Abstract
Neurovascular coupling studies are widely conducted in anesthetized animals using functional magnetic resonance imaging (fMRI). In this study, the dose-dependent effects of isoflurane on the neurovascular coupling were examined with concurrent recordings of the local field potential (FP) and cerebral blood flow (CBF) in the rat somatosensory cortex. Electrical forepaw stimulation was used and consisted of either a single pulse or ten pulses at various frequencies. We observed that the FP response to single-pulse stimulation remained unaffected across the different levels of isoflurane tested (1.1% to 2.1%), while the CBF response to single-pulse stimulation increased dose-dependently (7 ± 3% to 17 ± 4%). The isoflurane dose did not affect the vascular reactivity induced by a hypercapnic challenge. These findings suggest that the action site of isoflurane affects the neurovascular mechanisms. For ten-pulse stimulation, the sum-FP responses monotonically decreased with an increase in the isoflurane dose, possibly due to an enhancement in the FP adaptation. In contrast, the dose-dependent effect on the CBF response varied depending on the stimulus frequency; a dose-dependent decrease in the CBF response was observed for high-frequency stimulation, whereas a dose-dependent increase was observed for low-frequency stimulation. Further, a linear time-invariant model composed of the single-pulse hemodynamic impulse response convoluted with ten-pulse FP recordings showed that the neurovascular transfer function was altered by the isoflurane dose for high-frequency stimulation. These results indicate that a careful and consistent maintenance of the anesthetic depth is required when comparing fMRI data obtained from different animals or physiological and pharmacological manipulations.
Keywords: fMRI, hemodynamic response function, laser-Doppler flowmetry, local field potential, somatosensory cortex
Introduction
Functional magnetic resonance imaging (fMRI) measures brain activity via the changes in oxygen metabolism and hemodynamics that are associated with neural activity (i.e., neurometabolic and neurovascular couplings). However, the neural basis of the fMRI signal is still a subject of controversy (Mukamel et al., 2005; Raichle and Mintun, 2006; Viswanathan and Freeman, 2007; Nir et al., 2008). It has been shown that the fMRI signal correlates better with local field potential (FP) activity, than local spiking activity (Logothetis et al., 2001; Nir et al., 2007; Rauch et al., 2008). Direct recordings of neural activity and hemodynamic signals from anesthetized rodents have been conducted to reveal the relationships between these signals. In these studies, a linear relationship was observed between the integrated FP activity and hemodynamic signals induced by varying the strength or frequency of a sensory stimulus (Ngai et al., 1999; Matsuura et al., 2001; Sheth et al., 2003), while a nonlinear relationship has also been reported (Nielsen and Lauritzen, 2001; Devor et al., 2003; Sheth et al., 2004; Hewson-Stoate et al., 2005). However, these experiments were conducted under various types of anesthesia (e.g., α-chloralose, enflurane, and urethane), which makes it difficult to reconcile the discrepancies among these studies.
The depth of anesthesia can also modulate the neurovascular relationships via actions on the neural response, vascular reactivity, and/or neurovascular transfer mechanisms. Austin et al. (2005) showed that fMRI responses to somatosensory stimulation were unaffected by halothane anesthesia within a range of 0.7% to 1.5%, whereas Schulte and Hudetz (2006) showed that changes in cerebral blood flow (CBF) induced by visual stimulation were dose-dependently attenuated by halothane anesthesia over a range of 0.4% to 1.4%. Although these studies reported conflicting effects with anesthetic dose, the effects of anesthetic dose on neural activity were not directly compared. In our previous study, the neurovascular coupling properties were tested in the rat somatosensory cortex under 1.3% isoflurane, and a nonlinear relationship was found depending on the stimulus pulse width, current and frequency (Masamoto et al., 2007). As a result of that report, questions arose of whether the observed neurovascular properties at 1.3% isoflurane could be generalized over different levels of isoflurane anesthesia.
In the present study, we aimed to determine the dose-dependent effects of isoflurane anesthesia on the neurovascular coupling in the rat somatosensory cortex. By manipulating the end-tidal concentrations of isoflurane (between 1.1% and 2.1%), the isoflurane dose effect on the neural activity, vascular reactivity, and hemodynamic responses were examined. First, the dose-dependent effect on the vascular reactivity was assessed by measuring the change in CBF induced by a hypercapnic challenge, independent of neural activity. Second, using single-pulse stimulation, the neural and hemodynamic impulse responses (HIR) were determined, which provide an ‘intrinsic’ neurovascular transfer function. Third, neurovascular responses to ten-pulse stimuli were also measured with variable stimulus frequencies. Finally, to generalize the neurovascular transfer function, the apparent HIRs were calculated for each isoflurane dose condition and compared to the single-pulse HIR to examine the isoflurane dose effects on the neurovascular transfer mechanisms.
Materials and methods
Animal preparation
Animal use was in accordance with the standards for humane animal care and use as set by the Animal Welfare Act and the NIH Guide for the Care and Use of Laboratory Animals and the experimental protocol used was approved by the University of Pittsburgh Institutional Animal Care and Use Committee. A total of twelve male Sprague-Dawley rats (350 to 470 g; Charles River Laboratories, Wilmington, MA) were used for the single-pulse, impulse response study (N = 6), and the ten-pulse, frequency-dependent study (N = 6).
The animals were initially anesthetized with isoflurane (5% for induction, and 1.5 to 2% during surgery) in a mixture of oxygen (35 to 50%) and nitrous oxide (65 to 50%). Tracheal intubation was performed and a catheter was placed into the femoral artery. A 5 mm × 7 mm portion of the left skull, centered 3.5-mm lateral and 0.5-mm rostral from Bregma, was thinned. After completing the animal preparation, the inspired gas was converted to a mixture of air and pure oxygen (30 to 35% total O2) and the end-tidal concentration of isoflurane was adjusted to be 1.3 ± 0.1% (~1 MAC, Antognini et al., 1999). The administration of nitrous oxide, which was beneficial to stabilize the anesthetic depth for surgery, was replaced to air after surgery in all experiments to eliminate possible confounding effects. Preliminary data obtained showed that the evoked FP was greatly reduced when the animal was anesthetized with a mixture of isoflurane and nitrous oxide. End-tidal gas levels were monitored throughout the experiments with a multi-parameter airway gas monitor (ULT-i, CAPNOMAC ULTIMA™, Datex-Ohmeda, Inc., Madison, WI), while the minute ventilation and respiratory rate were adjusted as needed. The physiologic parameters, i.e., fraction of inspiratory oxygen, end-tidal carbon dioxide, isoflurane concentration, arterial blood pressure, and electrocardiogram, were all recorded using a polygraph data-acquisition software (ACK100W AcqKnowledge Software, BIOPAC systems, Inc., Goleta, CA). Rectal temperature was maintained at 37 °C with a DC temperature control module. Arterial blood gas was sampled every 0.5 to 1.5 hours and maintained within normal physiologic limits (PaO2 = 104 ± 5 mmHg, PaCO2 = 36.4 ± 1.2 mmHg, pH = 7.491 ± 0.012, Mean ± SD, N = 12 animals).
Isoflurane dose
The end-tidal isoflurane level was adjusted to be between 1.1 ± 0.1%, which minimally immobilizes the animals (i.e., low dosage), and 2.1 ± 0.1%, which is deep enough for surgery (i.e., high dosage). Two to five doses were tested over a range of 1.1% to 2.1% isoflurane in each animal. Whenever the isoflurane dose was adjusted, the spontaneous FP activity was monitored to determine when a steady-state was reached. We observed that the pattern of spontaneous FP activity changed shortly after the changes in isoflurane dose (< 5 min) and stabilized within 10 to 15 minutes. Therefore, the experiments were initiated 15 min after the adjustment of the isoflurane dose. This criterion is supported by previous reports that have shown that the isoflurane concentration in arterial blood and brain tissue equilibrates after approximately 15 min (Lin, 1994; Antognini et al., 1999). To minimize possible time-dependent effects, the experimental order of the various isoflurane doses was randomized and the experiments were completed within four hours from the beginning of the first recording in each animal. Preliminary experiments performed showed that the variation in the mean baseline LDF level over variable doses of isoflurane was 104 ± 13% at the beginning of the final session (set to 1.1% isoflurane) relative to that of the first session (set to 1.1% isoflurane) over a time period of 184 ± 13 minutes (N = 5). This shift in the baseline LDF level could be due to the slow clearance of isoflurane from fat tissue, but the effect was not consistent (e.g., LDF increased and decreased among subjects).
