Abstract
Cosmeceuticals are used for nourishing as well as improving the appearance of the skin, and are also documented as effective agents for treating various dermatologic conditions. Cosmeceutical preparations from herbal origin are most popular among consumers, as these agents are mostly non-toxic and possess strong antioxidant activity. Since oxidative stress is one of the major mechanisms for skin aging and dermatological conditions, the phytochemicals, such as silibinin, with proven antioxidant activity, could be useful for treating many dermatologic conditions as well as skin aging. Silibinin is a flavonolignan compound from milk thistle plant which possesses strong antioxidant activity and also modulates many molecular changes, caused by xenobiotics and ultraviolet radiation, to protect the skin. In this review we have provided an account of evidence generated from the laboratory studies to support the scientific rationale for the effective use of silibinin in cosmeceutical preparations.
Cosmeceuticals of herbal origin are becoming more popular than conventional cosmetics. It is now widely understood that what we eat directly influences our metabolism and health. Since skin is the most important protective barrier in our body and directly exposed to the harsh environment, most of the deleterious effects of the environmental toxicants are visibly manifested on the skin, including that of aging. Many anti-aging agents in cosmeceuticals are consumed orally; however, these are also known to work topically for improving the skin health. Due to the increased popularity of cosmeceuticals, these agents are being specifically manufactured and marketed in different categories such as body care, face care, skin care, hair care and growth and sun protection cosmeceuticals. These preparations usually contain ingredients that can prevent or reverse cutaneous aging, which is mainly caused by highly reactive oxygen molecules damaging the skin structure (1,2). In this regard, botanical agents are getting wide-spread attention due to the fact that many antioxidant phytochemicals are evolved as a protective mechanism in plants to counter the oxidative stress (1–3). There is an upsurge in discovering novel and effective botanical antioxidants that can quench reactive oxygen species including hydroxyl radicals, superoxide anions and fatty peroxy radicals, in order to protect the skin from oxidative damages.
There are many phytochemicals which have been commercialized for cosmeceutical preparations. Most of these agents belong to the categories of flavonoids, polyphenols, carotinoids and coumarins. Some commonly used botanicals in cosmeceuticals are kinetin, curcumin, teas, soy, pomegranate, date, grape seed, pycnogenol, horse chestnut, German chamomile, comfrey, allantoin, and aloe (3–5). There are many other pure phytochemicals which are found in different cosmeceutical preparations such as quercetin, hesperidin, diosmin, mangiferin, lutein, lycopene, rosmarinic acid, ellagic acid and chlorogenic acid (3,4). Despite a wide spread use of botanicals in cosmeceuticals, there are very few such cosmeceuticals which are supported by scientific evidence for their efficacy. A recent report indicates that published clinical trials have been done with only soy, green and black tea, pomegranate and date for the treatment of extrinsic aging (5). Due to the paucity of preclinical and clinical studies with phytochemicals present in cosmeceuticals for their efficacy as well as safety, there is an urgent need for scientific validation of such preparations. Though cosmeceuticals can be used as adjunct therapy to dermatological conditions, there is a great deal of confusion among dermatologists, as well as the consumers, about their use, as very little is known about the constituents (phytochemicals) in cosmeceuticals. Accordingly, greater emphasis should be given to the pharmaceutical research for evaluating the existing cosmeceutical components as well as for the development of safer and more effective phytochemicals for this purpose in both academic as well as industrial research laboratories. Here we have provided a brief description of a non-toxic phytochemical, silibinin, which has been studied extensively and found to protect the skin from the harmful effects of a number of toxicants including those by ultraviolet radiation.
Silibinin
Silibinin is a flavonolignan compound (Fig. 1A) which is abundantly found in the extract of milk thistle plant (Silybum marianum) (Fig. 1B) and also in artichoke (Cynara scolymus) (Fig. 1C); both belong to the family compositae. The ancient use of milk thistle is known in traditional European medicine. In the seventeenth century a flavonolignan complex silymarin, which contains silibinin, was isolated from milk thistle which has been clinically used to treat various liver ailments for over three decades (6). Recent studies suggest that silibinin, also known as silybin or silibin in literature, has many isomers, namely, silybin A, silybin B, isosilibinin A and isosilibinin B (7). Silibinin is a strong antioxidant and prevents oxidants-induced lipid peroxidation by scavenging free radicals (8). It has been shown to protect from liver injury in human and different animal models caused by various toxic compounds such as carbon tetrachloride, thioacetamide, ethanol, benzopyrene, alpha-amanitin and baterial endotoxins (9–11). Silibinin constitutes approximately 32% (w/w) of the popular dietary supplement milk thistle extract, which is sold as standardized preparation of about 80% (w/w) silymarin.
