Abstract
Secondary acute leukemia is a devastating complication in children and adolescents who have been treated for cancer. Secondary acute lymphoblastic leukemia (s-ALL) was previously rarely reported but can now be distinguished from recurrent primary ALL by comparison of immunoglobulin and T-cell receptor rearrangement. Secondary acute myeloid leukemia (s-AML) is much more common, and some cases may actually be second primary cancers. Treatment- and host-related characteristics and their interactions have been identified as risk factors for s-AML. The most widely recognized treatment-related risk factors are alkylating agents and topoisomerase II inhibitors (epipodophyllotoxins and anthracyclines). The magnitude of the risk associated with these factors depends on several variables, including the administration schedule, concomitant medications, and host factors. A high cumulative dose of alkylating agents is well known to predispose to s-AML. The prevalence of alkylator-associated s-AML has diminished among pediatric oncology patients with the reduction of cumulative alkylator dose and limited use of the more leukemogenic alkylators. The best-documented topoisomerase II inhibitor–associated s-AML is s-AML associated with epipodophyllotoxins. The risk of s-AML in these cases is influenced by the schedule of drug administration and by interaction with other antineoplastic agents but is not consistently related to cumulative dose. The unpredictable risk of s-AML after epipodophyllotoxin therapy may discourage the use of these agents even in patients at high risk of relapse, although the benefit of relapse prevention may outweigh the risk of s-AML. Studies in survivors of adult cancers suggest that contrary to previous beliefs, the outcome of s-AML is not necessarily worse than that of de novo AML when adjusted for cytogenetic features. More studies are needed to confirm this finding in the pediatric patient population.
Keywords: Secondary leukemia, cancer survivor, epipodophyllotoxins, alkylating agents, AML, MDS, ALL
INTRODUCTION
As contemporary therapy increases the increases the survival of patients with pediatric malignancies, the sequelae of cancer treatment are of increasing concern. The development of a second cancer is one of the most devastating and potentially life-threatening sequelae of childhood cancer. A second cancer may be of any histologic subtype, from benign, low-grade tumors to high-grade malignancies such as acute myeloid leukemia (AML)/myelodysplastic syndrome and acute lymphoblastic leukemia (ALL). There is compelling evidence that specific therapies are etiologic agents of secondary leukemogenesis.(1–4) In some cases, host factors may also contribute.(5;6) Here we review current knowledge about the incidence of second hematological malignancies and risk factors for their development in children and adolescents previously treated for cancer. Where information is not available from pediatric studies, we include data derived from adult cancer patients. In addition, we provide a novel mortality-based analysis of the risk-benefit ratio of epipodophyllotoxin administration in patients with ALL by comparing the risk of death from ALL relapse to the risk of death from secondary AML (s-AML).
AML AS A SECONDARY MALIGNANCY
“Secondary” AML or “second de novo” AML?
The terms “secondary AML” and “treatment-related AML” are often used interchangeably to describe AML for which previous cytotoxic therapy is considered to have contributed to its etiology. This designation includes cases of myelodysplastic syndrome (MDS) and chronic myeloproliferative disorder.(7) However, some investigators have reported cases of AML occurring as a second cancer that cannot be attributed to previous cancer chemotherapy or radiation. Examples include AML as a second cancer in patients whose sole therapy was surgical resection of the primary cancer.(8;9) These cases are now hypothesized to be “second de novo” cancers because leukemogenesis is likely to reflect genetic predisposition to multiple primary cancers, as opposed to genotoxicity caused by chemotherapy or radiotherapy. This hypothesis is supported by the high incidence of AML and other cancers in patients with specific genetic disorders like Down syndrome(10) and Fanconi anemia.(11) The incidence of cancer is also significantly higher in first-degree relatives of patients with s-AML than in those of patients with de novo AML.(8)
While the prognosis of s-AML is often considered to be less favorable than that of de novo AML, a similarly favorable prognosis is reported for de novo and secondary acute promyelocytic leukemia (APL).(9;12) However, because a significant proportion of “secondary” APL seems to be unrelated to prior therapies(8;9) and because of the clinical similarity of de novo APL and APL following other tumors, some cases of “secondary” APL are considered to be second primary malignancies.(12)
Risk factors
Chemotherapeutic agents
The combinations of cytotoxic and biologic agents and modalities used to treat pediatric cancer hinder elucidation of the factors that contribute to s-AML (Table 1). Moreover, unknown host factors may confound the calculated risk estimates and compromise their predictive accuracy. Nevertheless, compelling data indicate that treatment with alkylating agents and topoisomerase II inhibitors (epipodophyllotoxins and anthracyclines) increases the probability of s-AML.
Table 1.
Factors associated with the risk of s-AML
| Category | Specific factor | Reference |
|---|---|---|
| Chemotherapeutic agents | Topoisomerase II inhibitors | (3;95) |
| Alkylating agents | (4) | |
| Other medications | Dexrazoxane | (44) |
| Azathioprine | (50) | |
| G-CSF | (39;46;47) | |
| Radiotherapy | (96) | |
| Host factors | Predisposing genetic abnormalities | (97) |
| Original cancer | (24;98) |
G-CSF, granulocyte colony-stimulating factor
Alkylating agents
Alkylating agent–related s-AML is often preceded by MDS with losses or deletions of chromosome 5 or 7 (Table 2). This type of s-AML tends to occur late (typically 5 to 7 years after therapy); the timing between the onset of MDS and s-AML varies and may be explained by the requirement for subsequent genetic events after the loss of material from chromosome 5 or 7.(4) The French-American-British (FAB) type is most commonly M1 or M2, in contrast to the myelomonocytic subtypes of epipodophyllotoxin-induced s-AML (Table 2).
Table 2.
Characteristics of alkylating agent- and topoisomerase II–related s-AML
| Feature | Epipodophyllotoxins | Anthracyclines Mitoxantrone |
Alkylating Agents |
|---|---|---|---|
| Genetic aberrations |
MLL rearrangements (common) AML1-ETO CBFβ-MYH11 PML-RARα |
PML-RARα AML1-ETO CBFβ-MYH11 MLL rearrangements (rare) |
Monosomy or partial deletions of chromosome 7 and 5 (common) |
| Mean interval between diagnosis of primary malignancy and secondary AML |
2–3 years | 2–3 years | 5–7 years |
| Common presentation |
Acute onset AML M4, M5 APL |
Acute onset AML M4, M5 APL |
Protracted onset usually AML M1, M2 preceded by MDS |
| Additional Risk Factors |
See Table 3 | High cumulative dosage Concomitant use of alkylating agents |
High cumulative dosage Young age Concomitant use of epipodophyllotoxins |
Abbreviations: MDS, myelodysplastic syndrome
During the early 1970s, several groups reported an excess risk of s-AML in adults and children with Hodgkin lymphoma who received MOPP (mechlorethamine, vincristine, procarbazine, and prednisone) chemotherapy or similar alkylating-agent regimens.(13–15) The Late Effects Study Group (LESG) observed that survivors of Hodgkin lymphoma who had been treated with alkylating agents at age 16 years or younger had a relative risk of leukemia almost 80 times that of population controls (standardized incidence ratio [SIR], 78.8; 95% CI, 56.6–123.2). The relative risk of s-AML in this group was 321.3 (95% CI, 207.5–467.1).(16) German and Austrian investigators subsequently observed a decline in the SIR to 122 (95% CI, 36–254) after the introduction of protocols with lower cumulative doses of alkylating agents that substituted cyclophosphamide for the more leukemogenic mechlorethamine.(17) The use of ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) instead of MOPP also significantly reduced the risk of s-AML.(18)
Unlike s-AML associated with topoisomerase II inhibitors, alkylating agent–related s-AML(4;19) appears to be dependent on the dose, but not the schedule of administration.(4) Moreover, some alkylating agents are more leukemogenic than others.(17) For example, mechlorethamine is more leukemogenic than cyclophosphamide.(17) Host factors also seem to play a crucial role in the development of alkylating agent–induced s-AML.(4) For example, a high incidence of second malignancies, including s-AML, has been reported in individuals with neurofibromatosis 1 who develop a first cancer. The increased susceptibility to s-AML in patients with neurofibromatosis 1 who were treated previously with alkylating agents was verified in Nf1 knockout mouse.(20) Patients with other genetic syndromes, such as Fanconi anemia, also exhibit susceptibility to alkylating agent–induced s-AML and MDS,(4) as do individuals with genetic polymorphisms that affect glutathione transferase theta 1 activity.(21)
Epipodophyllotoxins
Epipodophyllotoxin-induced s-AML was first described in the late 1980s(22–24) and has since been the characteristic model of s-AML. This type of s-AML is usually of the FAB M4 or M5 subtype, although other subtypes have been reported (Table 2).(23) Unlike alkylating agent–related s-AML, which occurs relatively late and often has a pre-leukemic phase, epipodophyllotoxin-related s-AML commonly presents as overt AML after a brief (usually 2 to 3 years) latency period (Table 2). The risk varies as a function of the schedule, the cumulative total dose, concomitant administration of other chemotherapeutic or supportive drug regimens, and the genetic make-up of the host. Table 3 summarizes the risk factors that have been documented.