Hypercapnic challenge
The vascular reactivity was tested using a hypercapnic challenge performed in four of the six animals from the single-pulse experiment. A breathing mixture of 5% carbon dioxide and 95% air gas was administered while the end-tidal concentration of carbon dioxide and the CBF level were measured every three seconds from the onset of gas administration for up to five minutes. For this experiment, the baseline inspired gas mixture consisted of only air (without supplementary oxygen) to minimize potential influences from changes in the inspired oxygen fraction. To evaluate the vascular reactivity, the relative change in CBF was plotted against the end-tidal carbon dioxide level for each isoflurane dose condition tested. A linear least-squares fit was performed on all the data points to determine whether the vascular reactivity is affected by the isoflurane dose. The arterial blood gas level was also measured before and about five minutes after the onset of gas administration (PaCO2 = 37 ± 2 mmHg and 47 ± 3 mmHg before and after gas administration, respectively, Mean ± SD, N = 4 animals).
Forepaw stimulation
Two needle-electrodes were inserted under the skin of the right forepaw between digits two and four for delivery of the electrical stimulus (Silva et al., 1999). Electrical pulses were given using a pulse generator and isolator (Master 8 and ISO-Flex, A.M.P.I, Israel). For the single-pulse experiments, one-pulse stimulation (1.0-ms width and 1.5-mA current amplitude) was repeatedly applied every 30 seconds. For each condition, 50 to 100 runs were repeated to improve the signal-to-noise ratio. For the frequency-dependent study, ten-pulse stimuli (1.0-ms width and 1.0-mA current amplitude) were applied with different onset-to-onset intervals (50 to 500 ms) and a 40-sec interval between stimulation runs (i.e., train). Five onset-to-onset intervals (also referred to as inter-pulse interval) were tested: 50, 75, 100, 200, and 500 ms which correspond to frequencies of 20, 13, 10, 5 and 2 Hz, respectively. For each stimulus frequency condition, six to ten runs were performed and the order of stimulation frequency was randomized. A slightly higher stimulus current was used for the single-pulse experiment relative to ten-pulse study (1.5 mA vs. 1.0 mA) to improve the signal to noise ratio.
Concurrent recordings of FP and CBF
To localize the forepaw activation area prior to placing the FP electrode and LDF probe, optical imaging of intrinsic signals was performed using an experimental paradigm and a custom-made imaging system as previously reported (Masamoto et al., 2007). Cortical images (3.7 mm × 4.9 mm) were captured with a charge-coupled device camera (CS8310, Tokyo Electric Industry, Japan) and a 10-bit frame grabber board at a rate of 30 frames per second, while the cortical surface was illuminated with filtered light (620 ± 10 nm). Each six-second recording run was divided into one second of pre-stimulation baseline, three seconds of stimulation (1.0-ms width and 1.2 to 1.5 mA current, applied at 3 Hz), and two seconds of post-stimulation baseline, repeated 20 times. Temporal averaging (15 consecutive frames; 0.5 sec/image) and spatial binning (2 × 2 pixels) were performed during off-line analysis. The activation focus was then determined to be the largest decrease in light reflectance (i.e., an increase in light absorption) observed between 0.5 to 2.5 seconds after the onset of stimulation.
The FP and CBF were concurrently recorded at the activation focus with a tungsten microelectrode (<1 MΩ, FHC, Inc., Bowdoinham, ME) and laser-Doppler flowmetry (LDF; PeriFlux 4001 Master system, Perimed, Sweden), respectively, as described previously (Masamoto et al., 2007). The spread of the FP signals was reported to span between 250 µm to a few millimeters in the visual cortex (Kreiman et al., 2006; Liu and Newsome 2006; Katzner et al., 2009). The tip of the microelectrode was placed at a depth of 0.5 to 0.6 mm from the cortical surface, while a needle-type LDF probe (0.45-mm tip diameter and 0.15-mm fiber separation, Probe 411, Perimed, Sweden) was placed on the surface of the thinned skull preparation above the activation area. To minimize the invasiveness of the procedure around the recording area and potentially reduce motion artifacts, the thinned skull preparation was used. The microelectrode was inserted through a small slit made on the thinned skull just above the activation focus according to the optical imaging map, and then the dura was punctured by the electrode. The stereotaxic frame and the holders for the FP electrode and LDF probe were fixed to the table to minimize motion. The distance between the LDF probe and the insertion site of the electrode at the cortical surface was about 0.5 mm. The FP and CBF data were recorded using the BIOPAC data-acquisition software at a frequency of 1 kHz.
Data analysis
The FP data were averaged across all stimulation runs for each stimulation condition. The mean amplitude of the evoked FP was reported as the difference between the positive and negative peaks observed 5 to 20 ms after stimulation onset (Masamoto et al., 2007). For the ten-pulse experiments, the summation of the evoked FP (ΣFP) was also reported by summing the amplitude (minimum to maximum) of all ten evoked FPs. The CBF data were first down-sampled by temporal averaging of 100 consecutive sampling points (resulting in a 0.1 sec temporal resolution), and then averaged across all runs in each stimulation condition. The CBF response was normalized by the pre-stimulation baseline (i.e., mean over 5 sec before stimulation onset), and the peak amplitude was measured. The fluctuation of the baseline level was also reported by measuring the mean and standard deviation of the resting condition data over a period of 1 minute. To compare data across animals, ΣFP and peak CBF were normalized by their respective maximum in each animal. All of the measurements of the mean arterial blood pressure (MABP), LDF, evoked FP and CBF were compared with their corresponding isoflurane concentration using linear regression, and the correlation coefficient was calculated. The significance of the slope being different from zero was assessed, and the statistical significance of the values observed for low and high dose conditions were determined by paired Student's t-test between subjects. Data are represented as Mean ± SD, unless otherwise specified.
Linear time-invariant model
Hemodynamic responses induced by ten-pulse stimulation may be described as a linear combination of ten individual, time-invariant, single-pulse responses. To examine this issue for each stimulation frequency tested, the apparent HIR was determined by de-convolution of the ten-pulse CBF data with the evoked FPs (considering the exact time of each FP), assuming that
| [1] |
where ⊗ indicates the convolution function. The single-pulse and ten-pulse studies were conducted in different animal groups to achieve sufficient signal averaging for each experiment. To compare the single-pulse data obtained and increase the signal-to-noise ratio, the CBF responses and FPs (without normalization) were averaged across all animals under low (1.1%) and high (2.1%) isoflurane conditions. Considering the variation in the FP amplitude between the two studies, the measured CBF response was normalized by the individual FP amplitude, such that the apparent HIR was represented by the CBF response per mV of FP amplitude. The apparent HIR was then compared across stimulation frequencies under the low and high isoflurane dose conditions. The peak intensity and full-width at half-maximum (FWHM) of the HIR were calculated.
