There has been a longstanding concern with the fetal effects of psychoactive drug use by pregnant women. In this article we describe the effects of three drugs with similar molecular targets that involve monoaminergic transmitter systems. These stimulants include the illegal drugs cocaine and methamphetamine and the class of selective serotonin re-uptake inhibitors (SSRIs) used to treat maternal depression during pregnancy. We discuss the mechanisms of action of each drug, including a possible common epigenetic mechanism for their effects on the developing child. We also discuss fetal neurobehavioral techniques that may be useful in the early detection of the effects of in utero drug exposure.
In the past three decades, the concept of behavioral teratology1 expanded the field of teratology to examine behavioral effects in the neonate due to acute exposure to substances in utero, including environmental, nutritional, and drug exposures.2,3 The developmental consequences of prenatal exposure to a toxic substance may include central nervous system (CNS) insult related to the period during gestation of the exposure. In contrast to the effects of drugs on the adult brain, which result in deformation of the developed brain, fetal effects are more likely to produce malformation in which the developing brain is prevented from forming normally.4 The effects of exposure during the first half of gestation will impact processes related to cytogenesis and histogenesis whereas effects during the second half of gestation relate to brain growth and differentiation. During this organizational phase in the second half of gestation, progressive events (neuroblast proliferation and migration, axonal projection, and synaptogenesis) and regressive events (programmed cell death and selective elimination of processes) affect the maturation of brain circuitry. Thus toxic influences during this period may dramatically alter brain development but may also alter the regressive events that underlie the capacity of the developing brain to compensate for injury.4
Recent years have seen further expansion of the principles of behavioral teratology to examine exposure effects on the human fetus at the time of the exposure. Epigenetic and organismic models of developmental theory suggest that true understanding of a developing system can occur with the study of its organization of form and structure as it moves toward a teleological state.5,6 This study includes examination of the mutual influences of genes, physiology, and behavior as well as the physical, cultural, and social environments of the organism.6
Increasing evidence from preclinical, prospective clinical and epidemiological studies suggests that many biological factors acting during prenatal life are associated with adult disease as well as long term neurobehavioral abnormalities7–17 and behavioral disorders.18–21 The notion that the development of common adult cardiovascular and metabolic disorders are linked to factors during prenatal development was originally known as the “Barker” or “fetal origins hypothesis”.7,9,10 Although these early studies related low birthweight to adult disease, it is generally accepted that low birth weight per se is not at the heart of these disorders, but that there are common factors that influence intrauterine growth as well as adult physiological systems.22 The “fetal origins” observations are due, in part, to environmental factors acting early in life that affect developing systems, altering structure and function. It has been suggested that the biological purpose of this “programming” is to alter the set-points or “hard-wire” physiological systems to prepare the fetus for optimal adaptation to the postnatal environment.23
Common factors arising from prenatal exposure to psychoactive drugs could include mechanisms related to “fetal origins” and may also contribute to more long-term outcomes. Longitudinal study of the organism and contexts beginning before or at the time of exposure, as well as of long-term outcomes, is essential to understanding developmental trajectories related to substance exposures.
Cocaine
In the 1980s, cocaine became one of the most frequently abused illicit drugs during pregnancy and also one of the most studied with regard to its potential teratologic and neurodevelopmental effects on the developing fetus and child. While early catastrophic predictions about the long-term outcome of prenatally cocaine- exposed children were exaggerated, concern remains about more “subtle” effects24 that may affect development, particularly in childhood and adolescence.
The neurochemical and vasoconstrictive effects of cocaine have been well documented. Cocaine acts primarily at the presynaptic level to block reuptake of the monoaminergic neurotransmitters dopamine, norepinephrine and serotonin25,26 by specific, presynaptic plasma membrane transporters.27 These transporters are expressed in discrete pathways of the CNS, on postganglionic sympathetic neurons and in the adrenal medulla. These pharmacologic actions lead to elevated circulating catecholamine levels and exaggerated sympathetic responses including hypertension, tachycardia, vasoconstriction, agitation, euphoria, and excitation. These effects are particularly profound in the fetus in which elevated sympathetic tone has been demonstrated.28–31 Cocaine affects neuronal formation, proliferation and early connectivity,32–34 and disrupts neuronal migration and resulting cortical architecture.35–38 Cocaine also affects the expression of transcription factors (IEGs or immediate early genes) along dopaminergic39,40 and serotonergic pathways.41 Because monoamine neurotransmitter receptors (NA, 5-HT, and DA) are present early in corticogenesis, areas highly expressing these neurotransmitter systems may be especially susceptible to elevated synaptic monoamine neurotransmitter levels secondary to cocaine’s main effect of blocking catecholamine reuptake at the presynaptic level.42–44 Also, because monoamine neurotransmitters play a key trophic role in brain development,45 prenatal cocaine may alter normal mechanisms that modulate neuronal growth.42
The effects of cocaine on fetal development have also been attributed to vasoconstrictive mechanisms. Uptake inhibitors such as cocaine (as well asamphetamines and SSRIs), which block catecholamine transport28,46 and decrease placental blood flow, reduce the supply of oxygen and nutrients to the fetus. Fetal hypoxemia and possibly ischemic injury can compromise brain development. Blood flow to the developing brain can also be reduced by cocaine-related noradrenergic effects on the developing fetal vasculature.47,48 Norepinephrine and particularly the monoamine serotonin (5-HT) exert vasoconstrictive effects on the umbilical vein, thereby reducing blood flow from the placenta to the fetus.49,50 Furthermore, the vascular response to 5-HT is potentiated by uptake inhibition.51,52 Vasoconstriction at the uteroplacental complex coupled with anorexic effects of cocaine could explain the increase in intrauterine growth retardation (IUGR) that has been reported in cocaine-exposed infants.53
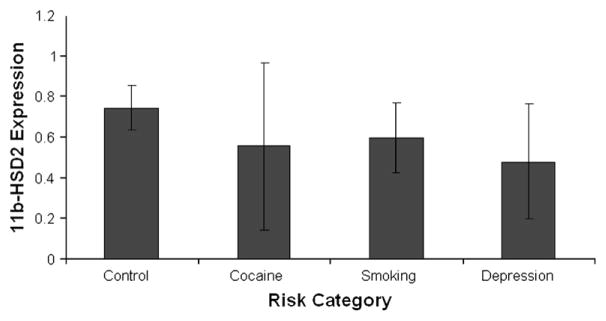
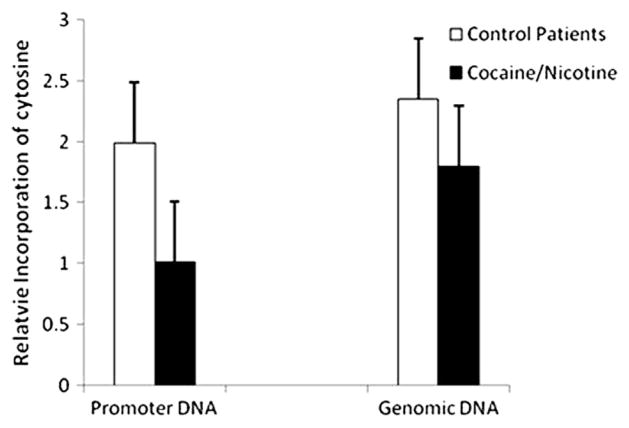
In addition to neurochemical and vasoconstrictive effects, cocaine may also act as an intrauterine stressor that alters “fetal programming” through epigenetic mechanisms that might alter the offspring’s developmental trajectory.54 In this model, cocaine alters the expression of key candidate genes and gene networks important to placental function in late gestation, specifically the norepinephrine transporter NET 55 and a steroid metabolic enzyme, 11β-HSD-2. Placental NET and 11β-HSD-2 protect the fetus from excess catecholamines and glucocorticoids, which have harmful effects on the fetus.56 11β-HSD-2 in particular converts maternal cortisol to inert cortisone, protecting the developing fetus from exposure to maternal cortisol.57 Placental expression of 11β-HSD-2 is downregulated by norepinephrine, which is in turn regulated by NET.58 Prenatal cocaine exposure is associated with downregulation of NET,59,60 which leads to increased circulating catecholamines, downregulation of 11β-HSD-2, and chronic fetal hypercortisolism. These changes in placental gene expression may be due to epigenetic mechanisms including DNA methylation, as suggested by the findings in Figures 1 and 2.54 Figure 1 shows decreased 11β-HSD-2 expression in mothers who used cocaine (n=4) or cigarettes (n=4) or were depressed (n=3), compared with 17 controls. The altered expression of these two key candidate genes is likely associated with changes in networks of genes involved in critical placental functions that maintain physiological homeostasis in utero and otherwise promote intrauterine growth, development and preparation for postnatal life. Figure 2 suggests that these changes inplacental gene expression are associated with methylation of placental genomic DNA, particularly in promoter regions. These findings in Figure 2 are based on the same group of subjects with pregnancies complicated by cocaine, nicotine, and depression, and controls, shown in Figure 1. The relative incorporation of cytosine used to measure methylation was comparable in promoter and genomic DNA in the cocaine and nicotine exposed subjects, suggesting hypermethylation of the promoter regions of DNA that contain CpG islands. This hypermethylation of DNA suggests gene silencing related to in utero cocaine exposure.
Figure 1.
Mean 11β-HSD-2 expression in risk groups.
Figure 2.
Hypermethylation of DNA in placentas from cocaine/nicotine exposed.
There is a substantial literature on the effects of cortisol on children61 including infants with prenatal cocaine exposure.62–64 Preclinical studies suggest effects of prenatal cocaine exposure on the developing monoaminergic system, resulting in both structural and functional changes to circuitry subserving functions such as arousal, regulation, and reactivity.43,65 Human infant studies show effects of prenatal cocaine exposure on arousal, hypertonicity and excitability, acoustic cry characteristics,66 and auditory brain response.67 At school age these children show more behavior problems68 and they are more likely referred for special education services.69 On functional neuroimaging (fMRI) they show differences in the right inferior frontal cortex and caudate during response inhibition, suggesting cocaine effects on brain systems involved in the regulation of attention and response inhibition.70 This set of cognitive abilities is referred to as executive function and is particularly important as children reach school age. A recent review of 42 follow-up studies of cocaine-exposed children suggested that executive function and behavior problems were major domains affected by prenatal cocaine exposure.71 Thus, fetal programming effects that alter the intrauterine neuroendocrine environment may be a marker for long-term behavioral consequences of prenatal cocaine exposure.
Methamphetamine
Methamphetamine (MA) is the dominant drug problem in the Western and Midwestern portions of the United States, second only to alcohol and marijuana,72 and is the most widely abused drug worldwide.73,74 The number of adults age 12 and over who have tried MA once in their lifetime has increased to 5.3% in 2007 from 4.3% in 1999 and 2.5% in 1997.75 This increase has led to the concern that MA is the growing drug of choice for adults in the United States, including pregnant women.76–78
Although there is controversy about the nature and extent of the MA problem in the U.S., including exaggerations reminiscent of the cocaine “epidemic,” there is little argument that MA is a dangerous drug that substantially challenges policymakers, health care professionals, social service providers, and the law enforcement community,79 and there is little information about MA use by pregnant women.