Table 3.
Factors reported to influence the risk of epipodophyllotoxin-related AML
| Factor | References | Note |
|---|---|---|
| Frequency of administration | (1;2;26) | Weekly or twice-weekly schedule causes greater risk than every-other-week schedule. Administration for 5 consecutive days causes less risk than intermittent schedule. |
| Prolonged administration of low dose | (29) | May reduce risk. |
| Cumulative dose | (2;26;35) | Available data are inconsistent. |
| Asparaginase | (31;32) | |
| Anti-metabolites | (2;22) | |
| Alkylating agents | (36) | |
| G-CSF | (19;39;46–48) | Available data are inconsistent. |
| Primary cancer | (24;98) | |
| Host factors | (97) | Polymorphism of CYP3A, GST1 TPMT genes |
G-CSF, granulocyte colony-stimulating factor
Cumulative dose and schedule of epipodophyllotoxins
Data about the impact of cumulative epipodophyllotoxin dose on the risk of s-AML are contradictory. Some groups(24;25) have observed a significant excess risk of s-AML in patients treated with higher cumulative etoposide doses, although no specific threshold has been shown to be necessary for induction of leukemogenesis. Retain et al(25) observed that a median cumulative etoposide dose of 6,795 mg/m2 was more leukemogenic than a 3,025 mg/m2 dose in adults with advanced non-small-cell lung cancer. Le Deley et al (24) reported a 7-fold (95% CI, 2.6 to 19) greater risk of s-AML in children treated for solid tumors who received between 1,200 and 6,000 mg/m2 of epipodophyllotoxins or more than 170 mg/m2 of anthracyclines than in those who received lower doses or none of these drugs. However, these dose relationships have not been confirmed by other investigators.(2;26)
The findings of several studies suggest that the schedule of administration of epipodophyllotoxins is more important than the cumulative dose.(1;2) St. Jude investigators compared frequent intermittent (once or twice weekly) administration of etoposide to other schedules (during induction therapy only or every other week) in children with ALL. The frequent intermittent schedule was associated with a greater risk of s-AML (6-year cumulative incidence [SE], 8.3% [3.0%] for weekly schedule and 7.1% [2.8%] for twice weekly schedule) than the other schedules (0%–2.0% [1.2%] for induction only or every other week)(P=.02).(1;2) A review by Cancer Therapy Evaluation Program (CTEP) investigators also determined that the likelihood of s-AML after treatment with epipodophyllotoxins is not dose-dependent. The 6-year cumulative incidence of s-AML in groups that received low (< 1.5 g/m2), moderate (1.5 to 2.99 g/m2), and higher (≥ 3.0 g/m2) cumulative doses of etoposide was 3.3%, (95% upper confidence limit, 5.9%), 0.7% (1.6%), and 2.2%, (4.6%), respectively. The authors also noted that patients with solid tumors had a lower frequency of s-AML than those with leukemia, which they attributed to the different dosing schedules of epipodophyllotoxins.(26) In regimens for solid tumors, etoposide is commonly administered for five consecutive days, whereas intermittent dosing schedules are used in treating leukemia. These investigators speculated that mutant cells that had undergone leukemogenic recombination did not survive the more protractedschedule.(26) It is also plausible that the hematopoietic cells of patients with leukemia are more vulnerable to genotoxic events that predispose to s-AML. Indeed, patients treated for certain solid tumors (e.g. retinoblastoma) appear to be less susceptible to s-AML than patients with other tumors despite the use of epipodophyllotoxins.(24;27) Regimens for relapsed tumors and for palliative care often call for continuous administration of low-dose etoposide.(28;29) Although the limited survival of this patient population obscures the true incidence of s-AML, this approach appears to confer a low risk of s-AML.(29) Consistent with this observation are the results of in vitro studies showing a greater ratio of cytotoxicity to genetic recombination after prolonged exposure to etoposide than after brief exposure.(30)
Concomitant chemotherapy agents
The results of a few studies suggest that asparaginase administration enhances the risk of epipodophyllotoxin-induced s-AML.(31;32) The precise mechanism is not known, but Relling speculated that the lower protein levels generated by asparaginase decrease the synthesis of some proteins involved in protection from etoposide-induced recombinogenesis.(33) If this premise is correct, the high incidence of s-AML in the Pediatric Oncology Group (POG) 8704 study may be explained by chronic exposure to high-dose asparaginase (25,000 IU/m2) administered weekly for 20 weeks.(32) Likewise, St. Jude investigators postulated that asparaginase exposure immediately before epipodophyllotoxin administration accounted for the increased incidence of s-AML.(31) In that study, asparaginase was given every 4 weeks, which probably resulted in consistent suppression of the plasma protein level. The combination of epipodophyllotoxins and alkylating agents (e.g. cisplatin) (25;34–36) or antimetabolites (e.g., mercaptopurine or methotrexate)(2;22) has also been associated with an increased incidence of s-AML.
Anthracyclines and mitoxantrone
The administration of topoisomerase II inhibitors other than epipodophyllotoxins is also associated with an increased risk of s-AML; these agents include anthracyclines and anthracenediones (mitoxantrone). Their impact tends to be underrated because of the substantial risk associated with epipodophyllotoxins, but their potential leukemogenic activity should be considered. In fact, it appears only 5 of the 24 patients treated on CCG 2891 who later had s-AML(37) received epipodophyllotoxins. Most of these patients had received anthracyclines and/or cyclophosphamide. A St. Jude series identified four patients with s-AML involving 11q23 and 21q23 abnormalities(34) among those whose prior therapy included doxorubicin, cyclophosphamide, and radiation therapy but not epipodophyllotoxins.
The clinical and cytogenetic features of anthracycline-related s-AML resemble those of epipodophyllotoxin-related s-AML (Table 2), but other chromosomal abnormalities have been reported. A large study of adults with APL treated with all-trans retinoic acid and anthracycline monochemotherapy identified cytogenetic abnormalities involving chromosomes 5 and 7 that are characteristic of alkylating agent-associated s-AML.(38) The role of the dosing schedule or cumulative dose of anthracyclines in the development of s-AML has not been established. (19;34)
Several studies of adults with cancer have demonstrated an excess risk of s-AML related to anthracenedione therapy. In a large case-control study of patients with breast cancer, those treated with an anthracenedione-based regimen featuring mitoxantrone had a much higher risk of s-AML than those who received an anthracycline-based regimen.(39) This finding has been confirmed by other groups.(40–42) Adults who developed s-AML after receiving mitoxantrone-based chemotherapy for acute leukemia had cytogenetic abnormalities most frequently involving chromosomes 7q, 20q, 1q, and 13q, but not the 11q23 abnormalities typically associated with topoisomerase-II inhibitors.(43)
Non-chemotherapeutic agents
Several non-chemotherapeutic agents may also contribute to the development of s-AML, either independently or through interaction with cytotoxic antineoplastic agents. POG studies 9426 and 9425 evaluated the impact of the cardioprotectant dexrazoxane on outcomes of children with Hodgkin lymphoma treated with standard chemotherapy (doxorubicin, bleomycin, vincristine, and etoposide ± prednisone and cyclophosphamide) and low-dose radiation. Participants were randomly assigned to receive either dexrazoxane or no cardioprotectant before anthracycline.(44) Six of eight patients who developed s-AML and two who developed secondary solid tumors were in the dexrazoxane arm. The 4-year cumulative incidence of s-AML was 2.55% ± 1.0% with dexrazoxane and 0.85% ± 0.6% in the non-dexrazoxane group (P = .160). The SIR for s-AML was 613.6 (95% CI, 225.2–1335.6) among patients receiving dexrazoxane (n=239) and 202.4 (95% CI, 24.5–731.0) among those not receiving dexrazoxane (n=239)(P = .099). Theinvestigators speculated that dexrazoxane, a topoisomerase II inhibitor with a mechanism distinct from that of either epipodophyllotoxins or anthracyclines, may have a synergistic adverse effect on DNA repair when combined with etoposide. Interestingly, most cases of s-AML in this study did not exhibit the typical 11q23 translocation but had other cytogenetic abnormalities (including monosomy 7 and trisomy 8) usually associated with alkylating agent–induced s-AML. Similar results were not found in a study of high-risk ALL in children at Dana-Farber Cancer Institute. With a median follow-up of 6.2 years, only one patient developed a second cancer (melanoma), and this patient did not receive dexrazoxane; the incidence of second malignancy did not differ statistically between the groups that did (n=105) and did not (n=100) receive dexrazoxane (P = .66).(45)
G-CSF may also increase the incidence of s-AML, although reports have not been consistent. Relling et al. observed an increased risk of s-AML in pediatric patients with ALL who received G-CSF plus a regimen including alkylating agents, anthracyclines, and epipodophyllotoxins,(46). Conversely, Bhatia et al. found no association between G-CSF administration and s-AML in a group of children with Ewing sarcoma who received doxorubicin, vincristine, cyclophosphamide, and dactinomycin (regimen A) or these 4 drugs alternating with etoposide and ifosfamide (regimen B).(19) In adults, a few studies found G-CSF to be associated with an increased risk of s-AML in breast cancer patients,(39;47) but this finding was not replicated in a study of patients who were older (the median age 75.6 years, range 66 to 104 years) at breast cancer diagnosis.(48) Therefore, it is not clear whether G-CSF treatment induces s-AML, and it is not clear whether G-CSF enhances leukemogenesis associated with alkylating agents or topoisomerase II inhibitors.(49)
Immunosuppression associated with solid organ transplantation is a well known risk factor for lymphoma, but myeloid leukemia has been reported only rarely in transplant recipients. Offman et al. examined data from 170,000 recipients of solid organ transplants at more than 300 centers participating in the Collaborative Transplant Study.(50) The relative risk of s-AML in transplant recipients vs. age-, sex-, and geographically-matched controls was 5.5 (95% CI, 4.0–7.7; P < .0001) for heart/lung recipients and 2.1 (95% CI, 1.6–2.7; P < .0001) for kidney recipients. In that study, the incidence of s-AML was significantly higher in patients who received 2.0 to 3.0 mg/kg per day of azathioprine than in patients who received less than 1.0 mg/kg per day (P = .031).