Figure 1.
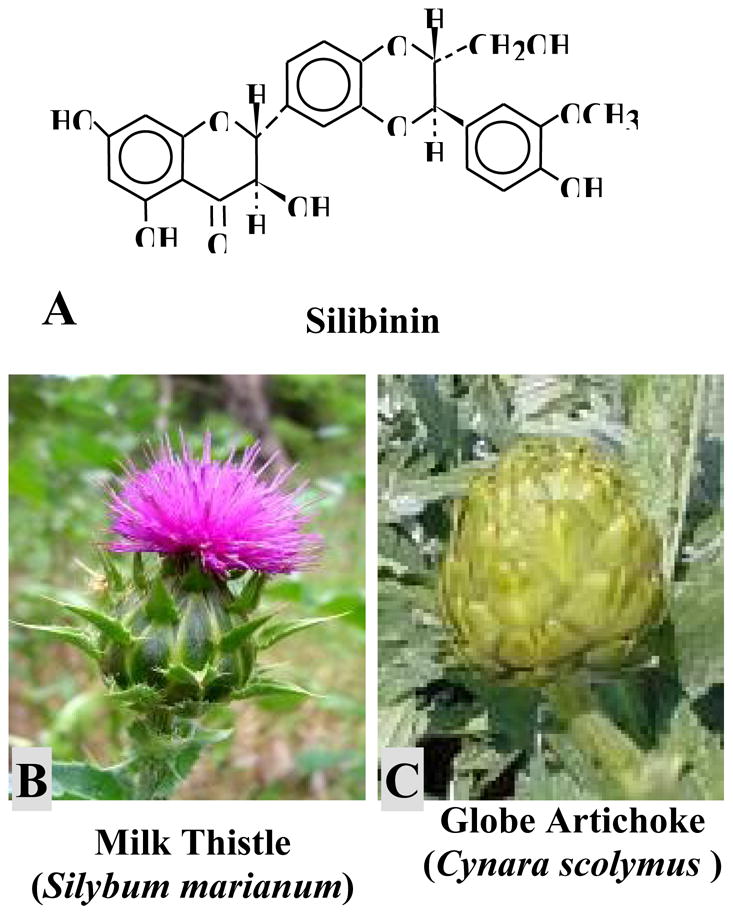
Chemical structure of (A) silibinin, a naturally occurring flavonolignan which is abundantly present in (B) milk thistle (Silybum marianum) and (C) artichoke (Cynara scolymus).
Recent studies suggest that silibinin possesses strong cancer chemopreventive properties in various animal models of carcinogenesis, including skin cancer (12). It has been shown to have strong efficacy against prostate and lung cancers in vivo (13, 14), though it is not clearly known how antioxidant activity of silibinin contributes to its anticancer efficacy in these two in vivo models. However, experimental findings in mouse skin carcinogenesis models clearly suggest that antioxidant activity of silibinin plays an important role in protecting against chemically or radiation-induced skin injuries as well as carcinogenesis (15). The protective effect of silibinin against ultraviolet B-induced skin injuries in the mouse model is clinically relevant as sun exposure, particularly ultraviolet radiation, is one of the major etiologic factors in skin aging and cancer (16). It is anticipated that skin protecting effects of silibinin could be utilized for humans through its cosmeceutical preparations.
Potential Mechanisms of Cosmeceutical Benefits of Silibinin
In the beginning it was observed that silibinin, or its parent flavonolignan mixture silymarin, possesses strong antioxidant activity and protects from liver injuries (8–11). Later it was identified that these agents can also modulate immune system to protect from the deleterious effects of various xenotoxic chemicals (17). Furthermore, recent studies suggest that silibinin can interfere with many signaling pathways which are aberrantly altered by toxic compounds as well as ultraviolet radiation (12, 15). We have provided an account of such studies where silibinin is shown to adopt different mechanisms to neutralize the toxic effects of different chemicals and ultraviolet-B radiation in skin.