MA is a CNS stimulant of the sympathetic nervous system with neurotoxicpotential for developing monoaminergic systems. As the “first cousin” of amphetamine with the addition of a methyl radical, MA exerts its action by releasing dopamine and serotonin, blocking monoamine reuptake mechanisms, and inhibiting monoamine oxidase.80 The mechanism of action most likely occurs by increasing synaptic concentrations of the neurotransmitters dopamine and norepinephrine80 either by direct release from storage vesicles or by inhibition of reuptake.81,82 MA may enhance synaptic catecholamine levels by inhibiting monoamine oxidase, the enzyme responsible for the oxidation of norepinephrine and serotonin.83 MA acts on the dopamine transporter (DAT) that mediates the inward transmission of dopamine in the neuron. The action of MA on DAT releases dopamine and inhibits reuptake of dopamine from the presynaptic terminals, thus increasing dopamine activity.84 MA also decreases serotonin (5HT) uptake and densities of binding sites.85 MA has been shown to be neurotoxic to mature dopaminergic and serotonergic axons and axon terminal arbors,86 and potentially neurotoxic to mature glutaminergic axons.87 The cellular and molecular mechanisms implicated in the neurotoxicity induced by MA on mature neurons include the production of reactive oxygen species and nitric oxide, p53 activation resulting in apoptosis, and mitochondrial dysfunction.88 Less is known about the mechanisms involved in MA-induced toxicity in the developing CNS; however, the early and widespread influence of serotonergic, dopaminergic and glutaminergic systems on neuronal growth and connectivity suggests that prenatal exposure to MA may result in alterations in developing neural circuitry.87
Amphetamines are considered noncatecholamine sympathomimetics because they lack catecholamine structure yet have sympathomimetic actions.89 These structural characteristics are important because they account for the wide distribution and long duration of action of amphetamine. MA also has vasoconstrictive effects90,91 resulting in decreased uteroplacental blood flow and fetal hypoxia.92 In addition, MA has anorexic effects on the mother. These maternal/placental effects could affect fetal development to the above monoaminergic effects. Weight control may also help explain the popularity of MA with women, including pregnant women.
Unfortunately, the scant human literature that is available on the effects of prenatal MA exposure is beset by methodological problems.93 Recent, more reliable findings showed that MA-exposed infants, although born at term, are more likely to be small for gestational age.94 Newborn neurobehavioral effects were reminiscent of cocaine-exposed infants showing effects on arousal and physiological stress.95 In addition, these findings showed a dose response relationship between amphetamine metabolites in meconium and newborn neurobehavior.
In preclinical work, administration of MA to laboratory animals results in profound and long-lasting toxicity to the developing CNS. Brain studies in the ovine model have found MA increases fetal blood pressure and decreases fetal oxyhemoglobin saturation and arterial pH.96,97 In rodents, MA is toxic to dopaminergic and serotonergic neurons.98,99 Damage to dopamine (DA) terminals100,101 are thought to reflect irreversible terminal degeneration.102 Positron emission tomography (PET) studies in abstinent MA users demonstrated decreased dopamine transporters, suggesting long-lasting neurotoxicity due to MA abuse.103
Neurotoxic effects of prenatal MA exposure on serotonergic neurons produce neurochemical alternations in the CNS104,105 thought to be associated with learning impairment, behavioral deficits,105 increased motor activity,106 enhanced conditioned avoidance responses,107 and postural motor movements108 seen in MA-exposed animals. Rhesus monkeys showed reduced brain monoamines 4 years after the last drug exposure.109
Administration of MA to laboratory animals also results in motor110 and learning and memory impairment.111 Studies with rats have shown a range of physical, motor, neurotransmitter, and behavioral effects in MA-exposed offspring. These include increased maternal and offspring mortality, retinal eye defects,106,112,113 cleft palate and rib malformations,113 and decreased rate of physical growth and delayed motor development.107,112 MA exposure to pregnant dams showed effects on spatial learning in their adult offspring.108 Spatial learning and attenuated corticosterone response was found in rats with prenatal MA exposure.114 In pregnant mice, MA caused dopaminergic nerve terminal degeneration and long-term motor deficits in offspring.115
Consistent with the “fetal programming” model described above, the human placenta may also be a direct target for MA. MA causes inhibition of the norepinephrine and serotonin transporters, suggesting cellular mechanisms by which MA could affect the developing fetus.116 Blockage of these transporters would increase the concentrations of norepinephrine and serotonin, resulting in constriction of blood vessels and decrease blood flow to the placenta. Also, placental norepinephrine transporter (NET) downregulation resulting from MA could lead to increases in circulating catecholamines, downregulation of 11β-hydroxysteroid dehydrogenase-2 (11β-HSD-2), and chronic fetal hypercortisolism,59,117 which could affect behavior through alteration of the HPA axis, especially arousal regulation and attention.43
SSRIs
Each year at least 600,000 infants born in the United States are exposed to maternal Major Depressive Disorder (MDD) during gestation, which is associated with newborn medical and neurobehavioral deficits and long-term emotional, behavioral, and social problems in the child. Pharmacological treatment of MDD during pregnancy remains the most common form of treatment. The current first-line choice of clinicians for somatic therapies during pregnancy is selective serotonin reuptake inhibitors (SSRIs) and dual-action serotonin and norepinephrine reuptake inhibitors (SNRIs)(referred to collectively here as SRIs) due to their lower side-effect profiles and relatively low risk to the fetus.118,119 Over the past two decades, the use of newer antidepressants has dramatically increased over the use of tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors. A survey of national patterns of antidepressant medication prescribed by physicians from 1987–2001 reported that SRIs were prescribed in 69% of office visits for depression in 2001.120 Similarly, another study reported that in 2000, 65% of all antidepressants prescribed by primary care providers were SSRIs (fluoxetine, sertraline, paroxetine, fluvoxamine and citalopram), and newer antidepressants, including SNRIs such asvenlafaxine, comprised an additional 17% of antidepressants prescribed.121 A recent study examining antidepressant treatment rates during pregnancy found that at least 37% of depressed pregnant women choose to take antidepressant medications during pregnancy.122 Another large study conducted retrospectively from 1993 to 2007 showed the use of SRIs during pregnancy to have a steady increase from 0.44% in 1993 to 6.61% of all pregnant women in 2007.
SRIs block the presynaptic reuptake of serotonin (5-HT) by binding to theserotonin transporter SERT. Some of the SRIs also bind to the norepinephrine (NE) transporter gene, NET. SERT and NET are responsible for the reuptake and transport of 5-HT and NE out of the synapse. Inhibition of SERT and NET activity by SRIs prolongs neurotransmitter signaling. Fluoxetine has also recently been found to antagonize 5-HT2c receptors. The antidepressant mechanisms of SRIs and many other psychoactive drugs remain unclear, but are presumed to be a result of the enhanced serotonergic neurotransmission at postsynaptic receptors, the effect on intracellular signal transduction cascades, and the modulation of other neurotransmitters.123
SRIs and their metabolites have been detected in both umbilical cord blood and amniotic fluid. The potency of serotonin placental passage, expressed as a ratio of medication concentration in cord blood to maternal serum, ranged from 0.29 (sertraline and paroxetine) to 0.89 (citalopram and fluoxetine).124 A study of cord:maternal serum levels found roughly equivalent values for fluoxetine, sertraline, and paroxetine and their metabolites (.52–67), while the concentration for the SRI venlafaxine was 1.1.125 SRIs have also been found in amniotic fluid, representing another source of exposure as the fetus swallows an increasing amount of amniotic fluid throughout pregnancy.126 Administration of fluoxetine to pregnant ewes was associated with a transient decrease in uterine artery blood flow, perhaps due to serotonin activity following administration.127 Immediately following fluoxetine administration, there is an increase in plasma serotonin concentrations. However, with prolonged exposure, plasma serotonin levels decline. Serotonin acts as a vasoconstrictor; therefore, elevations in serotonin followed by a decline might explain the uterine artery blood flow findings. Decreases in serotonin levels in exposed offspring may be one mechanism by which SRI medication influencesadverse effects.
The serotonergic system develops early in gestation and is likely to be influenced by serotonin levels in all trimesters of pregnancy.128 Serotonin is widely distributed throughout the central and peripheral nervous systems and is involved in the development of multiple brain areas.128–131 Alterations in the 5HT system during development are associated with changes in somatosensory processing, motor output, and emotional responses.132,133 Recent evidence suggests that components of the serotonin system are critical to the development of neurobehavioral systems involved inmood, anxiety, aggression, and substance abuse.133,134
The serotonin system includes at least 14 receptors, multiple enzymes, and transporter proteins that exert influence on 5HT metabolism, release, and reuptake. The serotonin transporter gene, or 5-HTT (SLC6A4), encodes for the transporter protein. The promoter region of the gene contains two common polymorphic alleles, a short or “S” allele with 14 repeated elements, and a long or “L” allele with 16 repeated elements. The variant region has been labeled the serotonin transporter linked polymorphic region (5-HTTLPR).135 Functionally, the deletion polymorphism (“s”, or short allele) appears to cause a reduction in basal and stimulated transcription activity, 5-HTT mRNA, and 5-HT binding and uptake.136 Individuals with the S allele (S/S or S/L) have been more susceptible to stress, depression, anxiety, suicidal ideation, and irritable temperament. Longitudinal research suggests a gene × environment interaction, in that those with the S allele are more susceptible to depression given stressful conditions.137 Allelic differences have also been linked to responsiveness to antidepressant medication.138
The most recent meta-analysis of the effects of SRIs on pregnancy and fetalphysical development included published reports from 1995 through August of 2005.139 The findings of the meta-analysis are in agreement with most of the previous reviews that did not find an increased risk of major, cardiovascular or minor malformations, but did find an increased risk of spontaneous abortion.140,141 The findings are in disagreement with reports of a higher rate of major cardiac malformations and persistent pulmonary hypertension of the newborn for infants exposed to SRIs compared with other antidepressant medications and controls.142,143
Lower birth weight, younger gestational age at birth, and lower Apgar scoreshave been reported with SRI exposure.144 Similar results were seen with third trimester exposure of fluoxetine but not with first or second trimester exposure.140 Lower birth weight has been associated with higher doses of fluoxetine compared with lower doses or other SRIs,145 while other studies failed to find birth weight differences between early and late SRI exposed infants146 or between SRI exposed and nonexposed infants.147,148
Recent evidence supports acute effects of SRI exposure on neonatal neurobehavior. Several reviews were published in 2005 looking at recent case reports, database analyses, and cohort studies on the effects of SRI exposure on neonatal outcomes.149–152 In general, a cluster of symptoms was observed in newborns who were prenatally exposed to SRIs. These symptoms include irritability, tremors, jitteriness, trouble feeding, agitation, respiratory distress, and poor sleep. Other symptoms reported include convulsions, abnormal posturing, and shivering.153,154 These symptoms have been reported most often in infants exposed to paroxetine, but all SRIs have been indicated,140,151,152 and the symptoms were originally described as a syndrome called “poor neonatal adaptation” which included respiratory difficulties, jitteriness, poor motor tone, hypothermia, hypoglycemia, weak or absent cry, and trouble feeding.140 Since that time, frequent reports of “poor neonatal adaptation” have been seen in the literature. Data from a 2006 review suggest that 30% of SRI–exposed neonates have symptoms consistent with “neonatal abstinence syndrome,” a condition often described in newborns withdrawing from other (mostly opioid) prenatal drug exposures.155–158 The long-term outcomes associated with these apparently transient symptoms following delivery have only just begun to be studied. The few empirical studies have shown SRIs related to increased active sleep159 and decreased facial and behavioral responses to acute pain in the first week postnatally and at 2 months of age, suggesting a blunting of pain reactivity.160,161 SRI-exposed infants were found to have decreased basal cortisol levels in the early evening compared with non-exposed infants at 3 months of age, although this effect was not related to prenatal exposure level or current SRI level measured in infant plasma.162 A related paper suggested that third trimester maternal mood, rather than SRI exposure, is related to increased infant HPA reactivity and that this effect is mediated by increased methylation of NR3C1 (human glucocorticoid receptor gene).163 In a recent review of studies on the long-term development of children with prenatal SSRI exposure, 11 studies (306 children) suggested no impairment with exposure, and 2 studies (81 children) suggested mild adverse effects.150
It is also possible that SRIs affect fetal neurobehavior. Studies of SRI effects on sleep state development in the fetus are limited to the work of Morrison et al., who examined fetal sheep after fluoxetine exposure and found a significant decrease in fetal rapid eye movement (REM) sleep.164 By contrast, a study of sleep state in the first 2–3days postnatally reported more active sleep, more arousals, and more activity during sleep in SSRI-exposed newborns.159 It is possible that the increase in REM sleep reported after birth by Zeskind et al.159 was compensatory following REM sleep deprivation based on the fetal sheep data.165 This finding could be due to the fact that serotonergic neurons in the dorsal raphe nucleus appear to be involved in the “turning-off” of active sleep and are a central monoamine involved in the regulation of ultradian rhythms.166 The initial action of antidepressant medications is to enhance postsynaptic transmission of serotonin. Therefore, it is reasonable to expect that SRI exposure wouldinitially decrease the amount of active sleep. Another possibility is that the newborn effects are related to the discontinuation of the SRIs following delivery.