Hematopoietic stem cell transplantation
A variety of second malignancies have been reported in recipients of hematopoietic stem cell transplants. Oncogenesis in these cases is probably multi-factorial.(51;52) Several studies have observed a higher incidence of s-AML in patients with lymphoma treated with autologous hematopoietic cell transplantation than in those who received conventional chemotherapy.(53–55) Pre-transplant therapy is likely an important contributor to leukemogenesis; preparative conditioning chemotherapy and total body irradiation for autologous transplantation are contributors as well, as are polymorphisms that govern drug metabolism and DNA repair during the extensive cellular proliferation associated with engraftment.(52) S-AML in transplant survivors usually exhibits features of alkylating agent–associated disease,(55)although it is not clear whether alkylating agents or other factors play a causative role.(54)
Host factors
Certain host factors contribute to susceptibility to s-AML. Several studies have demonstrated that specific polymorphisms of detoxification enzymes play an important role in secondary oncogenesis. Polymorphisms that reduce the enzymatic activity of thiopurine methyltransferase,(56) a variant of CYP3A that affects production of a DNA-damaging metabolite of epipodophyllotoxin,(57) and polymorphisms in glutathione S-transferase P1(58) and NAD(P)H:quinine oxidoreductase (NQO1)(59) are also associated with increased risk of s-AML after chemotherapy. Increased susceptibility to s-AML has also been linked to polymorphisms of DNA repair genes.(60;61) Emerging genome-wide approaches such as gene expression profiling(62) and single nucleotide polymorphism (SNP) arrays(63) are being utilized to understand the pathogenesis of s-AML and identify patients at risk. Further studies are needed to confirm the predictive value of these methods.
A higher incidence of s-AML has been observed in association with certain primary malignancies. For example, breast cancer often precedes acute promyelocytic leukemia (APL).(64) Le Deley et al also reported a high incidence of s-AML after pediatric Hodgkin lymphoma or osteosarcoma.(24) However, various confounding factors may affect the interpretation of these findings. For instance, Smith et al hypothesized that the incidence of s-AML was lower in pediatric patients with solid tumors than in those with ALL because of the different dosing schedule of epipodophyllotoxins.(26) Non-ocular secondary solid tumors are common in patients with retinoblastoma, but s-AML is rare in this population despite the common use of alkylating agents and topoisomerase II inhibitors.(27) This may be because the genotype that results in the retinoblastoma phenotype does not include hematopoietic stem cell abnormalities.(65)
Treatment and outcome of s-AML
The prognosis of s-AML is generally considered to be poorer than that of de novo AML.(66) The disease tends to be refractory to chemotherapy, and patients’ tolerance of treatment is generally reduced because of prior therapies. For these reasons, clinicians have been reluctant to use curative (i.e., highly intensive) therapies. Further, the survival rates of patients with s-AML are difficult to predict, as they are often affected by recurrence of the primary cancer. The outcome of treatment has been reported only for small series of pediatric patients with s-AML.(67–69) Twenty-four patients with s-AML treated on the Children’s Cancer Group (CCG) 2891 study had lower rates of remission induction, survival, and event-free survival than patients with de novo AML.(37) In that study, outcomes were better among patients (including patients with s-AML) randomly assigned to receive intensively timed induction therapy than among those who received standard-timed induction.
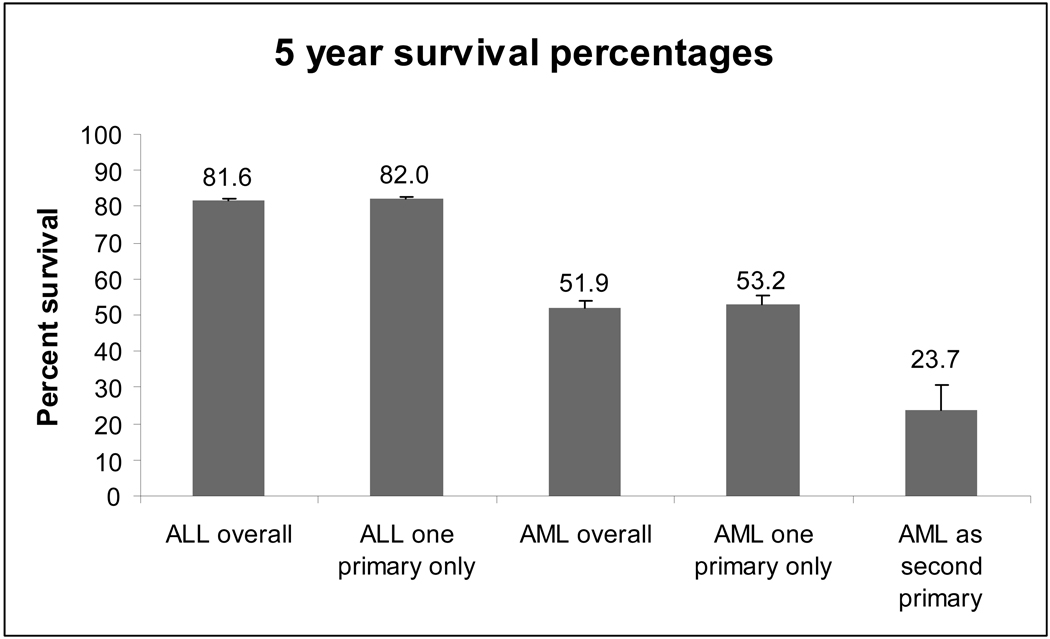
As a part of the current review, we used data from the Surveillance, Epidemiology and End Results (SEER) Cancer Registry to compare the outcomes of children with newly diagnosed ALL, AML, and s-AML.(70;71) Figure 1 shows the 2003 period estimate of 5-year survival by diagnostic category among children younger than 20 years at diagnosis. Children with s-AML had a 5-year survival rate (23.7%) significantly lower than that of children with AML as a first primary cancer (53.2%) (two-sample z-test comparing proportions=4.34, p < 0.001; Stata version 10.1).
Figure 1. SEER 2003 period estimates of survival at 5 years according to diagnosis.
The period method(99) was used in order to include only the most recent interval survival estimate of cases diagnosed in different calendar years (cross-sectional estimate of survival). Five years of data from the SEER17 registries(71) were used per survival cohort. SEER*Stat allows the calculation of period survival for only the latest possible year in the database. We calculated 5-year period survival by using 5-year cohorts for 2003. The first interval (1 year) used cases diagnosed in 1999–2003, the second interval (2 years) used cases diagnosed in 1998–2002, and so forth until there were 5 years of survival. This analysis therefore included cases diagnosed between 1995 and 2003.