Results
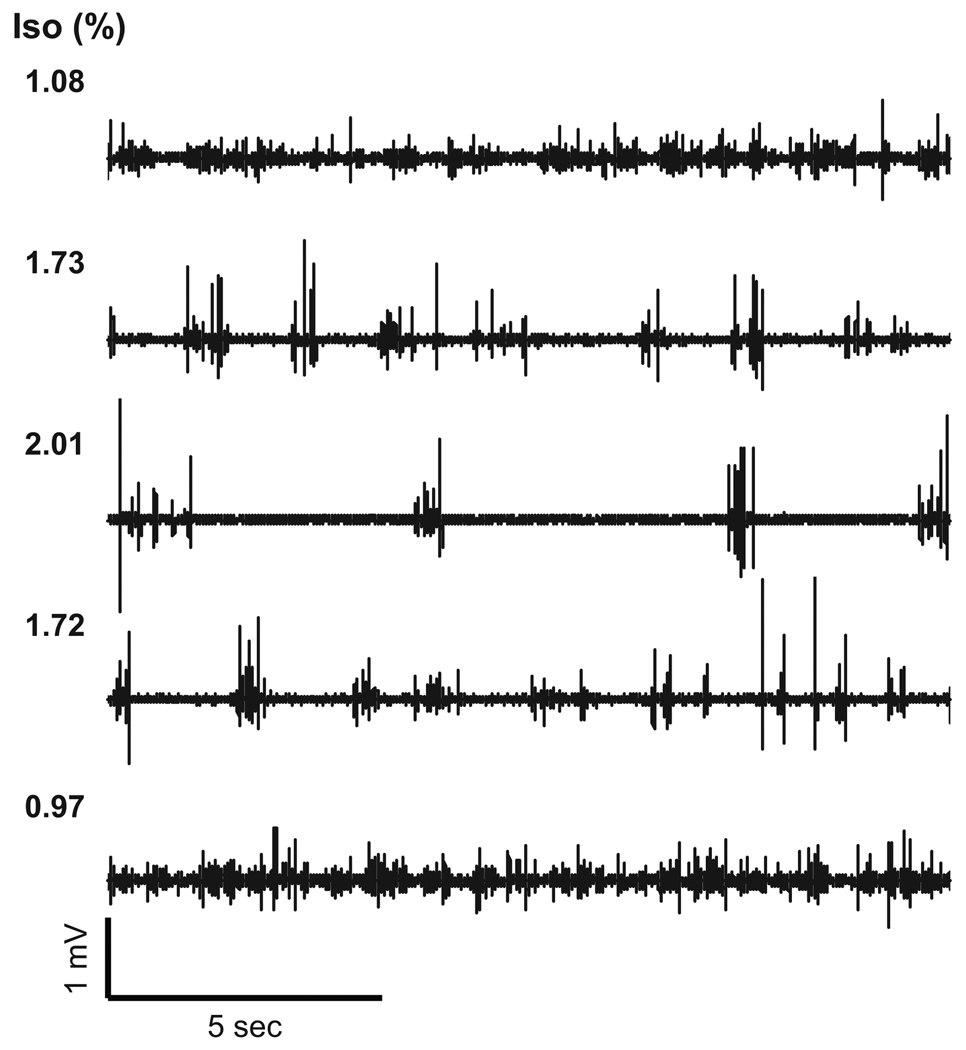
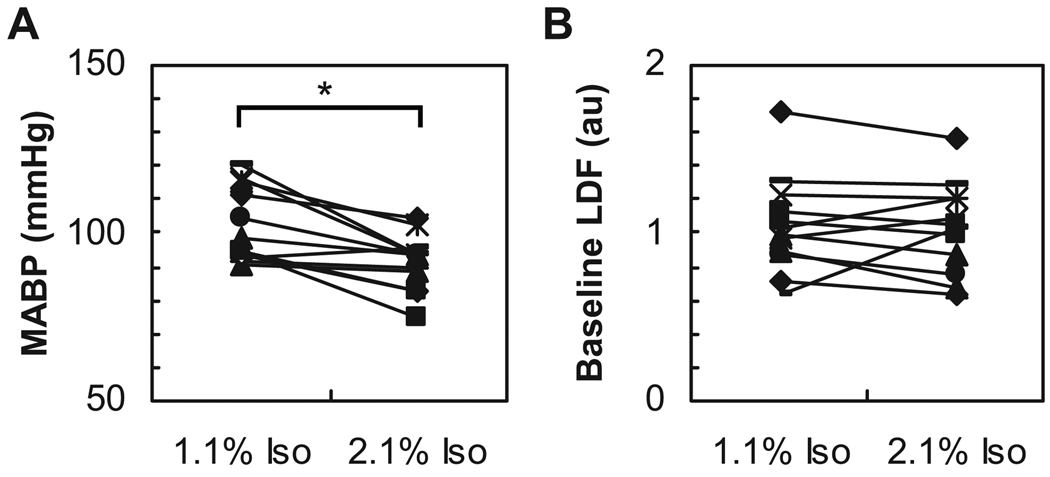
Figure 1 shows the dose-dependent effect of isoflurane on the spontaneous FP activity measured in the rat somatosensory cortex. Under high dose conditions (> 1.7% isoflurane), burst-suppression activity was evident, which is consistent with a previous report (Golanov et al., 1994). When the isoflurane dose was increased or decreased in a stepwise fashion, a similar pattern in the spontaneous FP activity was reproducibly observed. Increasing the dose of isoflurane caused the systemic blood pressure to decrease in a dose-dependent fashion. A statistically significant difference in the MABP was observed between the low and high dose conditions (t11 = 4.19, P = 0.0008, Fig. 2A). In contrast, the baseline CBF level measured by LDF showed no detectable changes between the low and high dose conditions (t11 = 0.38, P = 0.36, Fig. 2B). Also, the baseline fluctuation level in the LDF signal (= 1 SD) was ~ 9% of the mean irrespective of the isoflurane dose.
Figure 1. Baseline spontaneous field potentials induced by variable doses of isoflurane.
In this representative animal, the isoflurane concentration (Iso) was changed from 1.1% (top) to 2.0% (middle) and again adjusted to 1.0% (bottom). In each condition, the spontaneous FP activity varied depending on the isoflurane dose, and a consistent pattern was reproducibly observed in the respective doses. At high-dose conditions (≥ 1.7%), a burst-suppression pattern was evident. This pattern was consistently observed across all animals.
Figure 2. Mean arterial blood pressure and baseline blood flow level across variable doses of isoflurane.
A: Subject-to-subject comparison of MABP between low and high doses (N = 12). Note that the systemic blood pressure significantly decreased under high-dose conditions (2.1% Iso). *P < 0.05. B: Subject-to-subject comparison of the mean baseline LDF (N = 12). The baseline LDF level is presented as the raw LDF output value (a.u.). No significant differences between low and high doses were observed.