The effects of SRIs on fetal neurobehavior can be studied by incorporating fetal actocardiography with ultrasound observations of fetal behavior.167–170 The FEtal Neurobehavior coding System (FENS) is a method of fetal neurobehavioral observation and scoring that includes measurement of fetal heart rate, motor activity, behavioral state, and responsiveness to external or extra-uterine stimuli.171 A fetal actocardiograph provides measurement of fetal heart rate, fetal heart patterns, and motor activity. The use of ultrasound technology enables visualization of the fetus to observe specific fetal action patterns, quality and amplitude of movements, and eye movements. Thus, ultrasound technology coupled with fetal actocardiography allows for a comprehensive assessment of fetal neurobehavior. Table 1 presents the behavioral variables coded in the FENS coding system.
Table 1.
Fetal Behaviors Coded from Ultrasound Recordings in the FENS System
| Summary Variable | Variable | Description |
|---|---|---|
| Fetal Eye Movement | Present | Clear movement of the pupil or eyelid |
| Absent | A clear view of the eye is obtained and there is no movement | |
| Fetal Breathing Movements | Regular | Displacement of the diaphragm with outward movement of the abdomen |
| Vigorous | FBM’s that are large enough to move the entire fetus’ body | |
| Hiccup | Consists of a jerky, repetitive contraction of the diaphragm | |
| General Body Movements | Smooth | Pattern of movement involving smooth, simultaneous movement of a limb, trunk and head that results in a change in plane |
| Jerky | GBM that involves jerky movements of limbs or entire body | |
| Incomplete | GBM that is not fluid or coordinated and does not result in change | |
| Flexion | Flexion of the trunk | |
| Patterned Body Movements | Stretch | A single event including a back extension or upward movement of the shoulder with retroflexion of the head Typically includes a pause at the movement with subsequent relaxation |
| Backarche | Extension of the trunk and maintenance in this position for greater than 1 second | |
| Startle | A quick, generalized movement, involving abduction or extension of the limbs with or without movement of the trunk and head, followed by a return to a resting position. | |
| Fidget | Nearly continuous limb movements that are not part of a GBM or other patterned movement | |
| Head Movements | Rotation | Movement of the head in the lateral plane for at least a 30 degree angle from starting position |
| Extension | A small movement of the head that extends upward in the vertical plane | |
| General | Small Movement of the head that is not an extension or rotation | |
| Mouthing Movements | Rhythmic | Rhythmical bursts of jaw opening and closing at least 4 times in 5 seconds (sucking) |
| NonRhythmic | Mouth opening and closing that is isolated or limited to less than 4 at one time, often with tongue protrusion or lapping (drinking) | |
| Yawning | The timing of a yawn is similar to a stretch that includes prolonged wide opening of the jaws followed by relaxation. Often accompanied by a stretch or a subsequent GBM. | |
| Limb Movements | Smooth | Limb(s) moves from origin to destination without backtracking |
| Jerky | Limb movement is interrupted by backtracking | |
| Indeterminate | Unable to determine quality of movement | |
| Lower Limb | Lower limb is moving but unable to determine quality of movement | |
| Multiple | Repetitive limb movement in the same plane in a single epoch. | |
| Hand to Face | The hand slowly touches the face or mouth | |
| Tremor | Small rhythmic, jerky movement of an extremity | |
Effects of SRIs on FENS neurobehavioral measures are shown in Figures 3 and4. Fetal behavioral and physiological data were collected simultaneously by a research nurse certified in obstetrical ultrasound and fetal heart rate monitoring. The data were obtained using a Toshiba ultrasound machine model SSA-340A with a 3.75 MHz transducer and a Toitu MT325 actocardiograph. The time of the recordings was standardized to between 12 and 5:00 pm to account for possible variability in fetal activity levels at different times of the day.172 The participants were asked to fast for at 1 ½ hours before their scheduled appointment to increase their appetite. Upon arrival to the appointment, the participants were given a small meal, standardized for calories andcontent, to standardize the immediate nutritional influence on fetal activity. Participants were asked about their smoking as well as nutritional and caffeine intake on the day of the observation to account for the potential acute effects of caffeine and nicotine on fetal behavior. Baseline behaviors and heart rate were collected for the first 40 minutes. A single, 3-second single vibroacoustic stimulus (VAS) (Toitu) was applied to the maternal abdomen during the first quiescent period following the 40-minute baseline period. Recording continued for 20 minutes post VAS presentation. Additionally, a VAS-stimulus control trial was conducted in the baseline period to control for maternal reaction to the VAS. Research assistants trained on the FENS and blinded to maternal condition conducted the coding of fetal behaviors. Coders use the Mangold Interact Video Coding Software to score digital recording files for fetal movements and behaviors in 10-second epochs. The presence or absence of isolated limb and head movements, gross body movements, and behavior patterns were scored within each 10-second epoch. All movements were categorized as smooth, jerky, or indeterminate, with high inter-rater reliability. The actocardiograph data were scored for mean fetalheart rate (FHR), FHR accelerations, and fetal movement measures.169,173
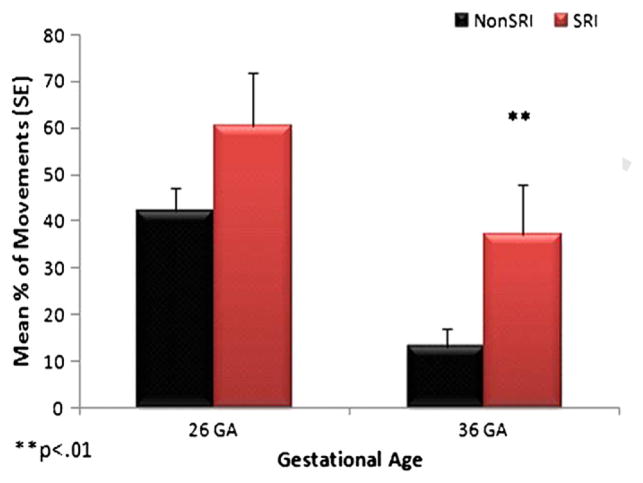
Figure 3.
Fetal jerky movements.
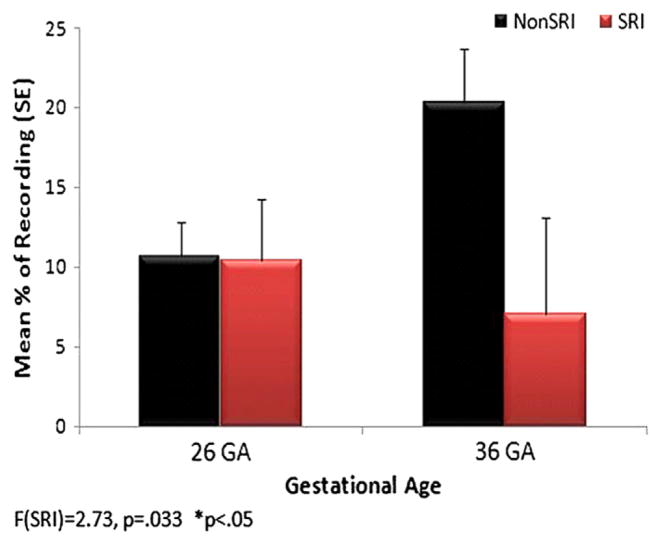
In Figures 3 and 4, the data are shown for fetuses (n=60) exposed and unexposed to SRIs, with maternal depression scores used as a covariate. Fetuses in both groups demonstrated the expected developmental decrease in jerky movements from 26 to 36 weeks’ gestational age; however, fetuses exposed to SRIs had more jerky movements at both gestational ages (Figure 3). The SRI-exposed fetuses did not show the anticipated developmental increase in fetal breathing movements at 36 weeks’ gestational age, and they had significantly fewer fetal breathing movements than nonSRI fetuses (Fig. 4).
Figure 4.
Fetal breathing movements.
Biogenic amine transporters
Some of the adverse effects on the fetus of the three uptake inhibitors that we have reviewed--cocaine, methamphetamine and SRIs--may be due to their action on blocking catecholamine transport, especially serotonin (5-HT). Catecholamines also exert vasoconstrictive effects on the umbilical vein, thereby reducing blood flow from the placenta to the fetus.49,50 Furthermore, the vascular response to 5-HT is potentiated by uptake inhibition.51,52 As the umbilical cord is not innervated, transporter-dependent uptake by the placenta is also protecting the umbilical-placental circulation from deleterious effects of these neurotransmitters.174,175
Nonetheless, regulation of the placental capacity for catecholamine uptake should not be viewed solely in the context of protecting the fetus from exaggerated elevations in catecholamines and/or serotonin. Endogenous catecholamines are critical to fetal and neonatal growth, development, and survival. This conclusion is supported by studies inmice in which the gene for either tyrosine hydroxylase176,177 or dopamine beta-hydroxylase178,179 has been disrupted. The majority of fetuses homozygous for the disruption of either gene die during embryonic development. Small proportions (5–10%) of fetuses survive, suggested to be rescue of the lethal phenotype by passage of maternal catecholamines across the placenta. Serotonin (5HT) is also important at critical stages of development. 5HT is present in early embryos and has been suggested to be maternal in origin.180 Mouse embryos grown in the presence of high concentrations of 5HT or serotonin uptake inhibitors develop cranio-facial and cardiac abnormalities of the 3rd-5th brachial arches.181 Similar abnormalities have been seen in rat and chick embryos.182 Thus, highly regulated mechanisms control the concentration of intrauterine biogenic amines, which are central to fetal growth and development. Administration of uptake inhibitors to mouse dams in early to mid-gestation during placentation and embryogenesis leads to a high incidence of fetal/placental resorption.183 In survivors, there is a significant reduction in birth weight and delay in maturational milestones (ear opening).183 Thus, the capacity for placental biogenic amine uptake and/or transport has a significant impact on intrauterine growth and development. Understanding the ontogeny and regulation of placental biogenic amine transport is important in understanding the way in which the intrauterine neuroendocrine milieu programs develop.