Protective effects of silibinin in skin from exposure to chemical oxidants. Some environmental toxins, including those which are skin tumor promoters, are generally non-mutagenic and their exposure to skin can lead to epigenetic alterations that are manifested in to various dermatological conditions (18, 19). Such exposure to chronic extent may facilitate the process of skin carcinogenesis, provided that the skin epidermis has initiated cells which are characterized by the genetic alteration in tumor suppressor or proto-oncogenes (19). Specifically, it facilitates the clonal expansion of initiated cells leading to the formation of benign tumors or papillomas which later become malignant (19). These toxicants use oxidative stress as one of their mechanisms to cause skin injury as well as to facilitate skin tumor promotion. Our studies suggest that silymarin can strongly inhibit tumor promotion caused by TPA (12-O-tetradecanoyl-13 phorbol acetate), benzoyl peroxide and mezerein in mouse skin carcinogenesis models (20, 21). Benzoyl peroxide primarily generates free radicals for tumor promotion. Ocadaic acid, a non-phorbol ester, is also a potent skin damaging agent and tumor promoter, and the topical application of silymarin/silibinin on mouse skin is found to strongly prevent its tumor promoting effect (22).
Many environmental toxins, such as TPA, are known to cause skin edema and epidermal hyperplasia involving oxidative stress. In this regard, silymarin almost completely blocks TPA-caused skin edema and induction of epidermal hyperplasia in mice (20). Silymarin also decreases TPA-induced epidermal cell proliferation in mouse skin (20). The lipid peroxidation in biological membranes is generally used to evaluate oxidant/antioxidant activity of environmental toxins (23). Silymarin is observed to strongly inhibit TPA-caused lipid peroxidation in mouse skin epidermis (20) which supports its strong in vivo antioxidant activity. Often, superoxide anions are formed by transfer of a single electron to oxygen during oxidative stress; however, these are scavenged by superoxide dismutase (SOD)-catalase/glutathione peroxidase (GPx) system (24). Silymarin is found to inhibit chemically-induced depletion of SOD, catalase and GPx enzyme activities in skin epidermis (25). These findings suggest that silymarin could be useful in preventing the oxidative stress and associated pathophysiological conditions caused by environmental toxicants in skin.
Anti-inflammatory mechanisms of silibinin in skin against toxic chemicals. Chemical oxidants induce neutrophil infiltration which is accompanied by an increase in myeloperoxidase activity in mouse skin, and both these parameters indicate intensity of skin inflammation (26). Usually, skin inflammation is mediated by increased metabolism of arachidonic acid which is induced by lipoxygenase and cyclooxygenase accompanied by the increased production of prostaglandins (27). In this regard, silymarin is found to inhibit oxidant-induced myeloperoxidase, lipoxygenase and cyclooxygenase (COX) activities in mouse skin (25). It is the inducible COX-2 which critically mediates inflammation and also contributes to the promotion stage of skin carcinogenesis in response to tumor promoters (28). Silymarin selectively inhibits chemically-induced COX-2 expression as well as activity without any effect on constitutive COX-1 expression in mouse epidermis. Many cytokines, such as tumor necrosis factor alpha (TNFa) and interleukin-1 alpha (IL-1a), are involved in skin inflammation as well as skin tumor promotion (22,25). We have observed that silymarin causes almost complete inhibition of chemically (TPA or ocadaic acid)-induced mRNA expression of TNFa and IL-1a in mouse skin (reviewed in ref. 12). Together, the findings that silymarin down-regulates chemically-induced lipoxygenase, cyclooxygenase, TNFa and IL-1a in mouse skin provides a scientific rationale for the strong anti-inflammatory and anti-tumor promoting activities of silymarin/silibinin.