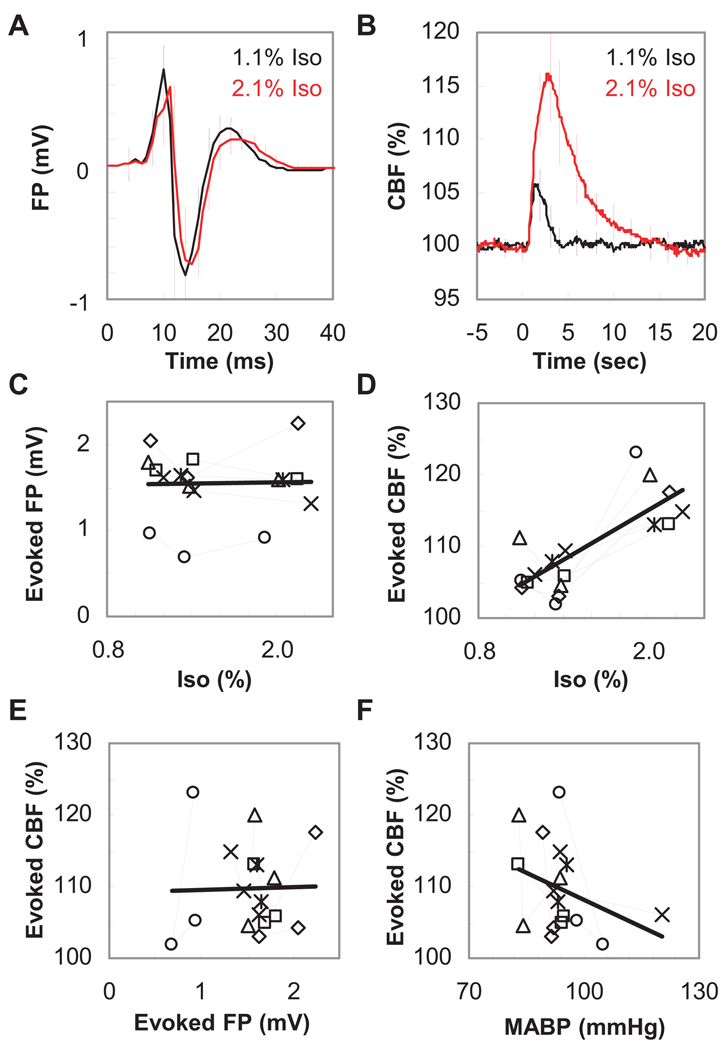
In the single-pulse experiment, similar evoked FP response amplitudes were observed irrespective of the dose of isoflurane (Fig. 3A). No significant differences in the amplitude of the positive peak (0.88 ± 0.23 mV vs. 0.83 ± 0.25 mV, t5 = 1.45, P = 0.10) and the negative peak (−0.74 ± 0.15 mV vs. −0.71 ± 0.22 mV, t5 = 0.47, P = 0.33) were found between the low and high dose conditions, respectively. In contrast, a higher CBF response was observed under the high dose condition (Fig. 3B). A significant difference in the peak amplitude of the evoked CBF (7 ± 3% vs. 17 ± 4%, t5 = 5.61, P = 0.0012) and in FWHM (1.7 ± 0.3 sec vs. 4.1 ± 0.8 sec, t5 = 6.52, P = 0.0006) was observed between low and high dose conditions, respectively. Population data (N = 6) consistently showed similar evoked FP amplitudes irrespective of the isoflurane level (r15 = 0.024, P = 0.93, Fig. 3C), while a significant correlation was observed between the peak amplitude of the evoked CBF and the isoflurane level (r15 = 0.78, P = 0.0002, Fig. 3D). Consequently, no correlation was observed between the evoked FP and CBF responses measured across different isoflurane doses (r15 = 0.030, P = 0.91, Fig. 3E). Also, it was confirmed that this variation in the evoked CBF was not solely due to the changes in MABP (r15 = −0.35, P = 0.16, Fig. 3F).
Figure 3. Evoked field potential and blood flow response induced by single-pulse stimulation.
A: Averaged evoked FPs obtained from all six animals tested. The comparisons between low (black) and high (red) isoflurane conditions showed that the amplitude of the evoked FP was relatively unaffected by the dose of isoflurane (Iso). One-pulse stimulation was given at time 0. Error bar: S.D. B: Averaged CBF time-course data obtained from the same six animals shown in panel A. The intensity of the CBF response was observed to increase at high isoflurane conditions (red) compared to low isoflurane conditions (black). One-pulse stimulation was given at time 0. Error bar: S.D. C: Population data of evoked FP (N = 6). Each symbol represents individual animal data. The amplitude of evoked FP was constant irrespective of the level of isoflurane. No significant correlation between FP amplitude and isoflurane level was observed (r15 = 0.024, P = 0.93). D: Population data of evoked CBF (N = 6). Identical symbols in panels C and D indicate data from the same animal. Note that the CBF response consistently increased in a dose-dependent manner (r15 = 0.78, P = 0.0002). E: Scatter plot of evoked FP and evoked CBF. No clear correlation was observed between evoked FP and CBF across all data obtained from six animals measured at different isoflurane levels (r15 = 0.030, P = 0.91). F: Population data of evoked CBF versus mean arterial blood pressure (N = 6). There are no significant correlations between mean arterial blood pressure (MABP) and evoked CBF (r15 = −0.35, P = 0.16).
The dose-dependent increase in the CBF response could be due to a change in the vascular reactivity. To test this possibility, the CBF response to a hypercapnic challenge was measured. The results showed that there were no detectable differences in the CBF response; 18 ± 6, 17 ± 4, and 18 ± 7 (LDF perfusion unit per end-tidal level of carbon dioxide), among the tested levels of isoflurane (1.1%, 1.4%, and 2.1%, respectively; N = 4 animals).
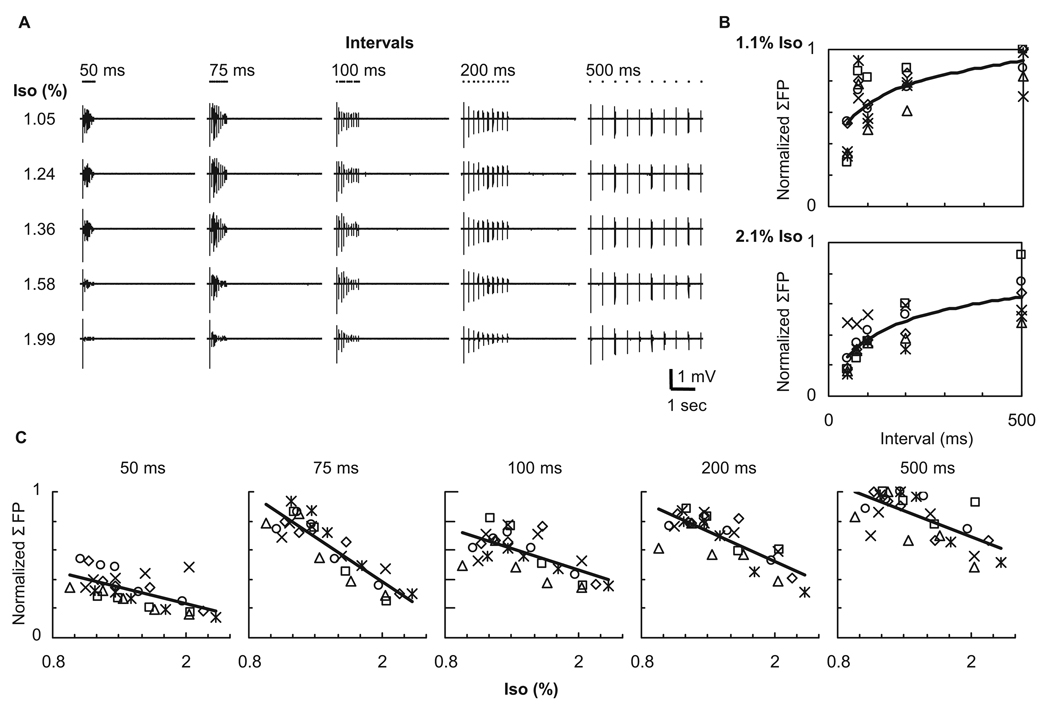
In the ten-pulse experiment, the adaptation of the FP response was increased in a dose-dependent fashion, which was consistently observed across all stimulation conditions (Fig. 4A). At a given isoflurane level, a lower frequency stimulus (i.e., a longer inter-pulse interval) induced less adaptation in the ten-pulse evoked FPs, and thus the larger ΣFP, compared to higher frequency stimulation (Fig. 4B). Population data (N = 6) showed a good linear correlation between the ΣFP and the isoflurane level irrespective of the stimulus interval (or frequency) (r27 = −0.61, −0.89, −0.63, −0.78, −0.66 and P = 0.0004, 0.0001, 0.0002, 0.0001, 0.0001 for 50, 75, 100, 200, and 500 ms intervals, respectively).
Figure 4. Evoked field potentials induced by ten-pulse stimuli.