Previous studies on the regulation of monoamine transporters suggest that drugs as well as maternal mood and anxiety are able to alter transporter activity.184 Male rats exposed to stress in early gestation were shown to have behavioral manifestations similar to major depression, including maladaptive behavioral reactivity and anhedonia with subsequent increased sensitivity to SRI medication. These rats were found to have decreased SERT in the hippocampus. The authors state that a potential mechanism could be increased 5-HT output and decreased reuptake by SERT. This mechanism may explain increased sensitivity to acute postnatal SRI administration.185
Other drugs that affect transporters, such as cocaine, given during fetal rat brain development have been related to increased norepinephrine turnover rate in older animals186 and altered cerebral glucose uptake.187,188 Interestingly, the cocaine-treated animals had enhanced acoustic startle responses to selective serotonin agonists whichwere attenuated by the fluoxetine exposure.188 This finding is consistent with our observations on the effects of antenatal cocaine exposure on auditory brain stem responses, newborn cry, fetal heart rate, and arousal responses.189,190 In other work, we showed that disorders linked to chronic intrauterine stress, including cocaine exposure and/or intrauterine growth retardation, are associated with decreased placental NET expression, directly proportional to the elevation in umbilical arterial plasma norepinephrine concentrations.59 The decreased placental NET gene expression we have observed, and resultant increases in circulating catecholamines, may explain the adverse effects on the fetus of drugs such as cocaine and methamphetamine, which block catecholamine transport and render the fetus more vulnerable to other physiologic derangements.
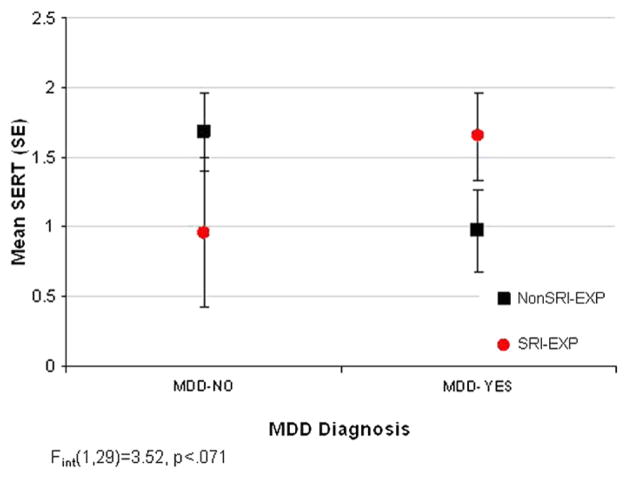
As described above, 11β-HSD is expressed in the placenta and converts biologically active cortisol to cortisone.191,192 11β-HSD-2 expression in the placenta is widely considered to protect the fetus from maternal hypercortisolism during pregnancy. We also know that placental 11β-HSD-2 is downregulated by norepinephrine and thatchronic intrauterine stress leads to downregulation of placental NET gene expression and increased circulating catecholamine levels in the fetus and the placental microenvironment. Dysregulation of norepinephrine levels in the placental microenvironment in turn leads to alterations in the placental neuroendocrine milieu. One potential mechanism for neurobehavioral effects of drugs such as cocaine, methamphetamine and SRIs involves the alteration of fetal placental monoamine transporter expression and the resulting alterations in neuroendocirne and neurotransmitter system development. As an example of how placental geneexpression may be related to substance exposure and maternal health conditions, we present in Figure 5 placental SERT data from 33 mothers. These data demonstrate an interaction for MDD/SRI use on SERT expression in the placenta. The MDD-exposed fetuses (no SRI exposure) had lower SERT levels than controls and MDD+SRI-exposed fetuses, but were not different from those of SRI-exposed fetuses whose mothers no longer met criteria for MDD. These data highlight how maternal diagnosis might change the outcome of substance exposure-related outcomes. We have also shown this same type of alteration in neurobehavioral outcomes in cocaine-exposed infants whose mothers were depressed in the first month of pregnancy.193
Figure 5.
Placental SERT levels.
Fetal Origins
The developmental trajectories of fetuses exposed to psychoactive substances may be altered by multiple factors. However, we suggest that drugs such as cocaine, methamphetamine and SRIs can be stressors that affect fetal programming, disrupt fetal placental monoamine transporter expression, and alter neuroendocrine and neurotransmitter system development. Stress hormones such as catecholamines and glucocorticoids can alter regulation of the neuroendocrine environment by acting on the hypothalamic-pituitary-adrenal (HPA) axis, which results in an altered set point for physiologic, metabolic, and behavioral outcomes.194 Because they are an important feature of the stress response, glucocorticoids have become prominent candidates as mediators of the effects of “programming.” Effects on gene expression through epigenetic mechanisms such as DNA methylation or chromatin remodeling could result in altered developmental trajectories.
Acknowledgments
This work was supported by NIH grants MH6547 (Salisbury) and P20RR018728 (Padbury).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Wilson JG. Current status of teratology: General principles and mechanisms derived from animal studies. In: Wilson JG, Fraser FC, editors. Handbook of Teratology, General Principles and Etiology. Vol. 1. New York: Plenum Press; 1977. pp. 47–74. [Google Scholar]
- 2.Wisner KL, Zarin DA, Holmboe ES, et al. Risk-benefit decision making for treatment of depression during pregnancy. Am J Psychiatry. 2000;157(12):1933–1940. doi: 10.1176/appi.ajp.157.12.1933. [DOI] [PubMed] [Google Scholar]
- 3.Yonkers KA, Wisner KL, Stowe Z, et al. Management of bipolar disorder during pregnancy and the postpartum period. Am J Psychiatry. 2004 Apr;161(4):608–620. doi: 10.1176/appi.ajp.161.4.608. [DOI] [PubMed] [Google Scholar]
- 4.Lester B, Kosofsky B. Neurobiology of Mental Illness. In: Charney D, Nestler E, editors. Effects of Drugs of Abuse on Brain Development. London: Oxford University Press; 2009. pp. 801–827. [Google Scholar]
- 5.Bertalanffy Lv. General Systems Theory. New York: Brazilier; 1968. [Google Scholar]
- 6.Gottleib G. Experiential canalization of behavioral development: Results. Developmental Psychobiology. 1991;27:35–39. [Google Scholar]
- 7.Barker D. Fetal programming of coronary heart disease. Trends Endocrinol Metab. 2002;13(9):364–368. doi: 10.1016/s1043-2760(02)00689-6. [DOI] [PubMed] [Google Scholar]
- 8.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005 Apr;85(2):571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 9.Barker DJ. The fetal origins of adult hypertension. J Hypertens Suppl. 1992 Dec;10(7):S39–44. [PubMed] [Google Scholar]
- 10.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, bloodpressure in childhood and adult life, and mortality from cardiovascular disease. Bmj. 1989 Mar 4;298(6673):564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falkner B. Birth weight as a predictor of future hypertension. Am J Hypertens. 2002 Feb;15(2 Pt 2):43S–45S. doi: 10.1016/s0895-7061(01)02297-x. [DOI] [PubMed] [Google Scholar]
- 12.Rich-Edwards JW, Colditz GA, Stampfer MJ, et al. Birthweight and the risk for type 2 diabetes mellitus in adult women. Ann Intern Med. 1999 Feb 16;130(4 Pt 1):278–284. doi: 10.7326/0003-4819-130-4_part_1-199902160-00005. [DOI] [PubMed] [Google Scholar]
- 13.Stein CE, Fall CH, Kumaran K, Osmond C, Cox V, Barker DJ. Fetal growth and coronary heart disease in south India. Lancet. 1996 Nov 9;348(9037):1269–1273. doi: 10.1016/s0140-6736(96)04547-3. [DOI] [PubMed] [Google Scholar]
- 14.Sallout B, Walker M. The fetal origin of adult diseases. J Obstet Gynaecol. 2003 Sep;23(5):555–560. doi: 10.1080/0144361031000156483. [DOI] [PubMed] [Google Scholar]
- 15.Phillips D, Barker D, Hales C, Hirst S, Osmond C. Thinness at birth and insulin resistance in adult life. Diabetologia. 1994;37(2):150–154. doi: 10.1007/s001250050086. [DOI] [PubMed] [Google Scholar]
- 16.Ong KK, Dunger DB. Birth weight, infant growth and insulin resistance. Eur J Endocrinol. 2004 Nov;151 (Suppl 3):U131–139. doi: 10.1530/eje.0.151u131. [DOI] [PubMed] [Google Scholar]
- 17.Hales CN, Barker DJ, Clark PM, et al. Fetal and infant growth and impaired glucose tolerance at age 64. Bmj. 1991 Oct 26;303(6809):1019–1022. doi: 10.1136/bmj.303.6809.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wals M, Reichart CG, Hillegers MH, et al. Impact of birth weight and genetic liability on psychopathology in children of bipolar parents. J Am Acad Child Adolesc Psychiatry. 2003 Sep;42(9):1116–1121. doi: 10.1097/01.CHI.0000070242.24125.78. [DOI] [PubMed] [Google Scholar]
- 19.Allin M, Rooney M, Cuddy M, et al. Personality in young adults who are born preterm. Pediatrics. 2006 Feb;117(2):309–316. doi: 10.1542/peds.2005-0539. [DOI] [PubMed] [Google Scholar]
- 20.Thompson C, Syddall H, Rodin I, Osmond C, Barker DJ. Birth weight and the risk of depressive disorder in late life. Br J Psychiatry. 2001 Nov;179:450–455. doi: 10.1192/bjp.179.5.450. [DOI] [PubMed] [Google Scholar]
- 21.Gale CR, Martyn CN. Birth weight and later risk of depression in a national birth cohort. Br J Psychiatry. 2004 Jan;184:28–33. doi: 10.1192/bjp.184.1.28. [DOI] [PubMed] [Google Scholar]
- 22.Welberg LA, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol. 2001 Feb;13(2):113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- 23.Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004 Sep 17;305(5691):1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 24.Lester BM, LaGasse LL, Seifer R. Cocaine exposure and children: the meaning of subtle effects. Science. 1998 Oct 23;282(5389):633–634. doi: 10.1126/science.282.5389.633. [DOI] [PubMed] [Google Scholar]
- 25.Gawin FH, Ellinwood EH., Jr Cocaine and other stimulants. Actions, abuse, and treatment. N Engl J Med. 1988 May 5;318(18):1173–1182. doi: 10.1056/NEJM198805053181806. [DOI] [PubMed] [Google Scholar]
- 26.Wise R. National Institute on Drug Abuse (Research Monograph) Vol. 50. 1984. Neural mechanisms of the reinforcing action of cocaine; pp. 15–33. [PubMed] [Google Scholar]
- 27.Goodman L. The Pharmacological Basis of Therapeutics. New York: MacMillan Publishing Company; 1985. [Google Scholar]
- 28.Bzoskie L, Blount L, Kashiwai K, Humme J, Padbury J. The contribution of transporter-dependent uptake to fetal catecholamine clearance. Biol Neonate. 1997;71:102–110. doi: 10.1159/000244403. [DOI] [PubMed] [Google Scholar]
- 29.Lau C, Burke S, Slotkin T. Maturation of sympathetic neurotransmission in the rat heart. IX. Development of transsynaptic regulation of cardiac adrenergic sensitivity. J Pharmacol Exp Ther. 1982;(223):675–680. [PubMed] [Google Scholar]