Silibinin protects from photocarcinogenesis. Ultraviolet (UV) radiation is regarded as a major cause of sunburn, skin aging and skin tumorigenesis, which is generally referred to as photocarcinogenesis. The most frequently used and reliable preclinical animal model to study the effect of ultraviolet radiation is the SKH-1 hairless mouse strain (15,29). UVB exposure is known to cause skin inflammation, edema and epidermal hyperplasia in this mouse strain, whereas chronic UVB exposure leads to the formation of benign papillomas and subsequently squamous cell carcinomas (SCC) similar to the non-melanoma skin cancer development in humans (15,29). In this regard, topical or dietary administration of silymarin or silibinin is found to strongly inhibit UVB-induced tumor initiation, promotion and complete carcinogenesis in SKH-1 hairless mouse skin (30,31). The anti-skin tumorigenic effect of the dietary administration of these botanicals suggests their action at molecular level rather than a sunscreen effect. Silibinin was found to inhibit tumor volume by up to 97% in a 25-week photocarcinogenesis study (31). Chronic administration of dietary or topical silibinin is well tolerated and did not show any adverse health effects in mice (31).
Silibinin protects from sunburn. Excess exposure to UVB can cause severe sunburn which is mediated via the induction of apoptosis leading to cell death (32,33). Even acute exposure of UVB can induce apoptosis in epidermal cells; this is regarded as a protective mechanism where cells escape from the mutagenic effect of UVB (32,33). Otherwise, cells harboring UVB-caused gene mutation may act as initiated cells for skin cancer growth and development. Our studies show the opposing effects of silibinin on apoptosis induction in UVB-exposed skin versus UVB-induced skin tumors in mice (34,35). In case of skin exposure of UVB, silibinin is found to decrease the apoptotic events whereas it enhances apoptotic cell death in skin tumors (34, 35). Topical and dietary administration of silibinin has been found to strongly protect from the sunburn and apoptotic cell formation in mouse skin caused by the acute exposure of UVB (31,35). Importantly, silibinin enhances apoptosis in mouse skin chronically exposed to UVB for 25 weeks (31). At molecular level, silibinin increased p53 activation, and decreased Akt activation and survivin expression during apoptosis induction, indicating multi molecular targets for its actions (31,34). In cell culture study, silibinin increases UVB-induced p53 protein by serine 15 phosphorylation and stabilization in oxidant/tumor promoter-sensitive epidermal JB6 cells, which was associated with apoptotic cell death (36). However, in another study and at lower dose of UVB, silibinin was found to protect epidermal HaCaT cells from apoptosis (37). These findings suggest that silibinin probably senses the extent of cell damage caused by UVB, and if the damage is moderate it protects from cell death whereas it promotes cell deletion when the damage is severe.
Silibinin protects from UVB-caused epidermal hyperplasia. UVB-initiated cells may acquire increased cell proliferation activity to form actinic keratoses in human or benign papillomas in mouse skin (38). Our studies show that silibinin strongly inhibits acute and chronic UVB-induced cell proliferation in mouse skin epidermis (31, 34). It also inhibits papilloma formation in chronic UVB exposure protocol (31). Mitogen-activated protein kinases (MAPKs) are known to be activated by a wide variety of extracellular stimuli, including UVB, and controlling cellular events such as proliferation (39). Topical or dietary silibinin is found to inhibit acute UVB-induced activation of MAPKs, including ERK1/2, JNK1/2 and p38 activation and was associated with decreased epidermal cell proliferation in SKH-1 hairless mouse skin (40). Similar MAPK inhibitory effects of silibinin have been observed in UVB and mitogenic ligand-activated mouse epidermal JB6 cells (41). These results suggest that inhibiting cell proliferation could be one of the mechanisms by which silibinin inhibits UVB-induced epidermal hyperplasia.
Silibinin alters cell cycle regulation in favor of maintaining cellular genetic integrity in response to UVB. Environmental genotoxins, including UVB radiation, present an ongoing challenge to the genetic integrity of the cell in which cell cycle regulation plays an important role in countering the challenge (42). Usually, an extended G1 phase with enhanced level of p53 and cyclin-dependent kinase inhibitor Cip1/p21 is a characteristic feature of UVB-induced DNA-damaged cells, which permits sufficient time for the cell to repair the damaged DNA (42–44). Therefore, agents causing G1 arrest after UVB exposure are likely to facilitate DNA repair. Our study shows that silibinin up-regulates acute UVB-induced levels of both p53 and Cip1/p21 protein in SKH-1 hairless mouse skin epidermis, and that could mediate its photoprotective effects (34). Further, silibinin is found to inhibit UVB-induced expression of G1 phase cyclins and cyclin-dependent kinases in mouse skin suggesting its role in delaying the G1 phase progression (31).