A: Evoked FPs obtained from one representative animal. The adaptation of the FP response was dose-dependently enhanced over all frequencies (inter-pulse intervals) tested. The dot shown above each FP trace indicates the onset time for each pulse; for each stimulus, ten dots indicate the time the ten pulses were delivered. B: Population data (N = 6) of the ΣFP against inter-pulse interval under the same isoflurane level (1.1%, top and 2.1%, bottom). Each symbol represents data from individual animals normalized by the highest ΣFP in each animal. As expected, the lower frequency stimuli (i.e., longer inter-pulse intervals) induced higher ΣFPs under low and high dose conditions. The curve fit was performed in logarithmic scale. C: Population data (N = 6) of the ΣFP plotted against isoflurane level at the same stimulation frequency. With increasing dose of isoflurane, the evoked FPs were suppressed over all frequency conditions, and thus significant negative correlations between ΣFP and isoflurane level were observed.
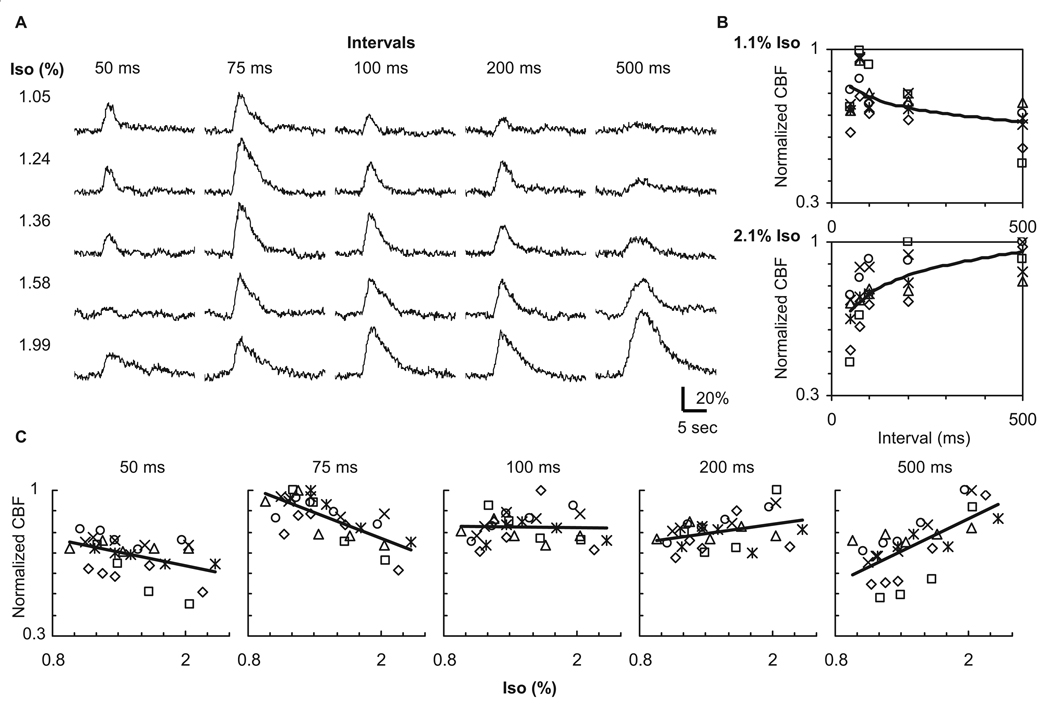
If a time-invariant linear relationship (Eq. [1]) is valid for the relationship between the evoked FP and CBF response, it is expected that, similar to the ΣFP activity, the CBF response to ten-pulse stimuli will monotonically decrease with an increase in the dose of isoflurane. This case was found only for high-frequency stimulation (e.g., 50 and 75 ms intervals) (Fig. 5A). In contrast, a dose-dependent increase in the CBF response was observed for low-frequency stimulation (e.g., 200 and 500 ms intervals) (Fig. 5A). Hence, the frequency-dependent pattern of the CBF response differed between the low and high dose conditions (Fig. 5B). Population data (N = 6) clearly showed the dose-dependent effects of isoflurane on the CBF response as a function of the stimulus frequency (Fig. 5C). A negative and positive correlation was observed depending on the stimulus frequency: r27 = −0.44, −0.72, −0.03, 0.38, 0.67, and P = 0.017, 0.0001, 0.90, 0.043, 0.0001 for 50, 75, 100, 200, and 500 ms intervals, respectively.
Figure 5. Evoked blood flow response induced by ten-pulse stimuli.
A: CBF data obtained from a representative animal. The intensity of the CBF response decreased for high-frequency stimuli (50 and 75 ms intervals) with increases in the isoflurane level (Iso), whereas the CBF response dose-dependently increased for low-frequency stimuli (200 and 500 ms intervals). Consequently, the effect of the isoflurane dose on the CBF response was opposite between low and high frequency stimuli. B: Population data (N = 6) of the CBF peak plotted against inter-pulse interval under low and high isoflurane conditions. Each symbol represents the data from individual animals normalized by their corresponding peak. The frequency-dependent CBF response also varied as a function of the isoflurane level; higher frequency stimuli (shorter intervals) induced larger CBF changes at low-dose condition (top), whereas lower frequency stimuli (longer intervals) induced larger CBF changes at high-dose condition (bottom). The curve fit was performed in a logarithmic scale. C: Population data (N = 6) of the CBF peak plotted against isoflurane level at the same stimulation frequency. The correlation between CBF peak and isoflurane level varied from negative to positive as a function of the inter-pulse interval.
Finally, a comparison between the apparent HIR and the measured single-pulse HIR revealed that the neurovascular transfer function was altered by the isoflurane dose as a function of the stimulation frequency (Figs. 6A and 6B). Under high-dose conditions, the peak intensity of the apparent HIR monotonically decreased with increases in the stimulation frequency, and reached half the intensity of the single-pulse HIR for the 50-ms stimulation interval condition (10% for single-pulse HIR vs. 5% to 9% for apparent HIR, Fig. 6C). However, the width of the apparent HIR at this stimulation interval (50 ms) was found to be the same as the single-pulse HIR (4.6 sec at FWHM). Under low-dose conditions, a slight decrease in the peak intensity of the apparent HIR was observed with decreasing in stimulation frequencies (5% to 3%, Fig. 6C). A comparison between the apparent HIR and the single-pulse HIR showed a reasonably good agreement in the peak intensity (4% for single-pulse HIR) and width (1.9 sec for single-pulse HIR), except for the 75-ms interval data (4.0 sec). The observed residue between model and data indicates that nonlinear components may act on the neurovascular transfer mechanism at this specific frequency.
Figure 6. Comparison of the apparent hemodynamic impulse responses determined using a linear time-invariant model.
A: The apparent HIR calculated from FP and CBF data obtained from ten-pulse experiments. B: The single-pulse HIR. The peak intensity was corrected by the measured FP amplitude. As compared to measured single-pulse HIR (B), the apparent HIR (A) was changed, especially for high-frequency stimuli (< 200-ms intervals) for both low (black) and high (red) isoflurane conditions. C: Peak intensity and full-width at half-maximum (FWHM) of the respective HIRs. For low frequency stimulation (≥ 200-ms intervals), a similar peak intensity was observed between the apparent HIR (closed circle) and the single-pulse HIR (open circle), indicating a good prediction by a linear time-invariant system (Eq. 1). Also, FWHM was observed to be in good agreement with that of single-pulse HIR, except for the 75-ms interval stimulation under low-dose condition.
Discussion
The present study shows that the neural impulse response as measured by local FP was independent of the dose of isoflurane administered (1.1% to 2.1%), while the HIR, measured by LDF, varied as a function of the isoflurane dose administered (Fig. 3). Since relatively constant neural responses and vascular reactivities tested with a hypercapnic challenge were observed, these findings indicate that the action site of isoflurane directly participates in the neurovascular transfer mechanism. For the ten-pulse stimuli, the evoked ΣFP decreased dose-dependently over all the frequency conditions tested due to dose-dependent adaptation (Fig. 4). However, the CBF response showed either a dose-dependent decrease or increase depending on the stimulus frequency (Fig. 5). Specifically, the dose-dependent effect was opposite between low and high frequency stimuli, which may indicate divergent effects of the anesthetic dose on the activity-induced hemodynamic responses (Hyder et al. 2002; Austin et al. 2005; Schulte and Hudetz, 2006). Further, the dose-dependent effect in the evoked CBF was found to be different from that in the evoked FP responses (Fig. 4 and Fig 5). These findings show that careful monitoring of the anesthetic depth and consistent use of stimulus paradigm are necessary for the accurate interpretation and reliable comparisons of hemodynamic-based functional studies (e.g., fMRI), both within and between anesthetized subjects.