Investigators in the German Cooperative Groups trials speculated that the comparatively low survival rate of s-AML patients (median age, 57 years; range, 16–82 years) resulted from the predominance of s-AML with unfavorable karyotypes. The presence of s-AML did not result in poor survival with standard intensive chemotherapy.(66;72) This finding has also been supported by the GIMEMA (Gruppo Italiano Malattie Ematologiche dell’ Adulto) study, in which poor survival was correlated with older age, lower performance status, and high co-morbidity after onset of s-AML during adulthood.(73) More recently, investigators at the Fred Hutchinson Cancer Research Center found that after hematopoietic stem cell transplantation, the outcome of pediatric and adult patients with s-AML was comparable to the outcome of patients with de novo AML, after adjustment for risk factors.(74) However, that study included patients with non-neoplastic primary diseases.(74)
Larson proposed a management algorithm for adult s-AML that uses performance status (age, co-morbidities, primary disease status, and complications of primary therapy) and karyotype.(75) According to the algorithm, patients with s-AML who have a good performance status should be treated similarly to patients with de novo AML who have the same cytogenetic abnormalities, i.e., chemotherapy alone for favorable cytogenetic features such as t(15;17), inv(16), and t(8;21); intensive chemotherapy and hematopoietic stem cell transplantation for other karyotypes; and more investigational therapy for unfavorable karyotypes. Supportive care alone may be warranted for those with poor performance status. Further studies are needed to determine whether this approach is applicable to pediatric patients.
When is the use of epipodophyllotoxins justified?
Epipodophyllotoxins are still commonly used to treat various solid tumors, Hodgkin lymphoma, and de novo AML in children. However, they have been eliminated from most frontline therapies for ALL.(76) Although clinicians are reluctant to use epipodophyllotoxins, almost all standard ALL regimens include anthracyclines and cyclophosphamide, which are also common causes of s-AML. Moreover, the contribution of epipodophyllotoxins to the overall success of frontline ALL therapies has not been established. Large clinical trials (77;78) have shown that most cases of pediatric ALL can be cured with regimens that do not include epipodophyllotoxins. A few frontline regimens for very high-risk ALL(78) have included epipodophyllotoxins but did not establish their efficacy. It is possible that specific groups of patients with high-risk disease features could benefit from these agents. As a preliminary exploration of this possibility, we used existing data to compare the relative risk of relapse of ALL to that of s-AML in children with ALL.
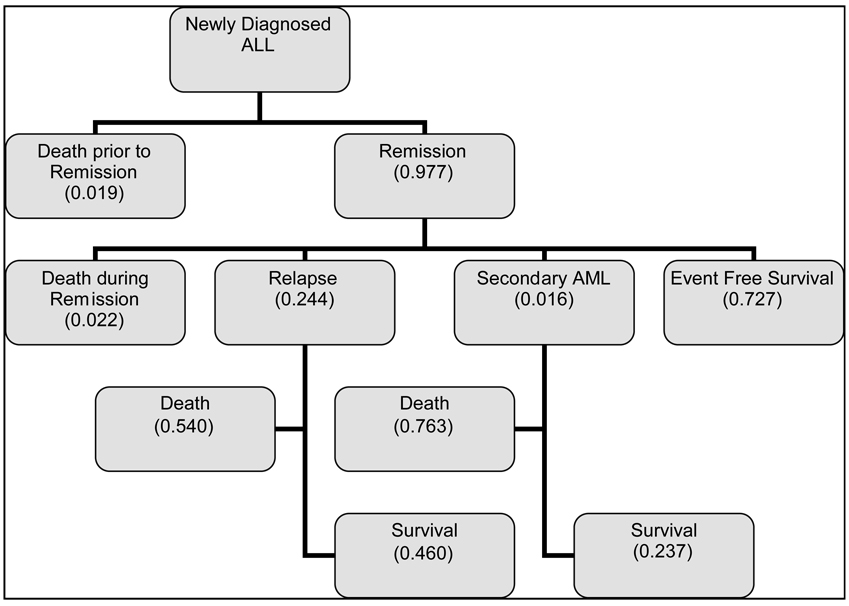
Figure 2 summarizes and estimates the proportions of potential outcomes of children within 5 years after diagnosis of ALL. Estimated proportions are either means calculated from reports of contemporary trials (79–89) or were derived from SEER data (survival after secondary AML).(90) These estimates indicate that the proportion of children who had a first remission but died because of relapse is approximately 0.13 (0.24*0.54). The proportion of children who had a first remission but died because of s-AML is estimated at 0.01 (0.016*0.763). The 12% (95% CI, 5.1–18.9%) difference between these two proportions is substantial and significant (P =.001).
Figure 2. Early outcomes and their proportions for children with newly diagnosed ALL.
Because many other host- and treatment-related factors may influence survival, we cannot identify the specific contributions of epipodophyllotoxins to the total risk of death from s-AML. However, while acknowledging confounding survival factors and differences between risk groups in published trials, we can use relapse-related and s-AML–related death rates to estimate the minimum event-free survival rate at which the risk of death from s-AML is less than the risk of death from relapse. At this point, clinicians considering the risks and benefits of treatment for an individual patient may wish to explore the role of epipodophyllotoxins given on an appropriate dose schedule to reduce the risk of relapse. Using the highest reported incidence of epipodophyllotoxin-related s-AML (0.083)(1) and the survival estimates from Figure 2, we estimate that the risk of death from s-AML exceeds the risk of death from relapse in a child with newly diagnosed ALL when the probability of relapse is 0.117 or higher.
This result indicates, in theory, that the risk of death from relapse of ALL exceeds the risk of death from s-AML when the survival rate of ALL is less than 88.3%. In reality, any pediatric oncologist would hesitate to use epipodophyllotoxins in a patient with an excellent (>80%) chance of survival. Moreover, most clinicians view s-AML as a treatment complication that should be avoided at all costs. Nonetheless, it appears that there is a subset of patients for whom treatment with epipodophyllotoxins may provide a benefit in optimizing disease control. Moreover, factors such as the administration schedule, combination with other agents, and host characteristics can modify the risk of s-AML.
ALL AS A SECONDARY MALIGNANCY
While clinicians are well aware of s-AML, ALL following primary cancer is considered very rare and usually receives little attention.(91–93) It is not known whether those cases are secondary to the primary cancer or represent a second primary cancer. In this section, we will term all ALL following cancer “secondary ALL (s-ALL).” Only 5% to 10% of all secondary acute leukemias are ALL.(93) S-ALL has been reported after primary ALL(1;91) as well as after various other cancers.(92;93)
Because cases of s-ALL after primary ALL are rare, most cases have probably been misdiagnosed as recurrent ALL. In 14 consecutive ALL studies at St. Jude over a 30-year period, only 2 of 2304 patients with primary ALL had a diagnosis of s-ALL.(1) In recent years, molecular detection of immunoglobulin and T-cell receptor rearrangements has facilitated identification of s-ALL. Using these methods, Zuna et al(91) estimated that 0.5%–1.5% of 366 cases of “relapsed” ALL were actually s-ALL. The malignant clones in all of these cases differed from those present at the time of diagnosis. The duration of first complete remission ranged from 1.7 to 6.5 years. These authors proposed diagnostic criteria for s-ALL while acknowledging the obstacles to its definitive diagnosis (Table 4). Another recent study(94) identified completely different T-cell receptor gene rearrangement at diagnosis and late “relapse” of T-ALL in 5 of 16 patients, suggesting the diagnosis of secondary T-cell ALL rather than relapse. Further, all patients remained in complete remission after retrieval therapy, which is an unusually good outcome for patients with relapsed T-ALL. Our knowledge will increase as more cases of s-ALL are identified using modern technologies.
Table 4.
Diagnostic criteria for secondary ALL proposed by Zuna et al(91) (Permission to cite the table was obtained from Dr Zuna and Nature Publishing Group.)
| A) Essential factor | No relationship between ALL clones at diagnosis and at recurrence (immunoglobulin/T-cell receptor gene arrangements, fusion genes at DNA level, cytogenetic markers) |
| B) Additional factors |
|
(A) plus at least one (B) criterion should be fulfilled for secondary ALL
SUMMARY
Numerous studies have confirmed that treatment with topoisomerase II inhibitors (epipodophyllotoxins and anthracyclines) and alkylating agents increases the probability of s-AML. The risk of s-AML is influenced by treatment factors, including the schedule of administration and concomitant medications. The role of host factors, such as polymorphisms of detoxification enzymes and primary tumors, should be considered as well. The risks and benefits of using epipodophyllotoxins in frontline pediatric cancer treatment regimens are often unclear. The benefit of epipodophyllotoxins may outweigh the risk of s-AML in some cases of high-risk childhood ALL, although more studies are needed to confirm this possibility. In addition, the probability of s-AML may be reduced by controlling or considering other risk factors, such as concomitantly administered drugs, administration schedule, and host characteristics. Recent studies have shown that the outcome of adults with s-AML does not differ from that of de novo AML when data are adjusted for unfavorable cytogenetic findings; therefore, the recommended treatment for adult patients with s-AML is the same as that used for de novo AML in the same cytogenetic risk group. More studies are needed to determine whether the same approach can be applied to pediatric patients. S-ALL has been reported very rarely, but more cases may be identified through modern technologies.