- 30.Padbury JF, Ludlow JK, Humme JA, Agata Y. Metabolic clearance and plasma appearance rates of catecholamines in preterm and term fetal sheep. Pediatr Res. 1986 Oct;20(10):992–995. doi: 10.1203/00006450-198610000-00020. [DOI] [PubMed] [Google Scholar]
- 31.Stein H, Oyama K, Martinez A, Chappell B, Padbury J. Plasma epinephrine appearance and clearance rates in fetal and newborn sheep. Am J Physiol. 1993 Oct;265(4 Pt 2):R756–760. doi: 10.1152/ajpregu.1993.265.4.R756. [DOI] [PubMed] [Google Scholar]
- 32.Garg UC, Turndorf H, Bansinath M. Effect of cocaine on macromolecular syntheses andcell proliferation in cultured glial cells. Neuroscience. 1993 Nov;57(2):467–472. doi: 10.1016/0306-4522(93)90079-u. [DOI] [PubMed] [Google Scholar]
- 33.Nassogne MC, Evrard P, Courtoy PJ. Selective neuronal toxicity of cocaine in embryonic mouse brain cocultures. Proc Natl Acad Sci U S A. 1995 Nov 21;92(24):11029–11033. doi: 10.1073/pnas.92.24.11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nassogne MC, Evrard P, Courtoy PJ. Selective direct toxicity of cocaine on fetal mouse neurons. Teratogenic implications of neurite and apoptotic neuronal loss. Ann N Y Acad Sci. 1998 Jun 21;846:51–68. [PubMed] [Google Scholar]
- 35.Akbari HM, Whitaker-Azmitia PM, Azmitia EC. Prenatal cocaine decreases the trophic factor S-100 beta and induced microcephaly: reversal by postnatal 5-HT1A receptor agonist. Neurosci Lett. 1994 Mar 28;170(1):141–144. doi: 10.1016/0304-3940(94)90259-3. [DOI] [PubMed] [Google Scholar]
- 36.Gressens P, Gofflot F, Van Maele-Fabry G, et al. Early neurogenesis and teratogenesis in whole mouse embryo cultures. Histochemical, immunocytological and ultrastructural study of the premigratory neuronal-glial units in normal mouse embryo and in mouse embryos influenced by cocaine and retinoic acid. J Neuropathol Exp Neurol. 1992 Mar;51(2):206–219. doi: 10.1097/00005072-199203000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Gressens P, Kosofsky BE, Evrard P. Cocaine-induced disturbances of corticogenesis in the developing murine brain. Neurosci Lett. 1992 Jun 8;140(1):113–116. doi: 10.1016/0304-3940(92)90694-3. [DOI] [PubMed] [Google Scholar]
- 38.Yablonsky-Alter E, Gleser I, Carter C, Juvan M. Effects of prenatal cocaine treatment onpostnatal development of neocortex in white mice: Immunocytochemistry of calbindin- and paralbumin-positive populations of gabaergic neurons. Society Neuroscience Abstract. 1992;18:367. [Google Scholar]
- 39.Steiner H, Gerfen CR. Dynorphin opioid inhibition of cocaine-induced, D1 dopamine receptor-mediated immediate-early gene expression in the striatum. J Comp Neurol. 1995 Mar 6;353(2):200–212. doi: 10.1002/cne.903530204. [DOI] [PubMed] [Google Scholar]
- 40.Steiner H, Gerfen CR. Cocaine-induced c-fos messenger RNA is inversely related to dynorphin expression in striatum. J Neurosci. 1993 Dec;13(12):5066–5081. doi: 10.1523/JNEUROSCI.13-12-05066.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhat RV, Baraban JM. Activation of transcription factor genes in striatum by cocaine: role of both serotonin and dopamine systems. J Pharmacol Exp Ther. 1993 Oct;267(1):496–505. [PubMed] [Google Scholar]
- 42.Levitt P, Harvey JA, Friedman E, Simansky K, Murphy EH. New evidence for neurotransmitter influences on brain development. Trends Neurosci. 1997 Jun;20(6):269–274. doi: 10.1016/s0166-2236(96)01028-4. [DOI] [PubMed] [Google Scholar]
- 43.Mayes LC. Developing brain and in utero cocaine exposure: effects on neural ontogeny. Dev Psychopathol. 1999 Fall;11(4):685–714. doi: 10.1017/s0954579499002278. [DOI] [PubMed] [Google Scholar]
- 44.Malanga CJ, 3rd, Kosofsky BE. Mechanisms of action of drugs of abuse on the developing fetal brain. Clin Perinatol. 1999 Mar;26(1):17–37. v–vi. [PubMed] [Google Scholar]
- 45.Meier E, Schousboe A. Neurotransmitters as developmental signals. Neurochem Int. 1991;19(1–2):1–15. [Google Scholar]
- 46.Bzoskie L, Blount L, Kashiwai K, Humme J, Padbury J. Placental norepinephrine tansporter development in the ovine fetus. Placenta. 1997;18:65–70. doi: 10.1016/s0143-4004(97)90072-2. [DOI] [PubMed] [Google Scholar]
- 47.Koegler SM, Seidler FJ, Spencer JR, Slotkin TA. Ischemia contributes to adverse effects of cocaine on brain development: suppression of ornithine decarboxylase activity in neonatal rat. Brain Res Bull. 1991 Dec;27(6):829–834. doi: 10.1016/0361-9230(91)90217-8. [DOI] [PubMed] [Google Scholar]
- 48.Woods JR, Jr, Plessinger MA, Clark KE. Effect of cocaine on uterine blood flow and fetal oxygenation. Jama. 1987 Feb 20;257(7):957–961. [PubMed] [Google Scholar]
- 49.Reviriego J, Fernandez-Alfonso MS, Marin J. Actions of vasoactive drugs on human placental vascular smooth muscle. Gen Pharmacol. 1990;21(5):719–727. doi: 10.1016/0306-3623(90)91024-l. [DOI] [PubMed] [Google Scholar]
- 50.Zhang L, Dyer DC. Characterization of alpha-adrenoceptors mediating contraction in isolated ovine umbilical vein. Eur J Pharmacol. 1991 May 2;197(1):63–67. doi: 10.1016/0014-2999(91)90365-w. [DOI] [PubMed] [Google Scholar]
- 51.Dyer DC. An investigation of the mechanism of potentiation by cocaine of responses to serotonin in sheep umbilical blood vessels. J Pharmacol Exp Ther. 1970 Dec;175(3):571–576. [PubMed] [Google Scholar]
- 52.Nair X, Dyer DC. Responses of guinea pig umbilical vasculature to vasoactive drugs. Eur J Pharmacol. 1974 Aug;27(3):294–304. doi: 10.1016/0014-2999(74)90004-1. [DOI] [PubMed] [Google Scholar]
- 53.Bauer CR, Langer JC, Shankaran S, et al. Acute neonatal effects of cocaine exposure during pregnancy. Arch Pediatr Adolesc Med. 2005 Sep;159(9):824–834. doi: 10.1001/archpedi.159.9.824. [DOI] [PubMed] [Google Scholar]
- 54.Lester B, Padbury J. The Third Pathophysiology of Prenatal Cocaine Exposure. Developmental Neuroscience. 2009;31:23–35. doi: 10.1159/000207491. [DOI] [PubMed] [Google Scholar]
- 55.Nguyen TT, Tseng YT, McGonnigal B, et al. Placental biogenic amine transporters: in vivo function, regulation and pathobiological significance. Placenta. 1999 Jan;20(1):3–11. doi: 10.1053/plac.1998.0348. [DOI] [PubMed] [Google Scholar]
- 56.Meyer JS. Biochemical effects of corticosteroids on neural tissues. Physiol Rev. 1985 Oct;65(4):946–1020. doi: 10.1152/physrev.1985.65.4.946. [DOI] [PubMed] [Google Scholar]
- 57.Lopez-Bernal A, Craft IL. Corticosteroid metabolism in vitro by human placenta, fetal membranes and decidua in early and late gestation. Placenta. 1981 Oct–Dec;2(4):279–285. doi: 10.1016/s0143-4004(81)80025-2. [DOI] [PubMed] [Google Scholar]
- 58.Sarkar S, Tsai SW, Nguyen TT, Plevyak M, Padbury JF, Rubin LP. Inhibition of placental 11beta-hydroxysteroid dehydrogenase type 2 by catecholamines via alpha-adrenergic signaling. Am J Physiol Regul Integr Comp Physiol. 2001 Dec;281(6):R1966–1974. doi: 10.1152/ajpregu.2001.281.6.R1966. [DOI] [PubMed] [Google Scholar]
- 59.Bzoskie L, Yen J, Tseng YT, Blount L, Kashiwai K, Padbury JF. Human placental norepinephrine transporter mRNA: expression and correlation with fetal condition at birth. Placenta. 1997 Mar–Apr;18(2–3):205–210. doi: 10.1016/s0143-4004(97)90094-1. [DOI] [PubMed] [Google Scholar]
- 60.Bottalico B, Larsson I, Brodszki J, et al. Norepinephrine transporter (NET), serotonin transporter (SERT), vesicular monoamine transporter (VMAT2) and organic cation transporters (OCT1, 2 and EMT) in human placenta from pre-eclamptic and normotensive pregnancies. Placenta. 2004 Jul;25(6):518–529. doi: 10.1016/j.placenta.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 61.Gunnar M, Prudhomme-White B. Salivary Cortisol Measures in Infant and Child Assessment. In: Twarog-Singer L, Zeskind PS, editors. Biobehavioral Assessment of the Infant. New York, NY: The Guilford Press; 2001. [Google Scholar]
- 62.Magnano CL, Gardner JM, Karmel BZ. Differences in salivary cortisol levels in cocaine-exposed and noncocaine-exposed NICU infants. Dev Psychobiol. 1992 Mar;25(2):93–103. doi: 10.1002/dev.420250203. [DOI] [PubMed] [Google Scholar]
- 63.Jacobson SW, Bihun JT, Chiodo LM. Effects of prenatal alcohol and cocaine exposure on infant cortisol levels. Dev Psychopathol. 1999 Spring;11(2):195–208. doi: 10.1017/s0954579499002011. [DOI] [PubMed] [Google Scholar]
- 64.Scafidi FA, Field TM, Wheeden A, et al. Cocaine-exposed preterm neonates show behavioral and hormonal differences. Pediatrics. 1996 Jun;97(6 Pt 1):851–855. [PubMed] [Google Scholar]
- 65.Harvey JA. Cocaine effects on the developing brain: current status. Neurosci Biobehav Rev. 2004 Jan;27(8):751–764. doi: 10.1016/j.neubiorev.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 66.Lester BM, Tronick EZ, LaGasse L, et al. The maternal lifestyle study: effects of substance exposure during pregnancy on neurodevelopmental outcome in 1-month-old infants. Pediatrics. 2002 Dec;110(6):1182–1192. doi: 10.1542/peds.110.6.1182. [DOI] [PubMed] [Google Scholar]
- 67.Lester BM, Lagasse L, Seifer R, et al. The Maternal Lifestyle Study (MLS): effects of prenatal cocaine and/or opiate exposure on auditory brain response at one month. J Pediatr. 2003 Mar;142(3):279–285. doi: 10.1067/mpd.2003.112. [DOI] [PubMed] [Google Scholar]
- 68.Bada HS, Das A, Bauer CR, et al. Impact of prenatal cocaine exposure on child behavior problems through school age. Pediatrics. 2007 Feb;119(2):e348–359. doi: 10.1542/peds.2006-1404. [DOI] [PubMed] [Google Scholar]
- 69.Levine T, Liu J, Das A, et al. Effects of Prenatal Cocaine Exposure on Special Education in School Age Children. Pediatrics. 2008;122(1):e83–e91. doi: 10.1542/peds.2007-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sheinkopf SJ, Lester BM, Sanes JN, et al. Functional MRI and response inhibition in children exposed to cocaine in utero. Preliminary findings. Dev Neurosci. 2009;31(1–2):159–166. doi: 10.1159/000207503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lester B, LaGasse L. Children of Addicted Women. Journal of Addictive Diseases. In Press. [Google Scholar]
- 72.U.S. Drug Enforcement Administration. [accessed: March 11, 2009];Methamphetamine. http://www.usdoj.gov/dea/concern/meth.html.