Impact of isoflurane dose on the neurovascular transfer mechanisms
Isoflurane is widely used for physiological studies because it provides a stable condition, it is easy to control the targeted depth of anesthesia, and it allows repeated use for survival experiments in the same animal (Lukasik and Gillies, 2003; Masamoto et al., 2007). In addition, the action site of isoflurane is relatively well-studied compared to other, more conventional injectable anesthetics (for a review see Campagna et al., 2003). It has been shown that isoflurane acts at intracortical sites and directly depresses cortical activity (Hentschke et al., 2005). Previous studies have consistently shown that isoflurane dose-dependently depresses excitatory transmission via inhibition of glutamate release (Haseneder et al., 2004; Sandstrom, 2004; Wu et al., 2004). At the same time, isoflurane is thought to affect ligand-gated ion channels, including the inhibition of N-methyl-D-aspartate (NMDA) receptors and potentiation of gamma-aminobutyric acid (GABA) type A receptors (Ranft et al., 2004; Hentschke et al., 2005; Dickinson et al., 2007; Kelly et al., 2007). Further, a dose-dependent enhancement of glutamate uptake by astrocytes has been reported (Miyazaki et al., 1997). Although no detectable changes in the single-pulse FP were observed in the present study (Fig. 3), these known actions of isoflurane may contribute to the dose-dependent change in the neurovascular transfer function. A recent study showed that some receptor functions that do not participate in the evoked FP components can affect the intensity of hemodynamic changes (Hoffmeyer et al., 2007). Future studies are necessary to identify the exact site(s) of isoflurane action that would participate in the neurovascular transfer mechanism.
Since isoflurane is also known to be a potent vasodilator (Flynn et al., 1992; Farber et al., 1997), it is likely that the dose of isoflurane affects the cerebrovascular reactivity. In our study, however, we did not observe consistent effects of isoflurane dose on the baseline CBF level measured with LDF, although dose-dependent decreases in the systemic blood pressure were observed (Fig. 2). One possible explanation is that the slow change in anesthetic dose may preserve the experimental condition within the autoregulatory adjustment regime of the local CBF (Schulte and Hudetz, 2006). Since 15 min was allotted after the adjustment of the isoflurane dose prior to recording, it may have been sufficient time for autoregulation to adjust local blood flow. Supporting this notion, previous studies have consistently reported that the autoregulatory control is preserved under 0.7 to 2.8% isoflurane anesthesia (Lee et al. 1994; Hudetz et al. 1994). These studies performed with LDF measurements have also shown an anesthesia dose-dependent increase in the baseline CBF under either halothane (0.5 to 2.2%) or isoflurane (0.7 to 2.8%) anesthesia conditions (Lee et al. 1994; Hudetz et al. 1994). But, a later study from one of the groups reported no dependencies of the halothane dose (0.4 to 1.4%) on the baseline CBF measured with LDF (Schulte and Hudetz, 2006), irrespective of a constant maintenance of the systemic blood pressure. This discrepancy could be due to differences in the location and size of the LDF probe, physiological variability, and/or the experimental protocol used. A recent report also showed that isoflurane induced the breakdown of the blood-brain barrier, which was accompanied by a local increase in CBF (Tétrault et al., 2008). We did not observe detectable increases in the CBF baseline, suggesting that a disruption of the blood-brain barrier was unlikely in our experimental condition. In addition, we observed that the vascular reactivity tested with a hypercapnia challenge was similarly preserved (1.1% to 2.1% isoflurane), which is also consistent with previous reports (Hudetz et al. 1994; Sicard et al. 2003). In summary, the dose-dependent effect of isoflurane on the vascular physiology is not the major factor contributing to the dose-dependent effects presented here on the HIR.
Nonlinearity in stimulation frequency-dependent neurovascular responses
Previous studies have proposed that the neurovascular transfer function can be explained by a linear time-invariant system (Boynton et al., 1996; Dale and Buckner, 1997), which potentially provides a formula to compute neural activities from hemodynamic signals. However, later studies have shown that the neurovascular coupling has nonlinear components (Vazquez and Noll, 1998; Glover, 1999; Miller et al., 2001; Devor et al., 2003; Nemoto et al., 2004; Martindale et al., 2005), and claimed that nonlinear relationships capture a wider range of neurovascular behaviors (Sheth et al., 2004; Hewson-Stoate et al., 2005). In the present study, the neurovascular relationship during variable stimulation frequencies was also altered by the dose of isoflurane (Fig. 4 and Fig 5). This finding can be explained by two major factors; i) a dose-dependent effect of isoflurane on the neurovascular transfer function, and ii) a stimulus frequency-dependent nonlinear component. Since our results of a linear time-invariant model showed good correspondence between the measured single-pulse HIR and the apparent HIR at lower frequency conditions for both low and high isoflurane doses (Fig. 6), our findings suggest that isoflurane action further contributes to frequency-dependent nonlinear behavior at higher frequency stimulation. The frequency-dependent mechanism of neurovascular transfer function was actually shown by a recent study that showed that the NMDA receptor activity only contributes to the CBF response for high frequency stimulation (> 7Hz) (Hoffmeyer et al., 2007). Further, a comparison of the neurovascular couplings in anesthetized and alert animals is essential to extend our findings to human studies. There have been several reports on the differences in magnitude and localization of the hemodynamic-based neuroimaging signals in anesthetized vs. awake states (Lahti et al., 1998; Shtoyerman et al., 2000; Peeters et al., 2001; Martin et al., 2002; Berwick et al., 2002; Sicard et al., 2003; Sachdev et al,. 2003; Chen et al., 2005; Fukuda et al., 2005; Martin et al., 2006; Zhao et al., 2007). These state-dependent variations could be explained by the direct interference of the anesthetic action on the cortical excitability, but also on the neurovascular transfer mechanisms at intracortial sites where the imaging signals were obtained.
Acknowledgments
This study was supported by the National Institutes of Health (EB003375, EB003324 and NS044589). We thank Ms. Michelle Tasker for assisting animal preparations.