Silibinin protects from UVB-caused DNA damage in skin epidermal cells. Photocarcinogenesis is initiated with DNA damage where up to 90% of carcinogenic dose of sunlight is resulted from UVB radiation (45). UVB directly interacts with DNA forming a variety of photoproducts, including thymine dimers, 6-4 photoproducts, DNA strand breaks etc (45,46). The formation of thymine dimers is common and frequently occurs in the tumor suppressor p53 gene in non-melanoma skin cancer, actinic keratoses and sun/UV-damaged skin (38,45). Our studies show that silibinin administration, topical pre- or post-UVB exposure or in diet, can decrease thymine dimmer formation by up to 85% in UVB-exposed SKH-1 hairless mouse skin epidermis (34). These observations suggest that inhibition of UVB-induced DNA damage by botanical agents could reduce UVB-caused DNA damage as well as the incidence of skin cancer.
DNA repair is a dynamic mechanism and primary biological response to DNA damage in living cells (47). If DNA repair system is inefficient, the UVB exposure will increase the rate of mutation and subsequently the risk of skin cancer, as observed in the hereditary form of human disease called xeroderma pigmentosum (48). In an animal study, silibinin decreased UVB-caused thymine dimers in skin epidermis assessed one hour after UVB irradiation (34). In our ongoing studies, silibinin showed an enhanced rate of repair of thymine dimers after UVB exposure in mouse skin. These observations suggest a potential role of silibinin in repair of UVB-induced epidermal DNA damage which might be unique to this phytochemical in providing a rare cosmeceutical benefit as compared to other botanical cosmeceuticals.
Cosmeceutical Preparations with Silibinin
Silymarin/silibinin is found in a number of high-end moisturizers to prevent cutaneous oxidative damage and photoaging. For examples, RosaCure+ is sold as an anti-redness cream with silymarin to reduce the appearance of facial redness as in rosacea-prone skin, sooth reactive skin and even out skin tone. SkinCeuticals Antioxidant Lip Repair contains milk thistle extract as one of its ingredients which claims a powerful treatment in preventing premature signs of aging while restoring moisture and smoothing the surface of the lips. There are very few cosmeceuticals which contain silibinin as an ingredient. However, based on our extensive studies in mouse skin model with silymarin/silibinin showing strong protective effects from environmental toxicants as well as UVB radiation with scientific rationale, it could be suggested that silibinin has immense potential to offer cosmeceutical benefits.
Conclusions
There are many cosmeceuticals containing synthetic chemicals or botanical agents or a mixture. In most cases, there is either little or no information about the scientific rationale for their efficacy, mechanisms, side effects and other complications. Recently, cosmeceutical companies have started investing a great deal of money in discovering potential and safe agents for skin care. In this regard, many phytochemicals have antioxidant property and are relatively safer as compared to synthetic chemicals. Investigators continue to search for phytochemicals with greater efficacy in the ability to protect against various deleterious agents to skin, such as radiation. The scientific data available for silibinin’s skin protective efficacy against a range of toxic chemicals and ultraviolet radiation with no side effects suggest that silibinin could be an ideal compound for cosmeceutical preparation. This is also further supported by the fact that our experimental findings of skin-related studies with silibinin in the last ten years have generated a momentum of increased use of silibinin in different cosmeceutical preparations. Based on preclinical observations, clinical studies with silibinin in various dermatological conditions, including that of skin aging and cancer, are warranted to assess its efficacy and adverse effects, if any. The positive outcomes of such studies may be useful for the cosmeceutical as well as clinical applications of silibinin in humans against different dermatological conditions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pinnell SR. Cutaneous photodamage, oxidative stress, and topical antioxidant protection. J Am Acad Dermatol. 