- 73.Rawson R, Huber A, Brethen P, Obert J, Gulati V, Shoptaw S, Ling W. Methamphetamine and Cocaine Users: Differences in Characteristics and Treatment Retention. Journal of Psychoactive Drugs. 2000;32(2):233–238. doi: 10.1080/02791072.2000.10400234. [DOI] [PubMed] [Google Scholar]
- 74.United Nations Office on Drugs and Crime. World Drug Report, Analysis. Vol. 1. United Nations Publication; Vienna: 2004. [Google Scholar]
- 75.Substance Abuse and Mental Health Services Administration (SAMSA). Office of Applied Studies, National Survey on Drug Use and Health, 2004, 2005, 2006 and 2007. 2008.
- 76.Arria AM, Derauf C, Lagasse LL, et al. Methamphetamine and other substance use during pregnancy: preliminary estimates from the Infant Development, Environment, and Lifestyle (IDEAL) study. Matern Child Health J. 2006 May;10(3):293–302. doi: 10.1007/s10995-005-0052-0. [DOI] [PubMed] [Google Scholar]
- 77.Substance Abuse and Mental Health Services Administration (SAMSA) Preliminary Results from the 1997 National Household Survey on Drug Abuse. 1998. [Google Scholar]
- 78.Substance Abuse and Mental Health Services Administration (SAMSA) National Household Survey on Drug Abuse (NHSDA) National Institute on Drug Abuse. 1999. [Google Scholar]
- 79.King RS. The Next Big Thing: Methamphetamine In The United States. Washington, D.C.: The Sentencing Project: Research and Advocacy for Reform; Jun, 2006. [Google Scholar]
- 80.Heller A. Effects of in Utero Exposure to Methamphetamines. Bethesda, MD: National Institute on Drug Abuse; 2000. Neurotoxicology and developmental effects of meth and MDMA. [Google Scholar]
- 81.Karch S. The pathology of drug abuse. Boca Raton: CRC Press; 1993. [Google Scholar]
- 82.Catanzarite VA, Stein DA. ‘Crystal’ and pregnancy--methamphetamine-associated maternal deaths. West J Med. 1995;162(5):454–458. [PMC free article] [PubMed] [Google Scholar]
- 83.Bennett BA, Hyde CE, Pecora JR, Clodfelter JE. Differing neurotoxic potencies of methamphetamine, mazindol, and cocaine in mesencephalic cultures. J Neurochem. 1993 Apr;60(4):1444–1452. doi: 10.1111/j.1471-4159.1993.tb03307.x. [DOI] [PubMed] [Google Scholar]
- 84.Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci. 1998 Mar 15;18(6):1979–1986. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ricaurte GA, Schuster CR, Seiden LS. Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res. 1980 Jul 7;193(1):153–163. doi: 10.1016/0006-8993(80)90952-x. [DOI] [PubMed] [Google Scholar]
- 86.McCann UD, Ricaurte GA. Amphetamine neurotoxicity: accomplishments and remaining challenges. Neurosci Biobehav Rev. 2004 Jan;27(8):821–826. doi: 10.1016/j.neubiorev.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 87.Frost DO, Cadet JL. Effects of methamphetamine-induced neurotoxicity on the development of neural circuitry: a hypothesis. Brain Res Brain Res Rev. 2000 Dec;34(3):103–118. doi: 10.1016/s0165-0173(00)00042-4. [DOI] [PubMed] [Google Scholar]
- 88.Quinton MS, Yamamoto BK. Causes and consequences of methamphetamine and MDMA toxicity. AAPS J. 2006;8(2):E337–347. doi: 10.1007/BF02854904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Plessinger MA. Prenatal exposure to amphetamines. Risks and adverse outcomes in pregnancy. Obstetrics and Gynecology Clinics of North America. 1998;25(1):119–138. doi: 10.1016/s0889-8545(05)70361-2. [DOI] [PubMed] [Google Scholar]
- 90.Stek A, Fisher BK, Baker RS, Lang U, Tseng C, Clark KE. Maternal and fetal cardiovascular responses to methamphetamine in the pregnant sheep. Am J Obstete Gynecol. 1993;169(4):888–897. doi: 10.1016/0002-9378(93)90022-b. [DOI] [PubMed] [Google Scholar]
- 91.Burchfield DJ, Lucas VW, Abrams RM, Miller RL, DeVane CL. Disposition and pharmacodynamics of methamphetamine administration in sheep. JAMA. 1991;265:1968–1973. [PubMed] [Google Scholar]
- 92.Stek AM, Baker RS, Fisher BK, Lang U, Clark KE. Fetal responses to maternal and fetal methamphetamine administration in sheep. Am J Obstet Gynecol. 1995;173:1592–1598. doi: 10.1016/0002-9378(95)90654-1. [DOI] [PubMed] [Google Scholar]
- 93.Wouldes T, LaGasse L, Sheridan J, Lester B. Maternal Methamphetamine Use During Pregnancy and Child Outcome: What Do We Know? New Zealand Medical Journal. 2004;117(1206):U1180. [PubMed] [Google Scholar]
- 94.Smith LM, LaGasse LL, Derauf C, et al. The infant development, environment, and lifestyle study: effects of prenatal methamphetamine exposure, polydrug exposure, and poverty on intrauterine growth. Pediatrics. 2006 Sep;118(3):1149–1156. doi: 10.1542/peds.2005-2564. [DOI] [PubMed] [Google Scholar]
- 95.Smith LM, Lagasse LL, Derauf C, et al. Prenatal methamphetamine use and neonatal neurobehavioral outcome. Neurotoxicol Teratol. 2008 Jan–Feb;30(1):20–28. doi: 10.1016/j.ntt.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Burchfield DJ, Lucas VW, Abrams RM, Miller RL, DeVane CL. Disposition and pharmacodynamics of methamphetamine in pregnant sheep. JAMA. 1991 Apr 17;265(15):1968–1973. [PubMed] [Google Scholar]
- 97.Stek AM, Baker RS, Fisher BK, Lang U, Clark KE. Fetal responses to maternal and fetal methamphetamine administration in sheep. Am J Obstet Gynecol. 1995 Nov;173(5):1592–1598. doi: 10.1016/0002-9378(95)90654-1. [DOI] [PubMed] [Google Scholar]
- 98.Fuller R, Hemrick-Luecke S. Further studies on the long-term depletion of striatal dopamine in iprindole-treated rats by amphetamine. Neuropharmacology. 1992;21(5):433–438. doi: 10.1016/0028-3908(82)90027-2. [DOI] [PubMed] [Google Scholar]
- 99.Pu C, Vorhees CV. Developmental dissociation of astrocyte reaction in rat striatum. Brain Reseach Developmental Brain Research. 1993;72(2):325–328. doi: 10.1016/0165-3806(93)90201-k. [DOI] [PubMed] [Google Scholar]
- 100.Seiden LS, Sabol KE. Methamphetamine and methylenedioxymethamphetamine neurotoxicity: possible mechanisms of cell destruction. NIDA Research Monograph. 1996;163:251–276. [PubMed] [Google Scholar]
- 101.Gibb JW, Johnson M, Elayan I, Lim HK, Matsuda L, Hanson GR. Neurotoxicity of amphetamines and their metabolites. NIDA Research Monograph. 1997;173:128–145. [PubMed] [Google Scholar]
- 102.Ricaurte GA, McCann UD. Neurotoxic amphetamine analogues: effects in monkeys and implications for humans. Ann N Y Acad Sci. 1992;648:371–382. doi: 10.1111/j.1749-6632.1992.tb24586.x. [DOI] [PubMed] [Google Scholar]
- 103.Volkow ND, Chang L, Wang GJ, et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001 Mar;158(3):377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- 104.Cabrera TM, Levy AD, Li Q, van de Kar LD, Battaglia G. Prenatal methamphetamine attenuates serotonin mediated renin secretion in male and female rat progeny: evidence for selective long-term dysfunction of serotonin pathways in brain. Synapse 1993. 1993;15(3):198–208. doi: 10.1002/syn.890150305. [DOI] [PubMed] [Google Scholar]
- 105.Weissman AD, Caldecott-Hazard S. Developmental neurotoxicity to methamphetamines. Clinical and Experimental pharmacology and physiology. 1995;22(5):372–374. doi: 10.1111/j.1440-1681.1995.tb02022.x. [DOI] [PubMed] [Google Scholar]
- 106.Acuff-Smith KD, George M, Lorens SA, Vorhees CV. Preliminary evidence for methamphetamine-induced behavioral and ocular effects in rat offspring following exposure during early organogenesis. Psychopharmacology. 1992;109(3):255–263. doi: 10.1007/BF02245871. [DOI] [PubMed] [Google Scholar]
- 107.Cho Dh, Lyu HM, Lee HB, Kim PY, Chin K. Behavioral teratogenicity of methamphetamine. Journal of Toxicological Sciences. 1991;(Suppl 1):37–49. doi: 10.2131/jts.16.supplementi_37. [DOI] [PubMed] [Google Scholar]
- 108.Slamberova R, Pometlova M, Charousova P. Postnatal development of rat pups is altered by prenatal methamphetamine exposure. Prog Neuropsychopharmacol Biol Psychiatry. 2006 Jan;30(1):82–88. doi: 10.1016/j.pnpbp.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 109.Woolverton WL, Ricaurte GA, Forno LS, Seiden LS. Long-term effects of chronic methamphetamine administration in rhesus monkeys. Brain Res. 1989 May 1;486(1):73–78. doi: 10.1016/0006-8993(89)91279-1. [DOI] [PubMed] [Google Scholar]
- 110.Wallace TL, Gudelsky GA, Vorhees CV. Methamphetamine-induced neurotoxicity alters locomotor activity, stereotypic behavior, and stimulated dopamine release in the rat. J Neurosci. 1999;19:9141–9148. doi: 10.1523/JNEUROSCI.19-20-09141.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Itoh J, Nabeshima T, Kameyama T. Utility of an elevated plusmaze for dissociation of amnesic and behavioral effects of drugs in mice. Eur J Pharmacol. 1991;1991(194):71–76. doi: 10.1016/0014-2999(91)90125-a. [DOI] [PubMed] [Google Scholar]
- 112.Acuff-Smith KD, Schilling MA, Fisher JE, Vorhees CV. Stage-specific effects of prenatal d-methamphetamine exposure on behavioral and eye development in rats. Neurotoxicology and Teratology. 1996;18(2):199–215. doi: 10.1016/0892-0362(95)02015-2. [DOI] [PubMed] [Google Scholar]
- 113.Yamamoto Y, Yamanoto K, Fukui Y, Kurishita A. Teratogenic effects of methamphetamine in mice. Japanese Journal of Legal Medicine. 1992;46(2):126–131. [PubMed] [Google Scholar]