Abbreviations
- CBF
cerebral blood flow
- fMRI
functional magnetic resonance imaging
- FP
field potential
- FWHM
full-width at half-maximum
- GABA
gamma-aminobutyric acid
- HIR
hemodynamic impulse response
- LDF
laser-Doppler flowmetry
- MABP
mean arterial blood pressure
- NMDA
N-methyl-D-aspartate
References
- Antognini JF, Wang XW, Carstens E. Quantitative and qualitative effects of isoflurane on movement occurring after noxious stimulation. Anesthesiology. 1999;91:1064–1071. doi: 10.1097/00000542-199910000-00027. [DOI] [PubMed] [Google Scholar]
- Austin VC, Blamire AM, Allers KA, Sharp T, Styles P, Matthews PM, Sibson NR. Confounding effects of anesthesia on functional activation in rodent brain: a study of halothane and alpha-chloralose anesthesia. Neuroimage. 2005;24:92–100. doi: 10.1016/j.neuroimage.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Berwick J, Martin C, Martindale J, Jones M, Johnston D, Zheng Y, Redgrave P, Mayhew J. Hemodynamic response in the unanesthetized rat: intrinsic optical imaging and spectroscopy of the barrel cortex. J. Cereb. Blood. Flow. Metab. 2002;22:670–679. doi: 10.1097/00004647-200206000-00005. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J. Neurosci. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagna JA, Miller KW, Forman SA. Mechanisms of actions of inhaled anesthetics. N. Engl. J. Med. 2003;348:2110–2124. doi: 10.1056/NEJMra021261. [DOI] [PubMed] [Google Scholar]
- Chen LM, Friedman RM, Roe AW. Optical imaging of SI topography in anesthetized and awake squirrel monkeys. J. Neurosci. 2005;25:7648–7659. doi: 10.1523/JNEUROSCI.1990-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Buckner RL. Selective averaging of rapidly presented individual trials using fMRI. Hum. Brain. Mapp. 1997;5:329–340. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Devor A, Dunn AK, Andermann ML, Ulbert I, Boas DA, Dale AM. Coupling of total hemoglobin concentration, oxygenation, and neural activity in rat somatosensory cortex. Neuron. 2003;39:353–359. doi: 10.1016/s0896-6273(03)00403-3. [DOI] [PubMed] [Google Scholar]
- Dickinson R, Peterson BK, Banks P, Simillis C, Martin JC, Valenzuela CA, Maze M, Franks NP. Competitive inhibition at the glycine site of the N-methyl-D-aspartate receptor by the anesthetics xenon and isoflurane: evidence from molecular modeling and electrophysiology. Anesthesiology. 2007;107:756–767. doi: 10.1097/01.anes.0000287061.77674.71. [DOI] [PubMed] [Google Scholar]
- Farber NE, Harkin CP, Niedfeldt J, Hudetz AG, Kampine JP, Schmeling WT. Region-specific and agent-specific dilation of intracerebral microvessels by volatile anesthetics in rat brain slices. Anesthesiology. 1997;87:1191–1198. doi: 10.1097/00000542-199711000-00024. [DOI] [PubMed] [Google Scholar]
- Flynn NM, Buljubasic N, Bosnjak ZJ, Kampine JP. Isoflurane produces endothelium-independent relaxation in canine middle cerebral arteries. Anesthesiology. 1992;76:461–467. doi: 10.1097/00000542-199203000-00021. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Rajagopalan UM, Homma R, Matsumoto M, Nishizaki M, Tanifuji M. Localization of activity-dependent changes in blood volume to submillimeter-scale functional domains in cat visual cortex. Cereb. Cortex. 2005;15:823–833. doi: 10.1093/cercor/bhh183. [DOI] [PubMed] [Google Scholar]
- Glover GH. Deconvolution of impulse response in event-related BOLD fMRI. Neuroimage. 1999;9:416–429. doi: 10.1006/nimg.1998.0419. [DOI] [PubMed] [Google Scholar]
- Golanov EV, Yamamoto S, Reis DJ. Spontaneous waves of cerebral blood flow associated with a pattern of electrocortical activity. Am. J. Physiol. 1994;266:R204–R214. doi: 10.1152/ajpregu.1994.266.1.R204. [DOI] [PubMed] [Google Scholar]
- Haseneder R, Kurz J, Dodt HU, Kochs E, Zieglgänsberger W, Scheller M, Rammes G, Hapfelmeier G. Isoflurane reduces glutamatergic transmission in neurons in the spinal cord superficial dorsal horn: evidence for a presynaptic site of an analgesic action. Anesth. Analg. 2004;98:1718–1723. doi: 10.1213/01.ANE.0000112309.80017.3F. [DOI] [PubMed] [Google Scholar]
- Hentschke H, Schwarz C, Antkowiak B. Neocortex is the major target of sedative concentrations of volatile anaesthetics: strong depression of firing rates and increase of GABAA receptor-mediated inhibition. Eur. J. Neurosci. 2005;21:93–102. doi: 10.1111/j.1460-9568.2004.03843.x. [DOI] [PubMed] [Google Scholar]
- Hewson-Stoate N, Jones M, Martindale J, Berwick J, Mayhew J. Further nonlinearities in neurovascular coupling in rodent barrel cortex. Neuroimage. 2005;24:565–574. doi: 10.1016/j.neuroimage.2004.08.040. [DOI] [PubMed] [Google Scholar]
- Hoffmeyer HW, Enager P, Thomsen KJ, Lauritzen MJ. Nonlinear neurovascular coupling in rat sensory cortex by activation of transcallosal fibers. J. Cereb. Blood. Flow. Metab. 2007;27:575–587. doi: 10.1038/sj.jcbfm.9600372. [DOI] [PubMed] [Google Scholar]
- Hudetz AG, Lee JG, Smith JJ, Bosnjak ZJ, Kampine JP. Effects of volatile anesthetics on cerebrocortical laser Doppler flow: hyperemia, autoregulation, carbon dioxide response, flow oscillations, and role of nitric oxide. Adv. Pharmacol. 1994;31:577–593. doi: 10.1016/s1054-3589(08)60643-2. [DOI] [PubMed] [Google Scholar]
- Hyder F, Rothman DL, Shulman RG. Total neuroenergetics support localized brain activity: implications for the interpretation of fMRI. Proc. Natl. Acad. Sci. U. S. A. 2002;99:10771–10776. doi: 10.1073/pnas.132272299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzner S, Nauhaus I, Benucci A, Bonin V, Ringach DL, Carandini M. Local origin of field potentials in visual cortex. Neuron. 2009;61:35–41. doi: 10.1016/j.neuron.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly EW, Solt K, Raines DE. Volatile aromatic anesthetics variably impact human gamma-aminobutyric acid type A receptor function. Anesth. Analg. 2007;105:1287–1292. doi: 10.1213/01.ane.0000282829.21797.97. [DOI] [PubMed] [Google Scholar]
- Kreiman G, Hung CP, Kraskov A, Quiroga RQ, Poggio T, DiCarlo JJ. Object selectivity of local field potentials and spikes in the macaque inferior temporal cortex. Neuron. 2006;49:433–445. doi: 10.1016/j.neuron.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Lahti KM, Ferris CF, Li F, Sotak CH, King JA. Comparison of evoked cortical activity in conscious and propofol-anesthetized rats using functional MRI. Magn. Reson. Med. 1999;41:412–416. doi: 10.1002/(sici)1522-2594(199902)41:2<412::aid-mrm28>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Lee JG, Hudetz AG, Smith JJ, Hillard CJ, Bosnjak ZJ, Kampine JP. The effects of halothane and isoflurane on cerebrocortical microcirculation and autoregulation as assessed by laser-Doppler flowmetry. Anesth. Analg. 1994;79:58–65. [PubMed] [Google Scholar]
- Lin CY. Uptake of anaesthetic gases and vapours. Anaesth. Intensive. Care. 1994;22:363–373. doi: 10.1177/0310057X9402200406. [DOI] [PubMed] [Google Scholar]
- Liu J, Newsome WT. Local field potential in cortical area MT: stimulus tuning and behavioral correlations. J. Neurosci. 2006;26:7779–7790. doi: 10.1523/JNEUROSCI.5052-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Lukasik VM, Gillies RJ. Animal anaesthesia for in vivo magnetic resonance. NMR. Biomed. 2003;16:459–467. doi: 10.1002/nbm.836. [DOI] [PubMed] [Google Scholar]
- Martin C, Berwick J, Johnston D, Zheng Y, Martindale J, Port M, Redgrave P, Mayhew J. Optical imaging spectroscopy in the unanaesthetised rat. J. Neurosci. Methods. 2002;120:25–34. doi: 10.1016/s0165-0270(02)00185-1. [DOI] [PubMed] [Google Scholar]