Acknowledgement
The authors thank Sharon Naron for expert editorial review.
REFERENCES
- 1.Hijiya N, Hudson MM, Lensing S, Zacher M, Onciu M, Behm FG, et al. Cumulative incidence of secondary neoplasms as a first event after childhood acute lymphoblastic leukemia. JAMA. 2007;297:1207–1215. doi: 10.1001/jama.297.11.1207. [DOI] [PubMed] [Google Scholar]
- 2.Pui CH, Ribeiro RC, Hancock ML, Rivera GK, Evans WE, Raimondi SC, et al. Acute myeloid leukemia in children treated with epipodophyllotoxins for acute lymphoblastic leukemia. N.Engl.J.Med. 1991;325:1682–1687. doi: 10.1056/NEJM199112123252402. [DOI] [PubMed] [Google Scholar]
- 3.Felix CA. Leukemias related to treatment with DNA topoisomerase II inhibitors. Med.Pediatr.Oncol. 2001;36:525–535. doi: 10.1002/mpo.1125. [DOI] [PubMed] [Google Scholar]
- 4.Davies SM. Therapy-related leukemia associated with alkylating agents. Med.Pediatr.Oncol. 2001;36:536–540. doi: 10.1002/mpo.1126. [DOI] [PubMed] [Google Scholar]
- 5.Blanco JG, Edick MJ, Hancock ML, Winick NJ, Dervieux T, Amylon MD, et al. Genetic polymorphisms in CYP3A5, CYP3A4 and NQO1 in children who developed therapy-related myeloid malignancies. Pharmacogenetics. 2002;12:605–611. doi: 10.1097/00008571-200211000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Woo MH, Shuster JJ, Chen C, Bash RO, Behm FG, Camitta B, et al. Glutathione S-transferase genotypes in children who develop treatment-related acute myeloid malignancies. Leukemia. 2000;14:232–237. doi: 10.1038/sj.leu.2401660. [DOI] [PubMed] [Google Scholar]
- 7.Larson RA. Is secondary leukemia an independent poor prognostic factor in acute myeloid leukemia? Best.Pract.Res.Clin.Haematol. 2007;20:29–37. doi: 10.1016/j.beha.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Pagana L, Pulsoni A, Tosti ME, Avvisati G, Mele L, Mele M, et al. Clinical and biological features of acute myeloid leukaemia occurring as second malignancy: GIMEMA archive of adult acute leukaemia. Br.J.Haematol. 2001;112:109–117. doi: 10.1046/j.1365-2141.2001.02527.x. [DOI] [PubMed] [Google Scholar]
- 9.Pulsoni A, Pagano L, Lo CF, Avvisati G, Mele L, Di Bona E, et al. Clinicobiological features and outcome of acute promyelocytic leukemia occurring as a second tumor: the GIMEMA experience. Blood. 2002;100:1972–1976. doi: 10.1182/blood-2001-12-0312. [DOI] [PubMed] [Google Scholar]
- 10.Hasle H. Pattern of malignant disorders in individuals with Down's syndrome. Lancet Oncol. 2001;2:429–436. doi: 10.1016/S1470-2045(00)00435-6. [DOI] [PubMed] [Google Scholar]
- 11.Mathew CG. Fanconi anaemia genes and susceptibility to cancer. Oncogene. 2006;25:5874–5884. doi: 10.1038/sj.onc.1209878. [DOI] [PubMed] [Google Scholar]
- 12.Pollicardo N, O'Brien S, Estey EH, al Bitar M, Pierce S, Keating M, et al. Secondary acute promyelocytic leukemia. Characteristics and prognosis of 14 patients from a single institution. Leukemia. 1996;10:27–31. [PubMed] [Google Scholar]
- 13.Kushner BH, Zauber A, Tan CT. Second malignancies after childhood Hodgkin's disease. The Memorial Sloan-Kettering Cancer Center experience. Cancer. 1988;62:1364–1370. doi: 10.1002/1097-0142(19881001)62:7<1364::aid-cncr2820620721>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 14.Meadows AT, Baum E, Fossati-Bellani F, Green D, Jenkin RD, Marsden B, et al. Second malignant neoplasms in children: an update from the Late Effects Study Group. J.Clin.Oncol. 1985;3:532–538. doi: 10.1200/JCO.1985.3.4.532. [DOI] [PubMed] [Google Scholar]
- 15.Meadows AT, Obringer AC, Marrero O, Oberlin O, Robison L, Fossati-Bellani F, et al. Second malignant neoplasms following childhood Hodgkin's disease: treatment and splenectomy as risk factors. Med.Pediatr.Oncol. 1989;17:477–484. [PubMed] [Google Scholar]
- 16.Bhatia S, Robison LL, Oberlin O, Greenberg M, Bunin G, Fossati-Bellani F, et al. Breast cancer and other second neoplasms after childhood Hodgkin's disease. N.Engl.J.Med. 1996;334:745–751. doi: 10.1056/NEJM199603213341201. [DOI] [PubMed] [Google Scholar]
- 17.Schellong G, Riepenhausen M, Creutzig U, Ritter J, Harbott J, Mann G, et al. Low risk of secondary leukemias after chemotherapy without mechlorethamine in childhood Hodgkin's disease. German-Austrian Pediatric Hodgkin's Disease Group. J.Clin.Oncol. 1997;15:2247–2253. doi: 10.1200/JCO.1997.15.6.2247. [DOI] [PubMed] [Google Scholar]
- 18.Cimino G, Papa G, Tura S, Mazza P, Rossi Ferrini PL, Bosi A, et al. Second primary cancer following Hodgkin's disease: updated results of an Italian multicentric study. J.Clin.Oncol. 1991;9:432–437. doi: 10.1200/JCO.1991.9.3.432. [DOI] [PubMed] [Google Scholar]
- 19.Bhatia S, Krailo MD, Chen Z, Burden L, Askin FB, Dickman PS, et al. Therapy-related myelodysplasia and acute myeloid leukemia after Ewing sarcoma and primitive neuroectodermal tumor of bone: A report from the Children's Oncology Group. Blood. 2007;109:46–51. doi: 10.1182/blood-2006-01-023101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahgoub N, Taylor BR, Le Beau MM, Gratiot M, Carlson KM, Atwater SK, et al. Myeloid malignancies induced by alkylating agents in Nf1 mice. Blood. 1999;93:3617–3623. [PubMed] [Google Scholar]
- 21.Chen H, Sandler DP, Taylor JA, Shore DL, Liu E, Bloomfield CD, et al. Increased risk for myelodysplastic syndromes in individuals with glutathione transferase theta 1 (GSTT1) gene defect. Lancet. 1996;347:295–297. doi: 10.1016/s0140-6736(96)90468-7. [DOI] [PubMed] [Google Scholar]
- 22.Winick NJ, McKenna RW, Shuster JJ, Schneider NR, Borowitz MJ, Bowman WP, et al. Secondary acute myeloid leukemia in children with acute lymphoblastic leukemia treated with etoposide. J.Clin.Oncol. 1993;11:209–217. doi: 10.1200/JCO.1993.11.2.209. [DOI] [PubMed] [Google Scholar]
- 23.Pui CH, Behm FG, Raimondi SC, Dodge RK, George SL, Rivera GK, et al. Secondary acute myeloid leukemia in children treated for acute lymphoid leukemia. N.Engl.J.Med. 1989;321:136–142. doi: 10.1056/NEJM198907203210302. [DOI] [PubMed] [Google Scholar]
- 24.Le Deley MC, Leblanc T, Shamsaldin A, Raquin MA, Lacour B, Sommelet D, et al. Risk of secondary leukemia after a solid tumor in childhood according to the dose of epipodophyllotoxins and anthracyclines: a case-control study by the Societe Francaise d'Oncologie Pediatrique. J.Clin.Oncol. 2003;21:1074–1081. doi: 10.1200/JCO.2003.04.100. [DOI] [PubMed] [Google Scholar]
- 25.Ratain MJ, Kaminer LS, Bitran JD, Larson RA, Le Beau MM, Skosey C, et al. Acute nonlymphocytic leukemia following etoposide and cisplatin combination chemotherapy for advanced non-small-cell carcinoma of the lung. Blood. 1987;70:1412–1417. [PubMed] [Google Scholar]
