10.1109/10.855936. [DOI] [PubMed] [Google Scholar]
- 30.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 31.Reis CP, Ribeiro AJ, Houng S, Veiga F, Neufeld RJ. Nanoparticulate delivery system for insulin: Design, characterization and in vitro/in vivo bioactivity. European Journal of Pharmaceutical Sciences. 2007;30:392–397. doi: 10.1016/j.ejps.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Basu S, Campagnola PJ. Enzymatic activity of alkaline phosphatase inside protein and polymer structure fabricated via multiphoton excitation. Biomacromolecules. 2004;5:572–579. doi: 10.1021/bm0344194. [DOI] [PubMed] [Google Scholar]
- 33.Dowell-Mesfin NM, Abdul-Karim MA, Turner AMP, Schanz S, Craighead HG, Roysam B, Turner JN, Shain W. Topographically modified surfaces affect orientation and growth of hippocampal neurons. Journal of Neural Engineering. 2004;1:78–90. doi: 10.1088/1741-2560/1/2/003. [DOI] [PubMed] [Google Scholar]
- 34.Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. Journal of Neuroscience Research. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 35.Al-Kofahi KA, Can A, Lasek S, Szarowski DH, Dowell-Mesfin N, Shain W, Turner JN, Roysam B. Median-based robust algorithms for tracing neurons from noisy confocal microscope images. Information Technology in Biomedicine, IEEE Transactions on. 2003;7:302–317. doi: 10.1109/titb.2003.816564. [DOI] [PubMed] [Google Scholar]
- 36.Al-Kofahi Y, Dowell-Mesfin N, Pace C, Shain W, Turner JN, Roysam B. Improved detection of branching points in algorithms for automated neuron tracing from 3D confocal images. Cytometry A. 2008;73:36–43. doi: 10.1002/cyto.a.20499. [DOI] [PubMed] [Google Scholar]
- 37.Meijering E, Jacob M, Sarria J-CF, Steiner P, Hirling H, Unser M. Design and Validation of a tool for neurite tracing and analysis in fluorescence microscoppy images. Cytometry. 2004;58A:167–176. doi: 10.1002/cyto.a.20022. [DOI] [PubMed] [Google Scholar]
- 38.Nam Y, Branch DW, Wheeler BC. Epoxy-silane linking of biomolecules is simple and effective for patterning neuronal cultures. Biosensors and Bioelectronics. 2006;22:589–597. doi: 10.1016/j.bios.2006.01.027. [DOI] [PubMed] [Google Scholar]