- Martin C, Martindale J, Berwick J, Mayhew J. Investigating neural-hemodynamic coupling and the hemodynamic response function in the awake rat. NeuroImage. 2006;32:33–48. doi: 10.1016/j.neuroimage.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Martindale J, Berwick J, Martin C, Kong Y, Zheng Y, Mayhew J. Long duration stimuli and nonlinearities in the neural-haemodynamic coupling. J. Cereb. Blood. Flow. Metab. 2005;25:651–661. doi: 10.1038/sj.jcbfm.9600060. [DOI] [PubMed] [Google Scholar]
- Masamoto K, Kim T, Fukuda M, Wang P, Kim SG. Relationship between neural, vascular, and BOLD signals in isoflurane-anesthetized rat somatosensory cortex. Cereb. Cortex. 2007;17:942–950. doi: 10.1093/cercor/bhl005. [DOI] [PubMed] [Google Scholar]
- Matsuura T, Kanno I. Quantitative and temporal relationship between local cerebral blood flow and neuronal activation induced by somatosensory stimulation in rats. Neurosci. Res. 2001;40:281–290. doi: 10.1016/s0168-0102(01)00236-x. [DOI] [PubMed] [Google Scholar]
- Miller KL, Luh WM, Liu TT, Martinez A, Obata T, Wong EC, Frank LR, Buxton RB. Nonlinear temporal dynamics of the cerebral blood flow response. Hum. Brain. Mapp. 2001;13:1–12. doi: 10.1002/hbm.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki H, Nakamura Y, Arai T, Kataoka K. Increase of glutamate uptake in astrocytes: a possible mechanism of action of volatile anesthetics. Anesthesiology. 1997;86:1359–1366. doi: 10.1097/00000542-199706000-00018. [DOI] [PubMed] [Google Scholar]
- Mukamel R, Gelbard H, Arieli A, Hasson U, Fried I, Malach R. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science. 2005;309:951–954. doi: 10.1126/science.1110913. [DOI] [PubMed] [Google Scholar]
- Nemoto M, Sheth S, Guiou M, Pouratian N, Chen JW, Toga AW. Functional signal- and paradigm-dependent linear relationships between synaptic activity and hemodynamic responses in rat somatosensory cortex. J. Neurosci. 2004;24:3850–3861. doi: 10.1523/JNEUROSCI.4870-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai AC, Jolley MA, D'Ambrosio R, Meno JR, Winn HR. Frequency-dependent changes in cerebral blood flow and evoked potentials during somatosensory stimulation in the rat. Brain. Res. 1999;837:221–228. doi: 10.1016/s0006-8993(99)01649-2. [DOI] [PubMed] [Google Scholar]
- Nielsen A, Lauritzen M. Coupling and uncoupling of activity-dependent increases of neuronal activity and blood flow in rat somatosensory cortex. J. Physiol. 2001;533:773–785. doi: 10.1111/j.1469-7793.2001.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir Y, Fisch L, Mukamel R, Gelbard-Sagiv H, Arieli A, Fried I, Malach R. Coupling between neuronal firing rate, gamma LFP, and BOLD fMRI is related to interneuronal correlations. Curr. Biol. 2007;17:1275–1285. doi: 10.1016/j.cub.2007.06.066. [DOI] [PubMed] [Google Scholar]
- Nir Y, Dinstein I, Malach R, Heeger DJ. BOLD and spiking activity. Nat. Neurosci. 2008;11:523–524. doi: 10.1038/nn0508-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters RR, Tindemans I, De Schutter E, Van der Linden A. Comparing BOLD fMRI signal changes in the awake and anesthetized rat during electrical forepaw stimulation. Magn. Reson. Imaging. 2001;19:821–826. doi: 10.1016/s0730-725x(01)00391-5. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Mintun MA. Brain work and brain imaging. Annu. Rev. Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- Ranft A, Kurz J, Deuringer M, Haseneder R, Dodt HU, Zieglgänsberger W, Kochs E, Eder M, Hapfelmeier G. Isoflurane modulates glutamatergic and GABAergic neurotransmission in the amygdala. Eur. J. Neurosci. 2004;20:1276–1280. doi: 10.1111/j.1460-9568.2004.03603.x. [DOI] [PubMed] [Google Scholar]
- Rauch A, Rainer G, Logothetis NK. The effect of a serotonin-induced dissociation between spiking and perisynaptic activity on BOLD functional MRI. Proc. Natl. Acad. Sci. U. S. A. 2008;105:6759–6764. doi: 10.1073/pnas.0800312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdev RN, Champney GC, Lee H, Price RR, Pickens DR, 3rd, Morgan VL, Stefansic JD, Melzer P, Ebner FF. Experimental model for functional magnetic resonance imaging of somatic sensory cortex in the unanesthetized rat. Neuroimage. 2003;19:742–750. doi: 10.1016/s1053-8119(03)00093-4. [DOI] [PubMed] [Google Scholar]
- Sandstrom DJ. Isoflurane depresses glutamate release by reducing neuronal excitability at the Drosophila neuromuscular junction. J. Physiol. 2004;558:489–502. doi: 10.1113/jphysiol.2004.065748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte ML, Hudetz AG. Functional hyperemic response in the rat visual cortex under halothane anesthesia. Neurosci. Lett. 2006;394:63–68. doi: 10.1016/j.neulet.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Sheth S, Nemoto M, Guiou M, Walker M, Pouratian N, Toga AW. Evaluation of coupling between optical intrinsic signals and neuronal activity in rat somatosensory cortex. Neuroimage. 2003;19:884–894. doi: 10.1016/s1053-8119(03)00086-7. [DOI] [PubMed] [Google Scholar]
- Sheth SA, Nemoto M, Guiou M, Walker M, Pouratian N, Toga AW. Linear and nonlinear relationships between neuronal activity, oxygen metabolism, and hemodynamic responses. Neuron. 2004;42:347–355. doi: 10.1016/s0896-6273(04)00221-1. [DOI] [PubMed] [Google Scholar]
- Shtoyerman E, Arieli A, Slovin H, Vanzetta I, Grinvald A. Long-term optical imaging and spectroscopy reveal mechanisms underlying the intrinsic signal and stability of cortical maps in V1 of behaving monkeys. J. Neurosci. 2000;20:8111–8121. doi: 10.1523/JNEUROSCI.20-21-08111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard K, Shen Q, Brevard ME, Sullivan R, Ferris CF, King JA, Duong TQ. Regional cerebral blood flow and BOLD responses in conscious and anesthetized rats under basal and hypercapnic conditions: implications for functional MRI studies. J. Cereb. Blood. Flow. Metab. 2003;23:472–481. doi: 10.1097/01.WCB.0000054755.93668.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AC, Lee SP, Yang G, Iadecola C, Kim SG. Simultaneous blood oxygenation level-dependent and cerebral blood flow functional magnetic resonance imaging during forepaw stimulation in the rat. J. Cereb. Blood. Flow. Metab. 1999;19:871–879. doi: 10.1097/00004647-199908000-00006. [DOI] [PubMed] [Google Scholar]
- Tétrault S, Chever O, Sik A, Amzica F. Opening of the blood-brain barrier during isoflurane anaesthesia. Eur. J. Neurosci. 2008;28:1330–1341. doi: 10.1111/j.1460-9568.2008.06443.x. [DOI] [PubMed] [Google Scholar]
- Vazquez AL, Noll DC. Nonlinear aspects of the BOLD response in functional MRI. Neuroimage. 1998;7:108–118. doi: 10.1006/nimg.1997.0316. [DOI] [PubMed] [Google Scholar]
- Viswanathan A, Freeman RD. Neurometabolic coupling in cerebral cortex reflects synaptic more than spiking activity. Nat. Neurosci. 2007;10:1308–1312. doi: 10.1038/nn1977. [DOI] [PubMed] [Google Scholar]
- Wu XS, Sun JY, Evers AS, Crowder M, Wu LG. Isoflurane inhibits transmitter release and the presynaptic action potential. Anesthesiology. 2004;100:663–670. doi: 10.1097/00000542-200403000-00029. [DOI] [PubMed] [Google Scholar]
- Zhao F, Jin T, Wang P, Kim SG. Isoflurane anesthesia effect in functional imaging studies. Neuroimage. 2007;38:3–4. doi: 10.1016/j.neuroimage.2007.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]