- 114.Williams MT, Blankenmeyer TL, Schaefer TL, Brown CA, Gudelsky GA, Vorhees CV. Long-term effects of neonatal methamphetamine exposure in rats on spatial learning in the Barnes maze and on cliff avoidance, corticosterone release, and neurotoxicity in adulthood. Brain Res Dev Brain Res. 2003 Dec 30;147(1–2):163–175. doi: 10.1016/j.devbrainres.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 115.Jeng W, Wong AW, Ting AKR, Wells PG. Methamphetamine-enhanced embryonic oxidative DNA damage and neurodevelopmental deficits. Free Radic Biol Med. 2005 Aug 1;39(3):317–326. doi: 10.1016/j.freeradbiomed.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 116.Ramamoorthy JD, Ramamoorthy S, Leibach FH, Ganapathy V. Human placental monoamine transporters as targets for amphetamines. Am J Obstet Gynecol. 1995;173:1782–1787. doi: 10.1016/0002-9378(95)90427-1. [DOI] [PubMed] [Google Scholar]
- 117.Bzoskie L, Blount L, Kashiwai K, Humme J, Padbury JF. The contribution of transporter-dependent uptake to fetal catecholamine clearance. Biol Neonate. 1997;71(2):102–110. doi: 10.1159/000244403. [DOI] [PubMed] [Google Scholar]
- 118.Altshuler LL, Cohen LS, Moline ML, Kahn DA, Carpenter D, Docherty JP. Treatment of Depression in Women. Postgraduate Medicine. 2001 March;:1–28. Special Report(The Expert Consensus Guideline Series) [PubMed] [Google Scholar]
- 119.Swinkels JA, de Jonghe F. Safety of antidepressants. International Clinical Psychopharmacology. 1995;9(Suppl 4):19–25. doi: 10.1097/00004850-199501004-00003. [DOI] [PubMed] [Google Scholar]
- 120.Stafford RS, MacDonald EA, Finkelstein SN. National Patterns of Medication Treatment for Depression, 1987 to 2001. Prim Care Companion. J Clin Psychiatry. 2001 Dec;3(6):232–235. doi: 10.4088/pcc.v03n0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pirraglia PA, Stafford RS, Singer DE. Trends in Prescribing of Selective Serotonin Reuptake Inhibitors and Other Newer Antidepressant Agents in Adult Primary Care. Prim Care Companion. J Clin Psychiatry. 2003 Aug;5(4):153–157. doi: 10.4088/pcc.v05n0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Marcus SM, Flynn HA, Blow F, Barry K. A screening study of antidepressant treatment rates and mood symptoms in pregnancy. Arch Women Ment Health. 2005 May;8(1):25–27. doi: 10.1007/s00737-005-0072-1. [DOI] [PubMed] [Google Scholar]
- 123.Shelton R. The dual-action hypothesis: does pharmacology matter? Journal of Clinical Psychiatry. 2004;65(suppl 17):5–10. [PubMed] [Google Scholar]
- 124.Hendrick V, Stowe ZN, Altshuler LL, Hwang S, Lee E, Haynes D. Placental passage of antidepressant medications. Am J Psychiatry. 2003 May;160(5):993–996. doi: 10.1176/appi.ajp.160.5.993. [DOI] [PubMed] [Google Scholar]
- 125.Rampono J, Proud S, Hackett LP, Kristensen JH, Ilett KF. A pilot study of newer antidepressant concentrations in cord and maternal serum and possible effects in the neonate. Int J Neuropsychopharmacol. 2004 Sep;7(3):329–334. doi: 10.1017/S1461145704004286. [DOI] [PubMed] [Google Scholar]
- 126.Loughhead AM, Fisher AD, Newport DJ, et al. Antidepressants in amniotic fluid: another route of fetal exposure. Am J Psychiatry. 2006 Jan;163(1):145–147. doi: 10.1176/appi.ajp.163.1.145. [DOI] [PubMed] [Google Scholar]
- 127.Morrison JL, Chien C, Riggs KW, Gruber N, Rurak D. Effect of maternal fluoxetine administration on uterine blood flow, fetal blood gas status, and growth. Pediatr Res. 2002 Apr;51(4):433–442. doi: 10.1203/00006450-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 128.Sodhi MS, Sanders-Bush E. Serotonin and brain development. Int Rev Neurobiol. 2004;59:111–174. doi: 10.1016/S0074-7742(04)59006-2. [DOI] [PubMed] [Google Scholar]
- 129.Lesch KP. Serotonergic gene expression and depression: implications for developing novel antidepressants. J Affect Disord. 2001 Jan;62(1–2):57–76. doi: 10.1016/s0165-0327(00)00351-7. [DOI] [PubMed] [Google Scholar]
- 130.Herlenius E, Lagercrantz H. Neurotransmitters and neuromodulators during early human development. Early Hum Dev. 2001 Oct;65(1):21–37. doi: 10.1016/s0378-3782(01)00189-x. [DOI] [PubMed] [Google Scholar]
- 131.Gingrich JA, Ansorge MS, Merker R, Weisstaub N, Zhou M. New lessons from knockout mice: The role of serotonin during development and its possible contribution to the origins of neuropsychiatric disorders. Cns Spectrums. 2003;8(8):572–577. doi: 10.1017/s1092852900018848. [DOI] [PubMed] [Google Scholar]
- 132.Wurtman RJ. Genes, stress, and depression. Metabolism. 2005 May;54(5 Suppl 1):16–19. doi: 10.1016/j.metabol.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 133.Lesch KP, Mossner R. Genetically driven variation in serotonin uptake: is there a link to affective spectrum, neurodevelopmental, and neurodegenerative disorders? Biol Psychiatry. 1998 Aug 1;44(3):179–192. doi: 10.1016/s0006-3223(98)00121-8. [DOI] [PubMed] [Google Scholar]
- 134.Hamet P, Tremblay J. Genetics and genomics of depression. Metabolism. 2005 May;54(5 Suppl 1):10–15. doi: 10.1016/j.metabol.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 135.Heils A, Teufel A, Petri S, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996 Jun;66(6):2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 136.Lesch KP, Bengel D, Heils A, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996 Nov 29;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 137.Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003 Jul 18;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 138.Serretti A, Benedetti F, Zanardi R, Smeraldi E. The influence of Serotonin Transporter Promoter Polymorphism (SERTPR) and other polymorphisms of the serotonin pathway on the efficacy of antidepressant treatments. Prog Neuropsychopharmacol Biol Psychiatry. 2005 Jul;29(6):1074–1084. doi: 10.1016/j.pnpbp.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 139.Rahimi R, Nikfar S, Abdollahi M. Pregnancy outcomes following exposure to serotonin reuptake inhibitors: a meta-analysis of clinical trials. Reprod Toxicol. 2006 May 21; doi: 10.1016/j.reprotox.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 140.Chambers CD, Johnson KA, Dick LM, Felix RJ, Jones KL. Birth outcomes in pregnant women taking fluoxetine. N Engl J Med. 1996;335(14):1010–1015. doi: 10.1056/NEJM199610033351402. [DOI] [PubMed] [Google Scholar]
- 141.Einarson TR, Einarson A. Newer antidepressants in pregnancy and rates of major malformations: a meta-analysis of prospective comparative studies. Pharmacoepidemiol Drug Saf. 2005 Mar 1; doi: 10.1002/pds.1084. [DOI] [PubMed] [Google Scholar]
- 142.MedWatch F, editor. GlaxoSmithKline. Safety Alerts for Drugs, Biologics, Medical Devices, and Dietary Supplements: Paxil (paroxetine HCL) and Paxil CR (Posted 09/27/2005) 2005. [Google Scholar]
- 143.Chambers CD, Hernandez-Diaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006 Feb 9;354(6):579–587. doi: 10.1056/NEJMoa052744. [DOI] [PubMed] [Google Scholar]
- 144.Simon GE, Cunningham ML, Davis RL. Outcomes of prenatal antidepressant exposure. Am J Psychiatry. 2002 Dec;159(12):2055–2061. doi: 10.1176/appi.ajp.159.12.2055. [DOI] [PubMed] [Google Scholar]
- 145.Hendrick V, Smith LM, Suri R, Hwang S, Haynes D, Altshuler L. Birth outcomes after prenatal exposure to antidepressant medication. Am J Obstet Gynecol. 2003 Mar;188(3):812–815. doi: 10.1067/mob.2003.172. [DOI] [PubMed] [Google Scholar]
- 146.Cohen LS, Heller VL, Bailey JW, Grush L, Ablon JS, Bouffard SM. Birth outcomes following prenatal exposure to fluoxetine. Biol Psychiatry. 2000;48(10):996–1000. doi: 10.1016/s0006-3223(00)00877-5. [DOI] [PubMed] [Google Scholar]
- 147.Kulin NA, Pastuszak A, Sage SR, et al. Pregnancy outcome following maternal use of the new selective serotonin reuptake inhibitors: a prospective controlled multicenter study.[see comment] Journal of the American Medical Association. 1998;279(8):609–610. doi: 10.1001/jama.279.8.609. [DOI] [PubMed] [Google Scholar]
- 148.Suri R, Altshuler L, Hendrick V, Rasgon N, Lee E, Mintz J. The impact of depression and fluoxetine treatment on obstetrical outcome. Arch Women Ment Health. 2004 Jul;7(3):193–200. doi: 10.1007/s00737-004-0057-5. [DOI] [PubMed] [Google Scholar]
- 149.Lattimore KA, Donn SM, Kaciroti N, Kemper AR, Neal CR, Vazquez DM. Selective Serotonin Reuptake Inhibitor (SSRI) Use during Pregnancy and Effects on the Fetus and Newborn: A Meta-Analysis. J Perinatol. 2005 Sep;25(9):595–604. doi: 10.1038/sj.jp.7211352. [DOI] [PubMed] [Google Scholar]
- 150.Gentile S. The safety of newer antidepressants in pregnancy and breastfeeding. Drug Saf. 2005;28(2):137–152. doi: 10.2165/00002018-200528020-00005. [DOI] [PubMed] [Google Scholar]
- 151.Moses-Kolko EL, Bogen D, Perel J, et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors: literature review and implications for clinical applications. Jama. 2005 May 18;293(19):2372–2383. doi: 10.1001/jama.293.19.2372. [DOI] [PubMed] [Google Scholar]
- 152.Nordeng H, Spigset O. Treatment with selective serotonin reuptake inhibitors in the third trimester of pregnancy: effects on the infant. Drug Saf. 2005;28(7):565–581. doi: 10.2165/00002018-200528070-00002. [DOI] [PubMed] [Google Scholar]
- 153.Kallen B. Neonate characteristics after maternal use of antidepressants in late pregnancy. Arch Pediatr Adolesc Med. 2004 Apr;158(4):312–316. doi: 10.1001/archpedi.158.4.312. [DOI] [PubMed] [Google Scholar]
- 154.Sanz EJ, De-las-Cuevas C, Kiuru A, Bate A, Edwards R. Selective serotonin reuptake inhibitors in pregnant women and neonatal withdrawal syndrome: a database analysis. Lancet. 2005 Feb 5–11;365(9458):482–487. doi: 10.1016/S0140-6736(05)17865-9. [DOI] [PubMed] [Google Scholar]