- 26.Smith MA, Rubinstein L, Anderson JR, Arthur D, Catalano PJ, Freidlin B, et al. Secondary leukemia or myelodysplastic syndrome after treatment with epipodophyllotoxins. J.Clin.Oncol. 1999;17:569–577. doi: 10.1200/JCO.1999.17.2.569. [DOI] [PubMed] [Google Scholar]
- 27.Gombos DS, Hungerford J, Abramson DH, Kingston J, Chantada G, Dunkel IJ, et al. Secondary acute myelogenous leukemia in patients with retinoblastoma: is chemotherapy a factor? Ophthalmology. 2007;114:1378–1383. doi: 10.1016/j.ophtha.2007.03.074. [DOI] [PubMed] [Google Scholar]
- 28.Kushner BH, Kramer K, Cheung NK. Oral etoposide for refractory and relapsed neuroblastoma. J.Clin.Oncol. 1999;17:3221–3225. doi: 10.1200/JCO.1999.17.10.3221. [DOI] [PubMed] [Google Scholar]
- 29.Hijiya N, Gajjar A, Zhang Z, Sandlund JT, Ribeiro RC, Rubnitz JE, et al. Low-dose oral etoposide-based induction regimen for children with acute lymphoblastic leukemia in first bone marrow relapse. Leukemia. 2004;18:1581–1586. doi: 10.1038/sj.leu.2403467. [DOI] [PubMed] [Google Scholar]
- 30.Chen CL, Fuscoe JC, Liu Q, Pui CH, Mahmoud HH, Relling MV. Relationship between cytotoxicity and site-specific DNA recombination after in vitro exposure of leukemia cells to etoposide. J Natl.Cancer Inst. 1996;88:1840–1847. doi: 10.1093/jnci/88.24.1840. [DOI] [PubMed] [Google Scholar]
- 31.Pui CH, Relling MV, Behm FG, Hancock ML, Boyett JM, Raimondi SC, et al. L-asparaginase may potentiate the leukemogenic effect of the epipodophyllotoxins. Leukemia. 1995;9:1680–1684. [PubMed] [Google Scholar]
- 32.Amylon MD, Shuster J, Pullen J, Berard C, Link MP, Wharam M, et al. Intensive high-dose asparaginase consolidation improves survival for pediatric patients with T cell acute lymphoblastic leukemia and advanced stage lymphoblastic lymphoma: a Pediatric Oncology Group study. Leukemia. 1999;13:335–342. doi: 10.1038/sj.leu.2401310. [DOI] [PubMed] [Google Scholar]
- 33.Relling MV. Therapeutic and Pharmacokinetic Considerations. In: Pui CH, editor. Treatment of Acute Leukemias; New Directions for Clinical Research. 1 ed. Totowa, NJ: Humana Press, Inc.; 2003. pp. 421–427. [Google Scholar]
- 34.Sandoval C, Pui CH, Bowman LC, Heaton D, Hurwitz CA, Raimondi SC, et al. Secondary acute myeloid leukemia in children previously treated with alkylating agents, intercalating topoisomerase II inhibitors, and irradiation. J.Clin.Oncol. 1993;11:1039–1045. doi: 10.1200/JCO.1993.11.6.1039. [DOI] [PubMed] [Google Scholar]
- 35.Pedersen-Bjergaard J, Philip P, Larsen SO, Andersson M, Daugaard G, Ersboll J, et al. Therapy-related myelodysplasia and acute myeloid leukemia. Cytogenetic characteristics of 115 consecutive cases and risk in seven cohorts of patients treated intensively for malignant diseases in the Copenhagen series. Leukemia. 1993;7:1975–1986. [PubMed] [Google Scholar]
- 36.Kushner BH, Cheung NK, Kramer K, Heller G, Jhanwar SC. Neuroblastoma and treatment-related myelodysplasia/leukemia: the Memorial Sloan-Kettering experience and a literature review. J.Clin.Oncol. 1998;16:3880–3889. doi: 10.1200/JCO.1998.16.12.3880. [DOI] [PubMed] [Google Scholar]
- 37.Barnard DR, Lange B, Alonzo TA, Buckley J, Kobrinsky JN, Gold S, et al. Acute myeloid leukemia and myelodysplastic syndrome in children treated for cancer: comparison with primary presentation. Blood. 2002;100:427–434. doi: 10.1182/blood.v100.2.427. [DOI] [PubMed] [Google Scholar]
- 38.Montesinos P, Gonzalez JD, Rayon C, de la Serna J, Bergua J, Esteve J, Tormo M, Vellenga E, Amutio E, Deben G, Sanz M. Secondary Acute Myeloid Leukemia and Myelodysplastic Syndrome Following ATRA and Anthracycline Monochemotherapy Treatment for Acute Promyelocytic Leukemia. Blood. 2007;110(11):544a. [Google Scholar]
- 39.Le Deley MC, Suzan F, Cutuli B, Delaloge S, Shamsaldin A, Linassier C, et al. Anthracyclines, mitoxantrone, radiotherapy, and granulocyte colony-stimulating factor: risk factors for leukemia and myelodysplastic syndrome after breast cancer. J.Clin.Oncol. 2007;25:292–300. doi: 10.1200/JCO.2006.05.9048. [DOI] [PubMed] [Google Scholar]
- 40.Saso R, Kulkarni S, Mitchell P, Treleaven J, Swansbury GJ, Mehta J, et al. Secondary myelodysplastic syndrome/acute myeloid leukaemia following mitoxantrone-based therapy for breast carcinoma. Br.J.Cancer. 2000;83:91–94. doi: 10.1054/bjoc.2000.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Linassier C, Barin C, Calais G, Letortorec S, Bremond JL, Delain M, et al. Early secondary acute myelogenous leukemia in breast cancer patients after treatment with mitoxantrone, cyclophosphamide, fluorouracil and radiation therapy. Ann.Oncol. 2000;11:1289–1294. doi: 10.1023/a:1008375016038. [DOI] [PubMed] [Google Scholar]
- 42.Chaplain G, Milan C, Sgro C, Carli PM, Bonithon-Kopp C. Increased risk of acute leukemia after adjuvant chemotherapy for breast cancer: a population-based study. J.Clin.Oncol. 2000;18:2836–2842. doi: 10.1200/JCO.2000.18.15.2836. [DOI] [PubMed] [Google Scholar]
- 43.Seiter K, Feldman EJ, Sreekantaiah C, Pozzuoli M, Weisberger J, Liu D, et al. Secondary acute myelogenous leukemia and myelodysplasia without abnormalities of chromosome 11q23 following treatment of acute leukemia with topoisomerase II-based chemotherapy. Leukemia. 2001;15:963–970. doi: 10.1038/sj.leu.2402122. [DOI] [PubMed] [Google Scholar]
- 44.Tebbi CK, London WB, Friedman D, Villaluna D, De Alarcon PA, Constine LS, et al. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin's disease. J.Clin.Oncol. 2007;25:493–500. doi: 10.1200/JCO.2005.02.3879. [DOI] [PubMed] [Google Scholar]
- 45.Barry EV, Vrooman LM, Dahlberg SE, Neuberg DS, Asselin BL, Athale UH, et al. Absence of secondary malignant neoplasms in children with high-risk acute lymphoblastic leukemia treated with dexrazoxane. J.Clin.Oncol. 2008;26:1106–1111. doi: 10.1200/JCO.2007.12.2481. [DOI] [PubMed] [Google Scholar]
- 46.Relling MV, Boyett JM, Blanco JG, Raimondi S, Behm FG, Sandlund JT, et al. Granulocyte colony-stimulating factor and the risk of secondary myeloid malignancy after etoposide treatment. Blood. 2003;101:3862–3867. doi: 10.1182/blood-2002-08-2405. [DOI] [PubMed] [Google Scholar]
- 47.Hershman D, Neugut AI, Jacobson JS, Wang J, Tsai WY, McBride R, et al. Acute myeloid leukemia or myelodysplastic syndrome following use of granulocyte colony-stimulating factors during breast cancer adjuvant chemotherapy. J.Natl.Cancer Inst. 2007;99:196–205. doi: 10.1093/jnci/djk028. [DOI] [PubMed] [Google Scholar]
- 48.Patt DA, Duan Z, Fang S, Hortobagyi GN, Giordano SH. Acute myeloid leukemia after adjuvant breast cancer therapy in older women: understanding risk. J.Clin.Oncol. 2007;25:3871–3876. doi: 10.1200/JCO.2007.12.0832. [DOI] [PubMed] [Google Scholar]