2003;48:1–19. doi: 10.1067/mjd.2003.16. [DOI] [PubMed] [Google Scholar]
- 2.Gilchrest BA. A review of skin ageing and its medical therapy. Br J Dermatol. 1996;135:867–75. doi: 10.1046/j.1365-2133.1996.d01-1088.x. [DOI] [PubMed] [Google Scholar]
- 3.Choi CM, Berson DS. Cosmeceuticals. Semin Cutan Med Surg. 2006;25:163–8. doi: 10.1016/j.sder.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Pieroni A, Quave CL, Villanelli ML, et al. Ethnopharmacognostic survey on the natural ingredients used in folk cosmetics, cosmeceuticals and remedies for healing skin diseases in the inland Marches, Central-Eastern Italy. J Ethnopharmacol. 2004;91:331–44. doi: 10.1016/j.jep.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Thornfeldt C. Cosmeceuticals containing herbs: fact, fiction, and future. Dermatol Surg. 2005;31:873–80. doi: 10.1111/j.1524-4725.2005.31734. [DOI] [PubMed] [Google Scholar]
- 6.Wellington K, Jarwis B. Silymarin: a review of its clinical properties in the management of hepatic disorders. BioDrugs. 2001;15:465–89. doi: 10.2165/00063030-200115070-00005. [DOI] [PubMed] [Google Scholar]
- 7.Davis-Searles PR, Nakanishi Y, Kim NC, et al. Milk thistle and prostate cancer: differential effects of pure flavonolignans from Silybum marianum on antiproliferative end points in human prostate carcinoma cells. Cancer Res. 2005;65:4448–57. doi: 10.1158/0008-5472.CAN-04-4662. [DOI] [PubMed] [Google Scholar]
- 8.Carini R, Comoglio A, Albano E, et al. Lipid peroxidation and irreversible damage in the rat hepatocyte model: Protection by the silybin-phospholipid complex IdB 1016. Biochem Pharm. 1992;43:2111–15. doi: 10.1016/0006-2952(92)90168-i. [DOI] [PubMed] [Google Scholar]
- 9.Vogel G, Trost W, Braatz R. Studies on the pharmacodynamics, including site and mode of action, of silymarin: The antihepatotoxic principle from Silybum mar. (L.) Gaertn. Arzneimittelforsch. 1975;25:82–9. [PubMed] [Google Scholar]
- 10.Trinchet JC, Coste T, Levy VG, et al. Treatment of alcoholic hepatitis with silymarin. A double-blind comparative study in 116 patients. Gastroenterol Clin Biol. 1989;13:120–4. [PubMed] [Google Scholar]
- 11.Pares A, Planas R, Torres M, et al. Effects of silymarin in alcoholic patients with cirrhosis of the liver: results of a controlled, double-blind, randomized and multicenter trial. J Hepatol. 1998;28:615–21. doi: 10.1016/s0168-8278(98)80285-7. [DOI] [PubMed] [Google Scholar]
- 12.Singh RP, Agarwal R. Flavonoid antioxidant silymarin and skin cancer. Antioxid Redox Signal. 2002;4:655–63. doi: 10.1089/15230860260220166. [DOI] [PubMed] [Google Scholar]
- 13.Singh RP, Dhanalakshmi S, Tyagi AK, et al. Dietary feeding of silibinin inhibits advance human prostate carcinoma growth in athymic nude mice and increases plasma insulin-like growth factor-binding protein-3 levels. Cancer Res. 2002;62:3063–9. [PubMed] [Google Scholar]
- 14.Singh RP, Deep G, Chittezhath M, et al. Effect of silibinin on the growth and progression of primary lung tumors in mice. J Natl Cancer Inst. 2006;98:846–55. doi: 10.1093/jnci/djj231. [DOI] [PubMed] [Google Scholar]