- 155.Levinson-Castiel R, Merlob P, Linder N, Sirota L, Klinger G. Neonatal abstinence syndrome after in utero exposure to selective serotonin reuptake inhibitors in term infants. Arch Pediatr Adolesc Med. 2006 Feb;160(2):173–176. doi: 10.1001/archpedi.160.2.173. [DOI] [PubMed] [Google Scholar]
- 156.Lainwala S, Brown ER, Weinschenk NP, Blackwell MT, Hagadorn JI. A retrospective study of length of hospital stay in infants treated for neonatal abstinence syndrome with methadone versus oral morphine preparations. Adv Neonatal Care. 2005 Oct;5(5):265–272. doi: 10.1016/j.adnc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 157.Dean JC, Hailey H, Moore SJ, Lloyd DJ, Turnpenny PD, Little J. Long term health and neurodevelopment in children exposed to antiepileptic drugs before birth. J Med Genet. 2002 Apr;39(4):251–259. doi: 10.1136/jmg.39.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fulroth R, Phillips B, Durand DJ. Perinatal outcome of infants exposed to cocaine and/or heroin in utero. American Journal of Disease in Childhood. 1989 Aug;143(8):905–910. doi: 10.1001/archpedi.1989.02150200057018. [DOI] [PubMed] [Google Scholar]
- 159.Zeskind PS, Stephens LE. Maternal selective serotonin reuptake inhibitor use during pregnancy and newborn neurobehavior. Obstet Gynecol Surv. 2004;59(8):564–566. doi: 10.1542/peds.113.2.368. [DOI] [PubMed] [Google Scholar]
- 160.Oberlander TF, Eckstein Grunau R, Fitzgerald C, et al. Prolonged prenatal psychotropic medication exposure alters neonatal acute pain response. Pediatr Res. 2002 Apr;51(4):443–453. doi: 10.1203/00006450-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 161.Oberlander TF, Grunau RE, Fitzgerald C, Papsdorf M, Rurak D, Riggs W. Pain reactivity in 2-month-old infants after prenatal and postnatal serotonin reuptake inhibitor medication exposure. Pediatrics. 2005;115(2):411–425. doi: 10.1542/peds.2004-0420. [DOI] [PubMed] [Google Scholar]
- 162.Oberlander TF, Grunau R, Mayes L, et al. Hypothalamic-pituitary-adrenal (HPA) axis function in 3-month old infants with prenatal selective serotonin reuptake inhibitor (SSRI) antidepressant exposure. Early Hum Dev. 2008 Oct;84(10):689–697. doi: 10.1016/j.earlhumdev.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008 Mar–Apr;3(2):97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- 164.Morrison JL, Rurak DW, Chien C, et al. Maternal fluoxetine infusion does not alter fetal endocrine and biophysical circadian rhythms in pregnant sheep. J Soc Gynecol Investig. 2005 Jul;12(5):356–364. doi: 10.1016/j.jsgi.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 165.Vogel GW. A review of REM sleep deprivation. Arch Gen Psychiatry. 1975 Jun;32(6):749–761. doi: 10.1001/archpsyc.1975.01760240077006. [DOI] [PubMed] [Google Scholar]
- 166.Portas CM, Bjorvatn B, Ursin R. Serotonin and the sleep/wake cycle: special emphasis on microdialysis studies. Prog Neurobiol. 2000 Jan;60(1):13–35. doi: 10.1016/s0301-0082(98)00097-5. [DOI] [PubMed] [Google Scholar]
- 167.Maeda K, Tatsumura M, Utsu M. Analysis of fetal movements by Doppler actocardiogram and fetal B-mode imaging. Clin Perinatol. 1999 Dec;26(4):829–851. [PubMed] [Google Scholar]
- 168.Maeda K. Computerized analysis of cardiotocograms and fetal movements. Baillieres Clin Obstet Gynaecol. 1990 Dec;4(4):797–813. doi: 10.1016/s0950-3552(05)80345-1. [DOI] [PubMed] [Google Scholar]
- 169.DiPietro JA, Costigan KA, Pressman EK. Fetal movement detection: comparison of the Toitu actograph with ultrasound from 20 weeks gestation. J Matern Fetal Med. 1999;8(6):237–242. doi: 10.1002/(SICI)1520-6661(199911/12)8:6<237::AID-MFM1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 170.de Vries JIP, Visser GHA, Prechtl HFR. The emergence of fetal behavior, I. Qualitative aspects. Early Human Development. 1982;7:301–322. doi: 10.1016/0378-3782(82)90033-0. [DOI] [PubMed] [Google Scholar]
- 171.Salisbury AL, Fallone MD, Lester B. Neurobehavioral assessment from fetus to infant: The NICU network neurobehavioral scale and the fetal neurobehavior coding scale. Ment Retard Dev Disabil Res Rev. 2005;11(1):14–20. doi: 10.1002/mrdd.20058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pillai M, James DK, Parker M. The development of ultradian rhythms in the human fetus. Am J Obstet Gynecol. 1992;167(1):172–177. doi: 10.1016/s0002-9378(11)91654-8. [DOI] [PubMed] [Google Scholar]
- 173.DiPietro JA, Hodgson DM, Costigan KA, Hilton SC, Johnson TR. Development of fetal movement--fetal heart rate coupling from 20 weeks through term. Early Hum Dev. 1996;44(2):139–151. doi: 10.1016/0378-3782(95)01704-6. [DOI] [PubMed] [Google Scholar]
- 174.Fox SB, Khong TY. Lack of innervation of human umbilical cord. An immunohistological and histochemical study. Placenta. 1990 Jan–Feb;11(1):59–62. doi: 10.1016/s0143-4004(05)80443-6. [DOI] [PubMed] [Google Scholar]
- 175.Walker DW, McLean JR. Absence of adrenergic nerves in the human placenta. Nature. 1971 Jan 29;229(5283):344–345. doi: 10.1038/229344a0. [DOI] [PubMed] [Google Scholar]
- 176.Kobayashi K, Morita S, Sawada H, et al. Targeted disruption of the tyrosine hydroxylase locus results in severe catecholamine depletion and perinatal lethality in mice. J Biol Chem. 1995 Nov 10;270(45):27235–27243. doi: 10.1074/jbc.270.45.27235. [DOI] [PubMed] [Google Scholar]
- 177.Zhou QY, Quaife CJ, Palmiter RD. Targeted disruption of the tyrosine hydroxylase gene reveals that catecholamines are required for mouse fetal development. Nature. 1995 Apr 13;374(6523):640–643. doi: 10.1038/374640a0. [DOI] [PubMed] [Google Scholar]
- 178.Thomas SA, Matsumoto AM, Palmiter RD. Noradrenaline is essential for mouse fetal development. Nature. 1995 Apr 13;374(6523):643–646. doi: 10.1038/374643a0. [DOI] [PubMed] [Google Scholar]
- 179.Thomas SA, Palmiter RD. Examining adrenergic roles in development, physiology, and behavior through targeted disruption of the mouse dopamine beta-hydroxylase gene. Adv Pharmacol. 1998;42:57–60. doi: 10.1016/s1054-3589(08)60695-x. [DOI] [PubMed] [Google Scholar]
- 180.Yavarone MS, Shuey DL, Tamir H, Sadler TW, Lauder JM. Serotonin and cardiac morphogenesis in the mouse embryo. Teratology. 1993;47(6):573–584. doi: 10.1002/tera.1420470609. [DOI] [PubMed] [Google Scholar]
- 181.Shuey D, Sadler T, Tamir H, Lauder J. Transient expression of serotonin uptake and binding protein during craniofacial morphogenesis in the mouse. Anat Embryol (Berl) 1993;187(1):75–85. doi: 10.1007/BF00208198. [DOI] [PubMed] [Google Scholar]
- 182.Choi D, Ward S, Messaddeq N, Launay J, Maroteaux L. 5-HT2B receptor-mediated serotonin morphogenetic functions in mouse cranial neural crest and myocardiac cells. Development. 1997;124:1745–1755. doi: 10.1242/dev.124.9.1745. [DOI] [PubMed] [Google Scholar]
- 183.Church MW, Rauch HC. Prenatal cocaine exposure in the laboratory mouse: effects on maternal water consumption and offspring outcome. Neurotoxicol Teratol. 1992;14(5):313–319. doi: 10.1016/0892-0362(92)90037-b. [DOI] [PubMed] [Google Scholar]
- 184.Jayanthi LD, Ramamoorthy S. Regulation of monoamine transporters: influence of psychostimulants and therapeutic antidepressants. Aaps J. 2005;7(3):E728–738. doi: 10.1208/aapsj070373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008 Sep 3;28(36):9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Seidler FJ, Slotkin TA. Fetal cocaine exposure causes persistent noradrenergic hyperactivity in rat brain regions: effects on neurotransmitter turnover and receptors. J Pharmacol Exp Ther. 1992 Nov;263(2):413–421. [PubMed] [Google Scholar]
- 187.Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends in Neuroscience. 1997 Feb;20(2):78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 188.Hughes H, Grose E, Dow-Edwards D. Perinatal exposure to cocame or fluoxetine interacts with gender in predicting motor activity following quipazine injection in adult rats. Teratology. 1992;47 MBTS 22. [Google Scholar]
- 189.Lester BM, Boukydis CF, Garcia-Coll CT, et al. Developmental outcome as a function of the goodness of fit between the infant’s cry characteristics and the mother’s perception of her infant’s cry. Pediatrics. 1995;95(4):516–521. [PubMed] [Google Scholar]
- 190.Lester BM, Lagasse L, Seifer R, et al. The Maternal Lifestyle Study (MLS): effects of prenatal cocaine and/or opiate exposure on auditory brain response at one month. Journal of Pediatrics. 2003 Mar;142(3):279–285. doi: 10.1067/mpd.2003.112. [DOI] [PubMed] [Google Scholar]
- 191.Peters DA. Prenatal stress: effects on brain biogenic amine and plasma corticosterone levels. Pharmacology, Biochemistry and Behavior. 1982 Oct;17(4):721–725. doi: 10.1016/0091-3057(82)90353-7. [DOI] [PubMed] [Google Scholar]
- 192.Lakshmi V, Nath N, Muneyyirci-Delale O. Characterization of 11 beta-hydroxysteroid dehydrogenase of human placenta: evidence for the existence of two species of 11 beta-hydroxysteroid dehydrogenase. J Steroid Biochem Mol Biol. 1993 May;45(5):391–397. doi: 10.1016/0960-0760(93)90008-k. [DOI] [PubMed] [Google Scholar]
- 193.Salisbury AL, Lester BM, Seifer R, et al. Prenatal cocaine use and maternal depression: effects on infant neurobehavior. Neurotoxicol Teratol. 2007 May–Jun;29(3):331–340. doi: 10.1016/j.ntt.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Matthews SG. Antenatal glucocorticoids and the developing brain: mechanisms of action. Semin Neonatol. 2001 Aug;6(4):309–317. doi: 10.1053/siny.2001.0066. [DOI] [PubMed] [Google Scholar]