- 49.Smith RE, Bryant J, DeCillis A, Anderson S. Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: the National Surgical Adjuvant Breast and Bowel Project Experience. J.Clin.Oncol. 2003;21:1195–1204. doi: 10.1200/JCO.2003.03.114. [DOI] [PubMed] [Google Scholar]
- 50.Offman J, Opelz G, Doehler B, Cummins D, Halil O, Banner NR, et al. Defective DNA mismatch repair in acute myeloid leukemia/myelodysplastic syndrome after organ transplantation. Blood. 2004;104:822–828. doi: 10.1182/blood-2003-11-3938. [DOI] [PubMed] [Google Scholar]
- 51.Friedman DL, Leisenring W, Schwartz JL, Deeg HJ. Second malignant neoplasms following hematopoietic stem cell transplantation. Int.J.Hematol. 2004;79:229–234. doi: 10.1532/ijh97.03178. [DOI] [PubMed] [Google Scholar]
- 52.Hake CR, Graubert TA, Fenske TS. Does autologous transplantation directly increase the risk of secondary leukemia in lymphoma patients? Bone Marrow Transplant. 2007;39:59–70. doi: 10.1038/sj.bmt.1705547. [DOI] [PubMed] [Google Scholar]
- 53.Krishnan A, Bhatia S, Slovak ML, Arber DA, Niland JC, Nademanee A, et al. Predictors of therapy-related leukemia and myelodysplasia following autologous transplantation for lymphoma: an assessment of risk factors. Blood. 2000;95:1588–1593. [PubMed] [Google Scholar]
- 54.Lenz G, Dreyling M, Schiegnitz E, Haferlach T, Hasford J, Unterhalt M, et al. Moderate increase of secondary hematologic malignancies after myeloablative radiochemotherapy and autologous stem-cell transplantation in patients with indolent lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group. J.Clin.Oncol. 2004;22:4926–4933. doi: 10.1200/JCO.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 55.Micallef IN, Lillington DM, Apostolidis J, Amess JA, Neat M, Matthews J, et al. Therapy-related myelodysplasia and secondary acute myelogenous leukemia after high-dose therapy with autologous hematopoietic progenitor-cell support for lymphoid malignancies. J.Clin.Oncol. 2000;18:947–955. doi: 10.1200/JCO.2000.18.5.947. [DOI] [PubMed] [Google Scholar]
- 56.Relling MV, Yanishevski Y, Nemec J, Evans WE, Boyett JM, Behm FG, et al. Etoposide and antimetabolite pharmacology in patients who develop secondary acute myeloid leukemia. Leukemia. 1998;12:346–352. doi: 10.1038/sj.leu.2400928. [DOI] [PubMed] [Google Scholar]
- 57.Felix CA, Walker AH, Lange BJ, Williams TM, Winick NJ, Cheung NK, et al. Association of CYP3A4 genotype with treatment-related leukemia. Proc.Natl.Acad.Sci.U.S.A. 1998;95:13176–13181. doi: 10.1073/pnas.95.22.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allan JM, Wild CP, Rollinson S, Willett EV, Moorman AV, Dovey GJ, et al. Polymorphism in glutathione S-transferase P1 is associated with susceptibility to chemotherapy-induced leukemia. Proc.Natl.Acad.Sci.U.S.A. 2001;98:11592–11597. doi: 10.1073/pnas.191211198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Larson RA, Wang Y, Banerjee M, Wiemels J, Hartford C, Le Beau MM, et al. Prevalence of the inactivating 609C-->T polymorphism in the NAD(P)H:quinone oxidoreductase (NQO1) gene in patients with primary and therapy-related myeloid leukemia. Blood. 1999;94:803–807. [PubMed] [Google Scholar]
- 60.Seedhouse C, Faulkner R, Ashraf N, Das-Gupta E, Russell N. Polymorphisms in genes involved in homologous recombination repair interact to increase the risk of developing acute myeloid leukemia. Clin.Cancer Res. 2004;10:2675–2680. doi: 10.1158/1078-0432.ccr-03-0372. [DOI] [PubMed] [Google Scholar]
- 61.Leone G, Pagano L, Ben Yehuda D, Voso MT. Therapy-related leukemia and myelodysplasia: susceptibility and incidence. Haematologica. 2007;92:1389–1398. doi: 10.3324/haematol.11034. [DOI] [PubMed] [Google Scholar]
- 62.Yeoh EJ, Ross ME, Shurtleff SA, Williams WK, Patel D, Mahfouz R, et al. Classification, subtype discovery, and prediction of outcome in pediatric acute lymphoblastic leukemia by gene expression profiling. Cancer Cell. 2002;1:133–143. doi: 10.1016/s1535-6108(02)00032-6. [DOI] [PubMed] [Google Scholar]
- 63.Hartford C, Yang W, Cheng C, Fan Y, Liu W, Trevino L, et al. Genome scan implicates adhesion biological pathways in secondary leukemia. Leukemia. 2007;21:2128–2136. doi: 10.1038/sj.leu.2404885. [DOI] [PubMed] [Google Scholar]
- 64.Beaumont M, Sanz M, Carli PM, Maloisel F, Thomas X, Detourmignies L, et al. Therapy-related acute promyelocytic leukemia. J.Clin.Oncol. 2003;21:2123–2137. doi: 10.1200/JCO.2003.09.072. [DOI] [PubMed] [Google Scholar]
- 65.Weintraub M, Revel-Vilk S, Charit M, Aker M, Pe'er J. Secondary acute myeloid leukemia after etoposide therapy for retinoblastoma. J.Pediatr.Hematol.Oncol. 2007;29:646–648. doi: 10.1097/MPH.0b013e318142b561. [DOI] [PubMed] [Google Scholar]
- 66.Kern W, Haferlach T, Schnittger S, Hiddemann W, Schoch C. Prognosis in therapy-related acute myeloid leukemia and impact of karyotype. J.Clin.Oncol. 2004;22:2510–2511. doi: 10.1200/JCO.2004.99.301. [DOI] [PubMed] [Google Scholar]
- 67.Hale GA, Heslop HE, Bowman LC, Rochester RA, Pui CH, Brenner MK, et al. Bone marrow transplantation for therapy-induced acute myeloid leukemia in children with previous lymphoid malignancies. Bone Marrow Transplant. 1999;24:735–739. doi: 10.1038/sj.bmt.1701962. [DOI] [PubMed] [Google Scholar]
- 68.Leahey AM, Friedman DL, Bunin NJ. Bone marrow transplantation in pediatric patients with therapy-related myelodysplasia and leukemia. Bone Marrow Transplant. 1999;23:21–25. doi: 10.1038/sj.bmt.1701517. [DOI] [PubMed] [Google Scholar]
- 69.Sandler ES, Friedman DJ, Mustafa MM, Winick NJ, Bowman WP, Buchanan GR. Treatment of children with epipodophyllotoxin-induced secondary acute myeloid leukemia. Cancer. 1997;79:1049–1054. doi: 10.1002/(sici)1097-0142(19970301)79:5<1049::aid-cncr24>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 70.National Cancer Institute. Survellance Research Program, National Cancer Institute SEER*Stat software; SEER 9, 1990–2004, MP-SIR Event Analysis Event Record Frequencies. 2006 ( www.seer.cancer.gov/seerstat) version 6.3.6: Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 17 Regs Limited-Use, Nov 2006 Sub (1973–2004 varying) - Linked to County Attibutes - Total U.S., 1969–2004 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2007, based on the November 2006 submission.
- 71.National Cancer Institute. Survellance Research Program, National Cancer Institute SEER*Stat software; Overall period survival 2003 - ALL and AML Children < 20 years Allowing multiple primaries. 2006 ( www.seer.cancer.gov/seerstat) version 6.3.6: Surveillance, Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 17 Regs Limited-Use, Nov 2006 Sub (1973–2004 varying) - Linked to County Attibutes - Total U.S., 1969–2004 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2007, based on the November 2006 submission.