- 15.Singh RP, Agarwal R. Mechanisms and preclinical efficacy of silibinin in preventing skin cancer. Eur J Cancer. 2005;41:1969–79. doi: 10.1016/j.ejca.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 16.Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol. 2004;195:298–308. doi: 10.1016/j.taap.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Lang I, Nekam K, Gonzalez-Cabello R, et al. Hepatoprotective and immunological effects of antioxidant drugs. Tokai J Exp Clin Med. 1990;15:123–7. [PubMed] [Google Scholar]
- 18.Bowler RM, Gysens S, Hartney C, et al. Increased medication use in a community environmentally exposed to chemicals. Ind Health. 2002;40:335–44. doi: 10.2486/indhealth.40.335. [DOI] [PubMed] [Google Scholar]
- 19.Singh RP, Agarwal R. SENCAR mouse skin tumorigenesis model. In: Teicher BA, editor. Tumor models in cancer research. Humana Press; Totowa, New Jersey: 2001. pp. 359–80. [Google Scholar]
- 20.Lahiri-Chatterjee M, Katiyar SK, Mohan RR, et al. A flavonoid antioxidant, silymarin, affords exceptionally high protection against tumor promotion in the SENCAR mouse skin tumorigenesis model. Cancer Res. 1999;59:622–32. [PubMed] [Google Scholar]
- 21.Zhao J, Lahiri-Chatterjee M, Sharma Y, et al. Inhibitory effect of a flavonoid antioxidant silymarin on benzoyl peroxide-induced tumor promotion, oxidative stress and inflammatory responses in SENCAR mouse skin. Carcinogenesis. 2000;21:811–6. doi: 10.1093/carcin/21.4.811. [DOI] [PubMed] [Google Scholar]
- 22.Zi X, Mukhtar H, Agarwal R. Novel cancer chemopreventive effects of a flavonoid antioxidant silymarin: inhibition of mRNA expression of an endogenous tumor promoter TNFa. Biochem Biophys Res Commun. 1997;239:334–9. doi: 10.1006/bbrc.1997.7375. [DOI] [PubMed] [Google Scholar]
- 23.Harris RB, Alberts DS. Strategies for skin cancer prevention. Int J Dermatol. 2004;43:243–51. doi: 10.1111/j.1365-4632.2004.01966.x. [DOI] [PubMed] [Google Scholar]
- 24.Witz G. Active oxygen species as factors in multistage carcinogenesis. Proc Exp Biol Med. 1991;198:675–82. doi: 10.3181/00379727-198-43306. [DOI] [PubMed] [Google Scholar]
- 25.Zhao J, Sharma Y, Agarwal R. A Flavonoid antioxidant, silymarin, affords significant inhibition against 12-O-tetradecanoylphorbol 13-acetate-caused modulation of antioxidant and inflammatory enzymes, and cyclooxygenase 2 and interlukin-1a expression in SENCAR mouse epidermis: Implications in the prevention of stage I tumor. Mol Carcinog. 1999;26:321–33. [PubMed] [Google Scholar]
- 26.Bradley PP, Priebat DA, Christensen RD, et al. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–9. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 27.Marks F, Furstenberger G, Muller-Decker K. Arachidonic acid metabolism as a reporter of skin irritancy and target of cancer chemoprevention. Toxicol Lett. 1998;96–97:111–8. doi: 10.1016/s0378-4274(98)00057-5. [DOI] [PubMed] [Google Scholar]
- 28.Brecher AR. The role of cyclooxygenase-2 in the pathogenesis of skin cancer. J Drugs Dermatol. 2002;1:44–7. [PubMed] [Google Scholar]
- 29.Bowden GT. Prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signaling. Nat Rev Cancer. 2004;4:23–35. doi: 10.1038/nrc1253. [DOI] [PubMed] [Google Scholar]
- 30.Katiyar SK, Korman NJ, Mukhtar H, et al. Protective effects of silymarin against photocarcinogenesis in a mouse skin model. J Natl Cancer Inst. 1997;89:56–66. doi: 10.1093/jnci/89.8.556. [DOI] [PubMed] [Google Scholar]
- 31.Mallikarjuna G, Dhanalakshmi S, Singh RP, et al. Silibinin protects against photocarcinogenesis via modulation of cell cycle regulators, mitogen-activated protein kinases, and Akt signaling. Cancer Res. 2004;64:6349–56. doi: 10.1158/0008-5472.CAN-04-1632. [DOI] [PubMed] [Google Scholar]
- 32.Pourzand C, Tyrrell RM. Apoptosis, the role of oxidative stress and the example of solar UV radiation. Photochem Photobiol. 1999;70:380–90. [PubMed] [Google Scholar]