- 72.Schoch C, Kern W, Schnittger S, Hiddemann W, Haferlach T. Karyotype is an independent prognostic parameter in therapy-related acute myeloid leukemia (t-AML): an analysis of 93 patients with t-AML in comparison to 1091 patients with de novo AML. Leukemia. 2004;18:120–125. doi: 10.1038/sj.leu.2403187. [DOI] [PubMed] [Google Scholar]
- 73.Pulsoni A, Pagano L. Treatment of secondary acute myeloid leukemia. J.Clin.Oncol. 2005;23:926–927. doi: 10.1200/JCO.2005.05.202. [DOI] [PubMed] [Google Scholar]
- 74.Chang C, Storer BE, Scott BL, Bryant EM, Shulman HM, Flowers ME, et al. Hematopoietic cell transplantation in patients with myelodysplastic syndrome or acute myeloid leukemia arising from myelodysplastic syndrome: similar outcomes in patients with de novo disease and disease following prior therapy or antecedent hematologic disorders. Blood. 2007;110:1379–1387. doi: 10.1182/blood-2007-02-076307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Larson RA. Etiology and management of therapy-related myeloid leukemia. Hematology.Am.Soc.Hematol.Educ.Program. 2007;2007:453–459. doi: 10.1182/asheducation-2007.1.453. [DOI] [PubMed] [Google Scholar]
- 76.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N.Engl.J.Med. 2006;354:166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 77.Gaynon PS, Trigg ME, Heerema NA, Sensel MG, Sather HN, Hammond GD, et al. Children's Cancer Group trials in childhood acute lymphoblastic leukemia: 1983–1995. Leukemia. 2000;14:2223–2233. doi: 10.1038/sj.leu.2401939. [DOI] [PubMed] [Google Scholar]
- 78.Moricke A, Reiter A, Zimmermann M, Gadner H, Stanulla M, Dordelmann M, et al. Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood. 2008;111:4477–4489. doi: 10.1182/blood-2007-09-112920. [DOI] [PubMed] [Google Scholar]
- 79.Pui CH, Boyett JM, Rivera GK, Hancock ML, Sandlund JT, Ribeiro RC, et al. Long-term results of Total Therapy studies 11, 12 and 13A for childhood acute lymphoblastic leukemia at St Jude Children's Research Hospital. Leukemia. 2000;14:2286–2294. doi: 10.1038/sj.leu.2401938. [DOI] [PubMed] [Google Scholar]
- 80.Pui CH, Sandlund JT, Pei D, Campana D, Rivera GK, Ribeiro RC, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Total Therapy Study XIIIB at St Jude Children's Research Hospital. Blood. 2004;104:2690–2696. doi: 10.1182/blood-2004-04-1616. [DOI] [PubMed] [Google Scholar]
- 81.Vilmer E, Suciu S, Ferster A, Bertrand Y, Cave H, Thyss A, et al. Long-term results of three randomized trials (58831, 58832, 58881) in childhood acute lymphoblastic leukemia: a CLCG-EORTC report. Children Leukemia Cooperative Group. Leukemia. 2000;14:2257–2266. doi: 10.1038/sj.leu.2401960. [DOI] [PubMed] [Google Scholar]
- 82.Harms DO, Janka-Schaub GE. Co-operative study group for childhood acute lymphoblastic leukemia (COALL): long-term follow-up of trials 82, 85, 89 and 92. Leukemia. 2000;14:2234–2239. doi: 10.1038/sj.leu.2401974. [DOI] [PubMed] [Google Scholar]
- 83.Tsuchida M, Ikuta K, Hanada R, Saito T, Isoyama K, Sugita K, et al. Long-term follow-up of childhood acute lymphoblastic leukemia in Tokyo Children's Cancer Study Group 1981–1995. Leukemia. 2000;14:2295–2306. doi: 10.1038/sj.leu.2401937. [DOI] [PubMed] [Google Scholar]
- 84.Schrappe M, Reiter A, Zimmermann M, Harbott J, Ludwig WD, Henze G, et al. Long-term results of four consecutive trials in childhood ALL performed by the ALL-BFM study group from 1981 to 1995. Berlin-Frankfurt-Munster. Leukemia. 2000;14:2205–2222. doi: 10.1038/sj.leu.2401973. [DOI] [PubMed] [Google Scholar]
- 85.Gustafsson G, Schmiegelow K, Forestier E, Clausen N, Glomstein A, Jonmundsson G, et al. Improving outcome through two decades in childhood ALL in the Nordic countries: the impact of high-dose methotrexate in the reduction of CNS irradiation. Nordic Society of Pediatric Haematology and Oncology (NOPHO) Leukemia. 2000;14:2267–2275. doi: 10.1038/sj.leu.2401961. [DOI] [PubMed] [Google Scholar]
- 86.Eden OB, Harrison G, Richards S, Lilleyman JS, Bailey CC, Chessells JM, et al. Long-term follow-up of the United Kingdom Medical Research Council protocols for childhood acute lymphoblastic leukaemia, 1980–1997. Medical Research Council Childhood Leukaemia Working Party. Leukemia. 2000;14:2307–2320. doi: 10.1038/sj.leu.2401962. [DOI] [PubMed] [Google Scholar]
- 87.Kamps WA, Veerman AJ, van Wering ER, van Weerden JF, Slater R, van der Does-van den Berg Long-term follow-up of Dutch Childhood Leukemia Study Group (DCLSG) protocols for children with acute lymphoblastic leukemia, 1984–1991. Leukemia. 2000;14:2240–2246. doi: 10.1038/sj.leu.2401964. [DOI] [PubMed] [Google Scholar]
- 88.Conter V, Arico M, Valsecchi MG, Basso G, Biondi A, Madon E, et al. Long-term results of the Italian Association of Pediatric Hematology and Oncology (AIEOP) acute lymphoblastic leukemia studies, 1982–1995. Leukemia. 2000;14:2196–2204. doi: 10.1038/sj.leu.2401963. [DOI] [PubMed] [Google Scholar]
- 89.Maloney KW, Shuster JJ, Murphy S, Pullen J, Camitta BA. Long-term results of treatment studies for childhood acute lymphoblastic leukemia: Pediatric Oncology Group studies from 1986–1994. Leukemia. 2000;14:2276–2285. doi: 10.1038/sj.leu.2401965. [DOI] [PubMed] [Google Scholar]
- 90.National Cancer Institute. National Cancer Institute SEER*Stat software; Surveillance Research Program. 2006 ( www.seer.cancer.gov/seerstat) version 6.3.6. Surveillance Epidemiology, and End Results (SEER) Program ( www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 17 Regs Limited-Use, Nov 2006 Sub (1973–2004 varying) - Linked To County Attributes - Total U.S., 1969–2004 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2007, based on the November 2006 submission.
- 91.Zuna J, Cave H, Eckert C, Szczepanski T, Meyer C, Mejstrikova E, et al. Childhood secondary ALL after ALL treatment. Leukemia. 2007;21:1431–1435. doi: 10.1038/sj.leu.2404718. [DOI] [PubMed] [Google Scholar]
- 92.Geetha N, SreedeviAmma N, Kusumakumary P, Lali VS, Nair MK. Acute lymphoblastic leukemia occurring as a second malignancy: report of a case and review of literature. Pediatr.Hematol.Oncol. 1999;16:267–270. doi: 10.1080/088800199277344. [DOI] [PubMed] [Google Scholar]
- 93.Hunger SP, Sklar J, Link MP. Acute lymphoblastic leukemia occurring as a second malignant neoplasm in childhood: report of three cases and review of the literature. J.Clin.Oncol. 1992;10:156–163. doi: 10.1200/JCO.1992.10.1.156. [DOI] [PubMed] [Google Scholar]
- 94.Szezepanski T, van der Velden VHJ, Van Vlierberghe PV, Gruhn B, Panzer-Grumayer R, Spinelli M, Cave H, zur Stadt U, Campana D, Schrauder A, van Wering E, Meijerink JPP, van Dongen JJM. Late Relapses of Childhood T-ALL are Frequently Second T-ALL. Blood. 2007;110(11):430a. [Google Scholar]
- 95.Pui CH, Relling MV. Topoisomerase II inhibitor-related acute myeloid leukaemia. Br.J.Haematol. 2000;109:13–23. doi: 10.1046/j.1365-2141.2000.01843.x. [DOI] [PubMed] [Google Scholar]
- 96.Travis LB. The epidemiology of second primary cancers. Cancer Epidemiol.Biomarkers Prev. 2006;15:2020–2026. doi: 10.1158/1055-9965.EPI-06-0414. [DOI] [PubMed] [Google Scholar]
- 97.Bogni A, Cheng C, Liu W, Yang W, Pfeffer J, Mukatira S, et al. Genome-wide approach to identify risk factors for therapy-related myeloid leukemia. Leukemia. 2006;20:239–246. doi: 10.1038/sj.leu.2404059. [DOI] [PubMed] [Google Scholar]
- 98.Felix CA. Secondary leukemias induced by topoisomerase-targeted drugs. Biochim.Biophys.Acta. 1998;1400:233–255. doi: 10.1016/s0167-4781(98)00139-0. [DOI] [PubMed] [Google Scholar]
- 99.Cronin K, Mariotto A, Scoppa S, Green D, Clegg L. Statistical Research and Applications Branch, NCI; Differences between Brenner et al. and NCI methods for calculating period survival. 2008 Technical Report #2003-02-A. http://srab.cancer.gov/reports.