- 33.Ziegler A, Jonason AS, Leffell DJ, et al. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773–6. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- 34.Dhanalakshmi S, Mallikarjuna GU, Singh RP, et al. Silibinin prevents ultraviolet radiation-caused skin damages in SKH-1 hairless mice via a decrease in thymine dimer positive cells and an up-regulation of p53-p21/Cip1 in epidermis. Carcinogenesis. 2004;25:1459–65. doi: 10.1093/carcin/bgh152. [DOI] [PubMed] [Google Scholar]
- 35.Gu M, Dhanalakshmi S, Singh RP, et al. Dietary feeding of silibinin prevents early biomarkers of UVB radiation-induced carcinogenesis in SKH-1 hairless mouse epidermis. Cancer Epidemiol Biomarkers Prev. 2005;14:1344–9. doi: 10.1158/1055-9965.EPI-04-0664. [DOI] [PubMed] [Google Scholar]
- 36.Dhanalakshmi S, Agarwal C, Singh RP, et al. Silibinin up-regulates DNA-protein kinase-dependent p53 activation to enhance UVB-induced apoptosis in mouse epithelial JB6 cells. J Biol Chem. 2005;280:20375–83. doi: 10.1074/jbc.M414640200. [DOI] [PubMed] [Google Scholar]
- 37.Dhanalakshmi S, Mallikarjuna GU, Singh RP, et al. Dual-efficacy of silibinin in protecting or enhancing ultraviolet B radiation-caused apoptosis in human immortalized keratinocyte HaCaT cells. Carcinogenesis. 2004;25:99–106. doi: 10.1093/carcin/bgg188. [DOI] [PubMed] [Google Scholar]
- 38.Ramzi ST, Maruno M, Khaskhely NM, et al. An assessment of the malignant potential of actinic keratoses and Bowen’s disease: p53 and PCNA expression pattern correlate with the number of desmosomes. J Dermatol. 2002;29:562–72. doi: 10.1111/j.1346-8138.2002.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 39.Bode AM, Dong Z. Mitogen-activated protein kinase activation in UV-induced signal transduction. Sci STKE. 2003;2003:1–15. doi: 10.1126/stke.2003.167.re2. [DOI] [PubMed] [Google Scholar]
- 40.Gu M, Dhanalakshmi S, Mohan S, et al. Silibinin inhibits ultraviolet B radiation-induced mitogenic and survival signaling, and associated biological responses in SKH-1 mouse skin. Carcinogenesis. 2005;26:1404–13. doi: 10.1093/carcin/bgi096. [DOI] [PubMed] [Google Scholar]
- 41.Singh RP, Dhanalakshmi S, Mohan S, et al. Silibinin inhibits UVB- and epidermal growth factor-induced mitogenic and cell survival signaling involving activator protein-1 and nuclear factor-kappaB in mouse epidermal JB6 cells. Mol Cancer Ther. 2006;5:1145–53. doi: 10.1158/1535-7163.MCT-05-0478. [DOI] [PubMed] [Google Scholar]
- 42.Huang LC, Clarkin KC, Wahl GM. Sensitivity and selectivity of the DNA damage sensor responsible for activating p53-dependent G1 arrest. Proc Natl Acad Sci USA. 1996;93:4827–32. doi: 10.1073/pnas.93.10.4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ponten F, Berne B, Ren ZP, et al. Ultraviolet light induces expression of p53 and p21 in human skin: effect of sunscreen and constitutive p21 expression in skin appendages. J Invest Dermatol. 1995;105:402–6. doi: 10.1111/1523-1747.ep12321071. [DOI] [PubMed] [Google Scholar]
- 44.Fotedar R, Bendjennat M, Fotedar A. Role of p21WAF1 in the cellular response to UV. Cell Cycle. 2004;3:134–7. [PubMed] [Google Scholar]
- 45.Tornaletti S, Pfeifer GP. UV damage and repair mechanisms in mammalian cells. Bioessays. 1996;18:221–8. doi: 10.1002/bies.950180309. [DOI] [PubMed] [Google Scholar]
- 46.Cruz JR, Leverkus PDM, Dougherty I, et al. Thymidine dinucleotides inhibit contact hypersensitivity and activate the gene for tumor necrosis factor alpha1. J Invest Dermatol. 2000;114:253–8. doi: 10.1046/j.1523-1747.2000.00866.x. [DOI] [PubMed] [Google Scholar]
- 47.Li G, Ho VC. p53-dependent DNA repair and apoptosis respond differently to high- and low-dose ultraviolet radiation. Br J Dermatol. 1998;139:3–10. [PubMed] [Google Scholar]
- 48.de Gruijl FR, van Kranen HJ, Mullenders LH. UV-induced DNA damage, repair, mutations and oncogenic pathways in skin cancer. J Photochem Photobiol B. 2001;63:19–27. doi: 10.1016/s1011-1344(01)00199-3. [DOI] [PubMed] [Google Scholar]


